In Vitro Evaluation of NLS-DTX Activity in Triple-Negative Breast Cancer
Abstract
:1. Introduction
2. Results and Discussion
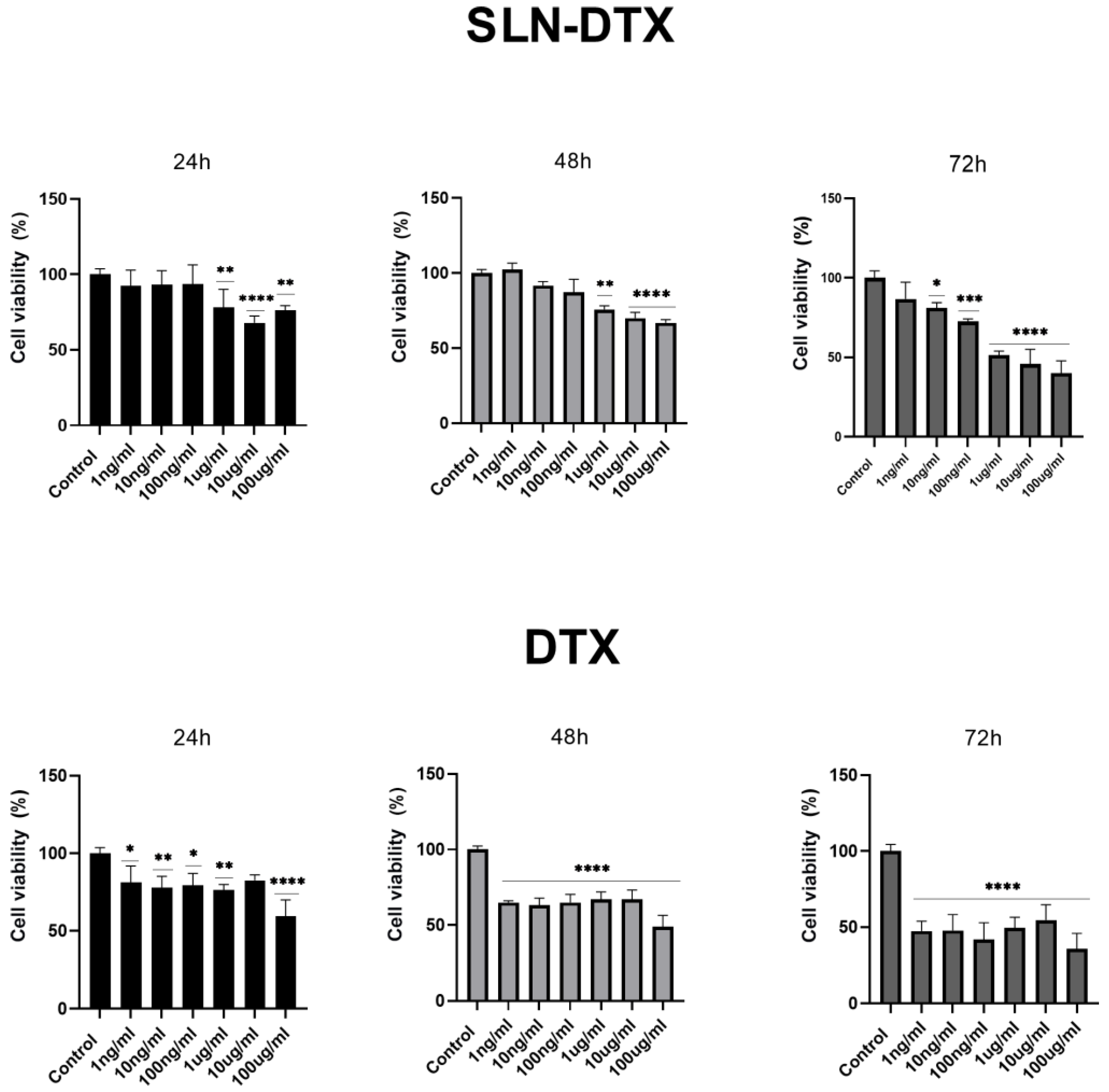
2.1. Cell Viability
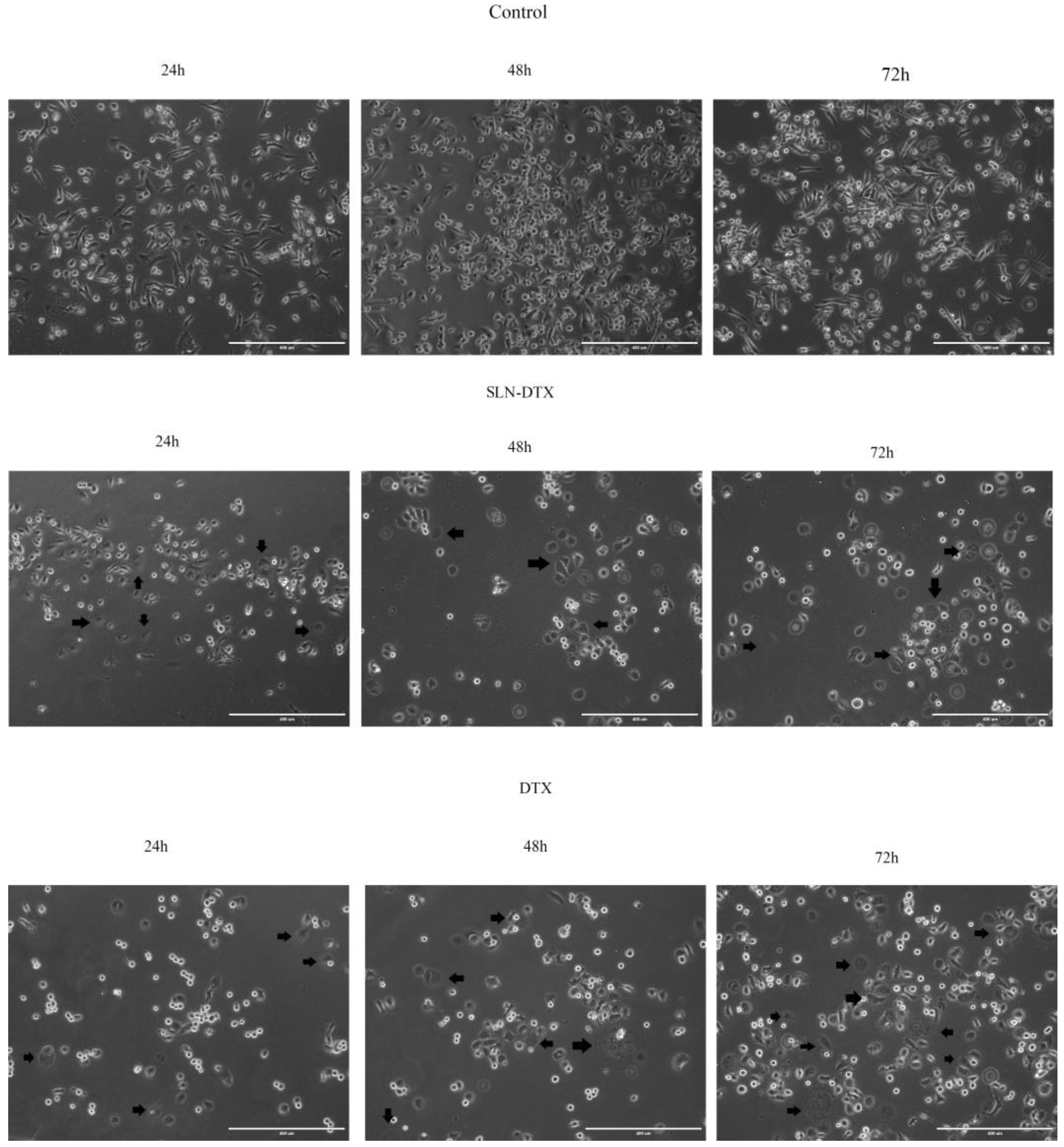
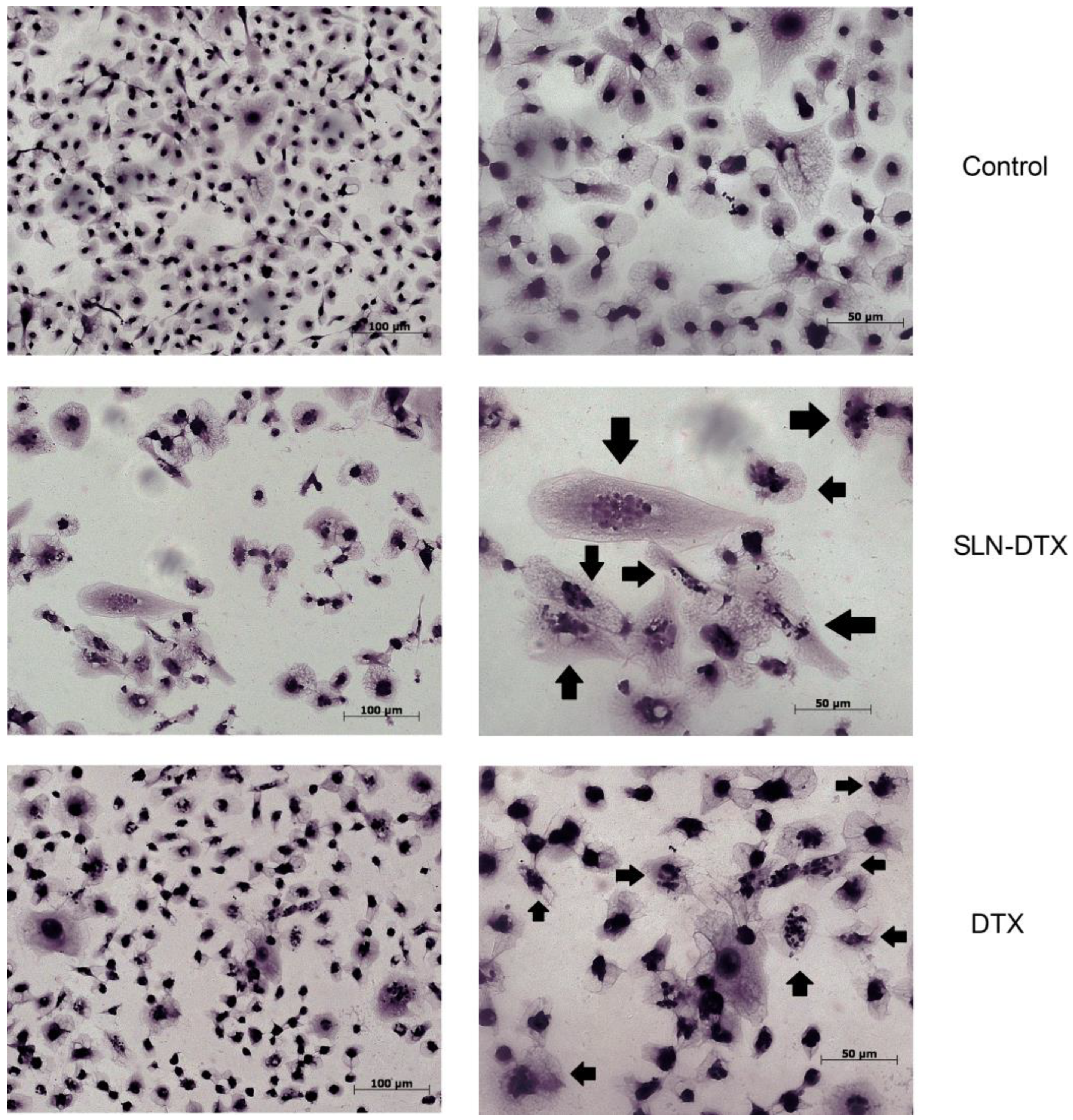
2.2. Cell Morphology
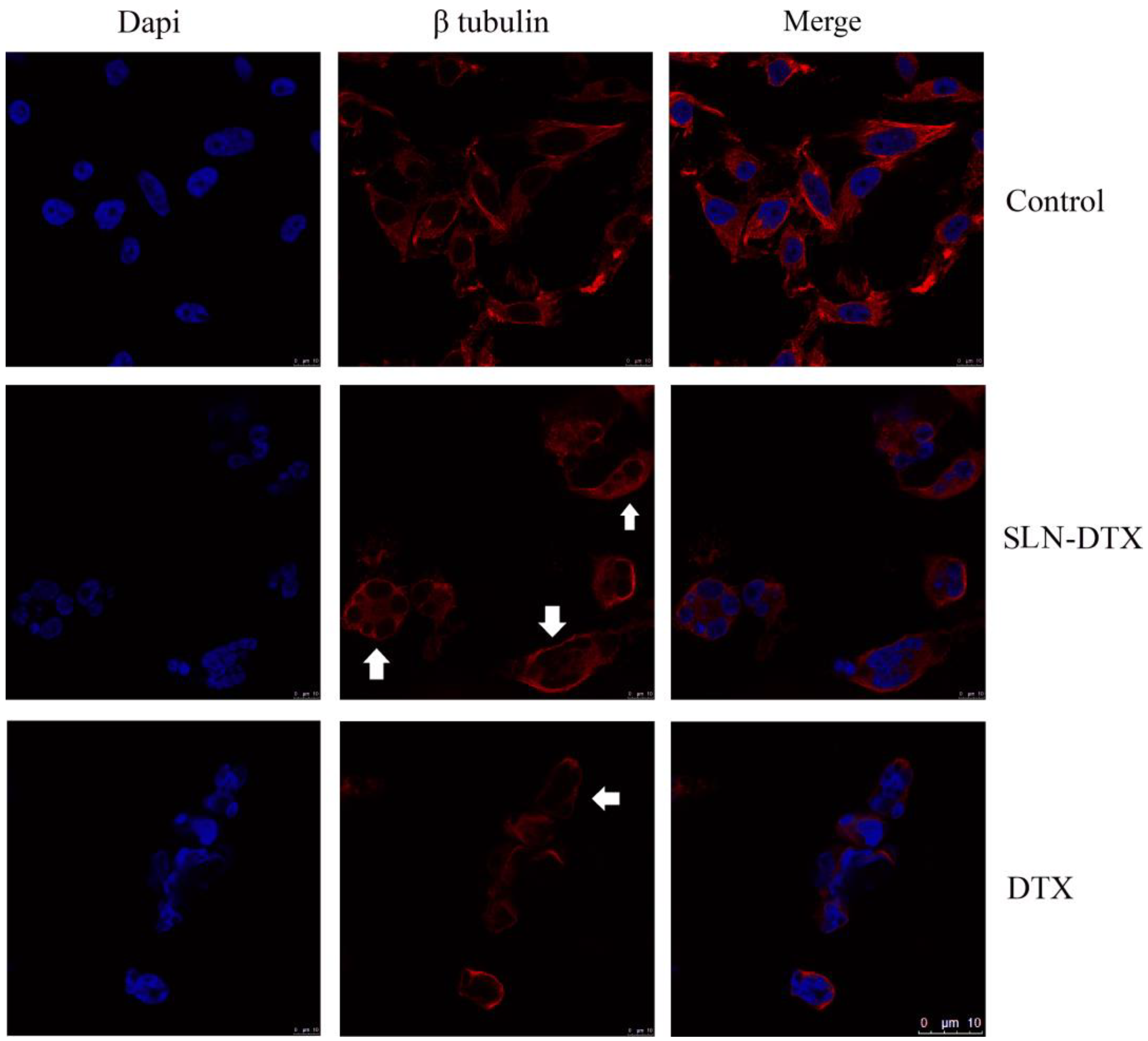
2.3. Immunostaining of Beta-Tubulin
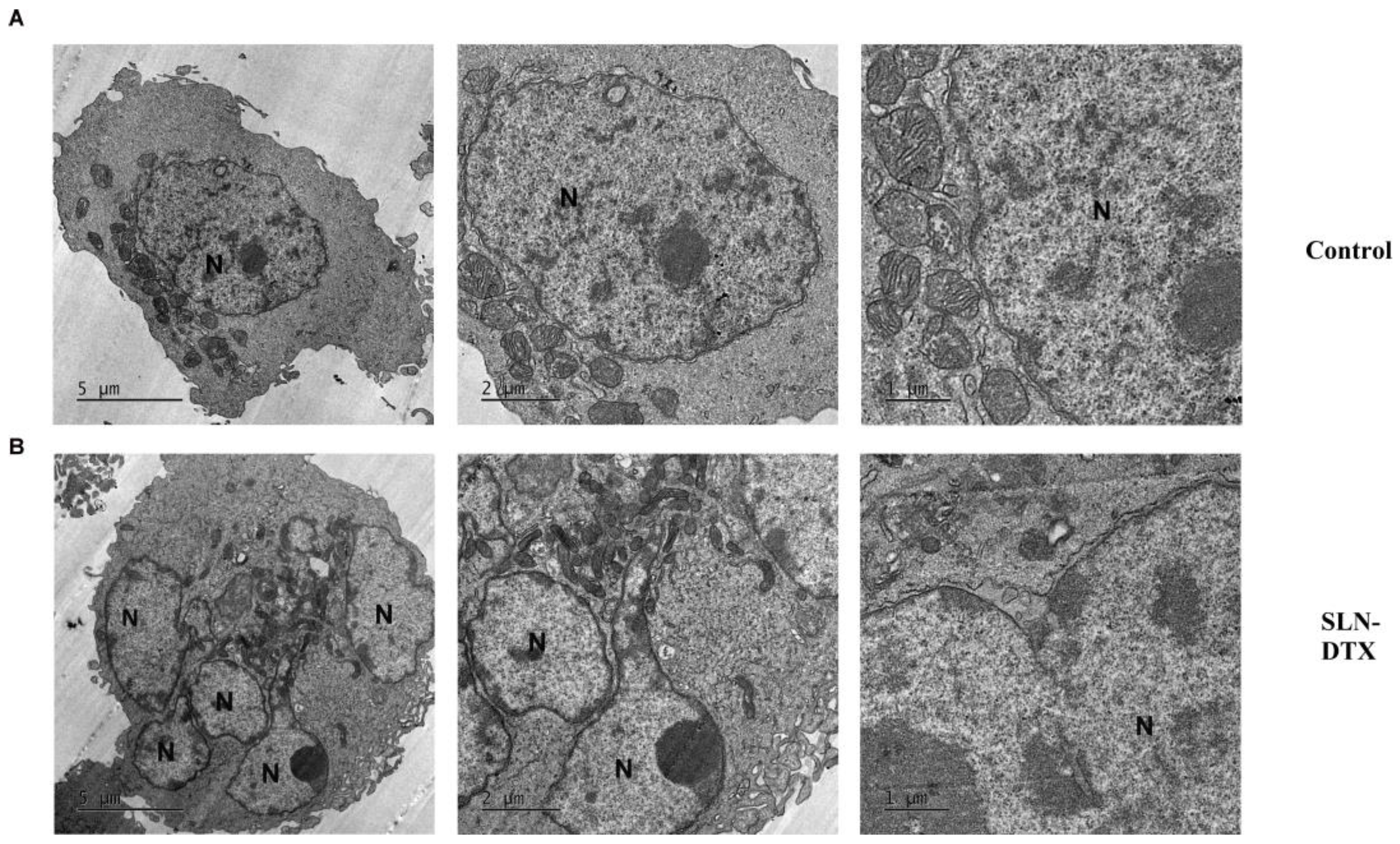
2.4. Ultra-Structural Analyses
2.5. Internalization of SNL by TEM and Confocal Microscopy
2.6. Wound Healing
2.7. Cell Cycle Analyses
2.8. Cell Death Analyses
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Preparation of Solid Lipid Nanoparticles (SLNs)
3.2.2. Cell Culture
3.2.3. Cell Viability Assay
3.2.4. Light Microscopy
3.2.5. SEM Microscopy
3.2.6. Immunostaining of Beta-Tubulin
3.2.7. TEM Microscopy
3.2.8. Confocal Microscopy
3.2.9. Wound Healing
3.2.10. Cell Cycle
3.2.11. Cell Death
3.2.12. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Nenclares, P.; Harrington, K.J. The biology of cancer. Medicine 2020, 48, 67–72. [Google Scholar] [CrossRef]
- Hassanpour, S.H.; Dehghani, M. Review of cancer from perspective of molecular. J. Cancer Res. Pract. 2017, 4, 127–129. [Google Scholar] [CrossRef]
- Hussain, A.; Oves, M.; Alajmi, M.; Hussain, I.; Amir, S.; Ahmed, J.; Rehman, M.T.; El-Seedi, H.R.; Ali, I. Biogenesis of ZnO nanoparticles using Pandanus odorifer leaf extract: Anticancer and antimicrobial activities. RSC Adv. 2019, 9, 15357–15369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da Silva, J.L.; Cardoso Nunes, N.C.; Izetti, P.; de Mesquita, G.G.; de Melo, A.C. Triple negative breast cancer: A thorough review of biomarkers. Crit. Rev. Oncol. /Hematol. 2020, 145, 102855. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Duan, J.J.; Bian, X.W.; Yu, S.C. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res. 2020, 22, 61. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Bauer, J.A.; Chen, X.; Sanders, M.E.; Chakravarthy, A.B.; Shyr, Y.; Pietenpol, J.A. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J. Clin. Investig. 2011, 121, 2750–2767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, P. Biology and Management of Patients with Triple-Negative Breast Cancer. Oncologist 2016, 21, 1050–1062. [Google Scholar] [CrossRef] [Green Version]
- Rodenak-Kladniew, B.; Islan, G.A.; de Bravo, M.G.; Durán, N.; Castro, G.R. Design, characterization and in vitro evaluation of linalool-loaded solid lipid nanoparticles as potent tool in cancer therapy. Colloids Surf. B Biointerfaces 2017, 154, 123–132. [Google Scholar] [CrossRef]
- Sharma, P.; López-Tarruella, S.; García-Saenz, J.A.; Ward, C.; Connor, C.S.; Gómez, H.L.; Prat, A.; Moreno, F.; Jerez-Gilarranz, Y.; Barnadas, A.; et al. Efficacy of Neoadjuvant Carboplatin plus Docetaxel in Triple-Negative Breast Cancer: Combined Analysis of Two Cohorts. Clin. Cancer Res. 2017, 23, 649–657. [Google Scholar] [CrossRef] [Green Version]
- Martin, M.; Ramos-Medina, R.; Bernat, R.; García-Saenz, J.A.; del Monte-Millan, M.; Alvarez, E.; Cebollero, M.; Moreno, F.; Gonzalez-Haba, E.; Bueno, O.; et al. Activity of docetaxel, carboplatin, and doxorubicin in patient-derived triple-negative breast cancer xenografts. Sci. Rep. 2021, 11, 7064. [Google Scholar] [CrossRef]
- Dawoud, M. Chitosan coated solid lipid nanoparticles as promising carriers for docetaxel. J. Drug Deliv. Sci. Technol. 2021, 62, 102409. [Google Scholar] [CrossRef]
- Zhang, E.; Xing, R.; Liu, S.; Li, P. Current advances in development of new docetaxel formulations. Expert Opin. Drug Deliv. 2019, 16, 301–312. [Google Scholar] [CrossRef]
- Mukhtar, E.; Adhami, V.M.; Mukhtar, H. Targeting Microtubules by Natural Agents for Cancer Therapy. Mol. Cancer Ther. 2014, 13, 275–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dumontet, C.; Jordan, M.A. Microtubule-binding agents: A dynamic field of cancer therapeutics. Nat. Rev. Drug Discov. 2010, 9, 790–803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, R.; Ni, S.; Chen, W.; Liu, M.; Feng, J.; Hu, K. Improved Anti-Triple Negative Breast Cancer Effects of Docetaxel by RGD-Modified Lipid-Core Micelles. Int. J. Nanomed. 2021, 16, 5265–5279. [Google Scholar] [CrossRef]
- Müller, R.H.; Mäder, K.; Gohla, S. Solid lipid nanoparticles (SLN) for controlled drug delivery—A review of the state of the art. Eur. J. Pharm. Biopharm. 2000, 50, 161–177. [Google Scholar] [CrossRef]
- Akanda, M.; Getti, G.; Nandi, U.; Mithu, M.S.; Douroumis, D. Bioconjugated solid lipid nanoparticles (SLNs) for targeted prostate cancer therapy. Int. J. Pharm. 2021, 599, 120416. [Google Scholar] [CrossRef] [PubMed]
- García-Pinel, B.; Porras-Alcalá, C.; Ortega-Rodríguez, A.; Sarabia, F.; Prados, J.; Melguizo, C.; López-Romero, J.M. Lipid-Based Nanoparticles: Application and Recent Advances in Cancer Treatment. Nanomaterials 2019, 9, 638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Chen, T.; Xu, H.; Ren, B.; Cheng, X.; Qi, R.; Liu, H.; Wang, Y.; Yan, L.; Chen, S.; et al. Curcumin-Loaded Solid Lipid Nanoparticles Enhanced Anticancer Efficiency in Breast Cancer. Molecules 2018, 23, 1578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amreddy, N.; Babu, A.; Muralidharan, R.; Panneerselvam, J.; Srivastava, A.; Ahmed, R.; Mehta, M.; Munshi, A.; Ramesh, R. Recent advances in nanoparticle-based Cancer Drug and Gene Delivery. Adv. Cancer Res. 2018, 137, 115–170. [Google Scholar]
- da Rocha, M.C.O.; da Silva, P.B.; Radicchi, M.A.; Andrade, B.Y.G.; de Oliveira, J.V.; Venus, T.; Merker, C.; Estrela-Lopis, I.; Longo, J.P.F.; Báo, S.N.; et al. Docetaxel-loaded solid lipid nanoparticles prevent tumor growth and lung metastasis of 4T1 murine mammary carcinoma cells. J. Nanobiotechnol. 2020, 18, 43. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, L.; Srivastava, S.; Panjeta, A.; Chaudhari, D.; Ghadi, R.; Kuche, K.; Malik, R.; Preet, S.; Jain, S.; Raza, K. Exploration of docetaxel palmitate and its solid lipid nanoparticles as a novel option for alleviating the rising concern of multi-drug resistance. Int. J. Pharm. 2020, 578, 119088. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, O.S.; Kim, H.S.; Zeb, A.; Choi, J.-S.; Kim, H.-S.; Kwon, J.-E.; Kim, M.-S.; Kang, J.-H.; Ryou, C.-S.; Park, J.-S.; et al. Sustained release docetaxel-incorporated lipid nanoparticles with improved pharmacokinetics for oral and parenteral administration. J. Microencapsul. 2017, 34, 250–261. [Google Scholar] [CrossRef]
- Mann, J.; Yang, N.; Montpetit, R.; Kirschenman, R.; Lemieux, H.; Goping, I.S. BAD sensitizes breast cancer cells to docetaxel with increased mitotic arrest and necroptosis. Sci. Rep. 2020, 10, 355. [Google Scholar] [CrossRef] [PubMed]
- Gadadhar, S.; Bodakuntla, S.; Natarajan, K.; Janke, C. The tubulin code at a glance. J. Cell Sci. 2017, 130, 1347–1353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, J.; Mitchison, T.J. Cell death response to anti-mitotic drug treatment in cell culture, mouse tumor model and the clinic. Endocr.-Relat. Cancer 2017, 24, T83–T96. [Google Scholar] [CrossRef] [Green Version]
- Tischer, J.; Gergely, F. Anti-mitotic therapies in cancer. J. Cell Biol. 2019, 218, 10–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vitale, I.; Galluzzi, L.; Castedo, M.; Kroemer, G. Mitotic catastrophe: A mechanism for avoiding genomic instability. Nat. Rev. Mol. Cell Biol. 2011, 12, 385–392. [Google Scholar] [CrossRef]
- Janssen, A.; Kops, G.J.P.L.; Medema, R.H. Elevating the frequency of chromosome missegregation as a strategy to kill tumor cells. Proc. Natl. Acad. Sci. USA 2009, 106, 19108–19113. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Qin, X.; Kong, F.; Chen, P.; Pan, G. Improving cellular uptake of therapeutic entities through interaction with components of cell membrane. Drug Deliv. 2019, 26, 328–342. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Chang, M.; Yu, Y.; Zhang, Y.; Liu, G.; Wei, T.; Zuo, T.; Guan, Y.; Lin, G.; Zhao, Z. Preparation and evaluation of lipid emulsified docetaxel-loaded nanoparticles. J. Pharm. Pharmacol. 2015, 67, 1546–1555. [Google Scholar] [CrossRef] [PubMed]
- Jadon, R.S.; Sharma, M. Docetaxel-loaded lipid-polymer hybrid nanoparticles for breast cancer therapeutics. J. Drug Deliv. Sci. Technol. 2019, 51, 475–484. [Google Scholar]
- Tao, J.; Tan, Z.; Diao, L.; Ji, Z.; Zhu, J.; Chen, W.; Hu, Y. Co-delivery of dihydroartemisinin and docetaxel in pH-sensitive nanoparticles for treating metastatic breast cancer via the NF-κB/MMP-2 signal pathway. RSC Adv. 2018, 8, 21735–21744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, N.; Guo, W.; Li, Y.; Zuo, H.; Zhang, H.; Wang, Z.; Zhao, Y.; Yang, F.; Ren, G.; Zhang, S. Construction and anti-tumor activities of disulfide-linked docetaxeldihydroartemisinin nanoconjugates. Colloids Surf. B Biointerfaces 2020, 191, 111018. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Wang, Y.; Liang, X.; Wusiman, Z.; Yin, Y.; Shen, Q. Synergistic inhibition of migration and invasion of breast cancer cells by dual docetaxel/quercetin-loaded nanoparticles via Akt/MMP-9 pathway. Int. J. Pharm. 2017, 523, 300–309. [Google Scholar] [CrossRef]
- Li, G.; Wang, Y.; Li, L.; Ren, Y.; Deng, X.; Liu, J.; Wang, W.; Luo, M.; Liu, S.; Chen, J. Design, synthesis, and bioevaluation of pyrazolo[1,5-a]pyrimidine derivatives as tubulin polymerization inhibitors targeting the colchicine binding site with potent anticancer activities. Eur. J. Med. Chem. 2020, 202, 112519. [Google Scholar] [CrossRef]
- Wang, E.C.; Sinnott, R.; Werner, M.E.; Sethi, M.; Whitehurst, A.W.; Wang, A.Z. Differential cell responses to nanoparticle docetaxel and small molecule docetaxel at a sub-therapeutic dose range. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 321–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, M.-H.; Zeng, R.-F.; Fang, S.; Dai, Q.-S.; Li, H.-P.; Long, J.-T. Liposome-based co-delivery of siRNA and docetaxel for the synergistic treatment of lung cancer. Int. J. Pharm. 2014, 474, 112–122. [Google Scholar] [CrossRef]
- Khodaverdi, E.; Tayarani-Najaran, Z.; Minbashi, E.; Alibolandi, M.; Hosseini, J.; Sepahi, S.; Kamali, H.; Hadizadeh, F. Docetaxel-Loaded Mixed Micelles and Polymersomes Composed of Poly (caprolactone)-Poly (ethylene glycol) (PEG-PCL) and Poly (lactic acid)-Poly (ethylene glycol) (PEG-PLA): Preparation and In-vitro Characterization. Iran. J. Pharm. Res. IJPR 2019, 18, 142–155. [Google Scholar]
- Singh, R.P.; Sharma, G.; Singh, S.; Kumar, M.; Pandey, B.L.; Koch, B.; Muthu, M.S. Vitamin E TPGS conjugated carbon nanotubes improved efficacy of docetaxel with safety for lung cancer treatment. Colloids Surf. B Biointerfaces 2016, 141, 429–442. [Google Scholar] [CrossRef]
- Liu, D.; Liu, Z.; Wang, L.; Zhang, C.; Zhang, N. Nanostructured lipid carriers as novel carrier for parenteral delivery of docetaxel. Colloids Surf. B Biointerfaces 2011, 85, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Maroufi, N.F.; Vahedian, V.; Mazrakhondi, S.A.M.; Kooti, W.; Khiavy, H.A.; Bazzaz, R.; Ramezani, F.; Pirouzpanah, S.M.; Ghorbani, M.; Akbarzadeh, M.; et al. Sensitization of MDA-MBA-231 breast cancer cell to docetaxel bymyricetin loaded into biocompatible lipid nanoparticles via sub-G1 cell cycle arrest mechanism. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020, 393, 1–11. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paiva, K.L.R.; Radicchi, M.A.; Báo, S.N. In Vitro Evaluation of NLS-DTX Activity in Triple-Negative Breast Cancer. Molecules 2022, 27, 4920. https://doi.org/10.3390/molecules27154920
Paiva KLR, Radicchi MA, Báo SN. In Vitro Evaluation of NLS-DTX Activity in Triple-Negative Breast Cancer. Molecules. 2022; 27(15):4920. https://doi.org/10.3390/molecules27154920
Chicago/Turabian StylePaiva, Karen L. R., Marina A. Radicchi, and Sônia N. Báo. 2022. "In Vitro Evaluation of NLS-DTX Activity in Triple-Negative Breast Cancer" Molecules 27, no. 15: 4920. https://doi.org/10.3390/molecules27154920
APA StylePaiva, K. L. R., Radicchi, M. A., & Báo, S. N. (2022). In Vitro Evaluation of NLS-DTX Activity in Triple-Negative Breast Cancer. Molecules, 27(15), 4920. https://doi.org/10.3390/molecules27154920





