An Overview of PDE4 Inhibitors in Clinical Trials: 2010 to Early 2022
Abstract
:1. Introduction
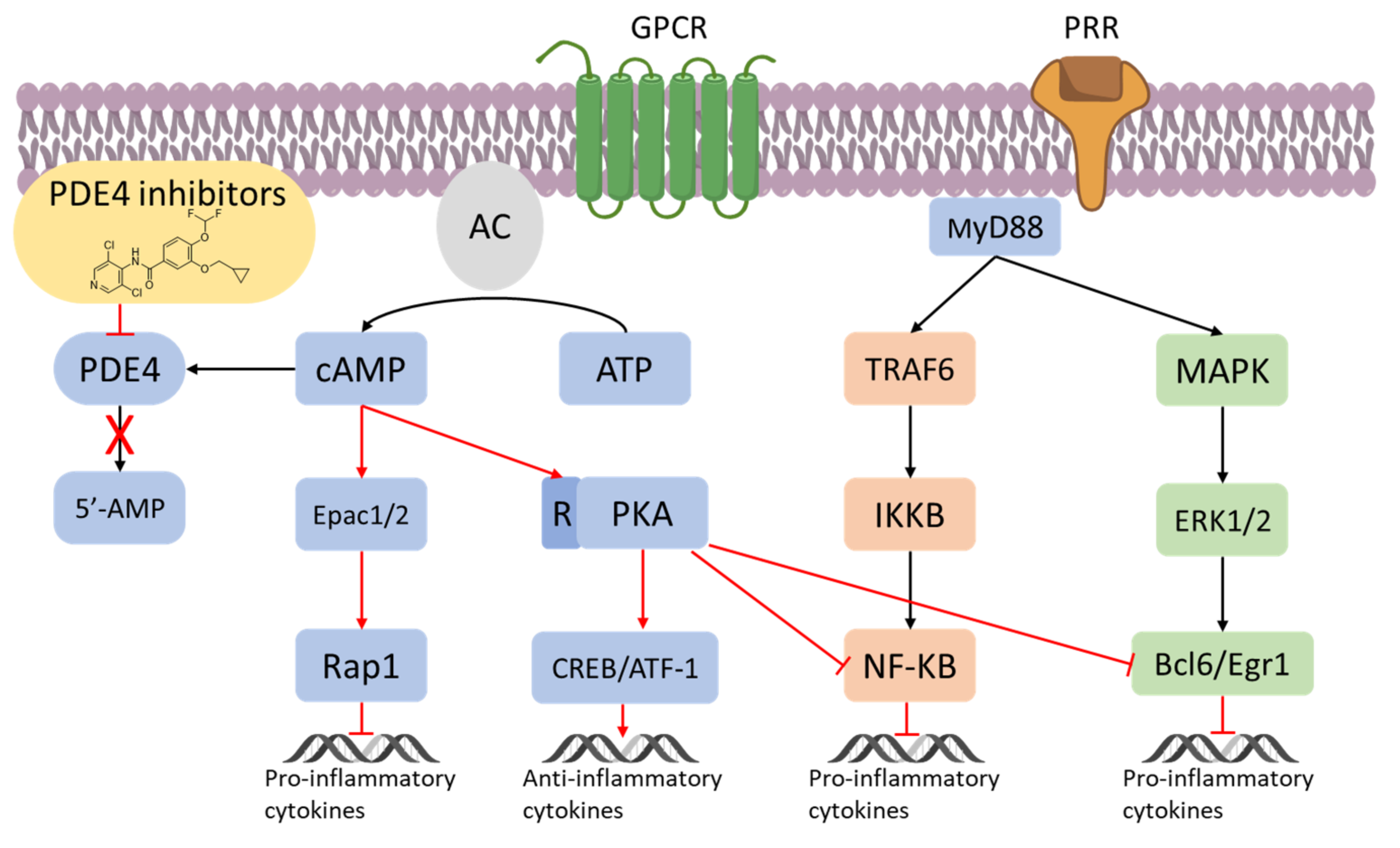
2. Overview of PDE4
3. Inflammation and PDE4
4. From In Vitro and Preclinical Profile of The First PDE 4 Inhibitors to Approved Drugs
5. PDE4 Inhibitors under Development
5.1. Asthma and COPD
5.2. Atopic Dermatitis (AD) and Psoriasis
5.3. Rheumatoid Arthritis and Lupus
5.4. Neurological Disorders
5.5. COVID-19 and PDE4
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sutherland, E.W.; Rall, T.W. Fractionation and characterization of a cyclic adenine ribonucleotide formed by tissue particles. J. Biol. Chem. 1958, 232, 1077–1091. [Google Scholar] [CrossRef]
- Newton, A.C.; Bootman, M.D.; Scott, J.D. Second messangers. Cold Spring Harb. Perspect. Biol. 2016, 8, a005926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raker, V.K.; Becker, C.; Steinbrink, K. The cAMP pathway as therapeutic target in autoimmune and inflammatory diseases. Front. Immunol. 2016, 7, 123. [Google Scholar] [CrossRef] [Green Version]
- Fajardo, A.M.; Piazza, G.A.; Tinsley, H.N. The role of cyclic nucleotide signaling pathways in cancer: Targets for prevention and treatment. Cancers. 2014, 6, 436–458. [Google Scholar] [CrossRef] [Green Version]
- Lorigo, M.; Oliveira, N.; Cairrao, E. PDE-mediated cyclic nucleotide compartmentation in vascular smooth muscle cells: From basic to a clinical perspective. J. Cardiovasc. Dev. Dis. 2022, 9, 4. [Google Scholar] [CrossRef]
- Hasegawa, G.R. Milrinone, a new agent for the treatment of congestive heart failure. Clin. Pharm. 1986, 5, 201–205. [Google Scholar] [CrossRef]
- Hilleman, D.E.; Forbes, W.P. Role of milrinone in the management of congestive heart failure. DICP 1989, 23, 357–362. [Google Scholar] [CrossRef]
- Varriale, P.; Ramaprasad, S. Short-term intravenous milrinone for severe congestive heart failure: The good, bad, and not so good. Pharmacotherapy 1997, 17, 371–374. [Google Scholar]
- Lewis, T.C.; Aberle, C.; Altshuler, D.; Piper, G.L.; Papadopoulos, J. Comparative effectiveness and safety between Milrinone or Dobutamine as initial inotrope therapy in cardiogenic shock. J. Cardiovasc. Pharmacol. Ther. 2019, 24, 130–138. [Google Scholar] [CrossRef]
- Little, W.N.; Park, G.T.; Patton, H.M. Sildenafil in the treatment of erectile dysfunction. N. Engl. J. Med. 1998, 339, 700. [Google Scholar]
- Goldstein, I.; Lue, T.F.; Padma-Nathan, H.; Rosen, R.C.; Steers, W.D.; Wicker, P.A. Oral sildenafil in the treatment of erectile dysfunction. Sildenafil study group. N. Engl. J. Med. 1998, 338, 1397–1404. [Google Scholar] [CrossRef]
- Cartledge, J.; Eardley, I. Sildenafil. Expert Opin. Pharmacother. 1999, 1, 137–147. [Google Scholar] [CrossRef]
- Antoniu, S.A. Sildenafil citrate for the treatment of pulmonary arterial hypertension. Expert Opin. Pharmacother. 2006, 7, 825–828. [Google Scholar] [CrossRef]
- Ahmed, W.S.; Geethakumari, A.M.; Biswas, K.H. Phosphodiesterase 5 (PDE5): Structure-function regulation and therapeutic applications of inhibitors. Biomed. Pharmacother. 2021, 134, 111128. [Google Scholar] [CrossRef]
- Dal Piaz, V.; Giovannoni, M.P. Phosphodiesterase 4 inhibitors, structurally unrelated to rolipram, as promising agents for the treatment of asthma and other pathologies. Eur. J. Med. Chem. 2000, 35, 463–480. [Google Scholar] [CrossRef]
- Li, H.; Zuo, J.; Tang, W. Phosphodiesterase-4 inhibitors for the treatment of inflammatory diseases. Front. Pharmacol. 2018, 9, 1048. [Google Scholar] [CrossRef] [Green Version]
- Salari-Sharif, P.; Abdollahi, M. Phosphodiesterase 4 inhibitors in inflammatory bowel disease: A comprehensive review. Curr. Pharm. Des. 2010, 16, 3661–3667. [Google Scholar] [CrossRef]
- Richter, W.; Menniti, F.S.; Zhang, H.-T.; Conti, M. PDE4 as a target for cognition enhancement. Expert Opin. Ther. Targets 2013, 17, 1011–1027. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Rajagopalan, S. Phosphodiesterase-4 inhibition as a therapeutic strategy for metabolic disorders. Obes. Rev. 2016, 17, 429–441. [Google Scholar] [CrossRef]
- Zebda, R.; Paller, A.S. Phosphodiesterase 4 inhibitors. J. Am. Acad. Dermatol. 2018, 78, S43–S52. [Google Scholar] [CrossRef]
- Mokry, J.; Urbanova, A.; Kertys, M.; Mokra, D. Inhibitors of phosphodiesterases in the treatment of cough. Respir. Physiol. Neurobiol. 2018, 257, 107–114. [Google Scholar] [CrossRef]
- Luo, J.; Yang, L.; Yang, J.; Yang, D.; Liu, B.-C.; Liu, D.; Liang, B.M.; Liu, C.-T. Efficacy and safety of phosphodiesterase 4 inhibitors in patients with asthma: A systematic review and meta-analysis. Respirology 2018, 23, 467–477. [Google Scholar] [CrossRef] [Green Version]
- Bhata, A.; Ray, B.; Mahalakshmi, A.M.; Tuladhar, S.; Nandakumar, D.N.; Srinivasan, M.; Essa, M.M.; Chidambaram, S.B.; Guillemin, G.J.; Sakharkar, M.K. Phosphodiesterase-4 enzyme as a therapeutic target in neurological disorders. Pharmacol. Res. 2020, 160, 105078. [Google Scholar] [CrossRef]
- Phillips, J.E. Inhaled phosphodiesterase 4 (PDE4) inhibitors for inflammatory respiratory diseases. Front. Pharmacol. 2020, 11, 259. [Google Scholar] [CrossRef] [Green Version]
- Peng, T.; Qi, B.; He, J.; Ke, H.; Shi, J. Advances in the development of phosphodiesterase-4 inhibitors. J. Med. Chem. 2020, 63, 10594–10617. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, M.; Cao, Z.; Qiu, P.; Song, G. Phosphodiesterase-4 inhibitors: A review of current developments (2013–2021). Expert Opin. Ther. Pat. 2022, 32, 261–278. [Google Scholar] [CrossRef]
- Lugnier, C. Cyclic nucleotide phosphodiesterase (PDE) superfamily: New target for the development of specific therapeutic agents. Pharmacol. Ther. 2006, 109, 366–398. [Google Scholar] [CrossRef]
- Soderling, S.H.; Beavo, J.A. Regulation of cAMP and cGMP signaling: New phosphodiesterases and new functions. Curr. Opin. Cell Biol. 2000, 12, 174–179. [Google Scholar] [CrossRef]
- Keravis, T.; Lugnier, C. Cyclic nucleotide phosphodiesterase (PDE) isoenzyme as targets of the intracellular signaling network: Benefits of PDE inhibitors in various diseases and perspective for future therapeutic developments. Br. J. Pharmacol. 2012, 165, 1288–1305. [Google Scholar] [CrossRef] [Green Version]
- Eskandari, N.; Mirmosayyeb, O.; Bordbari, G.; Bastan, R.; Yousefi, Z.; Andalib, A. A short review on structure and role of cyclic-3′,5′-adenosine monophosphate-specific phosphodiesterase 4 as a treatment tool. J. Pharm. Pract. Res. 2015, 4, 175–181. [Google Scholar] [CrossRef]
- Peng, T.; Gong, J.; Jin, Y.; Zhou, Y.; Tong, R.; Wei, X.; Bai, L.; Shi, J. Inhibitors of phosphodiesterase as cancer therapeutics. Eur. J. Med. Chem. 2018, 150, 742–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakkas, I.L.; Mavropoulos, A.; Bogdanos, P.D. Phosphodiesterase 4 inhibitors in immune-mediated diseases: Mode of action, clinical applications, current and future perspectives. Curr. Med. Chem. 2017, 24, 3054–3067. [Google Scholar] [CrossRef] [PubMed]
- Card, G.L.; England, B.P.; Suzuki, Y.; Fong, D.; Powell, B.; Lee, B.; Luu, C.; Tabrizizad, M.; Gillette, S.; Ibrahim, P.N.; et al. Structural basis for the activity of drugs that inhibit phosphodiesterases. Structure. 2004, 12, 2233–2247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biagini, P.; Biancalani, C.; Graziano, A.; Cesari, N.; Giovannoni, M.P.; Cilibrizzi, A.; Dal Piaz, V.; Vergelli, C.; Crocetti, L.; Del Canale, M.; et al. Functionalized pyrazoles and pyrazolo [3,4-d]pyridazinones: Synthesis and evaluation of their phosphodiesterase 4 inhibitory activity. Bioorg. Med. Chem. 2010, 18, 3506–3517. [Google Scholar] [CrossRef]
- Conti, M.; Richter, W.; Mehats, C.; Livera, G.; Park, J.-Y.; Jin, C. Cyclic AMP-specific PDE4 phosphodiesterases as critical components of cyclic AMP signaling. J. Biol. Chem. 2003, 278, 5493–5496. [Google Scholar] [CrossRef] [Green Version]
- Houslay, M.D.; Milligan, G. Tailoring cAMP-signaling responses through isoform multiplicity. Trends Biochem. Sci. 1997, 22, 217–224. [Google Scholar] [CrossRef]
- Atreya, I.; Atreya, R.; Neurath, M.F. NF-kappaB in inflammatory bowel disease. J. Intern. Med. 2008, 263, 591–596. [Google Scholar] [CrossRef]
- Nenci, A.; Becker, C.; Wullaert, A.; Gareus, R.; van Loo, G.; Danese, S.; Huth, M.; Nikolaev, A.; Neufert, C.; Madison, B.; et al. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature 2007, 446, 557–561. [Google Scholar] [CrossRef]
- Schafer, P.H.; Parton, A.; Capone, L.; Cedzik, D.; Brady, H.; Evans, J.F.; Man, H.W.; Muller, G.W.; Stirling, D.I.; Chopra, R. Apremilast is a selective PDE4 inhibitor with regulatory effects on innate immunity. Cell. Signal. 2014, 26, 2016–2029. [Google Scholar] [CrossRef] [Green Version]
- Ollivier, V.; Parry, G.C.; Cobb, R.R.; de Prost, D.; Mackman, N. Elevated cyclic AMP inhibits NF-kappaB-mediated transcription in human monocytic cells and endothelial cells. J. Biol. Chem. 1996, 271, 20828–20835. [Google Scholar] [CrossRef] [Green Version]
- Gobejishvili, L.; Barve, S.; Joshi-Barve, S.; McClain, C. Enhanced PDE4B expression augments LPS-inducible TNF expression in ethanol-primed monocytes: Relevance to alcoholic liver disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 295, G718–G724. [Google Scholar] [CrossRef]
- Eigler, A.; Siegmund, B.; Emmerich, U.; Baumann, K.H.; Hartmann, G.; Endres, S. Anti-inflammatory activities of cAMP-elevating agents: Enhancement of IL-10 synthesis and concurrent suppression of TNF production. J. Leukoc. Biol. 1998, 63, 101–107. [Google Scholar] [CrossRef]
- Essayan, D.M.; Huang, S.K.; Kagey-Sobotka, A.; Lichtenstein, L.M. Differential efficacy of lymphocyte- and monocyte-selective pretreatment with a type 4 phosphodiesterase inhibitor on antigen-driven proliferation and cytokine gene expression. J. Allergy Clin. Immunol. 1997, 99, 28–37. [Google Scholar] [CrossRef]
- Bopp, T.; Becker, C.; Klein, M.; Klein-Hessling, S.; Palmetshofer, A.; Serfling, E.; Heib, V.; Becker, M.; Kubach, J.; Schmitt, S.; et al. Cyclic adenosine monophosphate is a key component of regulatory T cell-mediated suppression. J. Exp. Med. 2007, 204, 1303–1310. [Google Scholar] [CrossRef] [Green Version]
- Bopp, T.; Dehzad, N.; Reuter, S.; Klein, M.; Ullrich, N.; Stassen, M.; Schild, H.; Buhl, R.; Schmitt, E.; Taube, C. Inhibition of cAMP degradation improves regulatory T cell-mediated suppression. J. Immunol. 2009, 182, 4017. [Google Scholar] [CrossRef]
- Houslay, M.D.; Schafer, P.; Zhang, K.Y. Keynote review: Phosphodiesterase-4 as a therapeutic target. Drug Disc. Today. 2005, 10, 1503–1519. [Google Scholar] [CrossRef]
- Jones, N.A.; Boswell-Smith, V.; Lever, R.; Page, C.P. The effect of selective phosphodiesterase isoenzyme inhibition on neutrophil function in vitro. Pulm. Pharmacol. Ther. 2005, 18, 93–101. [Google Scholar] [CrossRef]
- Schmiechen, R.; Horowski, R.; Palenschat, D.; Paschelke, G.; Wachtel, H.; Kehr, W. 4-(Polyalkoxyphenyl)-2-pyrrolidinones. U.S. Pat. 4,012,495 (to Sobering, A.G.), 15 March 1977. Available online: https://portal.unifiedpatents.com/patents/patent/US-4012495-A (accessed on 13 July 2022).
- Crossland, J. Rolipram. Drugs Future 1988, 13, 38–41. [Google Scholar] [CrossRef]
- Parkes, J.D.; Thompson, C.; Brennan, L.; Gajraj, N.; Howcroft, B.; Ruiz, J. Rolipram in Parkinson’s disease. Adv. Neurol. 1984, 40, 563–565. [Google Scholar]
- Schneider, H.H.; Schmiechen, R.; Brezinski, M.; Seidler, J. Stereospecific binding of the antidepressant rolipram to brain protein structures. Eur. J. Pharmacol. 1986, 127, 105–115. [Google Scholar] [CrossRef]
- Schudt, C.; Tenor, H.; Hatzelmann, A. PDE isoenzymes as targets for anti-asthma drugs. Eur. Respir. J. 1995, 8, 1179–1183. [Google Scholar] [CrossRef] [Green Version]
- Iona, S.; Cuomo, M.; Bushnik, T.; Naro, F.; Sette, C.; Hess, M.; Shelton, E.R.; Conti, M. Characterization of the rolipram-sensitive, cyclic AMP-specific phosphodiesterases: Identification and differential expression of immunologically distinct forms in the rat brain. Mol. Pharmacol. 1998, 53, 23–32. [Google Scholar] [CrossRef] [Green Version]
- Hartmann, G.; Bidlingmaier, C.; Siegmund, B.; Albrich, S.; Schulze, J.; Tschoep, K.; Eigler, A.; Lehr, H.A.; Endres, S. Specific type IV phosphodiesterase inhibitor rolipram mitigates experimental colitis in mice. J. Pharmacol. Exp. Ther. 2000, 292, 22–30. [Google Scholar]
- Gobejishvili, L.; Rodriguez, W.E.; Bauer, P.; Wang, Y.; Soni, C.; Lydic, T.; Barve, S.; McClain, C.; Maldonado, C. Novel liposomal rolipram formulation for clinical application to reduce emesis. Drug Des. Devel. Ther. 2022, 16, 1301–1309. [Google Scholar] [CrossRef]
- Maher, A.; El Sayed, N.; Nafea, H.; Gad, M. Rolipram rescues memory consolidation deficits caused by sleep deprivation: Implication of the cAMP/PKA and cAMP/Epac pathways. CNS Neurol. Disord. Drug Targets. 2022, 21, 631–639. [Google Scholar] [CrossRef]
- Brideau, C.; Van Staden, C.; Styhler, A.; Rodger, I.W.; Chan, C.C. The effects of phosphodiesterase type 4 inhibitors on tumour necrosis factor-alpha and leukotriene B4 in a novel human whole blood assay. Br. J. Pharmacol. 1999, 126, 979–988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCann, F.E.; Palfreeman, A.C.; Andrews, M.; Perocheau, D.P.; Inglis, J.J.; Schafer, P.; Feldmann, M.; Williams, R.O.; Brennan, F.M. Apremilast, a novel PDE4 inhibitor, inhibits spontaneous production of tumour necrosis factor-alpha from human rheumatoid synovial cells and ameliorates experimental arthritis. Arthritis Res. Ther. 2010, 12, R107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souness, J.E.; Aldous, D.; Sargent, C. Immunosuppressive and anti-inflammatory effects of cyclic AMP phosphodiesterase (PDE) type 4 inhibitors. Immunopharmacology 2000, 47, 127–162. [Google Scholar] [CrossRef]
- Harbinson, P.L.; MacLeod, D.; Hawksworth, R.; O’Toole, S.; Sullivan, P.J.; Heath, P.; Kilfeather, S.; Page, C.P.; Costello, J.; Holgate, S.T.; et al. The effect of a novel orally active selective PDE4 isoenzyme inhibitor (CDP840) on allergen-induced responses in asthmatic subjects. Eur. Respir. J. 1997, 10, 1008–1014. [Google Scholar] [CrossRef] [Green Version]
- Barnette, M.S.; Underwood, D.C. New phosphodiesterase inhibitors as therapeutics for the treatment of chronic lung disease. Curr. Opin. Pulm. Med. 2000, 6, 164–169. [Google Scholar] [CrossRef]
- Wollin, L.; Bundschuh, D.S.; Wohlsen, A.; Marx, D.; Beume, R. Inhibition of airway hyperresponsiveness and pulmonary inflammation by roflumilast and other PDE4 inhibitors. Pulm. Pharmacol. Ther. 2006, 19, 343–352. [Google Scholar] [CrossRef]
- Giembycz, M.A. Phosphodiesterase 4 inhibitors and the treatment of asthma: Where are we now and where do we go from here? Drugs 2000, 59, 193–212. [Google Scholar] [CrossRef]
- Souness, J.E.; Rao, S. Proposal for pharmacologically distinct conformers of PDE4 cyclic AMP phosphodiesterases. Cell Signal. 1997, 9, 227–236. [Google Scholar] [CrossRef]
- Duplantier, A.J.; Biggers, M.S.; Chambers, R.J.; Cheng, J.B.; Cooper, K.; Damon, D.B.; Eggler, J.F.; Kraus, K.G.; Marfat, A.; Masamune, H.; et al. Biarylcarboxylic acids and -amides: Inhibition of phosphodiesterase type IV versus [3H]rolipram binding activity and their relationship to emetic behavior in the ferret. J. Med. Chem. 1996, 39, 120–125. [Google Scholar] [CrossRef]
- Paes, D.; Schepers, M.; Rombaut, B.; van den Hove, D.; Vanmierlo, T.; Prickaerts, J. The molecular biology of phosphodiesterase 4 enzymes as pharmacological targets: An interplay of isoforms, conformational states, and inhibitors. Pharmacol. Rev. 2021, 73, 1016–1049. [Google Scholar] [CrossRef]
- Robichaud, A.; Stamatiou, P.B.; Jin, S.L.; Lachance, N.; Macdonald, D.; Laliberte, F.; Liu, S.; Huang, Z.; Conti, M.; Chan, C.C. Deletion of phosphodiesterase 4D in mice shortens alpha(2)- adrenoceptor-mediated anesthesia, a behavioral correlate of emesis. J. Clin. Invest. 2002, 110, 1045–1052. [Google Scholar] [CrossRef]
- Burgin, A.B.; Magnusson, O.T.; Singh, J.; Witte, P.; Staker, B.L.; Bjornsson, J.M.; Thorsteinsdottir, M.; Hrafnsdottir, S.; Hagen, T.; Kiselyov, A.S.; et al. Design of phosphodiesterase 4D (PDE4D) allosteric modulators for enhancing cognition with improved safety. Nat. Biotechnol. 2010, 28, 63–72. [Google Scholar] [CrossRef]
- Naganuma, K.; Omura, A.; Maekawara, N.; Saitoh, M.; Ohkawa, N.; Kubota, T.; Nagumo, H.; Kodama, T.; Takemura, M.; Ohtsuka, Y.; et al. Discovery of selective PDE4B inhibitors. Bioorg. Med. Chem. Lett. 2009, 19, 3174–3176. [Google Scholar] [CrossRef]
- Wedzicha, J.A.; Calverley, P.M.A.; Rabe, K.F. Roflumilast: A review of its use in the treatment of COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2016, 11, 81–90. [Google Scholar] [CrossRef] [Green Version]
- Woo, T.E.; Kuzel, P. Crisaborole 2% ointment (eucrisa) for atopic dermatitis. Skin Ther. Lett. 2019, 24, 4–6. [Google Scholar]
- Dozier, L.; Bartos, G.; Kerdel, F. Apremilast and psoriasis in the real world: A retrospective case series. J. Am. Acad. Dermatol. 2019, 83, 221–222. [Google Scholar] [CrossRef]
- Rolan, P.; Hutchinson, M.; Johnson, K. Ibudilast: A review of its pharmacology, efficacy and safety in respiratory and neurological disease. Expert Opin. Pharmacother. 2009, 10, 2897–2904. [Google Scholar] [CrossRef]
- Currie, G.P.; Butler, C.A.; Anderson, W.J.; Skinner, C. Phosphodiesterase 4 inhibitors in chronic obstructive pulmonary disease: A new approach to oral treatment. Br. J. Clin. Pharmacol. 2008, 65, 803–810. [Google Scholar] [CrossRef] [Green Version]
- Giembycz, M.A. Phosphodiesterase-4: Selective and dual-specificity inhibitors for the therapy of chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 2005, 2, 326–333. [Google Scholar] [CrossRef]
- Chong, J.; Leung, B.; Poole, P. Phosphodiesterase 4 inhibitors for chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2013, 9. [Google Scholar] [CrossRef]
- Wedzicha, J.A. Oral phosphodiesterase-4 inhibitors for chronic obstructive pulmonary disease “super exacerbators”. Am. J. Respir. Crit. Care Med. 2016, 194, 527–528. [Google Scholar] [CrossRef]
- Rhee, C.K.; Kim, D.K. Role of phosphodiesterase-4 inhibitors in chronic obstructive pulmonary disease. Korean J. Intern. Med. 2020, 35, 276–283. [Google Scholar] [CrossRef]
- National Center for Health Statistics. Number of deaths for leading causes of death. Available online: https://www.cdc.gov/nchs/fastats/leading-causes-of-death.htm (accessed on 13 January 2022).
- Hogg, J.C. A brief review of chronic obstructive pulmonary disease. Can. Respir. J. 2012, 19, 381–384. [Google Scholar] [CrossRef]
- Riley, C.M.; Sciurba, F.C. Diagnosis and outpatient management of chronic obstructive pulmonary disease: A review. JAMA 2019, 321, 786–797. [Google Scholar] [CrossRef]
- Yang, X.; Li, C.; Li, Y.; Du, F.; Gui, J.; Hu, J.; Bai, B.; Huang, C.; Chen, P.; Xu, G.; et al. Tiotropium in early-stage chronic obstructive pulmonary disease. N. Engl. J. Med. 2017, 377, 923–935. [Google Scholar]
- Dong, R.; Xie, L.; Zhao, K.; Zhang, Q.; Zhou, M.; He, P. Cigarette smoke-induced lung inflammation in COPD mediated via LTB4/BLT1/SOCS1 pathway. Int. J. Chron. Obstruct. Pulmon. Dis. 2016, 11, 31–41. [Google Scholar] [PubMed] [Green Version]
- Armani, E.; Amari, G.; Rizzi, A.; De Fanti, R.; Ghidini, E.; Capaldi, C.; Carzaniga, L.; Caruso, P.; Guala, M.; Peretto, I.; et al. Novel class of benzoic acid ester derivatives as potent PDE4 inhibitors for inhaled administration in the treatment of respiratory diseases. J. Med. Chem. 2014, 57, 793–816. [Google Scholar] [CrossRef] [PubMed]
- Moretto, N.; Caruso, P.; Bosco, R.; Marchini, G.; Pastore, F.; Armani, E.; Amari, G.; Rizzi, A.; Ghidini, E.; De Fanti, R.; et al. CHF6001 I: A novel highly potent and selective phosphodiesterase 4 inhibitor with robust anti-inflammatory activity and suitable for topical pulmonary administration. J. Pharmacol. Exp. Ther. 2015, 352, 559–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carzaniga, L.; Amari, G.; Rizzi, A.; Capaldi, C.; De Fanti, R.; Ghidini, E.; Villetti, G.; Carnini, C.; Moretto, N.; Facchinetti, F.; et al. Discovery and optimization of thiazolidinyl and pyrrolidinyl derivatives as inhaled PDE4 inhibitors for respiratory diseases. J. Med. Chem. 2017, 60, 10026–10046. [Google Scholar] [CrossRef]
- Mariotti, F.; Govoni, M.; Lucci, G.; Santoro, D.; Nandeuil, M.A. Safety, tolerability, and pharmacokinetics of single and repeat ascending doses of CHF6001, a novel inhaled phosphodiesterase-4 inhibitor: Two randomized trials in healthy volunteers. Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 3399–3410. [Google Scholar] [CrossRef]
- Singh, D.; Leaker, B.; Boyce, M.; Nandeuil, M.A.; Collarini, S.; Mariotti, F.; Santoro, D.; Barnes, P.J. A novel inhaled phosphodiesterase 4 inhibitor (CHF6001) reduces the allergen challenge response in asthmatic patients. Pulm. Pharmacol. Ther. 2016, 40, 1–6. [Google Scholar] [CrossRef]
- Singh, D.; Beeh, K.M.; Colgan, B.; Kornmann, O.; Leaker, B.; Watz, H.; Lucci, G.; Geraci, S.; Emirova, A.; Govoni, M.; et al. Effect of the inhaled PDE4 inhibitor CHF6001 on biomarkers of inflammation in COPD. Respir. Res. 2019, 20, 180. [Google Scholar] [CrossRef] [Green Version]
- Singh, D.; Petavy, F.; Macdonald, A.J.; Lazaar, A.L.; O’Connor, B.J. The inhaled phosphodiesterase 4 inhibitor GSK256066 reduces allergen challenge responses in asthma. Respir. Res. 2010, 11, 26. [Google Scholar] [CrossRef]
- Watz, H.; Mistry, S.J.; Lazaar, A.L.; IPC101939 investigators. Safety and tolerability of the inhaled phosphodiesterase 4 inhibitor GSK256066 in moderate COPD. Pulm. Pharmacol. Ther. 2013, 26, 588–595. [Google Scholar] [CrossRef]
- Nials, A.T.; Tralau-Stewart, C.J.; Gascoigne, M.H.; Ball, D.I.; Ranshaw, L.E.; Knowles, R.G. In vivo characterization of GSK256066, a high-affinity inhaled phosphodiesterase 4 inhibitor. J. Pharmacol. Exp. Ther. 2011, 337, 137–144. [Google Scholar] [CrossRef]
- Singh, D.; Abbott-Banner, K.; Bengtsson, T.; Newman, K. The short-term bronchodilator effects of the dual phosphodiesterase 3 and 4 inhibitor RPL554 in COPD. Eur. Respir. J. 2018, 52, 1801074. [Google Scholar] [CrossRef] [Green Version]
- Singh, D.; Martinez, F.J.; Watz, H.; Bengtsson, T.; Maurer, B.T. A dose-ranging study of the inhaled dual phosphodiesterase 3 and 4 inhibitor ensifentrine in COPD. Respir. Res. 2020, 21, 47. [Google Scholar] [CrossRef] [Green Version]
- Wunder, F.; Quednau, R.; Geerts, A.; Barg, M.; Tersteegen, A. Characterization of the cellular activity of PDE 4 inhibitors using two novel PDE 4 reporter cell lines. Mol. Pharm. 2013, 10, 3697–3705. [Google Scholar] [CrossRef]
- Martina, S.D.; Ismail, M.S.; Vesta, K.S. Cilomilast: Orally active selective phosphodiesterase-4 inhibitor for treatment of chronic obstructive pulmonary disease. Ann. Pharmacother. 2006, 40, 1822–1828. [Google Scholar] [CrossRef]
- Boot, J.D.; De Haas, S.L.; Van Gerven, J.M.A.; De Smet, M.; Leathem, T.; Wagner, J.; Denker, A.; Miller, D.; Van Doorn, M.B.A.; Schoemaker, R.C.; et al. MK-0873, a PDE4 inhibitor, does not influence the pharmacokinetics of theophylline in healthy male volunteers. Pulm. Pharmacol. Ther. 2008, 21, 573–577. [Google Scholar] [CrossRef]
- Balasubramanian, G.; Narayanan, S.; Andiappan, L.; Sappanimuthu, T.; Thirunavukkarasu, S.; Sundaram, S.; Natarajan, S.; Sivaraman, N.; Rajagopal, S.; Nazumudeen, F.A.A.; et al. In vivo effective dibenzo[b,d]furan-1-yl-thiazoles as novel PDE-4 inhibitors. Bioorg. Med. Chem. 2016, 24, 5702–5716. [Google Scholar] [CrossRef]
- Maliyar, K.; Sibbald, C.; Pope, E.; Gary Sibbald, R. Diagnosis and management of atopic dermatitis: A review. Adv. Skin. Wound. Care. 2018, 31, 538–550. [Google Scholar] [CrossRef]
- Weidinger, S.; Beck, L.A.; Bieber, T.; Kabashima, K.; Irvine, A.D. Atopic dermatitis. Nat. Rev. Dis. Primers. 2018, 4, 1. [Google Scholar] [CrossRef]
- Cury Martins, J.; Martins, C.; Aoki, V.; Gois, A.F.; Ishii, H.A.; da Silva, E.M. Topical tacrolimus for atopic dermatitis. Cochrane Database Syst. Rev. 2015, 2015, CD009864. [Google Scholar] [CrossRef]
- Greb, J.E.; Goldminz, A.M.; Elder, J.T.; Lebwohl, M.G.; Gladman, D.D.; Wu, J.J. Psoriasis. Nat. Rev. Dis. Primers. 2016, 2, 16082. [Google Scholar] [CrossRef]
- Armstrong, A.W.; Read, C. Pathophysiology, clinical presentation, and treatment of psoriasis: A review. JAMA 2020, 323, 1945–1960. [Google Scholar] [CrossRef] [PubMed]
- Chiricozzi, A.; Caposiena, D.; Garofalo, V.; Cannizzaro, M.V.; Chimenti, S.; Saraceno, R. A new therapeutic for the treatment of moderate-to-severe plaque psoriasis: Apremilast. Expert Rev. Clin. Immunol. 2016, 12, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Schafer, P.H.; Parton, A.; Gandhi, A.K.; Capone, L.; Adams, M.; Wu, L.; Bartlett, J.B.; Loveland, M.A.; Gilhar, A.; Cheung, Y.F.; et al. Apremilast, a cAMP phosphodiesterase-4 inhibitor, demonstrates anti-inflammatory activity in vitro and in a model of psoriasis. Br. J. Pharmacol. 2010, 159, 842–855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eirefelt, S.; Hummer, J.; Basse, L.H.; Bertelsen, M.; Johansson, F.; Birngruber, T.; Sinner, F.; Larsen, J.; Nielsen, S.F.; Lambert, M. Evaluating dermal pharmacokinetics and pharmacodynamics effect of soft topical PDE4 inhibitors: Open flow microperfusion and skin biopsies. Pharm. Res. 2020, 37, 243. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Z.; Zhang, H.; Guo, Y.; Yao, Z. Update on the pathogenesis and therapy of atopic dermatitis. Clin. Rev. Allergy Immunol. 2021, 61, 324–338. [Google Scholar] [CrossRef]
- Cauli, A.; Porru, G.; Piga, M.; Vacca, A.; Dessole, G.; Mathieu, A. Clinical potential of apremilast in the treatment of psoriatic arthritis. Immunotargets Ther. 2014, 3, 91–96. [Google Scholar] [CrossRef] [Green Version]
- Man, H.-W.; Schafer, P.; Wong, L.M.; Patterson, R.T.; Corral, L.G.; Raymon, H.; Blease, K.; Leisten, J.; Shirley, M.A.; Tang, Y.; et al. Discovery of (S)-N-{2-[1-(3-Ethoxy-4-methoxyphenyl)-2-methanesulfonylethyl]-1,3-dioxo-2,3-dihydro-1H-isoindol-4-yl}acetamide (Apremilast), a potent and orally active phosphodiesterase 4 and tumor necrosis factor-α inhibitor. J. Med. Chem. 2009, 52, 1522–1524. [Google Scholar] [CrossRef]
- Cada, D.J.; Ingram, K.; and Baker, D.E. Apremilast. Hosp. Pharm. 2014, 49, 752–762. [Google Scholar] [CrossRef] [Green Version]
- Paton, D.M. Crisaborole: Phosphodiesterase inhibitor for treatment of atopic dermatitis. Drugs Today 2017, 53, 239–245. [Google Scholar] [CrossRef]
- Akama, T.; Baker, S.J.; Zhang, Y.K.; Hernandez, V.; Zhou, H.; Sanders, V.; Freund, Y.; Kimura, R.; Maples, K.R.; Plattner, J.J. Discovery and structure-activity study of a novel benzoxaborole anti-inflammatory agent (AN2728) for the potential topical treatment of psoriasis and atopic dermatitis. Bioorg. Med. Chem. Lett. 2009, 19, 2129–2132. [Google Scholar] [CrossRef]
- Cheape, A.C.; Murrell, D.F. 2% Crisaborole topical ointment for the treatment of mild-to-moderate atopic dermatitis. Expert Rev. Clin. Immunol. 2017, 13, 415–423. [Google Scholar] [CrossRef]
- Ciaravino, V.; Coronado, D.; Lanphear, C.; Chanda, S. 2-Year animal carcinogenicity results for crisaborole, a novel phosphodiesterase 4 inhibitor for atopic dermatitis. J. Dermatol. Sci. 2017, 87, 116–122. [Google Scholar] [CrossRef] [Green Version]
- Stein Gold, L.F.; Spelman, L.; Spellman, M.C.; Hughes, M.H.; Zane, L.T. A phase 2, randomized, controlled, dose-ranging study evaluating Crisaborole topical ointment, 0.5% and 2% in adolescents with mild to moderate atopic dermatitis. J. Drugs Dermatol. 2015, 14, 1394–1399. [Google Scholar]
- Draelos, Z.D.; Stein Gold, L.F.; Murrell, D.F.; Hughes, M.H.; Zane, L.T. Post hoc analyses of the effect of Crisaborole topical ointment, 2% on atopic dermatitis: Associated pruritus from phase 1 and 2 clinical studies. J. Drugs Dermatol. 2016, 15, 172–176. [Google Scholar]
- Zane, L.T.; Chanda, S.; Jarnagin, K.; Nelson, D.B.; Spelman, L.; Gold, L.S. Crisaborole and its potential role in treating atopic dermatitis: Overview of early clinical studies. Immunotherapy. 2016, 8, 853–866. [Google Scholar] [CrossRef] [Green Version]
- Furue, M.; Kadono, T.; Tsuji, G.; Nakahara, T. Topical E6005/RVT-501, a novel phosphodiesterase 4 inhibitor, for the treatmentof atopic dermatitis. Expert Opin. Investig. Drugs 2017, 26, 1403–1408. [Google Scholar] [CrossRef]
- Ohba, F.; Matsuki, S.; Imayama, S.; Matsuguma, K.; Hojo, S.; Nomoto, M.; Akama, H. Efficacy of a novel phosphodiesterase inhibitor, E6005, in patients with atopic dermatitis: An investigator-blinded, vehicle-controlled study. J. Dermatolog. Treat. 2016, 27, 467–472. [Google Scholar] [CrossRef]
- Hanifin, J.M.; Ellis, C.N.; Frieden, I.J.; Folster-Holst, R.; Stein Gold, L.F.; Secci, A.; Smith, A.J.; Zhao, C.; Kornyeyeva, E.; Eichenfield, L.F. OPA-15406, a novel, topical, nonsteroidal, selective phosphodiesterase-4 (PDE4) inhibitor, in the treatment of adult and adolescent patients with mild to moderate atopic dermatitis (AD): A phase-II randomized, double-blind, placebo-controlled study. J. Am. Acad. Dermatol. 2016, 75, 297–305. [Google Scholar] [CrossRef] [Green Version]
- Felding, J.; Sorensen, M.D.; Poulsen, T.D.; Larsen, J.; Andersson, C.; Refer, P.; Engell, K.; Ladefoged, L.G.; Thormann, T.; Vinggaard, A.M.; et al. Discovery and early clinical development of 2-{6-[2-(3,5-dichloro-4-pyridyl)acetyl]-2,3-dimethoxyphenoxy}-N-propylacetamide (LEO 29102), a soft-drug inhibitor of phosphodiesterase 4 for topical treatment of atopic dermatitis. J. Med. Chem. 2014, 57, 5893–5903. [Google Scholar] [CrossRef]
- Takeiri, A.; Tanaka, K.; Harada, A.; Matsuzaki, K.; Yano, M.; Motoyama, S.; Katoh, S.; Mishima, M. Giemsa-stained pseudo-micronuclei in rat skin treated with vitamin D3 analog, pefcalcitol. Genes Environ. 2017, 39, 17. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Chen, R.; Zeng, G.; Gao, Y.; Liu, X.; Zhang, D.; Hu, P.; Wang, H.; Jiang, J. Determination of a PDE4 inhibitor Hemay005 in human plasma and urine by UPLC–MS/MS and its application to a PK study. Bioanalysis 2018, 10, 863–875. [Google Scholar] [CrossRef]
- Nielsen, S.F. Preparation of benzodioxole or benzodioxepine heterocyclic compounds as phosphodiesterase inhibitors. PCT WO2011/160632A1, 29 December 2011. [Google Scholar]
- Guay, D.; Boulet, L.; Friesen, R.W.; Girard, M.; Hamel, P.; Huang, Z.; Laliberté, F.; Laliberté, S.; Mancini, J.A.; Muise, E.; et al. Optimization and structure-activity relationship of a series of 1-phenyl-1,8-naphthyridin-4-one-3-carboxamides: Identification of MK-0873, a potent and effective PDE4 inhibitor. Bioorg. Med. Chem. Lett. 2008, 18, 5554–5558. [Google Scholar] [CrossRef]
- Bäumer, W.; Hoppmann, J.; Rundfeldt, C.; Kietzmann, M. Highly selective phosphodiesterase 4 inhibitors for the treatment of allergic skin diseases and psoriasis. Inflamm. Allergy Drug Targets. 2007, 6, 17–26. [Google Scholar] [CrossRef] [Green Version]
- Ahluwalia, J.; Udkoff, J.; Waldman, A.; Borok, J.; Eichenfield, L.F. Phosphodiesterase 4 inhibitor therapies for atopic dermatitis: Progress and outlook. Drugs 2017, 77, 1389–1397. [Google Scholar] [CrossRef]
- Aletaha, D.; Smolen, J.S. Diagnosis and management of rheumatoid arthritis: A review. JAMA 2018, 320, 1360–1372. [Google Scholar] [CrossRef]
- Horiuchi, A.C.; Pereira, L.H.C.; Kahlow, B.S.; Silva, M.B.; Skare, T.L. Rheumatoid arthritis in elderly and young patients. Rev. Bras. Reumatol. Engl. Ed. 2017, 57, 491–494. [Google Scholar] [CrossRef] [Green Version]
- Ometto, F.; Fedeli, U.; Botsios, C.; Punzi, L.; Grande, E. A long-term nationwide study on mortality associated to rheumatoid arthritis in Italy. Clin. Exp. Rheumatol. 2020, 38, 1223–1226. [Google Scholar]
- Crofford, L.J. Use of NSAIDs in treating patients with arthritis. Arthritis Res. Ther. 2013, 15 (Suppl. S3), S2. [Google Scholar] [CrossRef] [Green Version]
- Weinblatt, M.E. Methotrexate in rheumatoid arthritis: A quarter century of development. Trans. Am. Clin. Climatol. Assoc. 2013, 124, 16–25. [Google Scholar] [PubMed]
- Witte, T. Methotrexate as combination partner of TNF inhibitors and tocilizumab. What is reasonable from an immunological viewpoint? Clin. Rheumatol. 2015, 34, 629–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shams, S.; Martinez, J.M.; Dawson, J.R.D.; Flores, J.; Gabriel, M.; Garcia, G.; Guevara, A.; Murray, K.; Pacifici, N.; Vargas, M.V.; et al. The therapeutic landscape of rheumatoid arthritis: Current state and future directions. Front. Pharmacol. 2021, 12, 680043. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Goldminz, A.M.; Kim, N.; Gottlieb, A.B. Phosphodiesterase 4-targeted treatments for autoimmune diseases. BMC Medicine. 2013, 11, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spina, D. PDE4 inhibitors: Current status. Br. J. Pharmacol. 2008, 155, 308–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geiler, J.; Buch, M.; McDermott, M.F. Anti-TNF treatment in rheumatoid arthritis. Curr. Pharm. Des. 2011, 17, 3141–3154. [Google Scholar] [CrossRef]
- Sekut, L.; Yarnall, D.; Stimpson, S.A.; Noel, L.S.; Bateman-Fite, R.; Clark, R.L.; Brackeen, M.F.; Menius, J.A.; Connolly, K.M. Anti-inflammatory activity of phosphodiesterase (PDE)-IV inhibitors in acute and chronic models of inflammation. Clin. Exp. Immunol. 1995, 100, 126–132. [Google Scholar] [CrossRef]
- Crilly, A.; Robertson, S.E.; Reilly, J.H.; Gracie, J.A.; Lai, W.Q.; Leung, B.P.; Life, P.F.; Mclnnes, I.B. Phosphodiesterase 4 (PDE4) regulation of proinflammatory cytokine and chemokine release from rheumatoid synovial membrane. Ann. Rheum. Dis. 2011, 70, 1130–1137. [Google Scholar] [CrossRef]
- Pal, R.; Chaudhary, M.J.; Tiwari, P.C.; Babu, S.; Pant, K.K. Protective role of theophylline and their interaction with nitric oxide (NO) in adjuvant-induced rheumatoid arthritis in rats. Int. Immunopharmacol. 2015, 29, 854–862. [Google Scholar] [CrossRef]
- Clanchy, F.I.L.; Williams, R.O. Ibudilast inhibits chemokine expression in rheumatoid arthritis synovial fibroblasts and exhibits immunomodulatory activity in experimental arthritis. Arthritis Rheumatol. 2019, 71, 703–711. [Google Scholar] [CrossRef]
- Khairatkar-Joshi, N.; Anupindi, R.; Vaiyapuri, T.S. Pharmaceutical composition comprising the PDE4 enzyme inhibitor revamilast and a disease modifying agent, preferably methotrexate for treatment of autoimmune diseases. PCT Int. Appl. WO2012110946A1, 23 August 2012. [Google Scholar]
- Kiriakidou, M.; Ching, C.L. Systemic lupus erythematosus. Ann. Intern. Med. 2020, 172, ITC81–ITC96. [Google Scholar] [CrossRef]
- Durcan, L.; O’Dwyer, T.; Petri, M. Management strategies and future directions for systemic lupus erythematosus in adults. Lancet. 2019, 393, 2332–2343. [Google Scholar] [CrossRef]
- Kuhn, A.; Landmann, A.; Wenzel, J. Advances in the treatment of cutaneous lupus erythematosus. Lupus 2016, 25, 830–837. [Google Scholar] [CrossRef] [Green Version]
- Yougbare, I.; Morin, C.; Senouvo, F.Y.; Sirois, C.; Albadine, R.; Lugnier, C.; Rousseau, E. NCS 613, a potent and specific PDE4 inhibitor, displays anti-inflammatory effects on human lung tissues. Am. J. Physiol. Lung Cell. Mol. Physiol. 2011, 301, L441–L450. [Google Scholar] [CrossRef]
- Keravis, T.; Monneaux, F.; Yougbare, I.; Gazi, L.; Bourguignon, J.J.; Muller, S.; Lugnier, C. Disease progression in MRL/lpr lupus-prone mice is reduced by NCS 613, a specific cyclic nucleotide phosphodiesterase type 4 (PDE4) inhibitor. PLoS ONE 2012, 7, e28899. [Google Scholar] [CrossRef] [Green Version]
- Yougbare, I.; Boire, G.; Roy, M.; Lugnier, C.; Rouseau, E. NCS 613 exhibits anti-inflammatory effects on PBMCs from lupus patients by inhibiting p38 MAPK and NF-kappaB signalling pathways while reducing proinflammatory cytokine production. Can. J. Physiol. Pharmacol. 2013, 91, 353–361. [Google Scholar] [CrossRef]
- Yougbare, I.; Keravis, T.; Lugnier, C. NCS 613, a PDE4 inhibitor, by increasing cAMP level suppresses systemic inflammation and immune complexes deposition in kidney of MRL/lpr lupus- prone mice. BBA-Mol. Basis Dis. 2021, 1867, 166019. [Google Scholar] [CrossRef]
- Dobson, R.; Giovannoni, G. Multiple sclerosis a review. Eur. J. Neurol. 2019, 26, 27–40. [Google Scholar] [CrossRef] [Green Version]
- Murua, S.R.; Farez, M.F.; Quintana, F.J. The immune response in multiple sclerosis. Annu. Rev. Pathol. 2022, 17, 121–139. [Google Scholar] [CrossRef]
- Schepers, M.; Tiane, A.; Paes, D.; Sanchez, S.; Rombaut, B.; Piccart, E.; Rutten, B.P.F.; Brone, B.; Hellings, N.; Prickaerts, J.; et al. Targeting phosphodiesterases-towards a tailor-made approach in multiple sclerosis treatment. Front. Immunol. 2019, 10, 1727. [Google Scholar] [CrossRef]
- Sommer, N.; Loschmann, P.A.; Northoff, G.H.; Weller, M.; Steinbrecher, A.; Steinbach, J.P.; Lichtenfels, R.; Meyermann, R.; Riethmuller, A.; Fontana, A.; et al. The antidepressant rolipram suppresses cytokine production and prevents autoimmune encephalomyelitis. Nat. Med. 1995, 1, 244–248. [Google Scholar]
- Genain, C.P.; Roberts, T.; Davis, R.L.; Nguyen, M.H.; Uccelli, A.; Faulds, D.; Li, Y.; Hedgpeth, J.; Hauser, S.L. Prevention of autoimmune demyelination in non-human primates by a cAMP-specific phosphodiesterase inhibitor. Proc. Natl. Acad. Sci. USA 1995, 92, 3601–3605. [Google Scholar] [CrossRef] [Green Version]
- Bielekova, B.; Richert, N.; Howard, T.; Packer, A.N.; Blevins, G.; Ohayon, J.; McFarland, H.F.; Stürzebecher, C.-S.; Martin, R. Treatment with the phosphodiesterase type-4 inhibitor rolipram fails to inhibit blood–brain barrier disruption in multiple sclerosis. Mult. Scler. 2009, 15, 1206–1214. [Google Scholar] [CrossRef]
- Fox, R.J.; Coffey, C.S.; Conwit, R.; Cudkowicz, M.E.; Gleason, T.; Goodman, A.; Klawiter, E.C.; Matsuda, K.; McGovern, M.; Naismith, R.T.; et al. Phase 2 trial of ibudilast in progressive multiple sclerosis. N. Engl. J. Med. 2018, 379, 846–855. [Google Scholar] [CrossRef]
- Hardiman, O.; Al-Chalabi, A.; Chio, A.; Corr, E.M.; Logroscino, G.; Robberecht, W.; Shaw, P.J.; Simmons, Z.; van den Berg, L.H. Amyotrophic lateral sclerosis. Nat. Rev. Dis. Primers. 2017, 3, 17071. [Google Scholar] [CrossRef] [Green Version]
- Soriani, M.-H.; Desnuelle, C. Care management in amyotrophic lateral sclerosis. Rev. Neurol. 2017, 173, 288–299. [Google Scholar] [CrossRef]
- Rose, G.M.; Hopper, A.; De Vivo, M.; Tehim, A. Phosphodiesterase inhibitors for cognitive enhancement. Curr. Pharm. Des. 2005, 11, 3329–3334. [Google Scholar] [CrossRef]
- Garcia-Osta, A.; Cuadrado-Tejedor, M.; Garcia-Barroso, C.; Oyarzabal, J.; Franco, R. Phosphodiesterases as therapeutic targets for Alzheimer’s disease. ACS Chem. Neurosci. 2012, 3, 832–844. [Google Scholar] [CrossRef] [Green Version]
- Pearse, D.D.; Hughes, Z.A. PDE4B as a microglia target to reduce neuroinflammation. Glia 2016, 64, 1698–1709. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Ryder, J.; Ni, B. Inhibition of Abeta production and APP maturation by a specific PKA inhibitor. FEBS Lett. 2003, 546, 407. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Torres, S.; Cortés, R.; Tolnay, M.; Probst, A.; Palacios, J.M.; Mengod, G. Alterations on phosphodiesterase type 7 and 8 isozyme mRNA expression in Alzheimer’s disease brains examined by in situ hybridization. Exp. Neurol. 2003, 182, 322. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Yang, X.M.; Zhuo, Y.Y.; Zhou, H.; Lin, H.B.; Cheng, Y.F.; Xu, J.-P.; Zhang, H.-T. The phosphodiesterase-4 inhibitor rolipram reverses Aβ-induced cognitive impairment and neuroinflammatory and apoptotic responses in rats. Int. J. Neuropsychopharmacol. 2012, 15, 749. [Google Scholar] [CrossRef] [Green Version]
- Dowlatshahi, D.; MacQueen, G.M.; Wang, J.F.; Young, L.T. Increased temporal cortex CREB concentrations and antidepressant treatment in major depression. Lancet 1998, 352, 1754. [Google Scholar]
- Dwivedi, Y.; Conley, R.R.; Roberts, R.C.; Tamminga, C.A.; Pandey, G.N. [(3)H]cAMP binding sites and protein kinase a activity in the prefrontal cortex of suicide victims. Am. J. Psychiatry 2002, 159, 66–73. [Google Scholar] [CrossRef]
- Zhang, H.T.; Huang, Y.; Mishler, K.; Roerig, S.C.; O’Donnell, J.M. Interaction between the antidepressant-like behavioral effects of beta adrenergic agonists and the cyclic AMP PDE inhibitor rolipram in rats. Psychopharmacology 2005, 182, 104–115. [Google Scholar] [CrossRef]
- Bolger, G.B. The PDE4 cAMP-specific phosphodiesterases: Targets for drugs with antidepressant and memory-enhancing action. Adv. Neurobiol. 2017, 17, 63–102. [Google Scholar]
- Wyant, K.J.; Ridder, A.J.; Dayalu, P. Huntington’s disease-update on treatments. Curr. Neurol. Neurosci. Rep. 2017, 17, 33. [Google Scholar] [CrossRef]
- Ciacco, C.; Fontana, L.; Milani, D.; Tabano, S.; Miozzo, M.; Esposito, S. Fragile X syndrome: A review of clinical and molecular diagnoses. Ital. J. Pediatr. 2017, 43, 39. [Google Scholar] [CrossRef] [Green Version]
- Gurney, M.E.; Nugent, R.A.; Mo, X.; Sindac, J.A.; Hagen, T.J.; Fox, D.; O’Donnell, J.M.; Zhang, C.; Xu, Y.; Zhang, H.-T.; et al. Design and synthesis of selective phosphodiesterase 4D (PDE4D) allosteric inhibitors for the treatment of fragile X syndrome and other brain disorders. J. Med. Chem. 2019, 62, 4884–4901. [Google Scholar] [CrossRef]
- Rosenheck, M.; Sheeler, C.; Sarè, R.M.; Gurney, M.E.; Smith, C.B. Effects of chronic inhibition of phosphodiesterase-4D on behavior and regional rates of cerebral protein synthesis in a mouse model of fragile X syndrome. Neurobiol. Dis. 2021, 159, 105485. [Google Scholar] [CrossRef]
- Berry-Kravis, E.M.; Harnett, M.D.; Reines, S.A.; Reese, M.A.; Ethridge, L.E.; Outterson, A.H.; Michalak, C.; Furman, J.; Gurney, M.E. Inhibition of phosphodiesterase-4D in adults with fragile X syndrome: A randomized, placebo-controlled, phase 2 clinical trial. Nat. Med. 2021, 27, 862–870. [Google Scholar] [CrossRef]
- Lugnier, C.; Al-Kuraishy, H.M.; Rousseau, E. PDE4 inhibition as a therapeutic strategy for improvement of pulmonary dysfunctions in COVID-19 and cigarette smoking. Biochem. Pharmacol. 2021, 185, 114431. [Google Scholar] [CrossRef]
- Giorgi, M.; Cardarelli, S.; Ragusa, F.; Saliola, M.; Biagioni, S.; Poiana, G.; Naro, F.; Massimi, M. Phosphodiesterase inhibitors: Could they be beneficial for the treatment of COVID-19? Int. J. Mol. Sci. 2020, 21, 5338. [Google Scholar] [CrossRef]
- Bridgewood, C.; Damiani, G.; Sharif, K.; Watad, A.; Bragazzi, N.L.; Quartuccio, L.; Savic, S.; McGonagle, D. Rationale for evaluating PDE4 inhibition for mitigating against severe inflammation in COVID-19 pneumonia and beyond. Isr. Med. Assoc. J. 2020, 22, 335–339. [Google Scholar]



| Compound | Chemical Structure | Company | Phase | NCT Number a |
|---|---|---|---|---|
| CHF6001 (Tanimilast) | 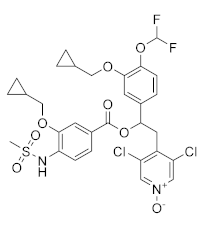 | Chiesi Farmaceutici | Phase III (COPD) | NCT04636801 (2022) NCT04636814 (2021) |
| Phase II (Asthma) | NCT01689571 (2017) | |||
| Phase II (COPD) | NCT03004417 (2020) NCT01730404 (2017) NCT02986321 (2019) | |||
| Phase I (COPD) | NCT04756960 (2021) NCT05373953 (2022) NCT04739774 (2021) NCT02386761 (2020) NCT05431426 (2022) NCT01703052 (2020) | |||
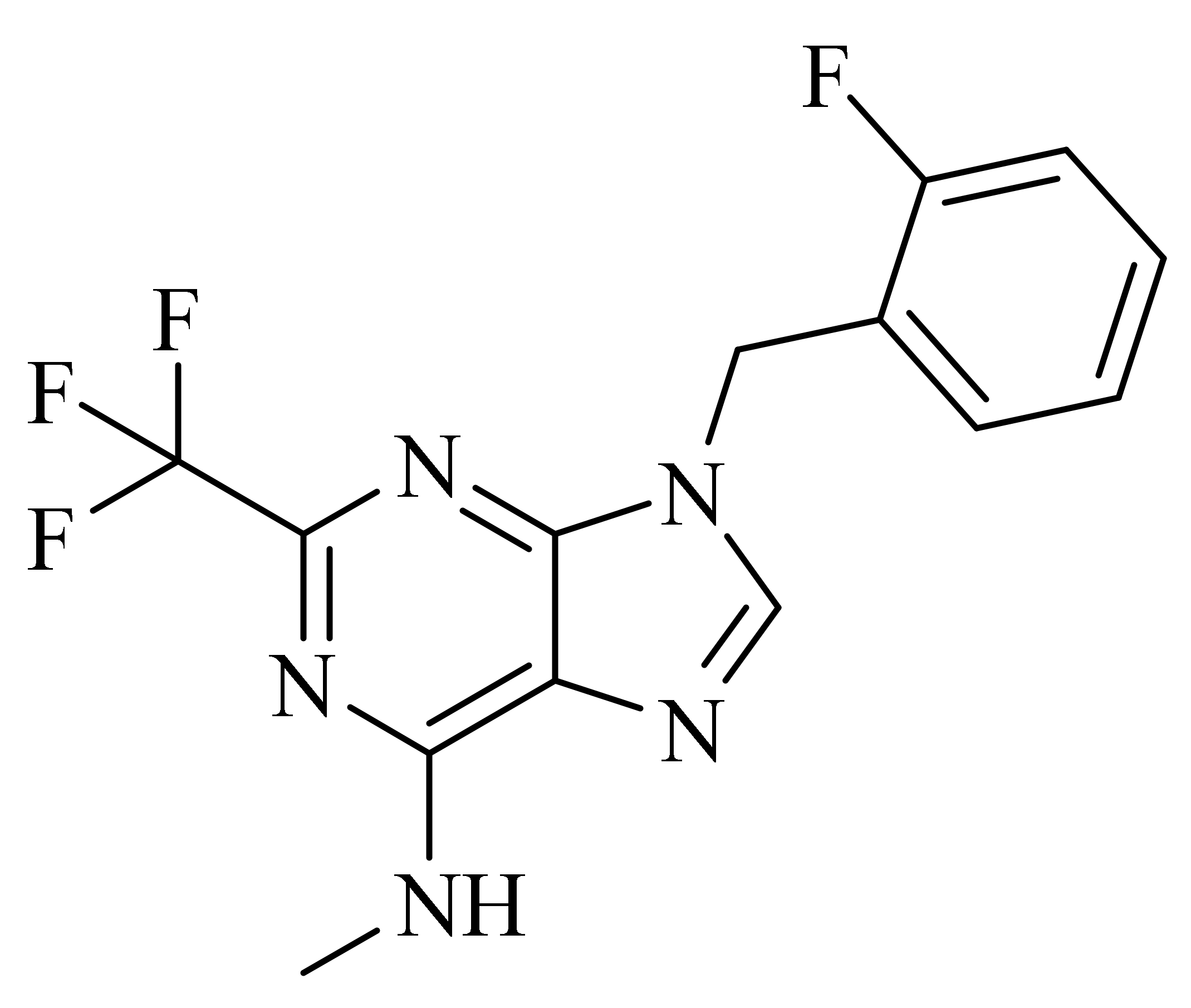
| GSK256066 | 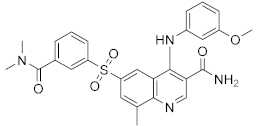 | GSK | Phase II (Asthma) | NCT00549744 (2017) |
| Phase II (COPD) | NCT00549679 (2017) NCT00515268 (2017) | |||
| RPL554 |  | Verona Pharma | Phase II (COPD) | NCT04027439 (2021) NCT03937479 (2020) NCT03673670 (2019) NCT03443414 (2019) NCT04091360 (2021) NCT05270525 (2022) |
| Oglemilast |  | Forest Laboratories | Phase II (COPD) | NCT00671073 (2019) |
| Cilomilast |  | GlaxoSmithKline | Phase III (COPD) | NCT00103922 (2016) |
| MK0873 | 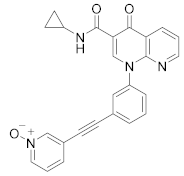 | Merck Sharp & Dohme LLC | Phase II (COPD) | NCT00132730 (2018) |
| Revamilast (GRC 4039) | 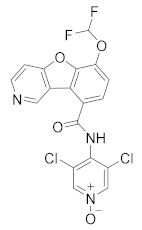 | Glenmark Pharmaceuticals Ltd. India | Phase II (Asthma) | NCT01436890 (2013) |
| Compound | Chemical Structure | Company | Phase | NCT Number a |
|---|---|---|---|---|
| E6005 | 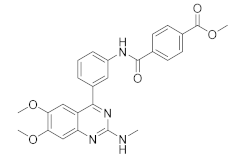 | Dermavant Sciences | Phase II (AD) | NCT01461941 (2018) |
| Phase I/II (AD) | NCT01179880 (2018) NCT02094235 (2018) | |||
| GW842470X | 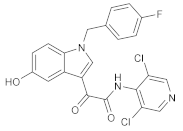 | GSK | Phase II (AD) | NCT00354510 (2012) |
| Phase I (AD) | NCT00356642 (2017) | |||
| OPA-15406 | 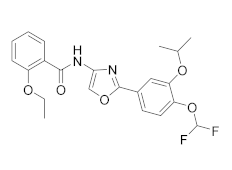 | Otsuka | Phase III (AD) | NCT05372653 (2022) NCT03961529 (2021) NCT03908970 (2021) NCT03911401 (2021) |
| Phase II (AD) | NCT02068352 (2021) NCT02914548 (2020) NCT03018691 (2020) NCT02945657 (2018) | |||
| Phase I (AD) | NCT02334787 (2016) NCT01702181 (2014) | |||
| Leo-29102 |  | Leo Pharma | Phase II (AD) | NCT01037881 (2019) |
| Phase II (Psoriasis Vulgaris) | NCT00875277 (2019) | |||
| Phase I (AD) | NCT00891709 (2016) NCT01447758 (2013) NCT01005823 (2013) NCT00958516 (2013) NCT01423656 (2013) | |||
| Phase I (Psoriasis Vulgaris) | NCT01466478 (2013) | |||
| DRM02 | Undisclosed | Dermira | Phase II (Rosacea) | NCT01993446 (2021) |
| Phase II (AD) | NCT01993420 (2021) | |||
| Phase II (Psoriasis) | NCT01993433 (2021) | |||
| Pefcalcitol (M518101) | 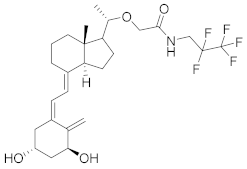 | Maruho | Phase III (Psoriasis) | NCT01908595 (2015) |
| Phase II (Psoriasis) | NCT02970331 (2019) | |||
| Hemay005 |  | Tianjin Hemay Pharmaceutical Co., Ltd. | Phase III (Psoriasis) | NCT04839328 (2022) |
| Phase II (Psoriasis) | NCT04102241 (2021) | |||
| Phase I (Psoriasis) | NCT04837235 (2021) NCT03007810 (2018) NCT03570346 (2018) | |||
| Orismilast (LEO-32731) |  | UNION therapeutics | Phase II (Psoriasis and Skin Diseases) | NCT05190419 (2022) |
| MK-0873 | 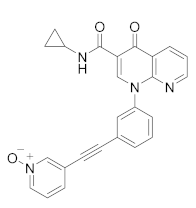 | Merck Sharp & Dohme LLC | Phase I (Psoriasis Plaque) | NCT01140061 (2019) NCT01235728 (2019) |
| Apremilast (CC-10004) | 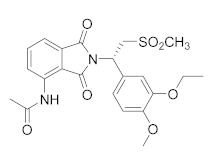 | Amgen | Phase IV (Psoriasis) | NCT03022617 (2021) NCT02400749 (2018) |
| Phase III (different type of Psoriasis) | NCT03777436 (2022) NCT04175613 (2022) NCT01194219 (2022) NCT03701763 (2022) NCT03930186 (2022) NCT05174065 (2022) NCT04804553 (2022) NCT03721172 (2021) | |||
| Phase II (Psoriasis) | NCT04572997 (2021) NCT00604682 (2020) NCT00606450 (2020) | |||
| Phase II (AD and eczema) | NCT04306965 (2021) NCT03160248 (2021) | |||
| Crisaborole (AN2728) | 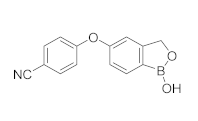 | Pzifer | Phase IV (AD and eczema) | NCT03832010 (2022) NCT04023084 (2021) |
| Phase IV (Seborrheic Dermatitis) | NCT03567980 (2021) | |||
| Phase II (AD and eczema) | NCT04091087 (2022) NCT01652885 (2017) | |||
| Phase II (Morphea) | NCT03351114 (2021) | |||
| Phase II (Psoriasis) | NCT00759161 (2017) NCT01300052 (2017) NCT00759161 (2017) NCT01029405 (2017) | |||
| Phase I (Psoriasis) | NCT00762658 (2019) NCT00763204 (2019) |
| Compound | Chemical Structure | Company | Phase | NCT Number a |
|---|---|---|---|---|
| Revamilast (GRC 4039) |  | Glenmark Pharmaceuticals Ltd. India | Phase II (RA) | NCT01430507 (2012) |
| MK0873 |  | Merck Sharp & Dohme LLC | Phase II (RA) | NCT00132769 (2015) |
| Apremilast (CC-10004) |  | Amgen | Phase II (RA) | NCT01285310 (2020) NCT01250548 (2014) |
| Phase I/II (lupus) | NCT00708916 (2021) |
| Compound | Chemical Structure | Company | Phase | NCT Number a |
|---|---|---|---|---|
| Ibudilast (MN-166) | 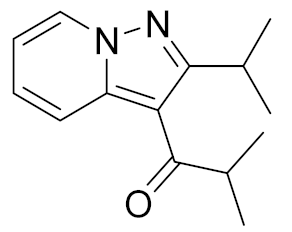 | Medicinova | Phase II (ASL) | NCT04057898 (2022) NCT02714036 (2020) NCT02238626 (2021) NCT01982942 (2020) |
| Phase II (Glioblastoma) | NCT03782415 (2022) | |||
| Phase I (Migraine Headache) | NCT01389193 (2015) | |||
| BPN14770 (Zatomilast) |  | Shionogi Pharma, Tetra Therapeutics | Phase III (Fragile X Syndrome) | NCT05367960 (2022) NCT05358886 (2022) NCT05163808 (2022) |
| Phase II (Fragile X Syndrome) | NCT03569631 (2020) | |||
| Phase II (Alzheimer disease) | NCT03817684 (2019) | |||
| Phase II (Depression) | NCT03861000 (2021) | |||
| Phase I (Alzheimer disease) | NCT02648672 (2017) NCT02840279 (2017) NCT03030105 (2018) | |||
| GSK356278 | 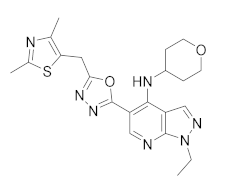 | GlaxoSmith Kline | Phase I (Huntington disease) | NCT01602900 (2017) NCT01573819 (2017) |
| Phase I (Depressive Disorder and Anxiety Disorders) | NCT01031186 (2017) | |||
| MK-0952 | 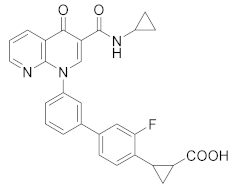 | Merck Sharp & Dohme LLC | Phase II (Alzheimer disease) | NCT00362024 (2016) |
| Roflumilast | 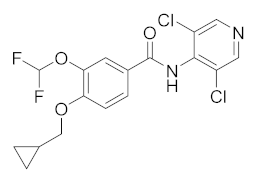 | AstraZeneca | Phase II (Dementia) | NCT04658654 (2022) NCT01433666 (2020) |
| Phase II (Cerebrovascular Disorder) | NCT04854811 (2021) | |||
| Phase II (Nervous System Disease) | NCT02743377 (2020) | |||
| Phase I (Major Depressive Disorder) | NCT04751071 (2022) | |||
| Phase I (Schizophrenia) | NCT02079844 (2016) | |||
| Phase I (Memory ImpairmentAlzheimer’s Disease) | NCT02051335 (2017) | |||
| Phase I (Fragile X Syndrome) | NCT05418049 (2022) | |||
| Rolipram | 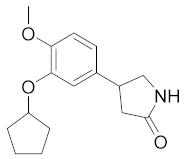 | Schering AG | Phase II (Nervous System Disease, PET) | NCT02743377 (2020) |
| Phase I (Major Depressive Disorder, PET) | NCT00369798 (2018) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crocetti, L.; Floresta, G.; Cilibrizzi, A.; Giovannoni, M.P. An Overview of PDE4 Inhibitors in Clinical Trials: 2010 to Early 2022. Molecules 2022, 27, 4964. https://doi.org/10.3390/molecules27154964
Crocetti L, Floresta G, Cilibrizzi A, Giovannoni MP. An Overview of PDE4 Inhibitors in Clinical Trials: 2010 to Early 2022. Molecules. 2022; 27(15):4964. https://doi.org/10.3390/molecules27154964
Chicago/Turabian StyleCrocetti, Letizia, Giuseppe Floresta, Agostino Cilibrizzi, and Maria Paola Giovannoni. 2022. "An Overview of PDE4 Inhibitors in Clinical Trials: 2010 to Early 2022" Molecules 27, no. 15: 4964. https://doi.org/10.3390/molecules27154964