MD Simulation Studies for Selective Phytochemicals as Potential Inhibitors against Major Biological Targets of Diabetic Nephropathy
Abstract
:1. Introduction
Current Treatment Problems
2. Methodology
2.1. Target Receptor Preparation
2.2. Ligand Preparation
2.3. Docking Protocol
2.4. In silico Pharmacokinetic Assessment of Investigated Compounds (ADMET)
2.5. Molecular Dynamics Simulation
3. Results and Discussion
3.1. Docking Study
3.2. ADMET Analysis
3.3. MMGBSA Analysis
3.4. RMSD Analysis
3.5. Ligand Properties
3.6. Protein-Ligand Interaction Contacts
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, X.; Xu, Y.; Pan, X.; Xu, J.; Ding, Y.; Sun, X.; Song, X.; Ren, Y.; Shan, P.F. Global, regional, and national burden and trend of diabetes in 195 countries and territories: An analysis from 1990 to 2025. Sci. Rep. 2020, 10, 14790. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.E.; Stevens, G.A.; Mathers, C.D.; Bonita, R.; Rehm, J.; Kruk, M.E.; Riley, L.M.; Dain, K.; Kengne, A.P.; Chalkidou, K.; et al. NCD Countdown 2030: Worldwide trends in non-communicable disease mortality and progress towards Sustainable Development Goal target 3.4. Lancet 2018, 392, 1072–1088. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.A.B.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Al Kaabi, J. Epidemiology of Type 2 Diabetes–Global Burden of Disease and Forecasted Trends. J. Epidemiol. Glob. Health 2020, 10, 107–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2009, 32 (Suppl. S1), S62–S67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Röder, P.V.; Wu, B.; Liu, Y.; Han, W. Pancreatic regulation of glucose homeostasis. Exp. Mol. Med. 2016, 48, e219. [Google Scholar] [CrossRef] [Green Version]
- Tabish, S.A. Is Diabetes Becoming the Biggest Epidemic of the Twenty-first Century? Int. J. Health Sci. 2007, 1, V–VIII. [Google Scholar]
- Roep, B.O.; Thomaidou, S.; van Tienhoven, R.; Zaldumbide, A. Type 1 diabetes mellitus as a disease of the β-cell (do not blame the immune system?). Nat. Rev. Endocrinol. 2020, 17, 150–161. [Google Scholar] [CrossRef]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar]
- Cade, W.T. Diabetes-related microvascular and macrovascular diseases in the physical therapy setting. Phys. Ther. 2008, 88, 1322–1335. [Google Scholar] [CrossRef] [Green Version]
- Altaf, Q.A.; Tahrani, A.A. Chapter 23-Obstructive Sleep Apnea and Diabetic Microvascular Complications. In Modulation of Sleep by Obesity, Diabetes, Age, and Diet; Watson, R.R., Ed.; Academic Press: San Diego, CA, USA, 2015; pp. 213–224. [Google Scholar]
- Jiao, H.; Xiao, E.; Graves, D.T. Diabetes and Its Effect on Bone and Fracture Healing. Curr. Osteoporos. Rep. 2015, 13, 327–335. [Google Scholar] [CrossRef] [Green Version]
- Cipriani, C.; Colangelo, L.; Santori, R.; Renella, M.; Mastrantonio, M.; Minisola, S.; Pepe, J. The Interplay Between Bone and Glucose Metabolism. Front. Endocrinol. 2020, 11, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, C.M.; Nee, R.; Ceckowski, K.A.; Knight, K.R.; Abbott, K.C. Diabetic nephropathy as the cause of end-stage kidney disease reported on the medical evidence form CMS2728 at a single center. Clin. Kidney J. 2016, 10, 257–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Persson, F.; Rossing, P. Diagnosis of diabetic kidney disease: State of the art and future perspective. Kidney Int. Suppl. 2018, 8, 2–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, A. Diabetic nephropathy–complications and treatment. Int. J. Nephrol. Renov. Dis. 2014, 7, 361–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallon, V.; Komers, R. Pathophysiology of the Diabetic Kidney. Compr. Physiol. 2011, 1, 1175–1232. [Google Scholar]
- Hofherr, A.; Williams, J.; Gan, L.-M.; Söderberg, M.; Hansen, P.B.L.; Woollard, K.J. Targeting inflammation for the treatment of Diabetic Kidney Disease: A five-compartment mechanistic model. BMC Nephrol. 2022, 23, 208. [Google Scholar] [CrossRef]
- Deshpande, A.D.; Harris-Hayes, M.; Schootman, M. Epidemiology of Diabetes and Diabetes-Related Complications. Phys. Ther. 2008, 88, 1254–1264. [Google Scholar]
- Gheith, O.; Farouk, N.; Nampoory, N.; A Halim, M.; Al-Otaibi, T. Diabetic kidney disease: World wide difference of prevalence and risk factors. J. Nephroph. 2015, 5, 49–56. [Google Scholar] [CrossRef]
- Gross, J.L.; de Azevedo, M.J.; Silveiro, S.P.; Canani, L.H.; Caramori, M.L.; Zelmanovitz, T. Diabetic Nephropathy: Diagnosis, Prevention, and Treatment. Diabetes Care 2005, 28, 164–176. [Google Scholar]
- Thomas, M.C.; Brownlee, M.; Susztak, K.; Sharma, K.; Jandeleit-Dahm, K.A.; Zoungas, S.; Rossing, P.; Groop, P.-H.; Cooper, M.E. Diabetic kidney disease. Nat. Rev. Dis. Primers 2015, 1, 15018. [Google Scholar] [CrossRef]
- Alicic, R.Z.; Rooney, M.T.; Tuttle, K.R. Diabetic Kidney Disease: Challenges, Progress, and Possibilities. Clin. J. Am. Soc. Nephrol. 2017, 12, 2032–2045. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Liu, Z.; Zeng, C.; Peng, A.; Chen, H.; Zhou, H.; Li, L. Amylin deposition in the kidney of patients with diabetic nephropathy. Kidney Int. 2007, 72, 213–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asif, M. The prevention and control the type-2 diabetes by changing lifestyle and dietary pattern. J. Educ. Health Promot. 2014, 3, 1. [Google Scholar] [CrossRef]
- Shahbazian, H. Diabetic kidney disease; review of the current knowledge. J. Ren. Inj. Prev. 2013, 2, 73–80. [Google Scholar] [PubMed]
- Pan, S.Y.; Zhou, S.F.; Gao, S.H.; Yu, Z.L.; Zhang, S.F.; Tang, M.K.; Sun, J.N.; Ma, D.L.; Han, Y.F.; Fong, W.F.; et al. New Perspectives on How to Discover Drugs from Herbal Medicines: CAM’s Outstanding Contribution to Modern Therapeutics. Evid.-Based Complement. Alternat. Med. 2013, 2013, 627375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Abhar, H.S.; Schaalan, M.F. Phytotherapy in diabetes: Review on potential mechanistic perspectives. World J. Diabetes 2014, 5, 176–197. [Google Scholar] [CrossRef]
- Salehi, B.; Ata, A.; Anil Kumar, N.V.; Sharopov, F.; Ramírez-Alarcón, K.; Ruiz-Ortega, A.; Ayatollahi, S.A.; Fokou, P.V.T.; Kobarfard, F.; Zakaria, Z.A.; et al. Antidiabetic Potential of Medicinal Plants and Their Active Components. Biomolecules 2019, 9, 551. [Google Scholar] [CrossRef] [Green Version]
- Röhrborn, D.; Wronkowitz, N.; Eckel, J. DPP4 in Diabetes. Fron. Immunol. 2015, 6, 386. [Google Scholar] [CrossRef] [Green Version]
- Seshadri, K.; Kirubha, M. Gliptins: A new class of oral antidiabetic agents. Indian J. Pharm. Sci. 2009, 71, 608–614. [Google Scholar] [CrossRef] [Green Version]
- Ajiboye, O.; Segal, J.B. National trends in the treatment of diabetic nephropathy in the United States. J Clin Pharm Ther. 2017, 42, 311–317. [Google Scholar] [CrossRef]
- Kim, Y.-G.; Byun, J.-H.; Yoon, D.; Jeon, J.Y.; Han, S.J.; Kim, D.J.; Lee, K.-W.; Park, R.W.; Kim, H.-J. Renal Protective Effect of DPP-4 Inhibitors in Type 2 Diabetes Mellitus Patients: A Cohort Study. J. Diabetes Res. 2016, 2016, 1423191. [Google Scholar] [CrossRef] [PubMed]
- Panchapakesan, U.; Pollock, C. The Role of Dipeptidyl Peptidase-4 Inhibitors in Diabetic Kidney Disease. Front. Immunol. 2015, 6, 443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, P.M.-K.; Zhang, Y.-Y.; Hung, J.S.-C.; Chung, J.Y.-F.; Huang, X.-R.; To, K.-F.; Lan, H.-Y. DPP4/CD32b/NF-κB Circuit: A Novel Druggable Target for Inhibiting CRP-Driven Diabetic Nephropathy. Mol. Ther. 2021, 29, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Alter, M.L.; Ott, I.M.; Websky, K.v.; Tsuprykov, O.; Sharkovska, Y.; Krause-Relle, K.; Raila, J.; Henze, A.; Klein, T.; Hocher, B. DPP-4 inhibition on top of angiotensin receptor blockade offers a new therapeutic approach for diabetic nephropathy. Kidney Blood Press Res. 2012, 36, 119–130. [Google Scholar] [CrossRef]
- Birnbaum, Y.; Bajaj, M.; Yang, H.-C.; Ye, Y. Combined SGLT2 and DPP4 Inhibition Reduces the Activation of the Nlrp3/ASC Inflammasome and Attenuates the Development of Diabetic Nephropathy in Mice with Type 2 Diabetes. Cardiovascular Drugs Ther. 2018, 32, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, L.; Qin, W.; Liu, Y. Near-infrared probe for early diagnosis of diabetic complications-nephropathy and in vivo visualization fluorescence imaging research. Anal. Chim. Acta 2022, 1221, 340147. [Google Scholar] [CrossRef]
- Zhang, X.X.; Pan, Y.-H.; Huang, Y.-M.; Zhao, H.-L. Neuroendocrine hormone amylin in diabetes. World J. Diabetes 2016, 7, 189–197. [Google Scholar] [CrossRef]
- Zhao, H.; Guan, J.; Lee, H.-M.; Sui, Y.; He, L.; Siu, J.J.; Tse, P.P.P.; Tong, P.C.Y.; Lai, F.M.M.; Chan, J.C.N. Up-regulated pancreatic tissue microRNA-375 associates with human type 2 diabetes through beta-cell deficit and islet amyloid deposition. Pancreas 2010, 39, 843–846. [Google Scholar] [CrossRef]
- Dember, L.M. Amyloidosis-associated kidney disease. J. Am. Soc. Nephrol. 2006, 17, 3458–3471. [Google Scholar] [CrossRef]
- Kanasaki, K. The role of renal dipeptidyl peptidase-4 in kidney disease: Renal effects of dipeptidyl peptidase-4 inhibitors with a focus on linagliptin. Clin. Sci. 2018, 132, 489–507. [Google Scholar] [CrossRef] [Green Version]
- Zardecki, C.; Dutta, S.; Goodsell, D.S.; Voigt, M.; Burley, S.K. RCSB Protein Data Bank: A Resource for Chemical, Biochemical, and Structural Explorations of Large and Small Biomolecules. J. Chem. Educ. 2016, 93, 569–575. [Google Scholar] [CrossRef] [Green Version]
- Schrödinger, Inc. Schrödinger Software Suite; Schrödinger: New York, NY, USA, 2021. [Google Scholar]
- Ioakimidis, L.; Thoukydidis, L.; Mirza, A.; Naeem, S.; Reynisson, J. Benchmarking the Reliability of QikProp. Correlation between Experimental and Predicted Values. QSAR Comb. Sci. 2008, 27, 445–456. [Google Scholar] [CrossRef]
- Lipinski, C.A. Lead-and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Ghahremanian, S.; Rashidi, M.M.; Raeisi, K.; Toghraie, D. Molecular dynamics simulation approach for discovering potential inhibitors against SARS-CoV-2: A structural review. J. Mol. Liq. 2022, 354, 118901. [Google Scholar] [CrossRef] [PubMed]
- Abedi, F.; Razavi, B.M.; Hosseinzadeh, H. A review on gentisic acid as a plant derived phenolic acid and metabolite of aspirin: Comprehensive pharmacology, toxicology, and some pharmaceutical aspects. Phytotherapy Res. 2019, 34, 729–741. [Google Scholar] [CrossRef]
- Nugraha, A.S.; Damayanti, Y.D.; Wangchuk, P.; Keller, P.A. Anti-Infective and Anti-Cancer Properties of the Annona Species: Their Ethnomedicinal Uses, Alkaloid Diversity, and Pharmacological Activities. Molecules 2019, 24, 4419. [Google Scholar] [CrossRef] [Green Version]
- Ekins, S.; Mestres, J.; Testa, B. In silico pharmacology for drug discovery: Methods for virtual ligand screening and profiling. Br. J. Pharmacol. 2007, 152, 9–20. [Google Scholar] [CrossRef] [Green Version]
- Rashid, H.U.; Ahmad, N.; Abdalla, M.; Khan, K.; Martines, M.A.U.; Shabana, S. Molecular docking and dynamic simulations of Cefixime, Etoposide and Nebrodenside A against the pathogenic proteins of SARS-CoV-2. J. Mol. Struct. 2022, 1247, 131296. [Google Scholar] [CrossRef]
- Qian, Y.; Sun, X.; Wang, X.; Yang, X.; Fan, M.; Zhong, J.; Pei, Z.; Guo, J. Mechanism of Cordyceps Cicadae in Treating Diabetic Nephropathy Based on Network Pharmacology and Molecular Docking Analysis. J. Diabetes Res. 2021, 2021, 5477941. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, S.; Liu, W.; Zhang, Z.; Liu, Y.; Li, S.; Sun, D.; Zhang, G.; Fang, J. Potential Molecular Mechanism of Yishen Capsule in the Treatment of Diabetic Nephropathy Based on Network Pharmacology and Molecular Docking. Diabetes Metab. Syndr. Obesity 2022, 15, 943–962. [Google Scholar] [CrossRef]
- Ramanjaneyulu, M.; Kumar, K.A.; Kumar, M.S.; Reddy, S.; Kara, D.; Mukthala, P.; Raj, R.; Madhu, C. Target identification and validation for diabetic nephropathy using molecular docking studies. Pharma Chem. 2013, 5, 353–363. [Google Scholar]
- Shree, P.; Yadav, D.; Singh, V.K.; Chaube, R. Modulation of mTOR Receptor in Diabetes Nephropathy by Santalina of Lalchandan: An In-Silico Assessment by Molecular Docking; Thermo Fisher Scientific: Waltham, MA, USA, 2019. [Google Scholar]

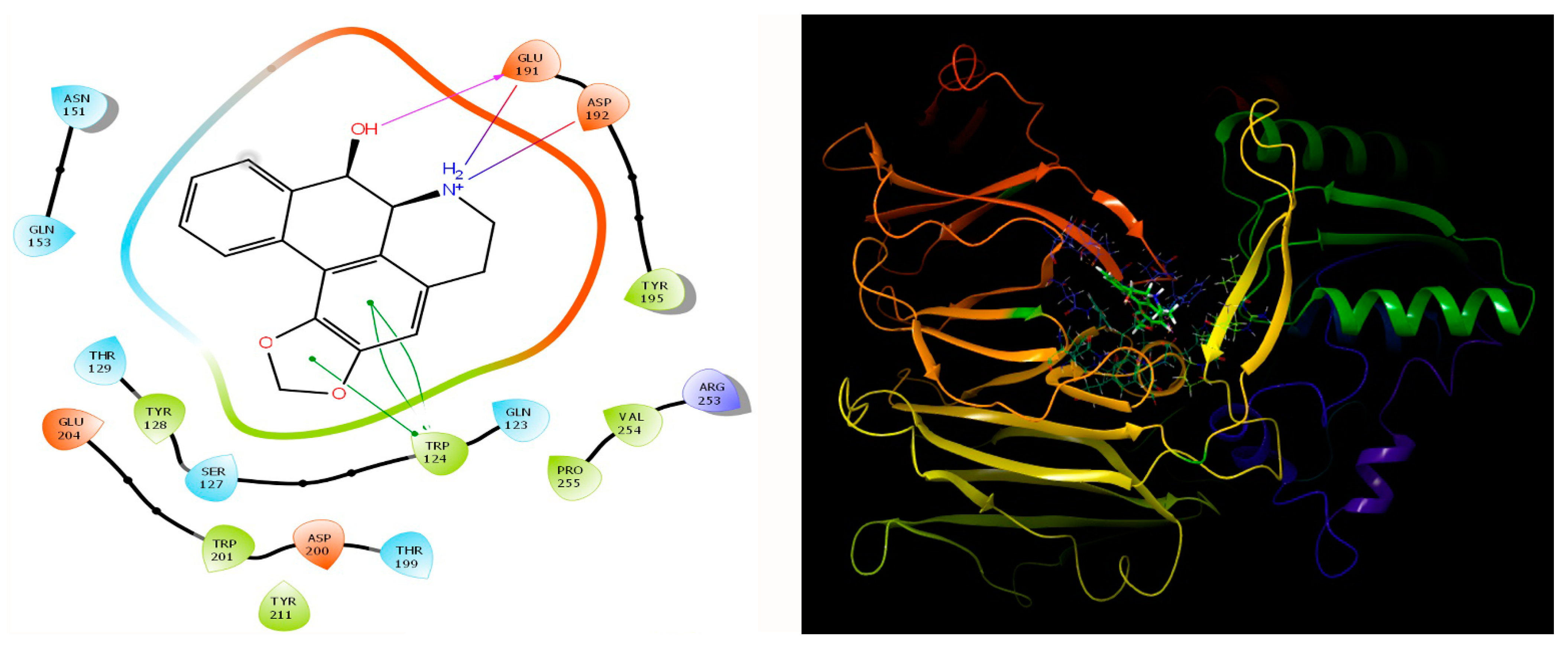

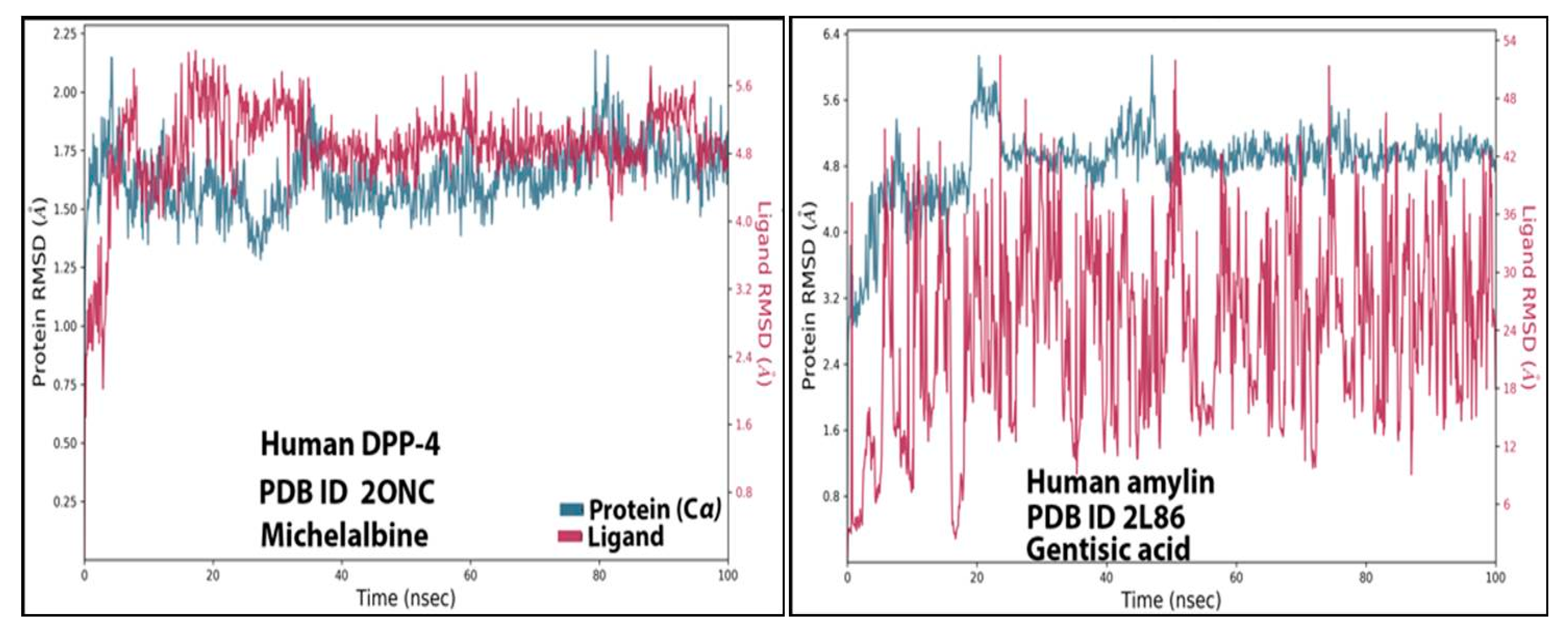
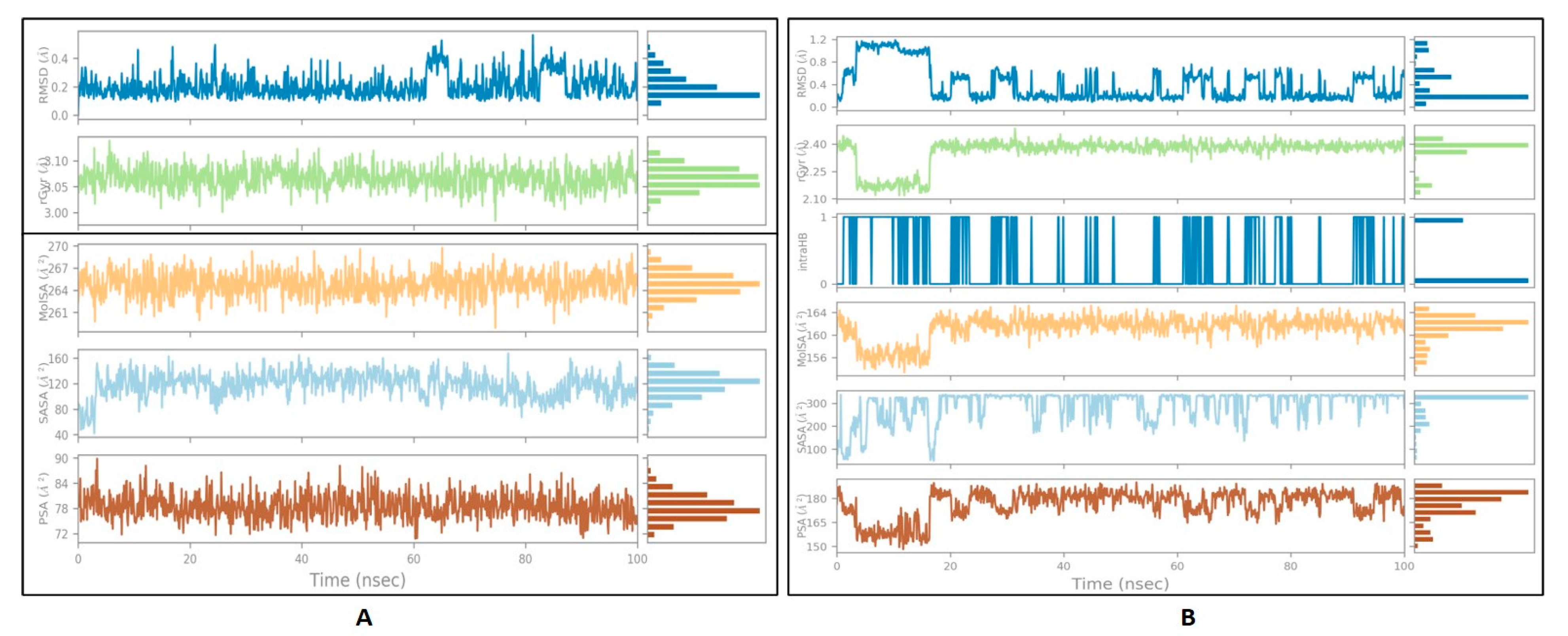
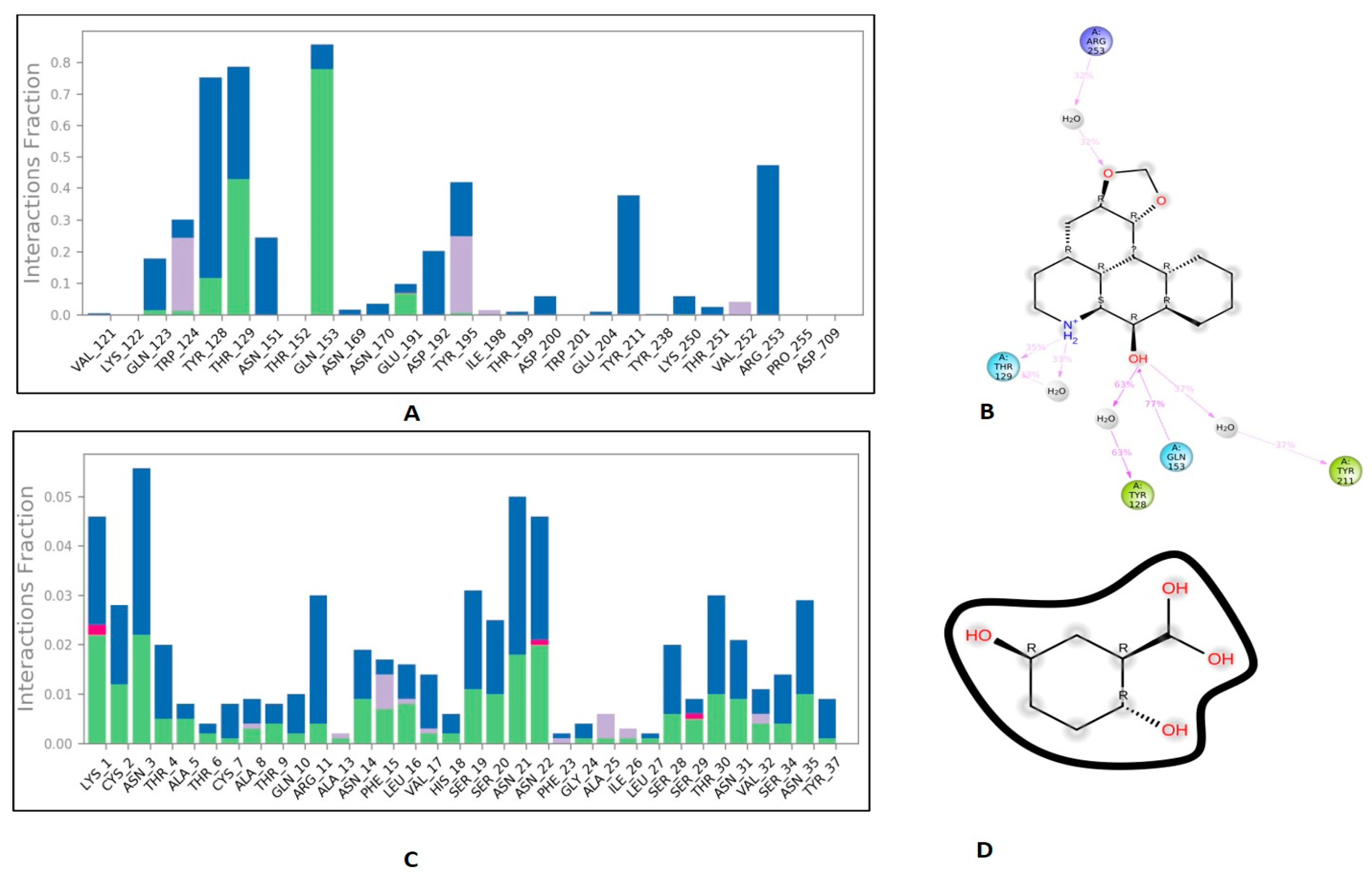
| S.No | Receptor | Best Molecule | mol_MW | donorHB | accptHB | PSA | BBB | Cytochrome p450 Inhibition/Substrate | Oral Acute Toxicity |
|---|---|---|---|---|---|---|---|---|---|
| 1 | DPP-4 | Michelalbine | 281.31 | 2 | 4.7 | 52.139 | 0.9739 | Substrate for CYP2D6 and CYP3A4/Only inhibit CYP2D6 | Category-III |
| 2 | Amylin | Gentisic acid | 154.122 | 2 | 2.5 | 91.598 | 0.9350 | Non-inhibitor/non- substrate | Category-III |
| Target | Phyto-Chemical | MMGBSA dG Bind | MMGBSA dG Bind Coulomb | MMGBSA dG Bind Covalent | MMGBSA dG Bind Solv GB | MMGBSA dG Bind vdW |
|---|---|---|---|---|---|---|
| Human amylin | Gentisic Acid | 0.057796044 | 0.015605996 | 0.030187095 | 0.043943182 | −0.031940229 |
| DPP-4 | Michelalbine | −38.17881278 | −50.49822686 | 0.999651095 | 54.78785013 | −29.32973714 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kausar, M.A.; Anwar, S.; Eltayb, W.A.; Kuddus, M.; Khatoon, F.; El-Arabey, A.A.; Khalifa, A.M.; Rizvi, M.R.; Najm, M.Z.; Thakur, L.; et al. MD Simulation Studies for Selective Phytochemicals as Potential Inhibitors against Major Biological Targets of Diabetic Nephropathy. Molecules 2022, 27, 4980. https://doi.org/10.3390/molecules27154980
Kausar MA, Anwar S, Eltayb WA, Kuddus M, Khatoon F, El-Arabey AA, Khalifa AM, Rizvi MR, Najm MZ, Thakur L, et al. MD Simulation Studies for Selective Phytochemicals as Potential Inhibitors against Major Biological Targets of Diabetic Nephropathy. Molecules. 2022; 27(15):4980. https://doi.org/10.3390/molecules27154980
Chicago/Turabian StyleKausar, Mohd Adnan, Sadaf Anwar, Wafa Ali Eltayb, Mohammed Kuddus, Fahmida Khatoon, Amr Ahmed El-Arabey, Amany Mohammed Khalifa, Moattar Raza Rizvi, Mohammad Zeeshan Najm, Lovnish Thakur, and et al. 2022. "MD Simulation Studies for Selective Phytochemicals as Potential Inhibitors against Major Biological Targets of Diabetic Nephropathy" Molecules 27, no. 15: 4980. https://doi.org/10.3390/molecules27154980









