5-Demethoxy-10′-ethoxyexotimarin F, a New Coumarin with MAO-B Inhibitory Potential from Murraya exotica L.
Abstract
1. Introduction
2. Results and Discussion
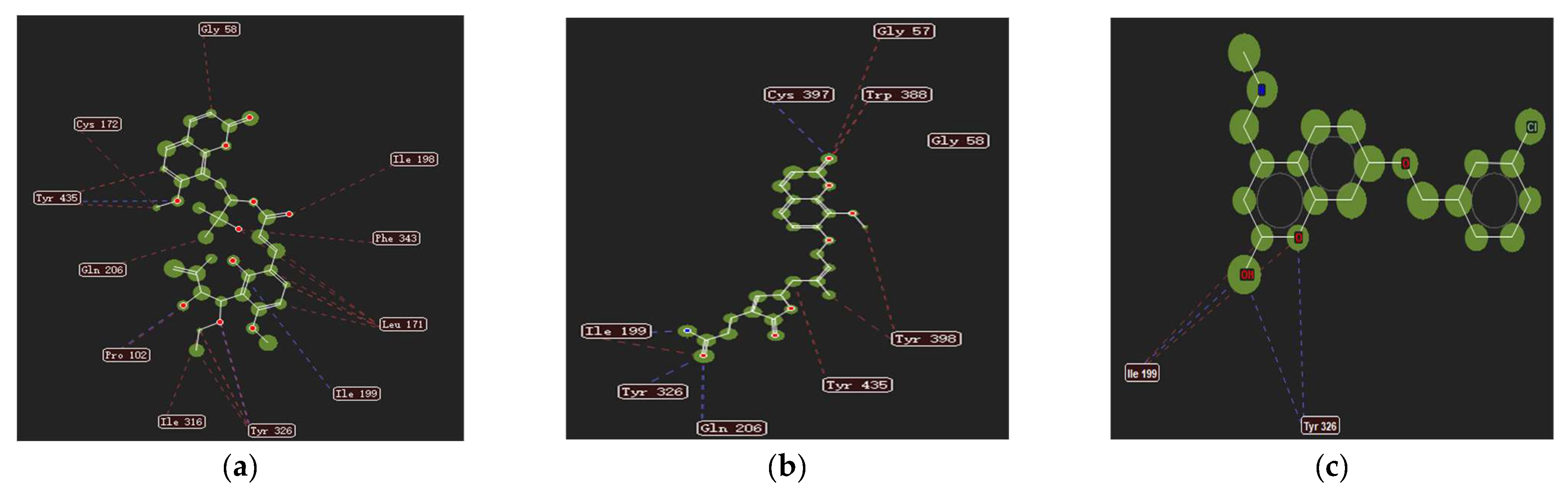
2.1. Molecular Docking of Coumarins in Clausena and Murraya
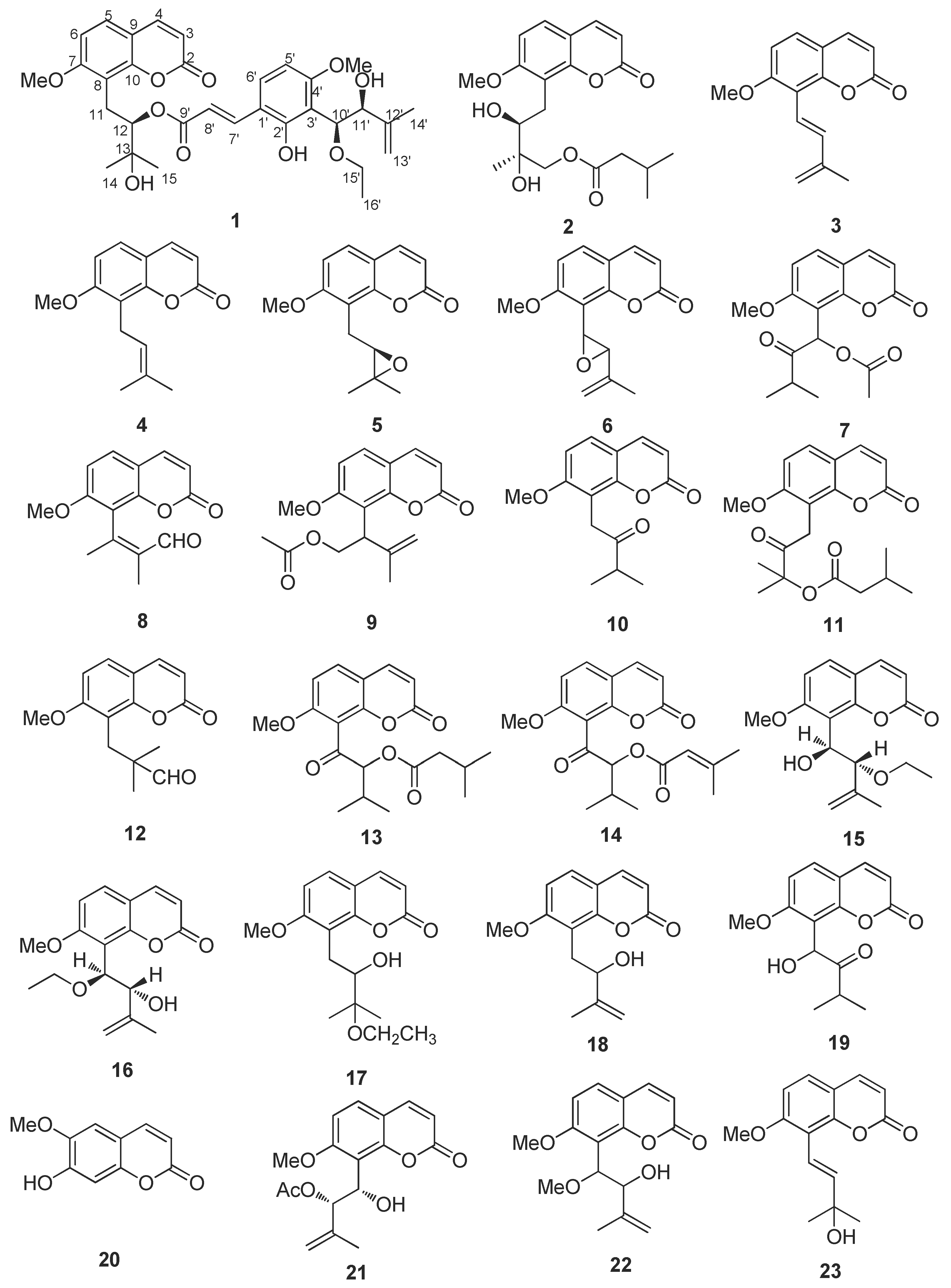
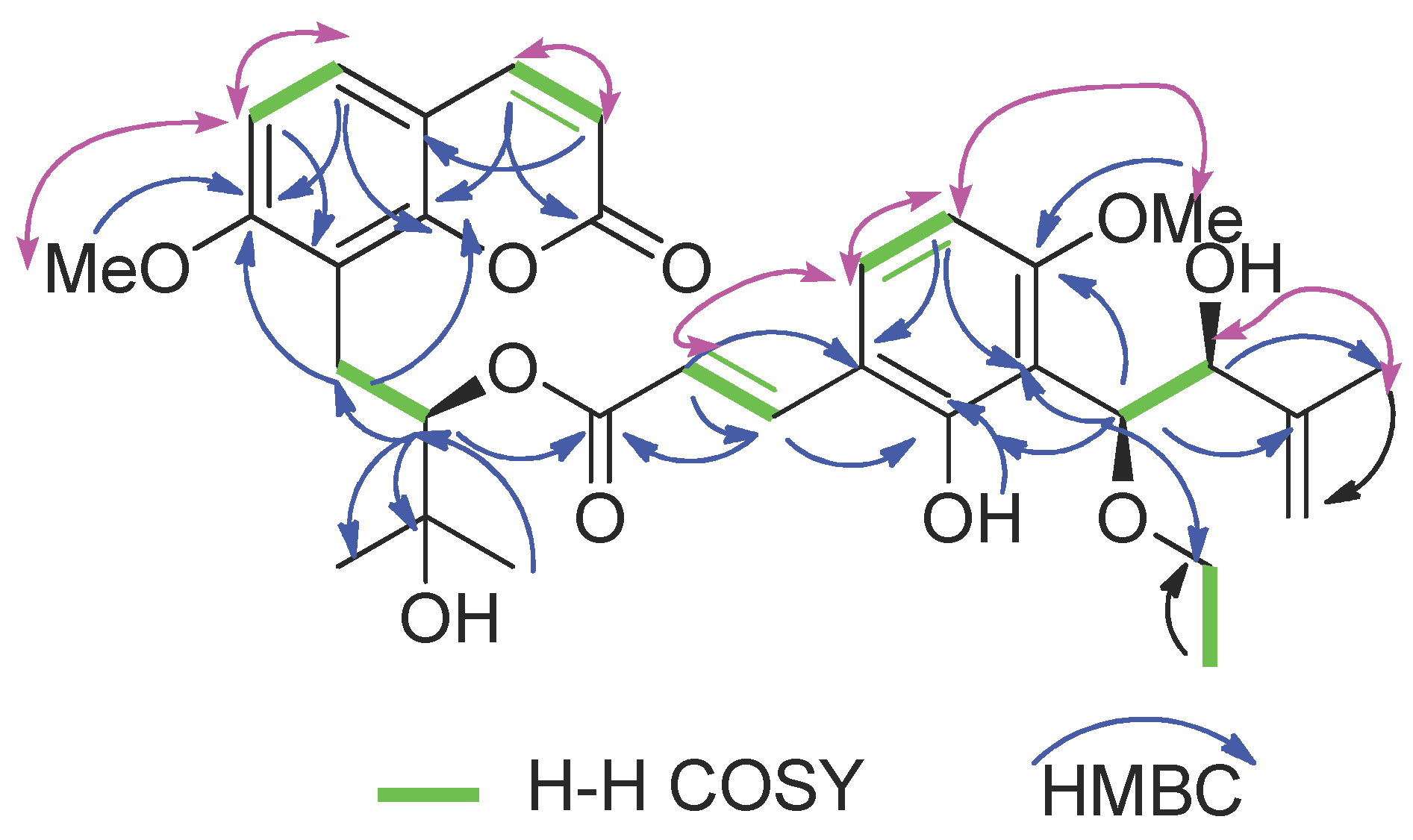
2.2. Structure Elucidation of New Coumarin 1
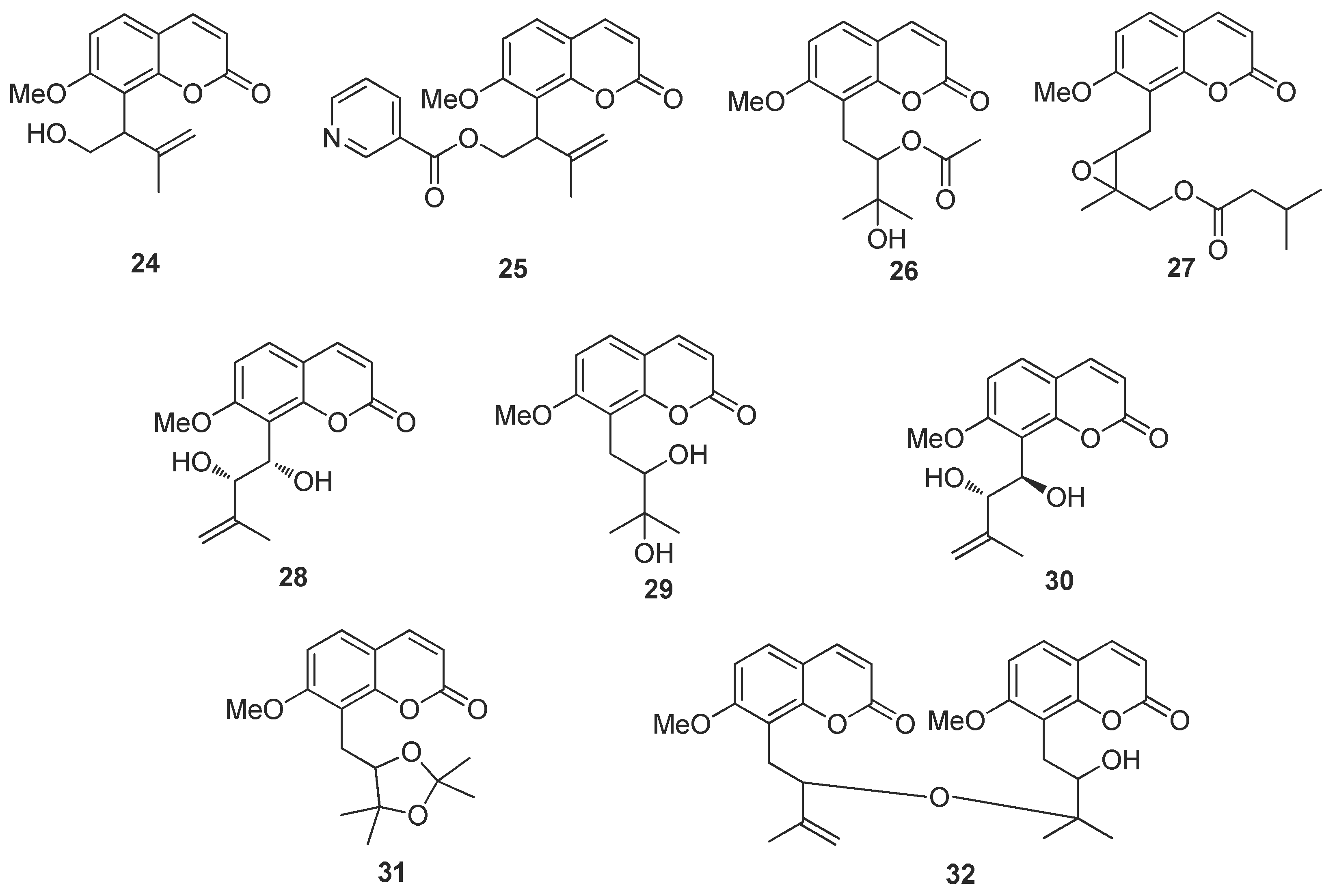
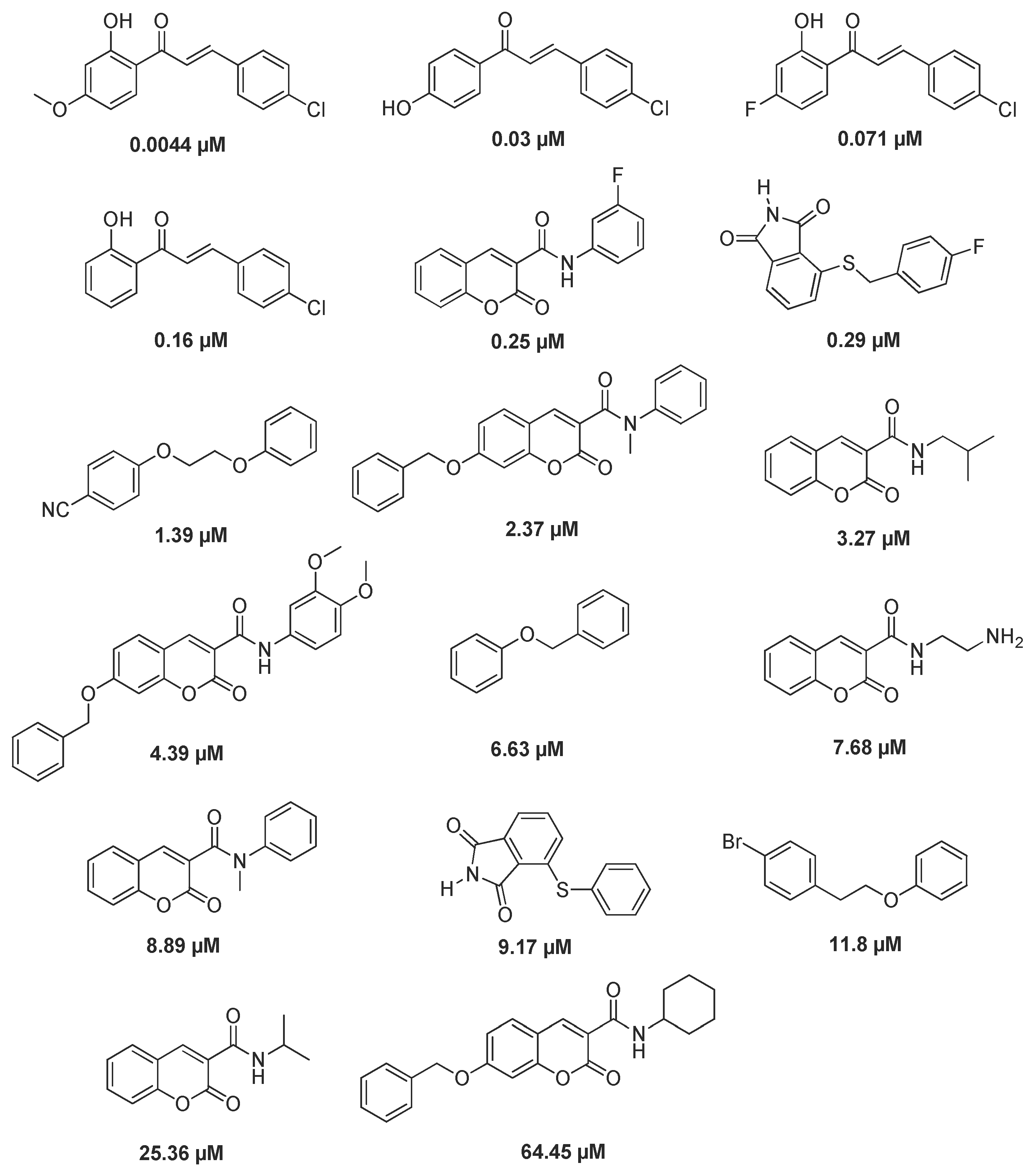
2.3. Virtual Screen of Coumarins Isolate from Murraya exotica L.
2.4. Biological Activity of Coumarin 1
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Materials
3.3. Extraction and Isolation
3.4. Structural Elucidation
3.5. Molecular Docking
3.6. Pharmacophore Model
3.7. In Vitro Bioassay
3.7.1. In Vitro MAO Inhibitory Assay
3.7.2. Anti-Inflammatory Assay
3.7.3. Acetylcholinesterase/Butrylcholinesterase Inhibitory Activity
3.7.4. α-Glucosidase Inhibition Assay
3.7.5. Antimicrobial Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Shih, J.C. Molecular basis of human MAO A and B. Neuropsychopharmacology 1991, 4, 1–7. [Google Scholar] [PubMed]
- Schwartz, T.L. A neuroscientific update on monoamine oxidase and its inhibitors. CNS Spectrums. 2013, 18 (Suppl. 1), 25–33. [Google Scholar] [CrossRef] [PubMed]
- Bonanno, L.; Ciurleo, R.; Marino, S.; Ruvolo, C.; Morabito, R.; Bramanti, A.; Corallo, F. Effect of MAO-B Inhibitors on Neurometabolic Profile of Patients Affected by Parkinson Disease: A Proton Magnetic Resonance Spectroscopy Study. J Clin. Med. 2022, 11, 1931. [Google Scholar] [CrossRef] [PubMed]
- Al Mamun, A.; Uddin, M.S. KDS2010: A Potent Highly Selective and Reversible MAO-B Inhibitor for Alzheimer’s Disease. Comb. Chem. High T. Scr. 2020, 23, 836–841. [Google Scholar] [CrossRef]
- Dhiman, P.; Malik, N.; Khatkar, A. 3D-QSAR and in-silico Studies of Natural Products and Related Derivatives as Monoamine Oxidase Inhibitors. Curr. Neuropharmacol. 2018, 16, 881–900. [Google Scholar] [CrossRef]
- Wang, Y.S.; Li, B.T.; Liu, S.X.; Wen, Z.Q.; Yang, J.H.; Zhang, H.B.; Hao, X.J. Anisucoumaramide, a Bioactive Coumarin from Clausena anisum-olens. J. Nat. Prod. 2017, 80, 798–804. [Google Scholar] [CrossRef]
- Liu, B.Y.; Zhang, C.; Zeng, K.W.; Li, J.; Guo, X.Y.; Zhao, M.B.; Tu, P.F.; Jiang, Y. Anti-inflammatory prenylated phenylpropenols and coumarin derivatives from Murraya exotica. J. Nat. Prod. 2018, 81, 22–33. [Google Scholar] [CrossRef]
- Liang, H.; Shi, Y.; Zeng, K.; Zhao, M.; Tu, P.; Jiang, Y. Coumarin derivatives from the leaves and twigs of Murraya exotica L. and their anti-inflammatory activities. Phytochemistry. 2020, 177, 112416. [Google Scholar] [CrossRef]
- Huang, L.; Feng, Z.L.; Wang, Y.T.; Lin, L.G. Anticancer carbazole alkaloids and coumarins from Clausena plants: A review. Chin. J. Nat. Med. 2017, 15, 881–888. [Google Scholar] [CrossRef]
- Lv, H.N.; Wang, S.; Zeng, K.W.; Li, J.; Guo, X.Y.; Ferreira, D.; Zjawiony, J.K.; Tu, P.F.; Jiang, Y. Anti-inflammatory coumarin and benzocoumarin derivatives from Murraya alata. J. Nat. Prod. 2015, 78, 279–285. [Google Scholar] [CrossRef]
- Wang, Y.S.; Huang, R.; Li, N.Z.; Yang, J.H. Coumarins from Clausena anisum-olens Merr. Biosci. Biotech. Bioch. 2010, 74, 1483–1484. [Google Scholar] [CrossRef]
- Takemura, Y.; Nakamura, K.; Hirusawa, T.; Ju-ichi, M.; Ito, C.; Furukawa, H. Four new furanone-coumarins from Clausena excavata. Chem. Pharm. Bull. 2000, 48, 582–584. [Google Scholar] [CrossRef]
- Zhang, D.X.; Hartley, T.G. Murraya J. Koenig ex Linnaeus. In Flora of China; Wu, Z.Y., Raven, P.H., Hong, D.Y., Eds.; Science Press: Beijing, China, 2008; Volume 11, pp. 85–87. [Google Scholar]
- Xu, S.K.; Peng, H.; Wang, N.; Zhao, M. Inhibition of TNF-α and IL-1 by compounds from selected plants for rheumatoid arthritis therapy: In vivo and in silico studies. Trop. J. Pharm. Res. 2018, 17, 277–285. [Google Scholar] [CrossRef]
- Raj, K.; Misra, S.C.; Kapil, R.S.; Popli, S.P. Coumarins from Murraya paniculata. Phytochemistry 1976, 15, 1787. [Google Scholar] [CrossRef]
- Wang, X.T.; Liang, H.Z.; Zeng, K.W.; Zhao, M.B.; Tu, P.F.; Li, J.; Jiang, Y. Panitins AG: Coumarin derivatives from Murraya paniculata from Guangxi Province, China shows variable NO inhibitory activity. Phytochemistry 2019, 162, 224–231. [Google Scholar] [CrossRef]
- Pescitelli, G. For a Correct Application of the CD Exciton Chirality Method: The Case of Laucysteinamide A. Mar. Drugs. 2018, 16, 388. [Google Scholar] [CrossRef]
- Steck, W. Paniculatin, a New Coumarin from Murraya paniculata (L.) Jack. Can. J. Chem. 1972, 50, 443–445. [Google Scholar] [CrossRef]
- You, C.X.; Guo, S.S.; Geng, Z.F.; Zhang, W.J.; Liang, J.Y.; Zhang, Z.; Wang, C.F.; Du, S.S.; Deng, Z.W. Repellent activity of compounds from Murraya alata Drake against Tribolium castaneum. Ind. Crop. Prod. 2017, 95, 460–466. [Google Scholar] [CrossRef]
- Yang, J.S.; Su, Y.L. Studeis on the constituents of Murraya paniculata (L.) Jack. Acta. Pharm. Sin. B. 1983, 18, 760–765. [Google Scholar]
- Saied, S.; Nizami, S.S.; Anis, I. Two new coumarins from Murraya paniculata. J. Asian. Nat. Prod. Res. 2008, 10, 515–519. [Google Scholar] [CrossRef]
- Ito, C.; Furukawa, H. Constituents of Murraya exotica L. Structure elucidation of new coumarins. Chem Pharm Bull. 1987, 35, 4277–4285. [Google Scholar] [CrossRef]
- Ito, C.; Furukawa, H. Two new coumarins from Murraya plants. Chem. Pharm. Bull. 1989, 37, 819–820. [Google Scholar] [CrossRef]
- Imai, F.; Kinoshita, T.; Sankawa, U. Constituents of the leaves of Murraya paniculata collected in Taiwan. Chem Pharm Bull. 1989, 37, 358–362. [Google Scholar] [CrossRef]
- Choudhary, M.I.; Azizuddin; Khalid, A.; Sultani, S.Z.; Atta-ur-Rahman. A New Coumarin from Murraya paniculate. Planta Med. 2002, 68, 81–83. [Google Scholar] [CrossRef]
- Lin, J.K.; Wu, T.S. Constituents of Flowers of Murraya Paniculata. J. Chin. Chem. Soc. Taip. 1994, 41, 213–216. [Google Scholar] [CrossRef]
- Wu, T.S.; Lin, C.N.; Yang, L.K.; Lin, S.T. Studies on the Constituents of Murraya Paniculata Jack (I). J. Chin. Chem. Soc. Taip. 1975, 22, 163–165. [Google Scholar] [CrossRef]
- Wu, T.S.; Liou, M.J.; Kuoh, C.S. Coumarins of the flowers of Murraya paniculate. Phytochemistry 1989, 28, 293–294. [Google Scholar] [CrossRef]
- Kikuchi, T.; Yokoi, T.; Umemoto, K.; Shingu, T. Constituents of Scaevola frutescens. Yaku gaku Zasshi. 1974, 94, 1616. [Google Scholar] [CrossRef][Green Version]
- Nikonov, G.K.; Saidkhodzhaev, A.I. Structure of pranferin, a new coumarin from Prangos ferulaceae roots. Khim. Prir. Soedin. 1971, 7, 255. [Google Scholar] [CrossRef]
- Teshima, N.; Tsugawa, M.; Tateishi, A.; Tokumaru, M.; Matsubara, R.; Kimachi, T.; Ju-ichi, M.; Ito, C.; Furukawa, H. Two new bicoumarins from the leaves of Murraya exotica. Heterocycles. 2004, 63, 2837–2843. [Google Scholar]
- Hubálek, F.; Binda, C.; Khalil, A.; Li, M.; Mattevi, A.; Castagnoli, N.; Edmondson, D.E. Demonstration of isoleucine 199 as a structural determinant for the selective inhibition of human monoamine oxidase B by specific reversible inhibitors. J. Biol. Chem. 2005, 280, 15761–15766. [Google Scholar] [CrossRef] [PubMed]
- Legoabe, L.J.; Petzer, A.; Petzer, J.P. Selected C7-substituted chromone derivatives as monoamine oxidase inhibitors. Bioorg Chem. 2012, 45, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.C.; Zhou, J.; Tang, Y.P.; Duan, J.A. Molecular Docking of COX-2 and Four Flavonoids from Scutellaria Baicalensis Georgi. Acta Chin. Med. Pharmacol. 2009, 37, 71–74. [Google Scholar]
- Rao, S.N.; Head, M.S.; Kulkarni, A.; Lalonde, J.M. Validation studies of the site-directed docking program LibDock. J. Chem. Inf. Model. 2007, 47, 2159–2171. [Google Scholar] [CrossRef]
- Binda, C.; Wang, J.; Pisani, L.; Caccia, C.; Carotti, A.; Salvati, P.; Edmondson, D.E.; Mattevi, A. Structures of human monoamine oxidase B complexes with selective noncovalent inhibitors: Safinamide and coumarin analogs. J. Med. Chem. 2007, 50, 5848–5852. [Google Scholar] [CrossRef]
- Suh, J.; Yum, E.K.; Cheon, H.G.; Cho, Y.S. Synthesis and biological evaluation of N-aryl-4-aryl-1,3-Thiazole-2-amine derivatives as direct 5-lipoxygenase inhibitors. Chem. Biol. Drug Des. 2012, 80, 90–99. [Google Scholar] [CrossRef]
- Chimenti, F.; Fioravanti, R.; Bolasco, A.; Chimenti, P.; Secci, D.; Rossi, F.; Yáñez, M.; Orallo, F.; Ortuso, F.; Alcaro, S. Chalcones: A valid scaffold for monoamine oxidases inhibitors. J. Med. Chem. 2009, 52, 2818–2824. [Google Scholar] [CrossRef]
- Chimenti, F.; Secci, D.; Bolasco, A.; Chimenti, P.; Bizzarri, B.; Granese, A.; Carradori, S.; Yáñez, M.; Orallo, F.; Ortuso, F.; et al. Synthesis, molecular modeling and selective inhibitory activity against human monoamine oxidases of 3-carboxamido-7-substituted coumarins. J. Med. Chem. 2009, 52, 1935–1942. [Google Scholar] [CrossRef]
- Manley-King, C.I.; Bergh, J.J.; Petzer, J.P. Monoamine oxidase inhibition by C4-substituted phthalonitriles. Bioorg. Chem. 2012, 40, 114–124. [Google Scholar] [CrossRef]
- Van der Walt, M.M.; Terre’Blanche, G.; Petzer, A.; Petzer, J.P. The adenosine receptor affinities and monoamine oxidase B inhibitory properties of sulfanylphthalimide analogues. Bioorg. Chem. 2015, 59, 117–123. [Google Scholar] [CrossRef]
- Yang, H. The research of constructing antivirus related pharmacophore and evaluating method. Beijing Univ. Chin. Med. 2010, 12, 83. [Google Scholar]
- Wang, X.; Xiang, Y.H.; Ren, Z.Z.; Zhang, Y.L.; Qiao, Y.J. Rational questing for inhibitors of endothelin converting enzyme-1 from Salvia miltiorrhiza by combining ligand and structure based virtual screening. Can. J. Chem. 2013, 91, 448–456. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.L.; Wang, Y.; Ren, Z.Z.; Bao, H.J.; Qiao, Y.J. Study on relations between transient receptor potential vanilloid 1 and pungent property of traditional Chinese medicines. Chin. J. Chin. Mater. Med. 2014, 39, 2422–2427. [Google Scholar]
- Wang, D.S.; Nie, W.; Jiang, T.T.; Ding, L.F.; Song, L.D.; Wu, X.D.; Zhao, Q.S.; Caesalpanins, A.-C. three dimeric cassane diterpenoids from the seeds of Caesalpinia sappan L. Chem. Biodiversity 2020, 17, 2–9. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.J.; Feather-Stone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Zhang, L.; Tu, Z.C.; Yuan, T.; Wang, H.; Xie, X.; Fu, Z.F. Antioxidants and α-glucosidase inhibitors from Ipomoea batatas leaves identified by bioassay-guided approach and structure-activity relationships. Food Chem. 2016, 208, 61–67. [Google Scholar] [CrossRef]

| Clausena | Murraya | ||
|---|---|---|---|
| Compound | Score | Compound | Score |
| clauslactone V | −149.541 | murradimerin A | −138.086 |
| 2′,3′-epoxyamsolactone | −136.470 | omphamurin isovalerate | −126.578 |
| clauslactone J | −134.113 | murratin L | −121.957 |
| anisocoumarin J | −132.726 | panitin C | −119.568 |
| excavacoumarin C | −131.813 | C18_1503 | −118.516 |
| clauslactone I | −129.619 | murratin D | −118.495 |
| clauslactone L | −128.073 | 6-(2′,3′-dihydroxy-3-methylbutyl)-8-prenylumbelliferone | −116.788 |
| clauslactone F | −127.125 | muralatin B | −114.919 |
| anisolactone | −125.489 | aurapten | −111.870 |
| clausenalansimin B | −125.462 | 7-(3-methyl-2-butenyloxy)-8(3-butenyl-3-methyl-2-oxo)-coumarin | −109.601 |
| clauslactone A | −125.350 | 5-methoxypanial | −108.623 |
| clauslactone S | −125.222 | 6-methoxy-7-geranyloxycoumarin | −106.905 |
| clauslactone R | −123.325 | murratin M | −106.550 |
| clauslactone K | −119.830 | isomurralonginol nicotinate | −105.839 |
| C18_1503 | −118.516 | murralonginol isovalerate | −105.761 |
| clauslactone O | −117.426 | mexoticin | −104.093 |
| 5-geranyloxy-7-hydroxycoumarin | −117.172 | isomurranganonsenecioate | −103.535 |
| hekumarin A | −116.313 | murpanicin | −102.054 |
| excavatin B | −116.078 | scopolin | −101.550 |
| anisucoumaramide | −115.707 | paniculin | −99.904 |
| indicolactone | −113.647 | isogosferol | −99.570 |
| excavacoumarin F | −111.570 | omphamurin | −98.948 |
| clauslactone C | −108.884 | isomexoticin | −97.931 |
| excavacoumarin E | −108.804 | murralongic acid | −97.607 |
| phellopterin | −108.137 | murpaniculol | −97.455 |
| clauslactone D | −102.058 | isomeranzin | −95.308 |
| excavatin M | −101.192 | braylin | −94.309 |
| 5-hydroxy-8-(3′-methyl-2′-butenyl) furocoumarin | −100.497 | yuehgesin-B | −93.991 |
| clausindine | −97.617 | murratin G | −93.762 |
| clauslactone W | −96.696 | muralatin L | −93.649 |
| (2″S)-isosaxalin | −95.188 | 7-methoxy-8-(5-(prop-1-en-2-yloxy) penta-1,3-dien-1-yl)-coumarin | −93.252 |
| (+)-elisin | −94.884 | osthenon | −92.408 |
| peucedanone | −94.700 | murraculatin | −92.231 |
| 7-methoxy-6-(2′R- methoxy-3′-hydroxy-3′-methylbutyl) coumarin | −93.072 | muralatin P | −91.867 |
| anisocoumarin F | −92.112 | 6-hydroxycoumurrayin | −91.595 |
| chalepensin | −91.600 | panitin G | −91.252 |
| xanthyletin | −91.432 | columbianetin acetate | −91.158 |
| (+)-trans-decursidinol | −90.820 | minumicrolin | −90.401 |
| heraclenol | −89.300 | auraptenol | −89.986 |
| xanthoxyletin | −86.778 | 5,7-dimethoxy-8-(3′-methyl-2′-oxobutyl) coumarin | −89.605 |
| excavatin F | −86.1468 | 8-(2′-oxo-3′-methyl)butoxy-7-methoxycoumarin | −89.181 |
| murratin I | −88.635 | ||
| murraxocin | −88.498 | ||
| (±)-murratin A | −88.132 | ||
| muralatin C | −87.348 | ||
| phebalosin | −86.790 | ||
| 10-methoxy-7-methyl-2H-benzo[g]chromen-2-one | −86.436 | ||
| murratin F | −85.589 | ||
| byakangelicin | −85.426 | ||
| Position | δH (J in Hz) | δC (Type) | COSY | HMBC |
|---|---|---|---|---|
| 2 | / | 161.2, C | ||
| 3 | 6.11, d (9.4) | 112.8, CH | H-4 | C-9 |
| 4 | 7.53, d (9.4) | 143.7, CH | H-3 | C-2, C-10 |
| 5 | 7.29, d (8.6) | 127.1, CH | H-6 | C-4, C-7, C-9, C-10 |
| 6 | 6.79, d (8.6) | 107.2, CH | H-5 | C-7, C-8 |
| 7 | / | 160.8, C | ||
| 8 | / | 114.7, C | ||
| 9 | / | 153.5, C | ||
| 10 | / | 110.3, C | ||
| 11a | 3.41, dd (13.6, 10.8) | 23.3, CH2 | H-12 | C-7, C-8, C-10 |
| 11b | 3.13, dd (13.6, 2.4) | |||
| 12 | 5.23, dd (10.6, 2.5) | 78.7, CH | H-11 | C-8, C-11, C-14, C-15, C-9′ |
| 13 | 72.7, C | |||
| 14 | 1.41, s | 25.3, CH3 | C-12, C-15 | |
| 15 | 1.35, s | 26.6, CH3 | C-12, C-14 | |
| 1′ | / | 116.0, C | ||
| 2′ | / | 156.8, C | ||
| 3′ | / | 110.3, C | ||
| 4′ | / | 160.0, C | ||
| 5′ | 6.38, d (8.7) | 102.4, CH | H-6′ | C-1′, C-3′ |
| 6′ | 7.31, d (8.7) | 130.0, CH | H-5′ | C-2′, C-4′, C-7′ |
| 7′ | 7.70, d (16.1) | 140.1, CH | H-8′ | C-2′, C-6′, C-9′ |
| 8′ | 6.28, d (16.1) | 115.8, CH | H-7′ | C-1′ |
| 9′ | / | 167.2, C | ||
| 10′ | 4.94, d (7.4) | 79.3, CH | C-2′, C-3′, C-4’, C-11′, C-12′, C-15′ | |
| 11′ | 4.27, d (7.4) | 77.1, CH | C-10′, C-12′, C-13′, C-14′ | |
| 12′ | / | 142.9, C | ||
| 13′a | 4.75, s | 113.6, CH2 | C-11′, C-12′, C-14′ | |
| 13′b | 4.62, s | C-11′, C-12′, C-14′ | ||
| 14′ | 1.75, s | 17.9, CH3 | C-11′, C-13′ | |
| 15′a | 3.68, dq (14.0, 7.0) | 66.5, CH2 | H-16′ | C-10′, C-16′ |
| 15′b | 3.59, dq (14.0, 7.0) | |||
| 16′ | 1.27, t (7.0) | 15.0, CH3 | H-15′ | C-15′ |
| 7-OCH3 | 3.92, s | 56.2, CH3 | C-7 | |
| 4′-OCH3 | 3.75, s | 55.4, CH3 | C-4′ | |
| 2′-OH | 8.98, s | C-2′ |
| Compound | Score |
|---|---|
| murradimerin A (32) | −138.086 |
| 5-demethoxy-10′-ethoxyexotimarin F (1) | −129.482 |
| C18_1503 | −118.516 |
| anisucoumaramide | −115.707 |
| isomurralonginol nicotinate (25) | −105.839 |
| isomurranganonsenecioate (14) | −103.535 |
| 7-methoxy-8-(2-formyl-2-methylpropyl)coumarin (12) | −99.9048 |
| Isomeranzin (10) | −95.3081 |
| muralatinP (27) | −91.8677 |
| minumicrolin (30) | −90.4016 |
| phebalosin (6) | −86.7904 |
| Model | Ht | Ha | A% | Y% | N | CAI |
|---|---|---|---|---|---|---|
| 01 | 44 | 40 | 80 | 90.9 | 2.73 | 2.18 |
| 02 | 43 | 39 | 78 | 90.7 | 2.72 | 2.12 |
| 03 | 43 | 39 | 78 | 90.7 | 2.72 | 2.12 |
| 04 | 48 | 41 | 82 | 85.4 | 2.56 | 2.10 |
| 05 | 48 | 40 | 80 | 83.3 | 2.50 | 2.00 |
| 06 | 54 | 40 | 80 | 74.1 | 2.22 | 1.78 |
| 07 | 54 | 40 | 80 | 74.1 | 2.22 | 1.78 |
| 08 | 51 | 40 | 80 | 78.4 | 2.35 | 1.88 |
| 09 | 52 | 40 | 80 | 76.9 | 2.31 | 1.85 |
| 10 | 66 | 40 | 80 | 60.6 | 1.82 | 1.45 |
| Compound | Fit Value |
|---|---|
| 5-demethoxy-10′-ethoxyexotimarin F (1) | 2.90864 |
| exotimarin G (2) | 2.76624 |
| isomurralonginol nicotinate (25) | 2.73866 |
| muralatin P (27) | 2.63347 |
| muralatin O (13) | 2.61354 |
| murradimerin A (32) | 2.61125 |
| isomurranganonsenecioate (14) | 2.50786 |
| yuehgesin-C (17) | 2.49509 |
| paniculonol isovalerate (11) | 2.16367 |
| 2′-O-ethylmurrangatin (15) | 1.8576 |
| muralatin K (16) | 1.70339 |
| hainanmurpanin (7) | 1.51473 |
| auraptenol (18) | 1.23251 |
| pranferin (31) | 1.2031 |
| murranganon (19) | 1.14868 |
| isomeranzin (10) | 0.912292 |
| murrangatin (28) | 0.79533 |
| murrangatin acetate (21) | 0.734945 |
| minumicrolin (30) | 0.725833 |
| meranzin (5) | 0.536345 |
| albiflorin-3 (22) | 0.476371 |
| osthol (4) | 0.316498 |
| isomurralonginol (24) | 0.265571 |
| phebalosin (6) | 0.244483 |
| isomurralonginol acetate (9) | 0.209117 |
| trans-dehydroosthol (3) | 0.104663 |
| 7-methoxy-8-(2-formyl-2-methylpropyl)coumarin (12) | 0.0957507 |
| Compounds | MAO-A IC50 | MAO-B IC50 | SI |
|---|---|---|---|
| (1) | 26.3 ± 1.03 μM | 153.25 ± 1.58 nM | 172 |
| selegiline | 67.24 ± 1.03 μM | 19.58 ± 0.83 nM | 3434 |
| iproniazide | 6.55 ± 0.75 μM | 7.52 ± 0.34 μM | 0.87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia-Hou, Z.-R.; Feng, X.-F.; Mei, Y.-F.; Zhang, Y.-Y.; Yang, T.; Pan, J.; Yang, J.-H.; Wang, Y.-S. 5-Demethoxy-10′-ethoxyexotimarin F, a New Coumarin with MAO-B Inhibitory Potential from Murraya exotica L. Molecules 2022, 27, 4950. https://doi.org/10.3390/molecules27154950
Xia-Hou Z-R, Feng X-F, Mei Y-F, Zhang Y-Y, Yang T, Pan J, Yang J-H, Wang Y-S. 5-Demethoxy-10′-ethoxyexotimarin F, a New Coumarin with MAO-B Inhibitory Potential from Murraya exotica L. Molecules. 2022; 27(15):4950. https://doi.org/10.3390/molecules27154950
Chicago/Turabian StyleXia-Hou, Zhen-Ru, Xiao-Fei Feng, Yu-Fei Mei, Yin-Yan Zhang, Tong Yang, Jie Pan, Jing-Hua Yang, and Yun-Song Wang. 2022. "5-Demethoxy-10′-ethoxyexotimarin F, a New Coumarin with MAO-B Inhibitory Potential from Murraya exotica L." Molecules 27, no. 15: 4950. https://doi.org/10.3390/molecules27154950
APA StyleXia-Hou, Z.-R., Feng, X.-F., Mei, Y.-F., Zhang, Y.-Y., Yang, T., Pan, J., Yang, J.-H., & Wang, Y.-S. (2022). 5-Demethoxy-10′-ethoxyexotimarin F, a New Coumarin with MAO-B Inhibitory Potential from Murraya exotica L. Molecules, 27(15), 4950. https://doi.org/10.3390/molecules27154950







