Antibacterial Potential of Bacopa monnieri (L.) Wettst. and Its Bioactive Molecules against Uropathogens—An In Silico Study to Identify Potential Lead Molecule(s) for the Development of New Drugs to Treat Urinary Tract Infections
Abstract
:1. Introduction
2. Results
2.1. Antimicrobial Activity of Crude Extracts of B. monnieri
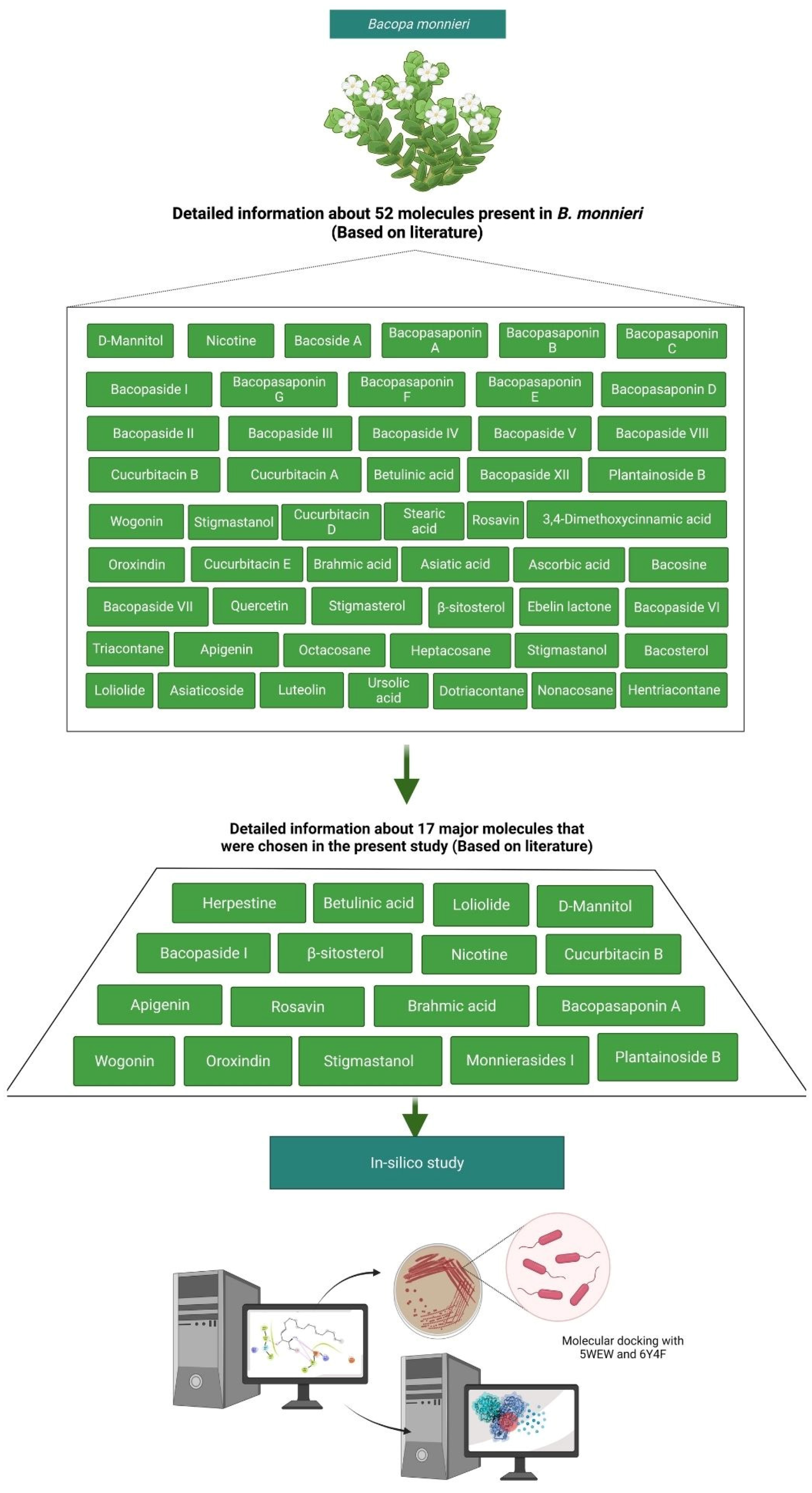
2.2. Phytochemical Screening
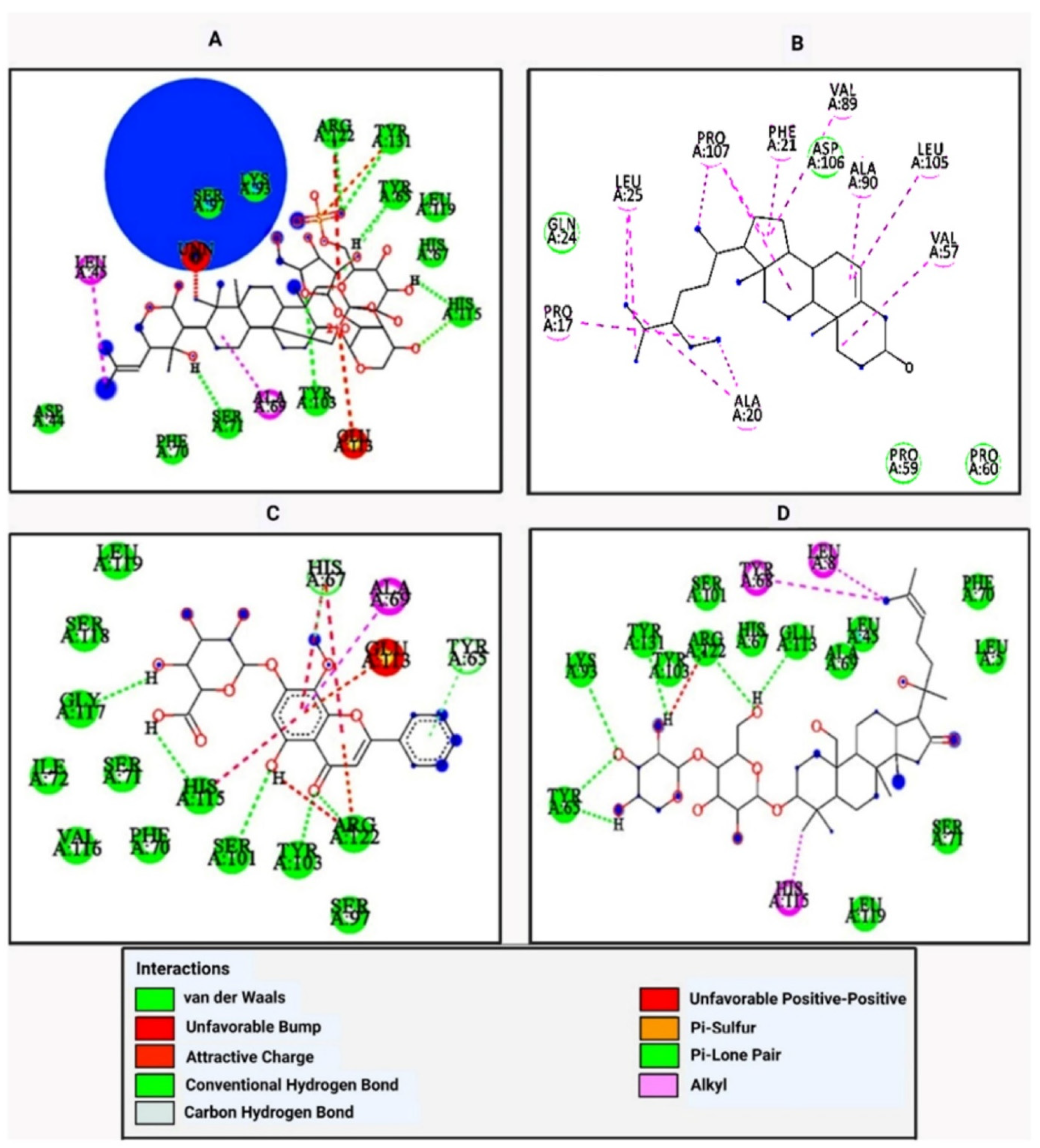
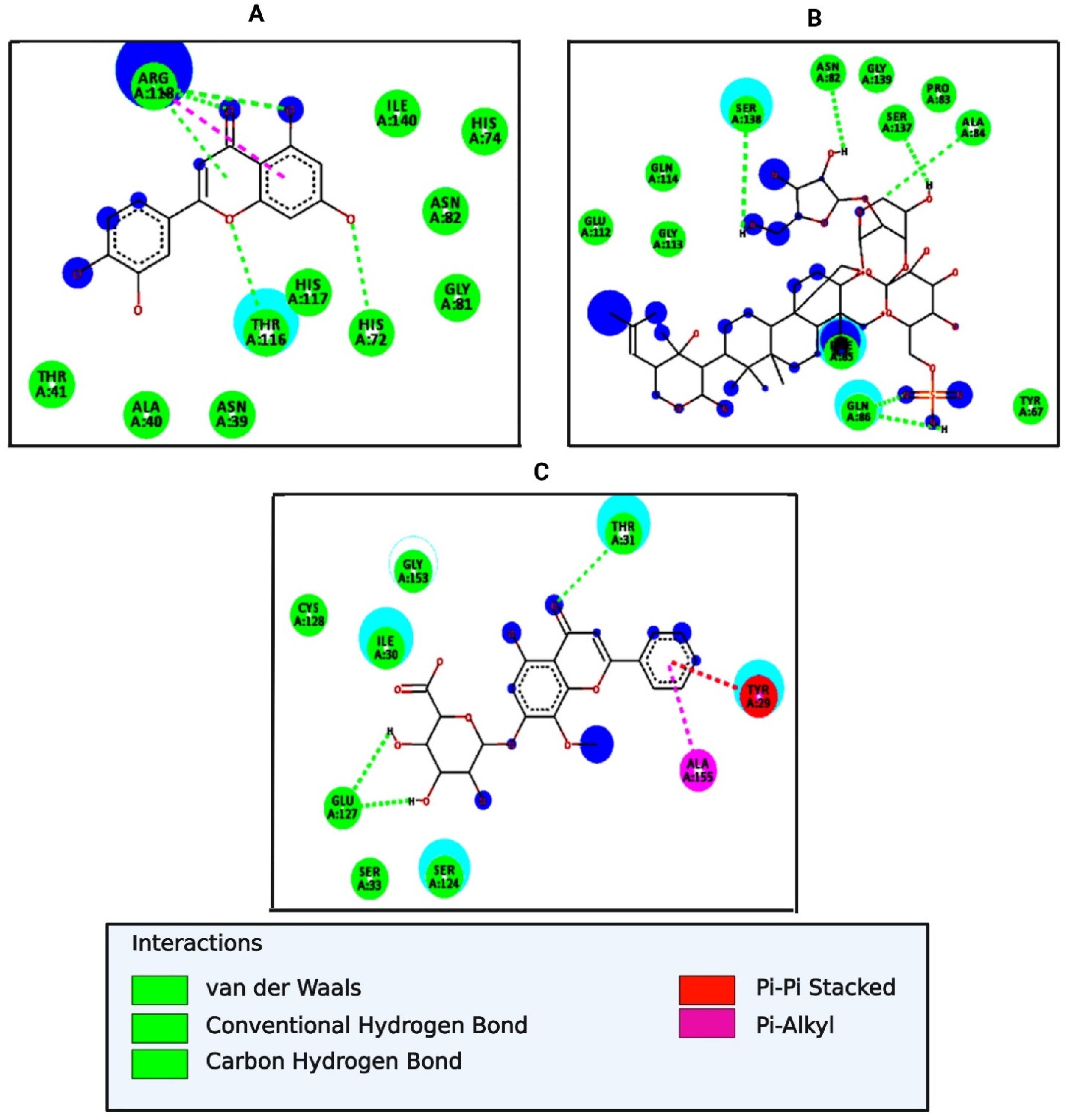
2.3. Molecular Docking Analysis of Major Bioactive Molecules from B. monnieri with UTI Proteins (5WEW and 6Y4F)
2.4. Drug-Likeness Predictions of Bioactive Molecules of B. monnieri
2.5. Toxicity Predictions for Trimethoprim and the Major Bioactive Molecules from B. monnieri
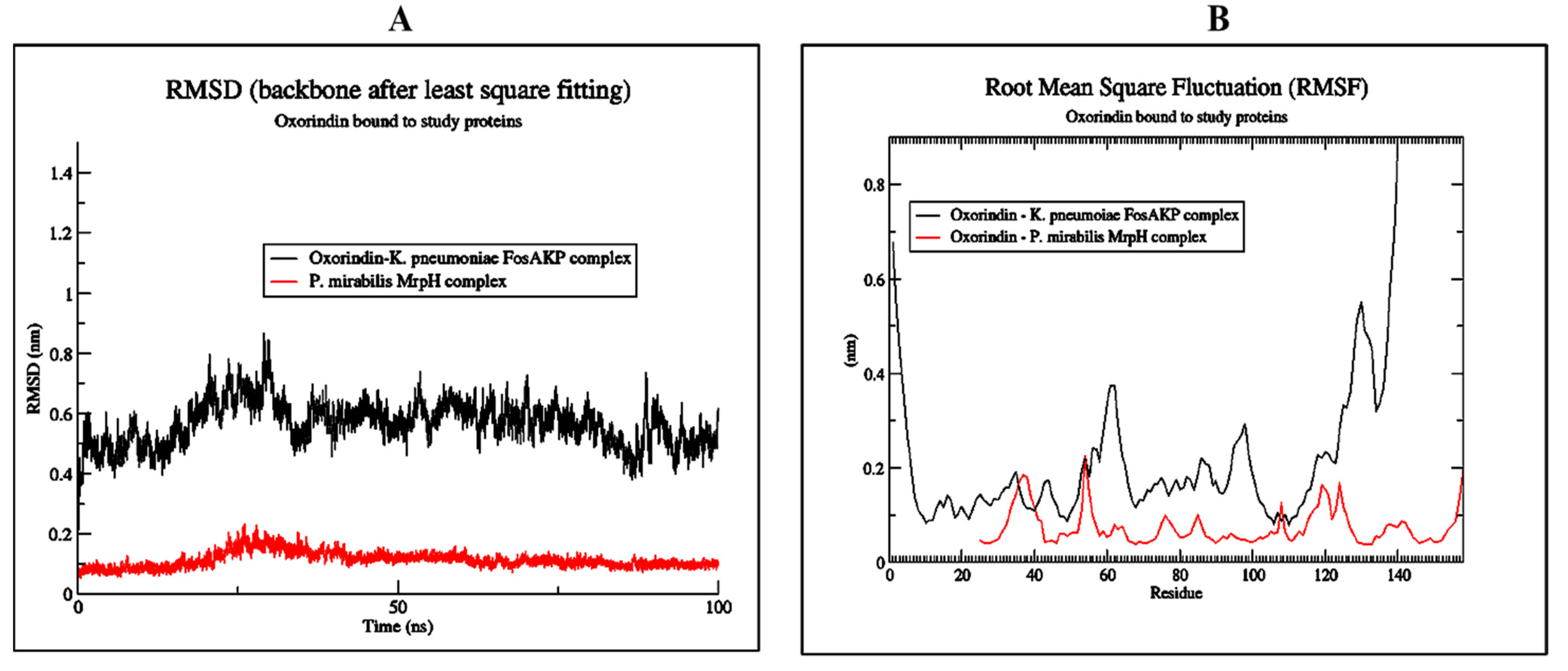
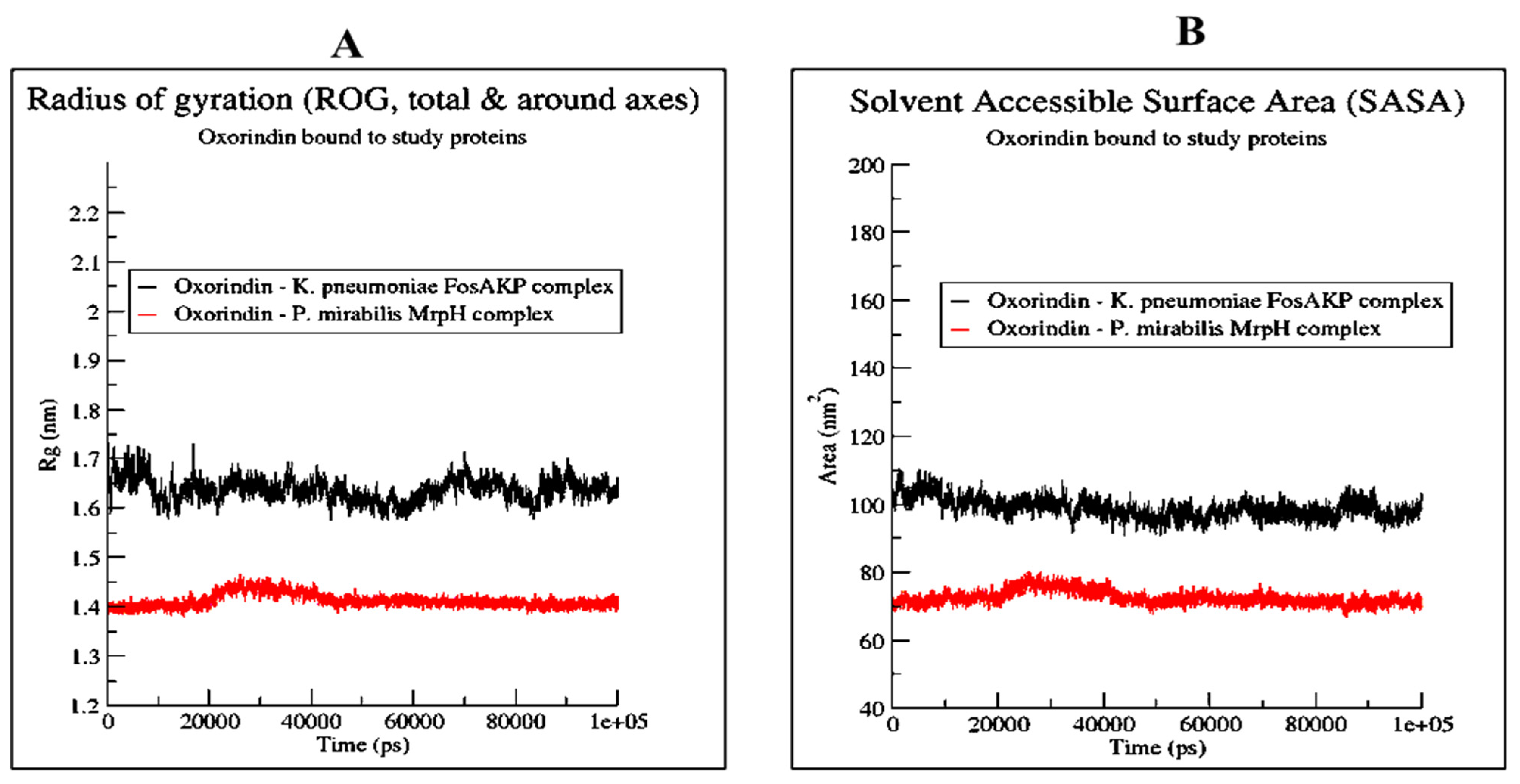
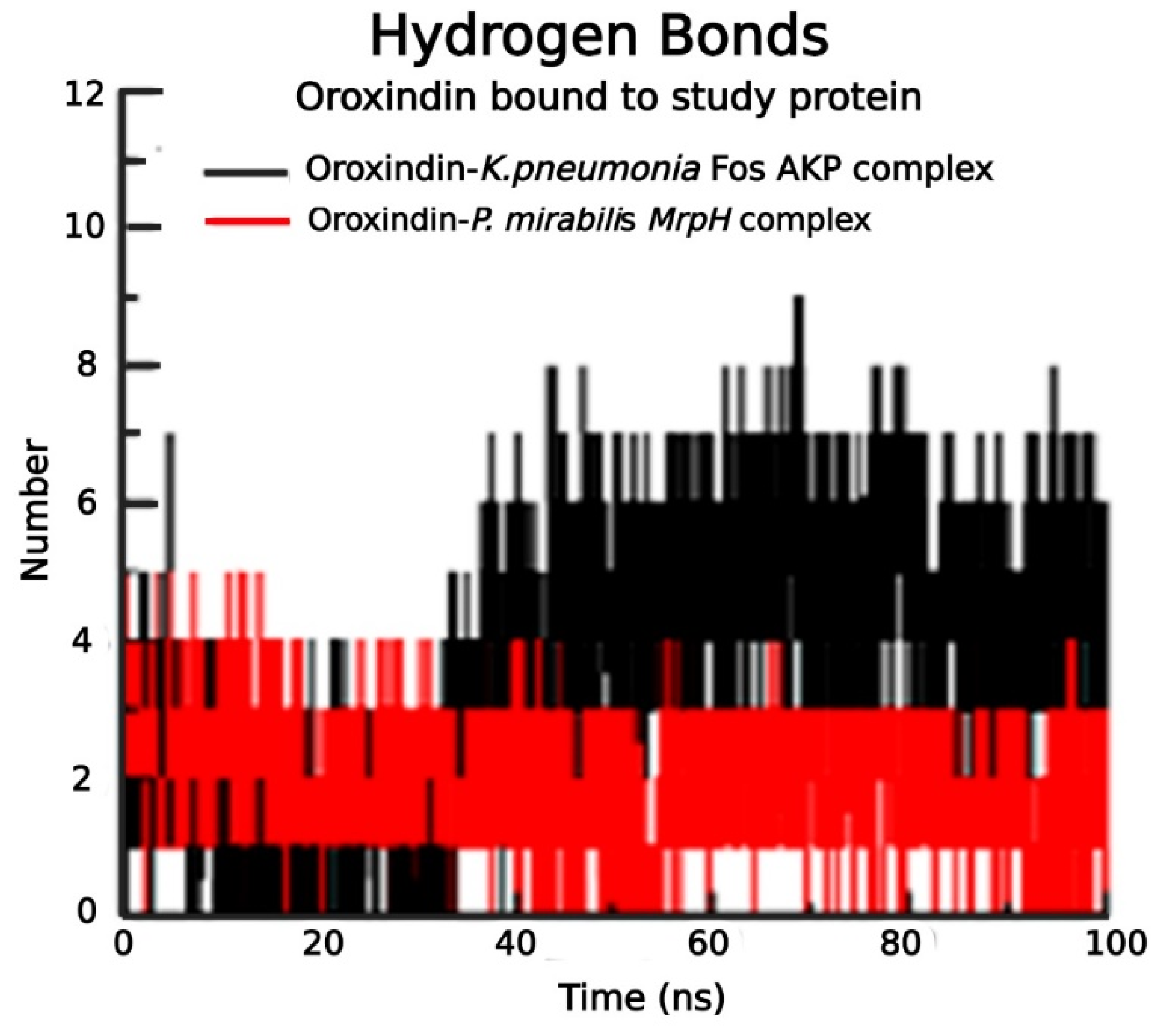
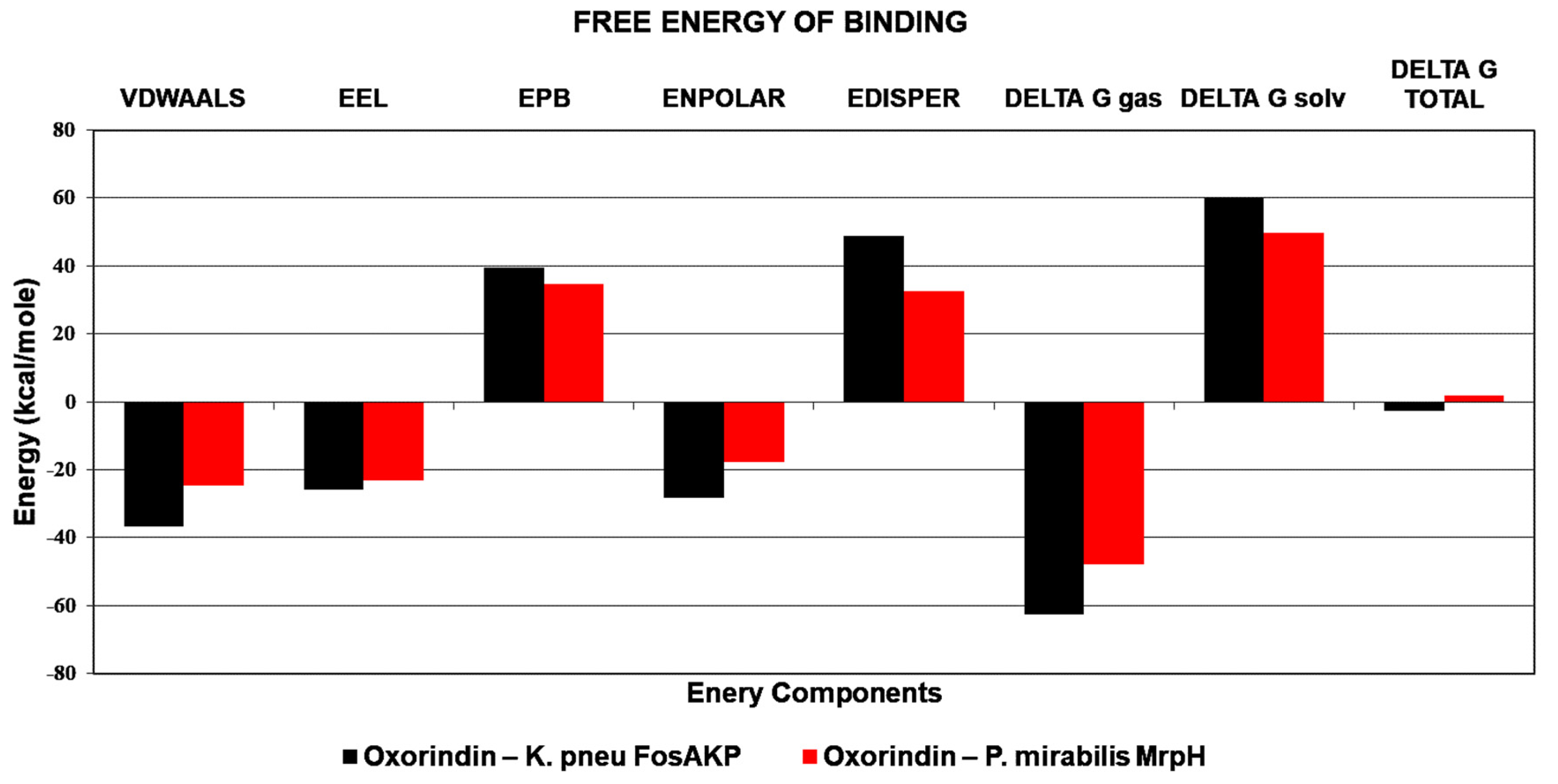
2.6. Molecular Dynamics (MD) Simulation of Best Protein Ligand Complexes
3. Discussion
4. Materials and Methods
4.1. Sample Collection of B. monnieri
4.2. Preparation of Aqueous Extracts of B. monnieri
4.3. Preparation of Methanolic and Ethanolic Extracts of B. monnieri
4.4. Antimicrobial Activity of Crude Extracts of B. monnieri
4.5. Qualitative Tests for Phytochemical Analysis of Crude Extracts of B. monnieri
4.5.1. Test for Flavonoid (Alkaline Reagent Test)
4.5.2. Test for Saponins (Frothing Test)
4.5.3. Test for Phenols (Ferric Chloride Test)
4.5.4. Test for Tannins (Gelatin Test)
4.5.5. Test for Steroids (Salkowski Test)
4.5.6. Test for Phytosterol (Salkowski Test)
4.6. In Silico Study of Selected Bioactive Molecules Present in B. monnieri
4.7. Drug-Likeness Calculations
4.8. ADMET Screening and Toxicity Prediction
4.9. MD Simulation of Protein Ligand Complexes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sheerin, N.S.; Glover, E.K. Urinary tract infection. Medicine 2019, 47, 546–550. [Google Scholar] [CrossRef]
- Parekh, J.; Chanda, S. Antibacterial and phytochemical studies on twelve species of Indian medicinal plants. Afr. J. Biomed. Res. 2010, 10, 175–181. [Google Scholar] [CrossRef]
- Sabir, N.; Ikram, A.; Zaman, G.; Satti, L.; Gardezi, A.; Ahmed, A.; Ahmed, P. Bacterial biofilm-based catheter-associated urinary tract infections: Causative pathogens and antibiotic resistance. Am. J. Infect. Control. 2017, 45, 1101–1105. [Google Scholar] [CrossRef]
- Azevedo, A.S.; Almeida, C.; Melo, L.F.; Azevedo, N.F. Impact of polymicrobial biofilms in catheter-associated urinary tract infections. Crit. Rev. Microbiol. 2017, 43, 423–439. [Google Scholar] [CrossRef] [Green Version]
- Kotaskova, I.; Obrucova, H.; Malisova, B.; Videnska, P.; Zwinsova, B.; Peroutkova, T.; Dvorackova, M.; Kumstat, P.; Trojan, P.; Ruzicka, F. Molecular techniques complement culture-based assessment of bacteria composition in mixed biofilms of urinary tract catheter-related samples. Front. Microbiol. 2019, 10, 462. [Google Scholar] [CrossRef]
- Armbruster, C.; Mobley, H.; Pearson, M. Pathogenesis of Proteus mirabilis Infection. EcoSal Plus 2018, 8. [Google Scholar] [CrossRef] [Green Version]
- Martin, R.M.; Bachman, M.A. Colonization, infection, and the accessory genome of Klebsiella pneumoniae. Front. Cell. Infect. Microbiol. 2018, 8, 4. [Google Scholar] [CrossRef] [Green Version]
- Sánchez, S.V.; Navarro, N.; Catalán-Figueroa, J.; Morales, J.O. Nanoparticles as potential novel therapies for urinary tract infections. Front. Cell. Infect. Microbiol. 2021, 11, 656496. [Google Scholar] [CrossRef]
- Ghani, A. Medicinal Plants of Bangladesh: Chemical Constituents and Uses; Asiatic Society of Bangladesh: Dhaka, Bangladesh, 1998. [Google Scholar]
- Chopra, R.N.; Nayar, S.L.; Chopra, I.C. Glossary of Indian Medicinal Plants; Council of Scientific and Industrial Research: New Dehli, India, 1956. [Google Scholar]
- Yarnell, E. Botanical medicines for the urinary tract. World J. Urol. 2002, 20, 285–293. [Google Scholar] [CrossRef]
- Amin, A.H.; Subbaiah, T.V.; Abbasi, K.M. Berberine sulfate: Antimicrobial activity, bioassay, and mode of action. Can. J. Microbiol. 1969, 15, 1067–1076. [Google Scholar] [CrossRef]
- Charoenphon, N.; Anandsongvit, N.; Kosai, P.; Sirisidthi, K.; Kangwanrangsan, N.; Jiraungkoorskul, W. Brahmi (Bacopa monnieri): Up-to-date of memory boosting medicinal plant: A review. Indian J. Agric. Res. 2016, 50, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Sivarajan, V.V.; Balachandran, I. Ayurvedic Drugs and Their Plant Sources; Oxford and IBH Publishing: New Delhi, India, 1994. [Google Scholar]
- Jeyasri, R.; Muthuramalingam, P.; Suba, V.; Ramesh, M.; Chen, J.T. Bacopa monnieri and their bioactive compounds inferred multi-target treatment strategy for neurological diseases: A cheminformatics and system pharmacology approach. Biomolecules 2020, 10, 536. [Google Scholar] [CrossRef] [Green Version]
- Phrompittayarat, W.; Wittaya-Areekul, S.; Jetiyanon, K.; Putalun, W.; Tanaka, H.; Ingkaninan, K. Stability studies of saponins in Bacopa monnieri dried ethanolic extracts. Planta Med. 2008, 74, 1756–1763. [Google Scholar] [CrossRef]
- Shinomol, G.; Bharath, M. Exploring the role of “Brahmi”(Bacopa monnieri and Centella asiatica) in brain function and therapy. Recent Pat. Endocr. Metab. Immune Drug Discov. 2011, 5, 33–49. [Google Scholar]
- Bammidi, S.R.; Volluri, S.S.; Chippada, S.C.; Avanigadda, S.; Vangalapati, M. A review on pharmacological studies of Bacopa monniera. J. Chem. Biol. Phys. Sci. 2011, 1, 250. [Google Scholar]
- Sampathkumar, P.; Dheeba, B.; Vidhyasagar, V.; Arulprakash, T.; Vinothkannan, R. Potential antimicrobial activity of various extracts of Bacopa monnieri (Linn.). Int. J. Pharmacol. 2008, 4, 230–232. [Google Scholar] [CrossRef] [Green Version]
- Usharani, K.; Jincy, K.; Neeraja, P.T. Screening and evaluation of potential bioactive compounds for antibacterial activity in Indian medicinal plants of Bacopa monnieri, Eclipta alba, Aegle marmelos and Centella asiatica. Int. J. Front. Chem. Pharm. Res. 2021, 1, 14–23. [Google Scholar] [CrossRef]
- López-Vallejo, F.; Caulfield, T.; Martínez-Mayorga, K.; Giulianotti, M.A.; Nefzi, A.; Houghten, R.A.; Medina-Franco, J.L. Integrating virtual screening and combinatorial chemistry for accelerated drug discovery. Comb. Chem. High Throughput Screen. 2011, 14, 475–487. [Google Scholar] [CrossRef]
- Gupta, M.; Sharma, R.; Kumar, A. Docking techniques in pharmacology: How much promising? Comput. Biol. Chem. 2018, 76, 210–217. [Google Scholar] [CrossRef]
- Mehta, J.; Rolta, R.; Dev, K. Role of medicinal plants from North Western Himalayas as an efflux pump inhibitor against MDR AcrAB-TolC Salmonella enterica serovar typhimurium: In vitro and In silico studies. J. Ethnopharmacol. 2022, 282, 114589. [Google Scholar] [CrossRef]
- Berghe, A.V.; Vlietinck, A.J. Screening methods for antibacterial and antiviral agents from higher plants. Methods Biochem. 1991, 6, 47–68. [Google Scholar]
- Urmila, J. A synergistic and efflux pump inhibitory activity of plant extracts and antibiotics on staphylococcus aureus strains. Asian J. Pharm. Clin. Res. 2016, 9, 277–282. [Google Scholar]
- Mehta, J.; Rolta, R.; Salaria, D.; Awofisayo, O.; Fadare, O.A.; Sharma, P.P.; Rathi, B.; Chopra, A.; Kaushik, N.; Choi, E.H. Phytocompounds from Himalayan medicinal plants as potential drugs to treat multidrug-resistant Salmonella Typhimurium: An in silico approach. Biomedicines 2021, 9, 1402. [Google Scholar] [CrossRef]
- Qureshi, K.A.; Bholay, A.D.; Rai, P.K.; Mohammed, H.A.; Khan, R.A.; Azam, F.; Jaremko, M.; Emwas, A.-H.; Stefanowicz, P.; Waliczek, M. Isolation, characterization, anti-MRSA evaluation, and in-silico multi-target anti-microbial validations of actinomycin X2 and actinomycin D produced by novel Streptomyces smyrnaeus UKAQ_23. Sci. Rep. 2021, 11, 14539. [Google Scholar] [CrossRef]
- Joshi, B.B.; Patel, M.G.H.; Dabhi, B.; Mistry, K.N. In vitro phytochemical analysis and anti-microbial activity of crude extract of Bacopa monniera. Bull. Pharm. Med. Sci. 2013, 1, 128–131. [Google Scholar]
- Alam, K.; Parvez, N.; Yadav, S.; Molvi, K.; Hwisa, N.; Sharif, S.; Pathak, D.; Murti, Y.; Zafar, R. Antimicrobial activity of leaf callus of Bacopa monnieri L. Der. Pharm. Lett. 2011, 3, 287–291. [Google Scholar]
- Hema, T.; As, A.; Suseelan, S.; Rk, J.C.; Pv, D. Antimicrobial activity of five South Indian medicinal plants against clinical pathogens. Int. J. Pharma Bio Sci. 2013, 4, 70–80. [Google Scholar]
- Mathur, A.; Verma, S.K.; Purohit, R.; Singh, S.K.; Mathur, D.; Prasad, G.; Dua, V. Pharmacological investigation of Bacopa monnieri on the basis of antioxidant, antimicrobial and anti-inflammatory properties. J. Chem. Pharm. Res. 2010, 2, 191–198. [Google Scholar]
- Sharath, R.; Krishna, V.; Sathyanarayana, B.; Harish, B. Antibacterial activity of Bacoside-A—An active constituent isolated of Bacopa monnieri (L.) Wettest. Pharmacol. Online 2008, 2, 517–528. [Google Scholar]
- Dar, K.B.; Bhat, A.H.; AmIN, S.; ANeeS, S.; Masood, A.; Zargar, M.I.; Ganie, S.A. Efficacy of aqueous and methanolic extracts of Rheum spiciformis against pathogenic bacterial and fungal strains. J. Clin. Diagn. Res. 2016, 10, BC18. [Google Scholar] [CrossRef]
- Bakkiyaraj, S.; Pandiyaraj, S. Evaluation of potential antimicrobial activity of some medicinal plants against common food-borne pathogenic microorganism. Int. J. Pharma Bio Sci. 2011, 2, 484–491. [Google Scholar]
- Kavishankar, G.; Lakshmidevi, N.; Murthy, S.M.; Prakash, H.; Niranjana, S. Diabetes and medicinal plants-A review. Int. J. Pharm. Biomed. Sci. 2011, 2, 65–80. [Google Scholar]
- Al-Zubaydi, S.R.; Al-Hmdany, M.A.; Raesan, S. Antibacterial effect of some medicinal plant extracts against some pathogenic bacteria strains. J. Duhok Univ. 2009, 12, 244–249. [Google Scholar]
- Bakht, J.; Muhammad, T.; Ali, H.; Islam, A.; Shafi, M. Effect of different solvent extracted sample of Allium sativum (Linn) on bacteria and fungi. Afr. J. Biotechnol. 2011, 10, 5910–5915. [Google Scholar]
- Mehta, J.; Jandaik, S.U.; Urmila, S. Evaluation of phytochemicals and synergistic interaction between plant extracts and antibiotics for efflux pump inhibitory activity against Salmonella enterica serovar typhimurium strains. Int. J. Pharm. Pharm. Sci. 2016, 8, 217–223. [Google Scholar] [CrossRef] [Green Version]
- Swamy, M.; Sudipta, K.; Lokesh, P.; Rashmi, W.; Vijay, R.; Ssn, K. Phytochemical screening and in vitro antimicrobial activity of Bougainvillea spectabilis flower extracts. Int. J. Phytomed. 2012, 4, 375. [Google Scholar]
- Kaur, B.; Rolta, R.; Salaria, D.; Kumar, B.; Fadare, O.A.; da Costa, R.A.; Ahmad, A.; Al-Rawi, M.B.A.; Raish, M.; Rather, I.A. An in silico investigation to explore anti-cancer potential of Foeniculum vulgare Mill. Phytoconstituents for the management of human breast cancer. Molecules 2022, 27, 4077. [Google Scholar] [CrossRef]
- Dey, D.; Ray, R.; Hazra, B. Antitubercular and antibacterial activity of quinonoid natural products against multi-drug resistant clinical isolates. Phytother. Res. 2014, 28, 1014–1021. [Google Scholar] [CrossRef]
- Dey, D.; Ray, R.; Hazra, B. Antimicrobial activity of pomegranate fruit constituents against drug-resistant Mycobacterium tuberculosis and β-lactamase producing Klebsiella pneumoniae. Pharm. Biol. 2015, 53, 1474–1480. [Google Scholar] [CrossRef] [Green Version]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Fuloria, S.; Mehta, J.; Chandel, A.; Sekar, M.; Rani, N.N.I.M.; Subramaniyan, V.; Nordin, R.B.; Djearamane, S.; Wu, Y.S.; Sathasivam, K.V. A Comprehensive Review on the Therapeutic Potential of Curcuma longa in Relation to its Major Active Constituent Curcumin. Front. Pharmacol. 2022, 13, 820806. [Google Scholar]
- Xue, W.-Y.; Qi, J.-C.; Du, L. Intervention effect and mechanism of curcumin in chronic urinary tract infection in rats. Asian Pac. J. Trop. Med. 2017, 10, 594–598. [Google Scholar] [CrossRef]
- Shaheen, G.; Akram, M.; Jabeen, F.; Ali Shah, S.M.; Munir, N.; Daniyal, M.; Riaz, M.; Tahir, I.M.; Ghauri, A.O.; Sultana, S. Therapeutic potential of medicinal plants for the management of urinary tract infection: A systematic review. Clin. Exp. Pharmacol. Physiol. 2019, 46, 613–624. [Google Scholar] [CrossRef]
- Ayyappan, S.; Srikumar, R.; Thangaraj, R. Phytochemical and antibacterial activity of Bacopa monniera against the common bacterial isolates from human. Int. J. Microbiol. Res. 2010, 1, 67–71. [Google Scholar]
- Sabina, E.; Chandel, S.; Rasool, M.K. Evaluation of analgesic, antipyretic and ulcerogenic effect of Withaferin A. Int. J. Integr. Biol. 2009, 6, 52–56. [Google Scholar]
- Leach, F.S. Anti-microbial properties of Scutellaria baicalensis and Coptis chinensis, two traditional Chinese medicines. Biosci. Horiz. 2011, 4, 119–127. [Google Scholar] [CrossRef] [Green Version]
- Rolta, R.; Salaria, D.; Sharma, P.; Sharma, B.; Kumar, V.; Rathi, B.; Verma, M.; Sourirajan, A.; Baumler, D.J.; Dev, K. Phytocompounds of Rheum emodi, Thymus serpyllum, and Artemisia annua inhibit spike protein of SARS-CoV-2 binding to ACE2 receptor: In silico approach. Curr. Pharmacol. Rep. 2021, 7, 135–149. [Google Scholar] [CrossRef]
- Rolta, R.; Yadav, R.; Salaria, D.; Trivedi, S.; Imran, M.; Sourirajan, A.; Baumler, D.J.; Dev, K. In silico screening of hundred phytocompounds of ten medicinal plants as potential inhibitors of nucleocapsid phosphoprotein of COVID-19: An approach to prevent virus assembly. J. Biomol. Struct. Dyn. 2021, 39, 7017–7034. [Google Scholar] [CrossRef]
- Salaria, D.; Rolta, R.; Patel, C.N.; Dev, K.; Sourirajan, A.; Kumar, V. In vitro and in silico analysis of Thymus serpyllum essential oil as bioactivity enhancer of antibacterial and antifungal agents. J. Biomol. Struct. Dyn. 2021, 1–20. [Google Scholar] [CrossRef]
- Salaria, D.; Rolta, R.; Sharma, N.; Patel, C.N.; Ghosh, A.; Dev, K.; Sourirajan, A.; Kumar, V. In vitro and in silico antioxidant and anti-inflammatory potential of essential oil of Cymbopogon citratus (DC.) Stapf. of North-Western Himalaya. J. Biomol. Struct. Dyn. 2021, 1–15. [Google Scholar] [CrossRef]
- Ramasamy, S.; Chin, S.P.; Sukumaran, S.D.; Buckle, M.J.C.; Kiew, L.V.; Chung, L.Y. In silico and in vitro analysis of bacoside A aglycones and its derivatives as the constituents responsible for the cognitive effects of Bacopa monnieri. PLoS ONE 2015, 10, e0126565. [Google Scholar] [CrossRef] [Green Version]
- Singh, H.; Rastogi, R.; Srimal, R.; Dhawan, B. Effect of bacosides A and B on avoidance responses in rats. Phytother. Res. 1988, 2, 70–75. [Google Scholar] [CrossRef]
- Emran, T.B.; Rahman, M.A.; Uddin, M.M.N.; Dash, R.; Hossen, M.F.; Mohiuddin, M.; Alam, M.R. Molecular docking and inhibition studies on the interactions of Bacopa monnieri’s potent phytochemicals against pathogenic Staphylococcus aureus. DARU J. Pharm. Sci. 2015, 23, 26. [Google Scholar] [CrossRef] [Green Version]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [Green Version]
- Kharat, M.; Aberg, J.; Dai, T.; McClements, D.J. Comparison of Emulsion and Nanoemulsion Delivery Systems: The Chemical Stability of Curcumin Decreases as Oil Droplet Size Decreases. J. Agric. Food Chem. 2020, 68, 9205–9212. [Google Scholar] [CrossRef]
- Priya James, H.; John, R.; Alex, A.; Anoop, K.R. Smart polymers for the controlled delivery of drugs—A concise overview. Acta Pharm. Sin. B 2014, 4, 120–127. [Google Scholar] [CrossRef] [Green Version]
- Su, S.; Kang, P.M. Recent Advances in Nanocarrier-Assisted Therapeutics Delivery Systems. Pharmaceutics 2020, 12, 837. [Google Scholar] [CrossRef]
- Grama, C.N.; Suryanarayana, P.; Patil, M.A.; Raghu, G.; Balakrishna, N.; Kumar, M.N.; Reddy, G.B. Efficacy of biodegradable curcumin nanoparticles in delaying cataract in diabetic rat model. PLoS ONE 2013, 8, e78217. [Google Scholar] [CrossRef] [Green Version]
- Sinjari, B.; Pizzicannella, J.; D’Aurora, M.; Zappacosta, R.; Gatta, V.; Fontana, A.; Trubiani, O.; Diomede, F. Curcumin/Liposome Nanotechnology as Delivery Platform for Anti-inflammatory Activities via NFkB/ERK/pERK Pathway in Human Dental Pulp Treated With 2-HydroxyEthyl MethAcrylate (HEMA). Front. Physiol. 2019, 10, 633. [Google Scholar] [CrossRef]
- Borrelli, A.; Tornesello, A.L.; Tornesello, M.L.; Buonaguro, F.M. cell penetrating peptides as molecular carriers for anti-cancer agents. Molecules 2018, 23, 295. [Google Scholar] [CrossRef] [Green Version]
- Harborne, J. Methods of plant analysis. In Phytochemical Methods; Springer: Berlin/Heidelberg, Germany, 1984; pp. 1–36. [Google Scholar]
- Aneja, K.R.; Joshi, R.; Sharma, C. Potency of Barleria prionitis L. bark extracts against oral diseases causing strains of bacteria and fungi of clinical origin. N. Y. Sci. J. 2010, 3, 5–12. [Google Scholar]
- Habsah, M.; Amran, M.; Mackeen, M.; Lajis, N.; Kikuzaki, H.; Nakatani, N.; Rahman, A.; Ali, A. Screening of Zingiberaceae extracts for antimicrobial and antioxidant activities. J. Ethnopharmacol. 2000, 72, 403–410. [Google Scholar] [CrossRef]
- Bergey, D.H.; Breed, R.S.; Murray, E.G.D.; Hitchens, A.P. Manual of Determinative Bacteriology, 5th ed.; Bailliere, Tindall & Cox: London, UK, 1939. [Google Scholar]
- Wayne, P.A. Methods for Antimicrobial Susceptibility Testing of Anaerobic Bacteria, 9th ed.; CLSI Standard M11; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Herborne, J.B. Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis, 2nd ed.; Springer: London, UK, 1973; Volume 2, pp. 5–11. [Google Scholar]
- Sofowora, A. Medicinal Plants and Traditional Medicine in Africa; Spectrum Books Ltd.: Karthala, Ibadan, 1996. [Google Scholar]
- Parekh, J.; Chanda, S. In vitro antimicrobial activity and phytochemical analysis of some Indian medicinal plants. Turk. J. Biol. 2007, 31, 53–58. [Google Scholar]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An open chemical toolbox. J. Cheminform. 2011, 3, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Ganzera, M.; Gampenrieder, J.; Pawar, R.S.; Khan, I.A.; Stuppner, H. Separation of the major triterpenoid saponins in Bacopa monnieri by high-performance liquid chromatography. Anal. Chim. Acta 2004, 516, 149–154. [Google Scholar] [CrossRef]
- Sivaramakrishna, C.; Rao, C.V.; Trimurtulu, G.; Vanisree, M.; Subbaraju, G.V. Triterpenoid glycosides from Bacopa monnieri. Phytochemistry 2005, 66, 2719–2728. [Google Scholar] [CrossRef]
- Rosell, R.; Crinó, L. Pemetrexed combination therapy in the treatment of non-small cell lung cancer. Semin. Oncol. 2002, 29, 23–29. [Google Scholar] [CrossRef]
- Banerjee, P.; Eckert, A.O.; Schrey, A.K.; Preissner, R. ProTox-II: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2018, 46, W257–W263. [Google Scholar] [CrossRef] [Green Version]
- Cheng, F.; Li, W.; Zhou, Y.; Shen, J.; Wu, Z.; Liu, G.; Lee, P.W.; Tang, Y. admetSAR: A Comprehensive Source and Free Tool for Assessment of Chemical ADMET Properties; ACS Publications: Washington, DC, USA, 2012. [Google Scholar]
- Yang, H.; Lou, C.; Sun, L.; Li, J.; Cai, Y.; Wang, Z.; Li, W.; Liu, G.; Tang, Y. admetSAR 2.0: Web-service for prediction and optimization of chemical ADMET properties. Bioinformatics 2019, 35, 1067–1069. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 2, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Kumari, R.; Kumar, R.; Open Source Drug Discovery Consortium; Lynn, A. g_mmpbsa—A GROMACS tool for high-throughput MM-PBSA calculations. J. Chem. Inf. Model. 2014, 54, 1951–1962. [Google Scholar] [CrossRef]
- Egan, W.J.; Merz, K.M.; Baldwin, J.J. Prediction of drug absorption using multivariate statistics. J. Med. Chem. 2000, 43, 3867–3877. [Google Scholar] [CrossRef]
- Salaria, D.; Rolta, R.; Mehta, J.; Awofisayo, O.; Fadare, O.A.; Kaur, B.; Kumar, B.; Araujo da Costa, R.; Chandel, S.R.; Kaushik, N.; et al. Phytoconstituents of traditional Himalayan Herbs as potential inhibitors of Human Papillomavirus (HPV-18) for cervical cancer treatment: An in silico approach. PLoS ONE 2022, 17, e0265420. [Google Scholar] [CrossRef]
| No. | Uropathogen | Concentration (μL) | Ethanolic Extract of B. monnieri | Methanolic Extract of B. monnieri | Aqueous Extract of B. monnieri | Negative Control (DMSO) |
|---|---|---|---|---|---|---|
| Zone of inhibition (in mm) | ||||||
| 1. | K. pneumoniae | 20 | 19.3 ± 0.8 | 22.3 ± 0.6 | - | - |
| 40 | 22.0 ± 0.5 | 24.0 ± 0.3 | - | - | ||
| 60 | 23.0 ± 0.4 | 25.0 ± 0.5 | 11.0 ± 0.2 | - | ||
| 2. | P. mirabilis | 20 | 17.0 ± 0.9 | 21.0 ± 0.8 | - | - |
| 40 | 21.0 ± 1.2 | 21.3 ± 0.9 | - | - | ||
| 60 | 23.0 ± 0.7 | 24.0 ± 0.6 | - | - | ||
| No. | Phytochemicals | Methanolic Extract of B. monnieri | Ethanolic Extract of B. monnieri | Aqueous Extract of B. monnieri |
|---|---|---|---|---|
| 1. | Carbohydrates | + | + | + |
| 2. | Flavonoids (Alkaline reagent test) | + | + | + |
| 3. | Tannins (Gelatin test) | + | + | − |
| 4. | Saponins (Frothing test) | + | + | + |
| 5. | Steroids (Salkowski test) | + | + | + |
| 6. | Phytosterols (Salkowski test) | + | + | + |
| 7. | Phenolic compounds (Ferric chloride test) | + | + | − |
| Bioactive Molecules/Antibiotic | Docking Score | Hydrogen Bonding | Hydrophobic Interactions | Alkyl or π-alkyl Interactions |
|---|---|---|---|---|
| Trimethoprim | −4.9 | Lys 111, Thr 66 | His 110 | - |
| Apigenin | −6.8 | - | Ala20, Gln24, Phe21, Ala87, Gly88, Leu105, Asp106 | Pro107, Val89 |
| Bacopasaponin A | −7.8 | |||
| Bacopaside I | −8.4 | Arg122, Tyr131, Tyr65, His115, Tyr103, Ser71 | Asp44, Phe70, His67, Leu119 | Leu45, Ala69 |
| β-sitosterol | −7.7 | - | Gln 24, Pro 59, Pro 60, Asp 106 | Ala 20, Leu 25, Phe 21, Val 57, Val 89, Ala 90, Leu 105, Pro 107 |
| Betulinic acid | −7.1 | |||
| Brahmic acid | −6.9 | Asp106 | Leu25, Gly88, Val89, Leu105, Asp108, Gly109, Val57 | Phe21, Pro107, Ala20, Pro17 |
| Cucurbitacin B | −6.7 | Thr58, Tyr68, Arg55 | Ser63, Lys111, His110, Gly109, Leu10, Thr9 | - |
| D-mannitol | −4.8 | Tyr68, Arg55, Gly109, Ser63, Asp64 | Thr58, Lys111, Tyr65, Thr66 | - |
| Herpestine | −7.0 | Ala69, Gly117, Ser71 | Phe70, Leu45, Tyr68, His67 | Leu5, Leu8 |
| Loliolide | −5.5 | Thr58, Arg55, Lys111, Thr66 | Asp64, Tyr68, His67, His110, Gly109, Ser63 | - |
| Monnierasides I | −6.9 | Tyr68, Lys111, Thr58, Arg55, Arg56 | His67, Thr66, His110, Gly109, Ser63, Gln54, Asp52 | Ala11 |
| Nicotine | −4.5 | Tyr68, His110 | Lys111, Thr66, Ser63, Thr58 | |
| Oroxindin | −7.5 | Gly117, His115, Ser101, Tyr103, Arg122, His67, Tyr65 | Leu119, Ser118, Ile72, Ser71, Val116, Phe70, Ser97 | Ala69 |
| Plantainoside B | −6.8 | Tyr65, Tyr103, Ser71, Leu119, Gly117, His115 | Glu113, His67, Lys93, Ser118 | Arg122, Ala69 |
| Rosavin | −6.9 | Gln54, Thr58, Arg56, Tyr68 | Asp64, Ser63, Thr66, Gly109, Thr9, Ser50, Asp52 | - |
| Stigmastanol | −7.0 | - | Gly109, Leu105, Pro60, Asp106, Ala90, Ala20, Gln24 | Pro107, Phe21, Leu25, Pro17, Val89, Ala87 |
| Wogonin | −6.2 | - | Asp44, Gly43, Ser41, His31 | - |
| Bacoside A | −7.5 | Tyr65, Lys93, Tyr131, Arg122, Glu113 | Tyr103, Ser101, His67, Ala69, Leu45, Phe70, Leu5, Ser71, Leu119 | Tyr68, Leu8, His115 |
| Luteolin | −7.0 | Ala69, Leu119, Tyr103 | Tyr65, Lys93, His67, Ser97, Ser101, Phe70, Ser71, Gly117, Ser118 | - |
| Bioactive Molecules/Antibiotic | Docking Score | Hydrogen Bonding | Hydrophobic Interactions | Alkyl or π-alkyl Interactions |
|---|---|---|---|---|
| Trimethoprim | −5.1 | Asn 82, Ala 84, Arg 107, Ser 137 | - | - |
| Apigenin | −6.2 | Ser124 | Ile30, Asn125, Gly153, Cys152, Cys128, Tyr29, Glu32, Ser33, | - |
| Bacopasaponin A | −7.0 | Cys152, Lys73, Tyr133, Leu146 | Ile 154, Val76, Asn77, Gly78, Leu147, Pro148, Gly149, Ser150, Leu151, Asn125 | Lys145 |
| Bacopaside I | −7.3 | Ser138, Asn82, Ser137, Gln86, Ala84, Phe85 | Glu112, Gly113, Gln114, Gly139, Pro83, Tyr67 | - |
| β-sitosterol | −6.5 | - | Glu 32, Ser 33, Ser 124, Asn 125, Glu 127, Cys 152, Glu 153 | Ile 30 |
| Betulinic acid | −6.8 | - | Arg100, Trp49, Ser108, Arg107 | Arg48, Phe109, Leu104, Lys105 |
| Brahmic acid | −6.5 | Leu146 | Cys152, Leu151, Ser150, Gly78, Asn77, Leu147, Pro148, Gly149, Ile154 | Lys73, Lys145 |
| Cucurbitacin B | −7.1 | Gln86, Arg107, Tyr44, Phe85 | Ser138, Ser137, Ala84, Thr115, Gly113, Glu112 | - |
| D-mannitol | −5.0 | Pro83, Gln86, Ser137, Ser138 | Gly139, Asn82, Ala84, Phe85 | - |
| Herpestine | −6.9 | Thr116, Asn82, Arg118 | Ile140, His117, Ala40, Asn39, Thr41 | His72, His74 |
| Loliolide | −5.4 | Cys128, Ile30 | Tyr29, Asn125, Gly153, Glu127, Cys152, Ser124, Val129 | - |
| Monnierasides I | −7.0 | Ser137, Gln86, Arg107, Ile87 | Ser138, Ala84, Phe85, Ala88 | - |
| Nicotine | −4.5 | Ile30 | Gly153, Ser124, Glu127, Thr31, Ser33, Glu32 | - |
| Oroxindin | −7.4 | Thr31, Glu127 | Cys128, Gly153, Ile30, Ser33, Ser124 | Ala155 |
| Plantainoside B | −7.1 | Ser137, Gln86, Arg107 Ile87 | Tyr67, Ala88, Arg89, Phe85, Ser138 | - |
| Rosavin | −7.1 | Ser33, Gly153, Ser124, Glu127, Ile30 | Thr31, Cys152, Glu32 | Ala155 |
| Stigmastanol | −6.4 | - | Ser33, Glu32, Thr31, Ser124, Glu127, Gly153, Pro156, Glu47 | Ile30, Tyr29, Ala155 |
| Wogonin | −6.3 | Gly149, Ser150 | Leu147, Lys73, Gly78, Asn77, Tyr133 | Pro59, Lys145 |
| Bacoside A | −7.1 | Gln86 | Phe85, Arg107, Ile87, Asn106, Ile90 | Ala88, Leu93, Val102, Arg89, Lys92 |
| Luteolin | −7.5 | Arg118, Thr116, His72 | Ile140, His74, Asn82, Gly81, His117, Asn39, Ala40, Thr41 | - |
| Bioactive Molecules | miLog P | TPSA (Å2) | Number of Atoms | Number of Nitrogen and Oxygen | Number of -OH and -NHn | Number of violations | Number of Rotations | MW |
|---|---|---|---|---|---|---|---|---|
| Trimethoprim | 0.99 | 105.53 | 21 | 7 | 4 | 0 | 5 | 290.32 |
| Apigenin | 2.46 | 90.89 | 20 | 5 | 3 | 0 | 1 | 270.24 |
| Bacopasaponin A | 3.86 | 176.77 | 52 | 12 | 6 | 3 | 5 | 736.94 |
| Bacopaside I | 2.54 | 215.00 | 54 | 13 | 8 | 3 | 10 | 768.98 |
| β-sitosterol | 8.62 | 20.23 | 30 | 1 | 1 | 1 | 6 | 414.72 |
| Betulinic acid | 7.04 | 57.53 | 33 | 3 | 2 | 1 | 2 | 456.71 |
| Brahmic acid | 3.78 | 118.21 | 36 | 6 | 5 | 1 | 2 | 504.71 |
| Cucurbitacin B | 2.83 | 138.20 | 40 | 8 | 3 | 1 | 6 | 558.71 |
| D-mannitol | −3.10 | 121.37 | 12 | 6 | 6 | 1 | 5 | 182.17 |
| Herpestine | −1.04 | 181.07 | 35 | 13 | 7 | 2 | 9 | 481.52 |
| Loliolide | 1.84 | 46.53 | 14 | 3 | 1 | 0 | 0 | 196.25 |
| Monnierasides I | −1.11 | 170.05 | 26 | 10 | 6 | 1 | 4 | 370.31 |
| Nicotine | 1.09 | 16.13 | 12 | 2 | 0 | 0 | 1 | 162.24 |
| Oroxindin | 0.82 | 176.12 | 33 | 11 | 5 | 1 | 5 | 460.39 |
| Plantainoside B | 0.69 | 186.37 | 34 | 11 | 7 | 2 | 9 | 478.45 |
| Rosavin | −0.95 | 158.30 | 30 | 10 | 6 | 1 | 7 | 428.43 |
| Stigmastanol | 8.71 | 20.23 | 30 | 1 | 1 | 1 | 6 | 416.73 |
| Wogonin | 2.96 | 79.90 | 21 | 5 | 2 | 0 | 2 | 284.27 |
| Bioactive Molecules | Protox-II | |||||
|---|---|---|---|---|---|---|
| LD50, (mg/kg) | Hepatotoxicity | Carcinogenicity | Immunotoxicity | Mutagenicity | Cytotoxicity | |
| Trimethoprim | 3500 (class 5) | Inactive | Active | Active | Inactive | Inactive |
| Apigenin | 2500 (class 5) | Inactive | Inactive | Inactive | Inactive | Inactive |
| Bacopasaponin A | 6000 (class 6) | Inactive | Inactive | Active | Inactive | Inactive |
| Bacopaside I | 1500 (class 4) | Inactive | Inactive | Active | Inactive | Inactive |
| β-sitosterol | 890 (Class 4) | Inactive | Inactive | Active | Inactive | Inactive |
| Betulinic acid | 2610 (Class 5) | Inactive | Active | Active | Inactive | Inactive |
| Brahmic acid | 2000 (Class 4) | Inactive | Inactive | Active | Inactive | Inactive |
| Cucurbitacin B | 1190 (Class 4) | Inactive | Inactive | Active | Inactive | Inactive |
| D-mannitol | 13500 (Class 6) | Inactive | Inactive | Inactive | Inactive | Inactive |
| Herpestine | 500 (Class 4) | Inactive | Inactive | Inactive | Inactive | Inactive |
| Loliolide | 34 (Class 2) | Inactive | Active | Inactive | Inactive | Inactive |
| Monnierasides I | 190 (Class 4) | Active | Inactive | Active | Inactive | Inactive |
| Nicotine | 3 (Class 1) | Inactive | Inactive | Active | Inactive | Inactive |
| Oroxindin | 5000 (Class 5) | Inactive | Inactive | Active | Inactive | Inactive |
| Plantainoside B | 5000 (Class 5) | Inactive | Inactive | Active | Inactive | Inactive |
| Rosavin | 2000 (Class 4) | N/A | N/A | N/A | N/A | Inactive |
| Stigmastanol | 500 (Class 4) | Inactive | Inactive | Active | Inactive | Inactive |
| Wogonin | 1190 (Class 4) | Active | Inactive | Active | Inactive | Inactive |
| No. | Test Pathogens | Potential Infections |
|---|---|---|
| 1 | K. pneumoniae | Pneumonia, UTIs, septicemia, and diarrhea |
| 2 | P. mirabilis | UTIs, including cystitis and pyelonephritis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehta, J.; Utkarsh, K.; Fuloria, S.; Singh, T.; Sekar, M.; Salaria, D.; Rolta, R.; Begum, M.Y.; Gan, S.H.; Rani, N.N.I.M.; et al. Antibacterial Potential of Bacopa monnieri (L.) Wettst. and Its Bioactive Molecules against Uropathogens—An In Silico Study to Identify Potential Lead Molecule(s) for the Development of New Drugs to Treat Urinary Tract Infections. Molecules 2022, 27, 4971. https://doi.org/10.3390/molecules27154971
Mehta J, Utkarsh K, Fuloria S, Singh T, Sekar M, Salaria D, Rolta R, Begum MY, Gan SH, Rani NNIM, et al. Antibacterial Potential of Bacopa monnieri (L.) Wettst. and Its Bioactive Molecules against Uropathogens—An In Silico Study to Identify Potential Lead Molecule(s) for the Development of New Drugs to Treat Urinary Tract Infections. Molecules. 2022; 27(15):4971. https://doi.org/10.3390/molecules27154971
Chicago/Turabian StyleMehta, Jyoti, Kumar Utkarsh, Shivkanya Fuloria, Tejpal Singh, Mahendran Sekar, Deeksha Salaria, Rajan Rolta, M. Yasmin Begum, Siew Hua Gan, Nur Najihah Izzati Mat Rani, and et al. 2022. "Antibacterial Potential of Bacopa monnieri (L.) Wettst. and Its Bioactive Molecules against Uropathogens—An In Silico Study to Identify Potential Lead Molecule(s) for the Development of New Drugs to Treat Urinary Tract Infections" Molecules 27, no. 15: 4971. https://doi.org/10.3390/molecules27154971
APA StyleMehta, J., Utkarsh, K., Fuloria, S., Singh, T., Sekar, M., Salaria, D., Rolta, R., Begum, M. Y., Gan, S. H., Rani, N. N. I. M., Chidambaram, K., Subramaniyan, V., Sathasivam, K. V., Lum, P. T., Uthirapathy, S., Fadare, O. A., Awofisayo, O., & Fuloria, N. K. (2022). Antibacterial Potential of Bacopa monnieri (L.) Wettst. and Its Bioactive Molecules against Uropathogens—An In Silico Study to Identify Potential Lead Molecule(s) for the Development of New Drugs to Treat Urinary Tract Infections. Molecules, 27(15), 4971. https://doi.org/10.3390/molecules27154971










