Layered Double Hydroxides as Rising-Star Adsorbents for Water Purification: A Brief Discussion
Abstract
:1. General Background
- (1)
- Physical adsorption. LDHs can have a high specific surface area, and hence present high adsorption capacities due to the presence and availability of active adsorption sites. Furthermore, the specific surface area of LDHs can be increased through calcinating or modifying/depositing on supports with three-dimensional structures.
- (2)
- Ion exchange. Strongly negative molecules can be easily changed for the original anions in LDHs. In addition, positive ions can also be exchanged in the intermediate layer of LDHs, if pre-interleaved by some chelators.
- (3)
- Interleaving. This starts from a preparation process, such as co-precipitation. Furthermore, the capture of molecules via the intercalation process is faster and more complete than ion exchange.
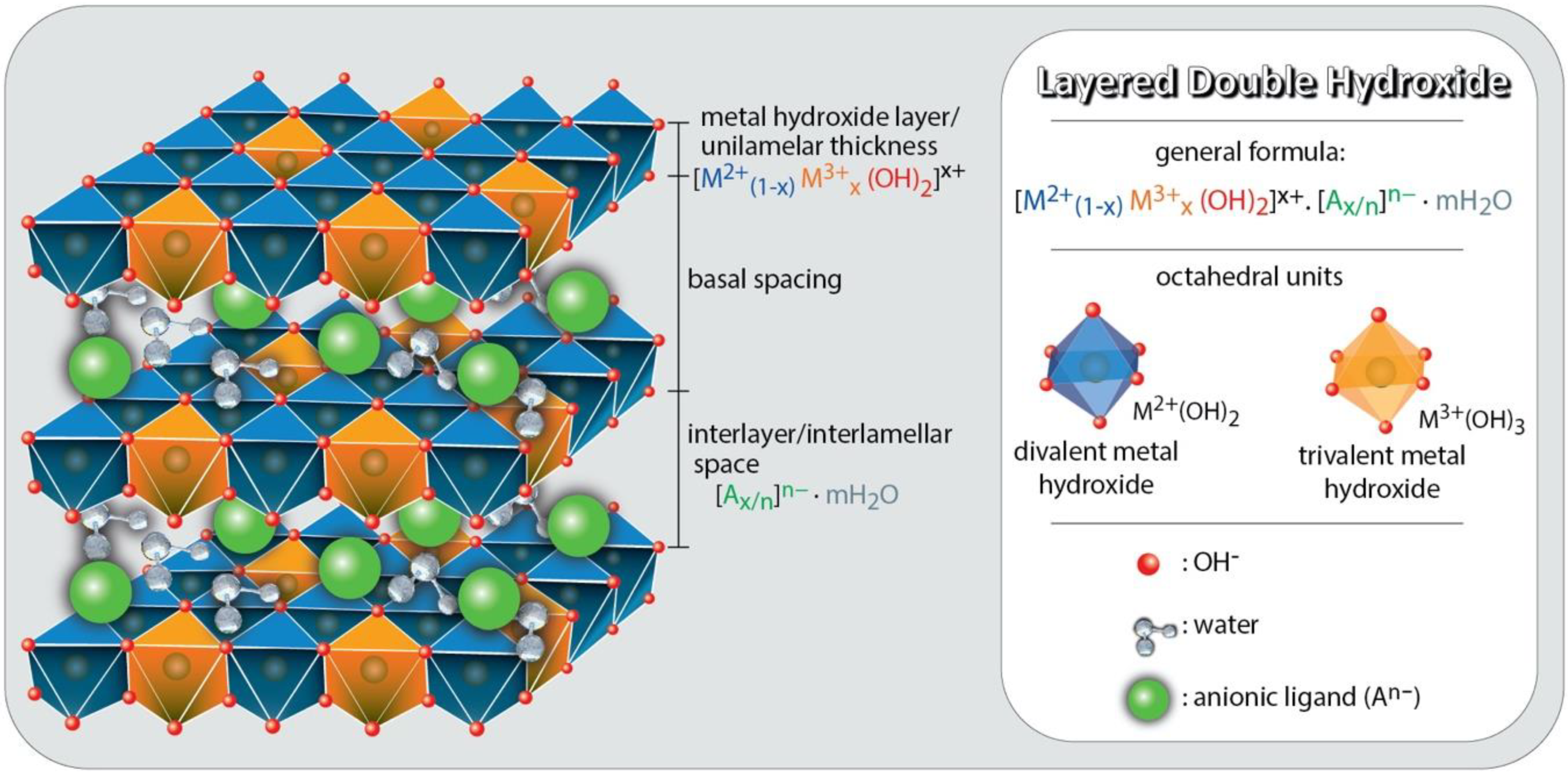
2. LDHs Physicochemical Characteristics
- (i)
- Direct methods: The preparation of LDHs occurs via direct precipitation from the addition of tri- and divalent cations, in a solution in alkaline pH with the main methods of coprecipitation, salt–oxide, sol–gel, induced hydrolysis, and hydrothermal synthesis.
- (ii)
- Indirect methods: involve replacing an interlamellar anion from a previously produced precursor LDH. Examples of this substitution method are ion exchange in solution, ion exchange in acidic medium, double phase replacement, and regeneration through the delaminate precursor [35,36,37]. Therefore, the supramolecular structure, the facile manipulation of adsorption sites at the atomic scale, the versatility of compositions, in addition to the possibility of morphological manipulation create the possibility of tuning the amount and accessibility of the active adsorption sites, and hence the adsorption kinetics, as well as the efficiency for a specifically targeted pollutant [34]. As in any case, LDHs have some specific characteristics that can complicate their use as adsorbents. The low mechanical resistance is a problem for continuous water treatment units and in certain regeneration processes, as LDHs can be exfoliated. Therefore, there is a series of studies in the literature proposing to support LDHs in larger and recalcitrant particles [38,39,40]. In addition, in acidic media, the removal capacity of LDHs is compromised due to low structural stability at low pH [26]. In Table 2, we collected characteristics of methods of synthesis which can be followed for the preparation of pure LDHs, as well as their composites and hybrids [18,25,41,42].
3. LDHs as Adsorbents
4. Discussion
5. Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Nimbalkar, M.N.; Bhat, B.R. Simultaneous adsorption of methylene blue and heavy metals from water using Zr-MOF having free carboxylic group. J. Environ. Chem. Eng. 2021, 9, 106216. [Google Scholar] [CrossRef]
- Yadav, A.; Bagotia, N.; Sharma, A.K.; Kumar, S. Advances in decontamination of wastewater using biomass-basedcomposites: A critical review. Sci. Total Environ. 2021, 784, 147108. [Google Scholar] [CrossRef]
- Yadav, A.; Bagotia, N.; Sharma, A.K.; Kumar, S. Simultaneous adsorptive removal of conventional and emerging contaminants in multi-component systems for wastewater remediation: A critical review. Sci. Total Environ. 2021, 799, 149500. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, D.N.; Naji, L.A.; Faisal, A.; Al-Ansari, N.; Naushad, M. Waste foundry sand/MgFe-layered double hydroxides composite material for efficient removal of Congo red dye from aqueous solution. Sci. Rep. 2020, 10, 2042. [Google Scholar] [CrossRef]
- Solehudin, M.; Sirimahachai, U.; Ali, G.A.; Chong, K.F.; Wongnawa, S. One-pot synthesis of isotype heterojunction g-C3N4-MU photocatalyst for effective tetracycline hydrochloride antibiotic and reactive orange 16 dye removal. Adv. Powder Technol. 2020, 31, 1891–1902. [Google Scholar] [CrossRef]
- Mureseanu, M.; Eliescu, A.; Ignat, E.-C.; Carja, G.; Cioatera, N. Different routes of MgAl–LDH synthesis for tailoring the adsorption of Pb(II) pollutant from water. Comptes Rendus. Chim. 2022, 25, 1–12. [Google Scholar] [CrossRef]
- Gayathri, R.; Gopinath, K.; Kumar, P.S. Adsorptive separation of toxic metals from aquatic environment using agro waste biochar: Application in electroplating industrial wastewater. Chemosphere 2021, 262, 128031. [Google Scholar] [CrossRef] [PubMed]
- Nava-Andrade, K.; Carbajal-Arízaga, G.; Obregón, S.; Rodríguez-González, V. Layered double hydroxides and related hybrid materials for removal of pharmaceutical pollutants from water. J. Environ. Manag. 2021, 288, 112399. [Google Scholar] [CrossRef]
- Manjunath, S.; Baghel, R.S.; Kumar, M. Antagonistic and synergistic analysis of antibiotic adsorption on Prosopis juliflora activated carbon in multicomponent systems. Chem. Eng. J. 2020, 381, 122713. [Google Scholar] [CrossRef]
- Somma, S.; Reverchon, E.; Baldino, L. Water Purification of Classical and Emerging Organic Pollutants: An Extensive Review. ChemEngineering 2021, 5, 47. [Google Scholar] [CrossRef]
- Kumari, P.; Pal, B.; Das, R.K. Superior adsorptive removal of eco-toxic drug diclofenac sodium by Zn–Al LDH·xBi2O3 layer double hydroxide composites. Appl. Clay Sci. 2021, 208, 106119. [Google Scholar] [CrossRef]
- Cheng, N.; Wang, B.; Wu, P.; Lee, X.; Xing, Y.; Chen, M.; Gao, B. Adsorption of emerging contaminants from water and wastewater by modified biochar: A review. Environ. Pollut. 2021, 273, 116448. [Google Scholar] [CrossRef]
- Quintela, D.U.; Henrique, D.C.; dos Santos Lins, P.V.; Ide, A.H.; Erto, A.; da Silva Duarte, J.L.; Meili, L. Waste of Mytella Falcata shells for removal of a triarylmethane biocide from water: Kinetic, equilibrium, regeneration and thermodynamic studies. Colloids Surf. B Biointerfaces 2020, 195, 111230. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Mu, B.; Yang, Y. Feasibility of industrial-scale treatment of dye wastewater via bio-adsorption technology. Bioresour. Technol. 2019, 277, 157–170. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, G.E.D.S.; dos Santos Lins, P.V.; de Magalhães Oliveira, L.M.T.; da Silva, E.O.; Anastopoulos, I.; Erto, A.; Giannakoudakis, D.A.; de Almeida, A.R.F.; da Silva Duarte, J.L.; Meili, L. Layered double hydroxides/biochar composites as adsorbents for water remediation applications: Recent trends and perspectives. J. Clean. Prod. 2021, 284, 124755. [Google Scholar] [CrossRef]
- Zhang, X.; Shan, R.; Li, X.; Yan, L.; Ma, Z.; Jia, R.; Sun, S. Effective removal of Cu(II), Pb(II) and Cd(II) by sodium alginate intercalated MgAl-layered double hydroxide: Adsorption properties and mechanistic studies. Water Sci. Technol. 2021, 83, 975–984. [Google Scholar] [CrossRef]
- Jung, K.-W.; Lee, S.Y.; Choi, J.-W.; Hwang, M.-J.; Shim, W.G. Synthesis of Mg–Al layered double hydroxides-functionalized hydrochar composite via an in situ one-pot hydrothermal method for arsenate and phosphate removal: Structural characterization and adsorption performance. Chem. Eng. J. 2021, 420, 129775. [Google Scholar] [CrossRef]
- Kostić, M.; Najdanović, S.; Velinov, N.; Vučić, M.R.; Petrović, M.; Mitrović, J.; Bojić, A. Ultrasound-assisted synthesis of a new material based on MgCoAl-LDH: Characterization and optimization of sorption for progressive treatment of water. Environ. Technol. Innov. 2022, 26, 102358. [Google Scholar] [CrossRef]
- Zubair, M.; Ihsanullah, I.; Aziz, H.A.; Ahmad, M.A.; Al-Harthi, M.A. Sustainable wastewater treatment by biochar/layered double hydroxide composites: Progress, challenges, and outlook. Bioresour. Technol. 2021, 319, 124128. [Google Scholar] [CrossRef]
- Zubair, M.; Manzar, M.S.; Mu’Azu, N.D.; Anil, I.; Blaisi, N.I.; Al-Harthi, M.A. Functionalized MgAl-layered hydroxide intercalated date-palm biochar for Enhanced Uptake of Cationic dye: Kinetics, isotherm and thermodynamic studies. Appl. Clay Sci. 2020, 190, 105587. [Google Scholar] [CrossRef]
- Mu’Azu, N.D.; Zubair, M.; Jarrah, N.; Alagha, O.; Al-Harthi, M.A.; Essa, M.H. Sewage Sludge ZnCl2-Activated Carbon Intercalated MgFe–LDH Nanocomposites: Insight of the Sorption Mechanism of Improved Removal of Phenol from Water. Int. J. Mol. Sci. 2020, 21, 1563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palapa, N.R.; Taher, T.; Rahayu, B.R.; Mohadi, R.; Rachmat, A.; Lesbani, A. CuAl LDH/Rice Husk Biochar Composite for Enhanced Adsorptive Removal of Cationic Dye from Aqueous Solution. Bull. Chem. React. Eng. Catal. 2020, 15, 525–537. [Google Scholar] [CrossRef]
- Alagha, O.; Manzar, M.S.; Zubair, M.; Anil, I.; Mu’Azu, N.D.; Qureshi, A. Comparative Adsorptive Removal of Phosphate and Nitrate from Wastewater Using Biochar-MgAl LDH Nanocomposites: Coexisting Anions Effect and Mechanistic Studies. Nanomaterials 2020, 10, 336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.; Ehite, E.; Houston, R.; Li, Y.; Pope, C.; Labbé, N.; Abdoulmoumine, N. Synthesis and evaluation of layered double hydroxide based sorbent for hot gas cleanup of hydrogen chloride. Mater. Sci. Energy Technol. 2021, 4, 46–53. [Google Scholar] [CrossRef]
- Daniel, S.; Thomas, S. Layered Double Hydroxides: Fundamentals to Applications; Woodhead Publishing: Sawston, UK, 2020; pp. 1–76. [Google Scholar] [CrossRef]
- Santos, L.C.; da Silva, A.F.; dos Santos Lins, P.V.; da Silva Duarte, J.L.; Ide, A.H.; Meili, L. Mg-Fe layered double hydroxide with chloride intercalated: Synthesis, characterization and application for efficient nitrate removal. Environ. Sci. Pollut. Res. 2020, 27, 5890–5900. [Google Scholar] [CrossRef]
- Tang, S.; Yao, Y.; Chen, T.; Kong, D.; Shen, W.; Lee, H.K. Recent advances in the application of layered double hydroxides in analytical chemistry: A review. Anal. Chim. Acta 2020, 1103, 32–48. [Google Scholar] [CrossRef] [PubMed]
- Dotto, G.L.; McKay, G. Current scenario and challenges in adsorption for water treatment. J. Environ. Chem. Eng. 2020, 8, 103988. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, Y.; Zheng, J.; Shang, L.; Shi, Y.; Wu, Q.; Liu, X.; Wang, Y.; Shi, L.; Shao, Q. Synthesis and characterization of ZnNiCr-layered double hydroxides with high adsorption activities for Cr(VI). Adv. Compos. Hybrid Mater. 2021, 4, 819–829. [Google Scholar] [CrossRef]
- Gabriel, R.; de Carvalho, S.H.; da Silva Duarte, J.L.; Oliveira, L.M.; Giannakoudakis, D.A.; Triantafyllidis, K.S.; Soletti, J.I.; Meili, L. Mixed metal oxides derived from layered double hydroxide as catalysts for biodiesel production. Appl. Catal. A Gen. 2021, 630, 118470. [Google Scholar] [CrossRef]
- Shao, M.; Wei, M.; Evans, D.G.; Duan, X. Layered Double Hydroxide Materials in Photocatalysis. Photofunct. Layer. Mater. 2015, 166, 105–136. [Google Scholar] [CrossRef]
- de Sousa, A.L.M.D.; dos Santos, W.M.; de Souza, M.L.; Silva, L.C.P.B.B.; Yun, A.E.H.K.; Aguilera, C.S.B.; de França Chagas, B.; Rolim, L.A.; da Silva, R.M.F.; Neto, P.J.R. Layered Double Hydroxides as Promising Excipients for Drug Delivery Purposes. Eur. J. Pharm. Sci. 2021, 165, 105922. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; O’Hare, D. Recent Advances in the Synthesis and Application of Layered Double Hydroxide (LDH) Nanosheets. Chem. Rev. 2012, 112, 4124–4155. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhang, F.; Evans, D.G.; Duan, X. Layered double hydroxide films: Synthesis, properties and applications. Chem. Eng. J. 2014, 46, 5197–5210. [Google Scholar] [CrossRef] [PubMed]
- Goh, K.-H.; Lim, T.-T.; Dong, Z. Application of layered double hydroxides for removal of oxyanions: A review. Water Res. 2008, 42, 1343–1368. [Google Scholar] [CrossRef] [PubMed]
- Rives, V.; Del Arco, M.; Martin, C. Intercalation of drugs in layered double hydroxides and their controlled release: A review. Appl. Clay Sci. 2014, 88–89, 239–269. [Google Scholar] [CrossRef]
- Conterosito, E.; Gianotti, V.; Palin, L.; Boccaleri, E.; Viterbo, D.; Milanesio, M. Facile preparation methods of hydrotalcite layered materials and their structural characterization by combined techniques. Inorganica Chim. Acta 2018, 470, 36–50. [Google Scholar] [CrossRef]
- Lins, P.V.S.; Henrique, D.C.; Ide, A.H.; da Silva Duarte, J.L.; Dotto, G.L.; Yazidi, A.; Sellaoui, L.; Erto, A.; e Silva Zanta, C.L.D.P.; Meili, L. Adsorption of a non-steroidal anti-inflammatory drug onto MgAl/LDH-activated carbon composite–Experimental investigation and statistical physics modeling. Colloids Surf. A Physicochem. Eng. Asp. 2020, 586, 124217. [Google Scholar] [CrossRef]
- Wan, S.; Wang, S.; Li, Y.; Gao, B. Functionalizing biochar with Mg–Al and Mg–Fe layered double hydroxides for removal of phosphate from aqueous solutions. J. Ind. Eng. Chem. 2017, 47, 246–253. [Google Scholar] [CrossRef]
- Meili, L.; Lins, P.V.; Zanta, C.L.P.S.; Soletti, J.I.; Ribeiro, L.M.O.; Dornelas, C.B.; Silva, T.L.; Vieira, M.G.A. MgAl-LDH/Biochar composites for methylene blue removal by adsorption. Appl. Clay. Sci. 2019, 168, 11–20. [Google Scholar] [CrossRef]
- Harizi, I. Synthèse et Caractérisation des Matériaux à Base de Zéolithe et D’hydroxydes Doubles Lamellaires: Application à L’élimination des Colorants. Ph.D. Thesis, Université Ferhat Abbas, Sétif, El Bez, 2020. [Google Scholar]
- Jijoe, P.S.; Yashas, S.R.; Shivaraju, H.P. Fundamentals, synthesis, characterization and environmental applications of layered double hydroxides: A review. Environ. Chem. Lett. 2021, 19, 2643–2661. [Google Scholar] [CrossRef]
- Rojas, R. Applications of Layered Double Hydroxides on Environmental Remediation; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2012; pp. 39–71. [Google Scholar]
- Daud, M.; Hai, A.; Banat, F.; Wazir, M.; Habib, M.; Bharath, G.; Al-Harthi, M.A. A review on the recent advances, challenges and future aspect of layered double hydroxides (LDH)—Containing hybrids as promising adsorbents for dyes removal. J. Mol. Liq. 2019, 288, 110989. [Google Scholar] [CrossRef]
- El-Abboubi, M.; Taoufik, N.; Mahjoubi, F.; Oussama, A.; Kzaiber, F.; Barka, N. Sorption of methyl orange dye by dodecyl-sulfate intercalated Mg-Al layered double hydroxides. Mater. Today Proc. 2021, 37, 3894–3897. [Google Scholar] [CrossRef]
- Fujii, S.; Sugie, Y.; Kobune, M.; Touno, A.; Touji, J. Uptakes of Cu2+, Pb2+ and Zn2+ on Synthetic Hydrotalcite in Aqueous Solution. J. Jpn. Chem. Soc. 1992, 1992, 1504–1507. [Google Scholar] [CrossRef]
- Liang, X.; Zang, Y.; Xu, Y.; Tan, X.; Hou, W.; Wang, L.; Sun, Y. Sorption of metal cations on layered double hydroxides. Colloids Surf. A Physicochem. Eng. Asp. 2013, 433, 122–131. [Google Scholar] [CrossRef]
- Dinari, M.; Neamati, S. Surface modified layered double hydroxide/polyaniline nanocomposites: Synthesis, characterization and Pb2+ removal. Colloids Surf. A Physicochem. Eng. Asp. 2020, 589, 124438. [Google Scholar] [CrossRef]
- Li, S.; Ma, X.; Ma, Z.; Dong, X.; Wei, Z.; Liu, X.; Zhu, L. Mg/Al-layered double hydroxide modified biochar for simultaneous removal phosphate and nitrate from aqueous solution. Environ. Technol. Innov. 2021, 23, 101771. [Google Scholar] [CrossRef]
- Zhou, H.; Tan, Y.; Gao, W.; Zhang, Y.; Yang, Y. Selective nitrate removal from aqueous solutions by a hydrotalcite-like absorbent FeMgMn-LDH. Sci. Rep. 2020, 10, 16126. [Google Scholar] [CrossRef]
- Motandi, M.K.; Zhang, Z.; Inkoua, S.; Yan, L. Application of zirconium modified layered double hydroxide and calcination product for adsorptive removal of phosphate from aqueous solution. Environ. Prog. Sustain. Energy 2022, 41, e13744. [Google Scholar] [CrossRef]
- dos Santos, G.E.D.S.; Ide, A.H.; Duarte, J.L.S.; McKay, G.; Silva, A.O.S.; Meili, L. Adsorption of anti-inflammatory drug diclofenac by MgAl/layered double hydroxide supported on Syagrus coronata biochar. Powder Technol. 2020, 364, 229–240. [Google Scholar] [CrossRef]
- Santamaría, L.; López-Aizpún, M.; García-Padial, M.; Vicente, M.; Korili, S.; Gil, A. Zn-Ti-Al layered double hydroxides synthesized from aluminum saline slag wastes as efficient drug adsorbents. Appl. Clay Sci. 2020, 187, 105486. [Google Scholar] [CrossRef]
- Zubair, M.; Daud, M.; McKay, G.; Shehzad, F.; Al-Harthi, M.A. Recent progress in layered double hydroxides (LDH)-containing hybrids as adsorbents for water remediation. Appl. Clay Sci. 2017, 143, 279–292. [Google Scholar] [CrossRef]
- de Sousa, F.W.; Moreira, S.A.; Oliveira, A.G.; Cavalcante, R.M.; Nascimento, R.F.; Rosa, M.F. Uso da casca de coco verde como adsorbente na remoção de metais tóxicos. Química Nova 2007, 30, 1153–1157. [Google Scholar] [CrossRef] [Green Version]
- Aksu, Z.; Gönen, F. Biosorption of phenol by immobilized activated sludge in a continuous packed bed: Prediction of breakthrough curves. Process Biochem. 2004, 39, 599–613. [Google Scholar] [CrossRef]

| Advantages | Disadvantages |
|---|---|
| Low cost | Few studies regarding their toxicity in the environment |
| Sustainable nature | Current methods limit the amount of LDHs produced |
| Can be engineered for specific purposes | Few studies on its application in real wastewater |
| Excellent thermal stability | Functional groups preferences for anionic dyes |
| High removal efficiency | Can be exfoliated during synthesis |
| Extensive specific surface area | Cannot be easily regenerated/reused |
| High number of active sites | |
| Easy to prepare | |
| Memory effect | |
| High anion exchange capacities | |
| Chemical stability |
| Methods of Synthesis | Characteristics |
|---|---|
| Coprecipitation | This method is based on the controlled and slow addition of a base (such as sodium hydroxide and/or bicarbonate, sodium carbonate or ammonium hydroxide) to a solution containing simultaneous divalent and trivalent metal cations. Since more than two cations can precipitate simultaneously, the process must be carried out under supersaturation conditions. It is recommended that the pH of the reaction medium be kept constant in the range of 7–10. Subsequently, the suspension is subjected to hydrothermal treatment to increase the yield or crystallinity. |
| Salt-oxide | This method was developed by Boehm in 1977 to prepare zinc and chromium LDHs, using an aqueous suspension of ZnO to react with excess CrCl3 in an aqueous solution. The salt–oxide method, in short, is a solid–liquid reaction in which the aqueous solution of the excess trivalent ion chloride salt is treated with an aqueous suspension of the divalent metal oxide. |
| Sol-gel | This synthetic protocol is widely used for the preparation of a plethora of metal oxides due to the possible high efficiency and purity of the final material. One important advantage of this method is the variety of compositions obtained through temperature adjustment. This process consists of the constant agitation of the component that transforms sol to gel. This sol–gel transformation occurs during the strong acid hydrolysis of metallic precursors, predominately using a strong acid such chloric acid or nitric acid. After the formation of the gel, the material is filtered and washed with distilled water, and later with ethanol. |
| Hydrothermal | The hydrothermal method is generally used when low-affinity anions need to be intercalated into the intermediate layers. This method uses gibbsite and brucite, double-layered hydroxide–deoxycholate intercalation compounds, which are not feasible to obtain easily via other syntheses. An aqueous suspension consists of two oxides, one trivalent metal ion and the other bivalent, which are placed in a pressurized container and subjected to hydrothermal treatment at high temperature for a few days. During this process, the hydrated amorphous precursor crystallizes in the presence of reactive basic oxide. |
| Ion exchange | This is an indirect method usually applied to pre-synthesized LDHs. This method is used when the anions or the divalent/trivalent metal cations are unstable in the alkaline solution, or when the LDHs have a greater affinity for the guest anions than for the intercalated anions of a pre-synthesized LDH. An aqueous suspension of the LDH precursors/pre-synthesized is mixed with a large excess of the salt of the anion to be intercalated. The reaction is carried out under an inert atmosphere to avoid excess carbonate in the intermediate layers. It is recommended the reaction not occur at pH lower than 4, due to the anion interaction in the LDH layers being weaker and presenting a high temperature in this pH range. |
| Regeneration/“memory effect” | One of the main properties of LDH is its ability to restructure. After being subjected to heat treatment or calcination (400 to 500 °C), the layered structure of LDH changes to mixed metallic oxides (water, anion, and hydroxyl groups are highlighted). When calcined LDH is placed in a solution containing guest anions, they can recover their original layered structure and form a new LDH phase. This procedure of retrieving its original form (rehydration) is called the “memory effect”, and must be carried out in an inert atmosphere, mostly comprised of nitrogen. |
| Pollutant | LDH | Synthesis Method | qmax (mg·g−1) | Reference |
|---|---|---|---|---|
| Dye methyl orange | Mg-Al-Ds | Coprecipitation | 185.06 | [45] |
| Mg-Al-CO3 | 97.50 | |||
| Dye RB19 | MgCoAl-CO3-LDH | Coprecipitation | 367.93 | [18] |
| Dye Congo red | Mg/Fe-LDHs | Precipitation | 9127.08 | [4] |
| Pb2+ | Ca/Fe LDH-Cit(NC10%) | Precipitation | 110.00 | [48] |
| Ca/Fe LDH-Cit(NC5%) | 56.00 | |||
| Cr6+ | ZnNiCr-LDHs | Hydrothermal | 28.20 | [29] |
| Cd2+ | MgAl-LDH (SA-LDH) | Coprecipitation | 60.00 | [16] |
| Pb2+ | 243.66 | |||
| Cu2+ | 95.55 | |||
| Phosphate | Zr-LDH | Coprecipitation | 99.35 | [51] |
| Zr-LDO | 80.33 | |||
| Arsenate (mono) | Mg-Al LDHs-FHC | Hydrothermal | 56.30 | [17] |
| Arsenate (mult) | 16.22 | |||
| Phosphate (mono) | 33.21 | |||
| Phosphate (mult) | 20.26 | |||
| Nitrate | FeMgMn-LDH | Co-precipitation | 10.56 | [50] |
| Diclofenac | ZnTiAl | Co-precipitation | 0.07 | [53] |
| Salicylic acid | 0.01 | |||
| Diclofenac | Zn-Al-LDH.xBi2O3 | Hydrothermal | 574.71 | [11] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gama, B.M.V.d.; Selvasembian, R.; Giannakoudakis, D.A.; Triantafyllidis, K.S.; McKay, G.; Meili, L. Layered Double Hydroxides as Rising-Star Adsorbents for Water Purification: A Brief Discussion. Molecules 2022, 27, 4900. https://doi.org/10.3390/molecules27154900
Gama BMVd, Selvasembian R, Giannakoudakis DA, Triantafyllidis KS, McKay G, Meili L. Layered Double Hydroxides as Rising-Star Adsorbents for Water Purification: A Brief Discussion. Molecules. 2022; 27(15):4900. https://doi.org/10.3390/molecules27154900
Chicago/Turabian StyleGama, Brígida Maria Villar da, Rangabhashiyam Selvasembian, Dimitrios A. Giannakoudakis, Konstantinos S. Triantafyllidis, Gordon McKay, and Lucas Meili. 2022. "Layered Double Hydroxides as Rising-Star Adsorbents for Water Purification: A Brief Discussion" Molecules 27, no. 15: 4900. https://doi.org/10.3390/molecules27154900
APA StyleGama, B. M. V. d., Selvasembian, R., Giannakoudakis, D. A., Triantafyllidis, K. S., McKay, G., & Meili, L. (2022). Layered Double Hydroxides as Rising-Star Adsorbents for Water Purification: A Brief Discussion. Molecules, 27(15), 4900. https://doi.org/10.3390/molecules27154900