The Genus Cetraria s. str.—A Review of Its Botany, Phytochemistry, Traditional Uses and Pharmacology
Abstract
:1. Introduction
2. General Features
2.1. Taxonomy/Phylogeny
2.2. Morphology and Distribution
| Morphology | |||||
|---|---|---|---|---|---|
| Type of Thallus | Color | Structures | Substrate | References | |
| Cetraria aculeata (Schreb.) Fr. | Fruticose | Thallus: brown to black. | Pseudocyphellae abundant Isidia and soredia absent | Terricolous | [35] |
| Cetraria annae Oxner | Foliose | Thallus: pale yellow Medulla: white | Soredia are white, granular No apothecia were seen Marginal black pycnidia Conidia absent | Terricolous | [30] |
| Cetraria australiensis W.A.Weber ex Kärnefelt | Fruticose | Thallus: Upper surface dark. Lower surface yellowish brown to dark brown. | Marginal pseudocyphellae Marginal projections with terminal pynidia Apothecia not known | Terricolous Corticolous | [30] |
| Cetraria crespoae (Barreno and Vázquez) Kärnefelt | Fruticose | Thallus: olive or brown (to almost black). | Soredia absent Abundant Pseudocyphellae | Corticolous | [39] |
| Cetraria ericetorum Opiz | Fruticose | Thallus: dark brown to paler brown Medulla: white | Pseudocyphellae abundant on the margins Apothecia frecuent Soredia absent Pycnidia laminal/marginal | Terricolous Corticolous Saxicolous | [39] |
| Cetraria islandica (L.) Ach. | Foliose | Thallus: upper surface greenish or greenish-brown, lower surface is greyish-white or light brownish. | Laminal pseudocyphellae present but sometimes badly visible Rarely discoid apothecia on terminal lobes Pycnidia present | Terricolous | [12,41] |
| Cetraria kamczatica Savicz | Fruticose | Thallus: dark brown. Medulla: white. | Apothecia not seen No pseudocyphellae or with few tiny pseudocyphellae on lower margins | Musciolous | [37] |
| Cetraria laevigata Rass. | Fruticose | Thallus: upper side pale brown, underside paler. | Apothecia not seen Marginal pseudocyphellae | Terricolous | [37] |
| Cetraria muricata (Ach.) Eckfeldt | Fruticose | Thallus: Brown to black. | Pseudocyphellae scattered, poorly visible, depressed Isidia and soredia absent | Terricolous | [41] |
| Cetraria nepalensis D.D. Awasthi | Fructicose | Thallus: brown to black. | Apothecia unknown | Terricolous | [45] |
| Cetraria nigricans Nyl. | Foliose | Thallus: upper side dark brown or olive-brown, lower side pale brown. | Apothecia present Pseudocyphellae absent or badly visible Pycnidial projections Marginal cilia numerous and long | Terricolous Saxicolous | [41,44] |
| Cetraria odontella (Ach.) Ach. | Fruticose | Thallus: olive or brown (to almost black). | Pseudocyphellae exclusively on the underside | Epilithic | [34] |
| Cetraria obtusata (Schaer.) Van den Boom and Sipman | Fruticose | Thallus: Dark brown. Medulla: Pale yellow. | Pseudocyphellae present Apothecia unknown Pycnidia dark Conidia clavate | Terricolous | [34,38,44] |
| Cetraria peruviana Kärnefelt and Thell | Fruticose | Thallus: reddish, brown to dark brown or almost black. | Apothecia usually absent Marginal cilia Pycnidial projections absent | Terricolous Saxicolous | [41] |
| Cetraria rassadinae Makryi | Fruticose | Thallus: brownish black | Pycnidium present Conidia oblong | Terricolous | [36] |
| Cetraria sepincola (Hoffm.) Ach. | Fruticose | Thallus: brown to almost black | Marginal pseudocyphellae absent. Absent soredia and isidia | Corticolous Lignicolous | [34] |
| Cetraria steppae (Savicz) Kärnefelt | Fruticose | Thallus: black, brown to light brown Medulla: white | Pseudocyphellae depressed or poorly visible Isidioid projections absent | Terricolous | [34,35] |
3. Ecological and Environmental Interest of Cetraria s. str. Species
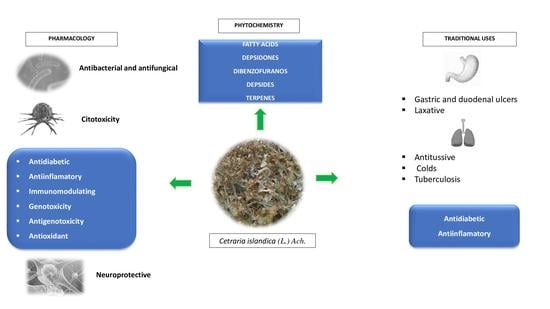
4. Traditional Uses
5. Phytochemistry
6. Therapeutic Potential
6.1. Antibacterial, Antifungal and Antitrypanosomal Activities
6.2. Antioxidant Activity
6.3. Immunomodulatory and Anti-Inflammatory Activities
6.4. Cytotoxic, Genotoxic and Antigenotoxic Activities
6.5. Cell Differentiation and Depigmentation Activities
| Lichen Species | Extracts/Active Compounds | Experimental Model | Activities | Results | References |
|---|---|---|---|---|---|
| Cetraria aculeata (Schreb.) Fr. | Diethyl ether extract Ethanol extract Acetone extract | Gram-positive: Bacillus cereus, Staphylococcus aureus, Bacillus subtilis, Streptococcus, Listeria monocytogenes Gram-negative: Escherichia coli, Proteus vulgaris, Pseudomonas aeruginosa, Pseudomonas syringae, Aeromonas hydrophila, Yersinia enterocolitica, Klebsiella pneumoniae | Antibacterial | Antimicrobial activity against B. cereus, S. aureus, E. coli, P. vulgaris, P. aeruginosa, Streptococcus, B. subtilis, A. hydrophila, L. monocytogenes | [13] |
| Diethyl ether extract Ethanol extract Acetone extract | Penicillum sp., Cladosporium sp., Fusarium oxysporum, F. culmorum, F. moniliforme, F. solani, Rhizopus sp, Aspergillus sp. | Antifungal | No antifungal activity detected | [13] | |
| Acetone extract | TA98 and TA100 strains of S. typhimurium | Antigenotoxicity | ↑ Inhibition of frameshift mutations in TA98 than in TA100 | [129] | |
| Methanol extract | Salmonella typhimurium TA1535 and TA1537 E. coli WP2uvrA Human lymphocyte cells | Antigenotoxicity | Antimutagenic activity against Salmonella typhimurium No activity against E. coli ↓ formation of SCE | [14] | |
| Methanol extract Ethyl acetate extract | Radical scavenging activity | Antioxidant | Methanol extract >>> ethyl acetate extract Methanol extract: DPPH (IC50 51.6 µg/mL); lipid peroxidation inhibition capacity (IC50 45.5 µg/mL); ferrous ion chelating capacity (IC50 50.4 µg/mL; hydroxyl radical scavenging activity (IC50 90.1 µg/mL) | [112] | |
| Methanol extract | Human lymphocytes cells | Antioxidant | ↑ SOD, GPX, MDA levels ↓ GSH | [14] | |
| Acetone extract | HeLa cells, A549 cells and 5RP7 cells | Cytotoxic | ↓ Cell viability | [129] | |
| Methanol extract | Salmonella typhimurium TA1535 E. coli WP2uvrA | Genotoxicity | No activity | [14] | |
| Cetraria islandica (L.) Ach | Methanol extract Acetone extract Light petrolatum extract Aqueous extract | Helicobacter pylori | Antibacterial | Light petrolatum extract > Acetone extract | [108] |
| Methanol extract | Gram-positive: Staphylococcus aureus, Bacillus subtilis, Bacillus cereus Gram negative: Escherichia coli, Proteus mirabilis | Antibacterial | Antimicrobial activity against all bacteria | [15] | |
| Methanol extract | Aspergillus flavus, Candida albicans, Fusarium oxysporum, Penicillium purpurescen, Trichoderma harsianum | Antifungal | Antifungical activity against all fungi | [15] | |
| Aqueous extract | Streptozotocin-induced Diabetes Mellitus type 1 Sprague-Dawley rats | Antidiabetic | Slight insulin increase No inhibition of glucose levels ↓ infiltration of immune cells, vacuolization, and intensity of fibrosis in the kidney ↑ SOD and GSH, ↓ MDA | [16] | |
| Aqueous extract | Streptozotocin-induced Diabetes Mellitus type 1 Sprague-Dawley rats | Antidiabetic | No body weight change ↓ glucose ↑ insulin levels ↑ SOD, CAT and GSH levels ↑ glycogen of hepatocytes ↓ intensity of fibrosis | [119] | |
| Aqueous Extract | Streptozotocin-induced Diabetes Mellitus type 1 Sprague-Dawley rats | Antidiabetic | ↓ TOS ↑ TAC ↑ regeneration and erythropoiesis ↑ MCV, MCH, MCHC | [118] | |
| Cetraria islandica (L.) Ach | Aqueous extract | BSA-induced arthritis in rats | Anti-inflammatory | ↓ reduction in the diameter between the right and left knee | [90] |
| Aqueous extract | Streptozotocin-induced Diabetes Mellitus type 1 Sprague-Dawley rats | Antioxidant | ↑ SOD and CAT ↓ MDA level Light prevention of pancreatic cells destruction | [120] | |
| Aqueous extract | Streptozotocin-induced Diabetes Mellitus type 1 Sprague-Dawley rats | Antioxidant | ↑ SOD, GSH ↓ MDA levels Prevention of renal cell destruction. | [16] | |
| Aqueous extract | Human erythrocytes with type 1 diabetes mellitus | Antioxidant | ↑ SOD, CAT and GPx ↓ MDA levels | [117] | |
| Methanol extract | Blood lymphocytes from human nonsmoking healthy volunteers | Antioxidant | ↑ SOD and GPx ↓ MDA | [113] | |
| Aqueous extract | Radical scavenging activity | Antioxidant | 96–100% inhibition upon lipid peroxidation of linoleic acid system ↑ Superoxide radical scavenging activity | [114] | |
| Ethanol extract | Radical scavenging activity | Antioxidant | DPPH, FRAP and ABTS | [115] | |
| Methanol extract | Radical scavenging activity | Antioxidant | DPPH (IC50 678.3 μg/mL) Superoxide anion scavenging activity (IC50 792.4 μg/mL) Reducing power range 0.0512 to 0.4562 μg/mL | [15] | |
| Melanin | Radical scavenging activity | Antioxidant | DPPH (IC50 405 μg/mL) | [105] | |
| Protolichesterinic acid, Lichesterinic acid, Protocetraric acid, Fumarprotocetraric acid | Trypanosoma brucei brucei | Antitrypanosomal | Protolichesterinic acid MIC value 12.5 µM Lichesterinic acid MIC value 6.30 µM Protocetraric acid and Fumarprotocetraric acid no antitrypanosomal activity detected | [109] | |
| β-1,3/1,4-Glucan lichenan | Keratinocytes (NHEK) cells HaCaT keratinocytes cells | Cellular differentiation | ↓ Proliferation ↑ CytoKeratin 10 (CK) in the cytoplasm ↑ Involucrin expression Dose-dependent CK gene expression regulation ↑ Involucrin transcription levels ↑ Transglutaminase gene expression Gene expression regulation of loricrin and filaggrin ↑ Gene group related to cellular differentiation | [97] | |
| Protolichesterinic acid | A549 cells | Cytotoxic | No change in 5-lipoxygenase activity ↓ LRRC8A expression ↓ cell viability | [126] | |
| Protolichesterinic acid | T-47D cells, K-562 cells and ZR-75-1 cells | Cytotoxic | Morphological changes in T-47D and K-562 ↓ Cell viability ↓ DNA synthesis Inhibition of 5-lipoxygenase | [125] | |
| Ethanol extract | MCF7 cells | Cytotoxic | ↓ Cell viability (IC50 9.2047 × 10−5 g/mL) ↓ PPAR-g levels ↑ AMPK-α1 and ERK1/2 levels ↑ Apoptotic cell percentage after 24 h ↓ P53, Caspase 3 and Bcl-2 dose dependent | [124] | |
| Methanol extract | FemX and LS174 cells | Cytotoxic | FemX (IC50 22.6 μg/mL) LS174 (IC50 33.7 μg/mL) | [15] | |
| Cetraria islandica (L.) Ach | Lichenan | U937 cells | Cytotoxic | No active | [128] |
| Methanol extract | MCF-7 and HepG2 cells | Cytotoxic | MCF-7 (IC50 181.0 µg/mL) HepG2 (IC50 19.5 µg/mL) | [121] | |
| Fumarprotocetraric acid | T-47D and Panc-1 | Cytotoxic | No antiproliferative effect | [127] | |
| Chloroform–methanol, extract | Developing zebrafish embryos | Depigmenting | ↓ Pigmentation (IC50 44 µg/mL) | [130] | |
| Chloroform–methanol extract | Radical scavenging activity MeWo | Depigmenting | Tyrosinase inhibition (IC50 86 µg/mL) Cell viability assay (IC50 264 µg/mL) ↓ Melanin levels | [130] | |
| Aqueous extract | Human erythrocytes with type 1 diabetes mellitus | Genotoxicity | ↑ Proliferation index ↓ DNA damage ↓ SCE | [117] | |
| Methanol extract | Peripheral venous blood | Genotoxicity | ↑ Number of BN cells containing MNi and number of MNi in BN cells | [15] | |
| Aqueous extract Fumarprotocetraric acid, Protolichesterinic acid, Lichenan and isolichenan | Human monocytes differentiated into mature dendritic cells. | Immunomodulating | Aqueous extract and lichenan were active ↑ CD86 and ↓ CD209 and IL-12p40/IL-10 | [90] | |
| (1 --> 3) -(1 --> 4)-α-D-Glucan polysaccharide Ci-3 | Whole blood | Immunomodulating | ↑ Granulocytic phagocytosis ↓ Complementarily induced hemolysis | [123] | |
| Fumarprotocetraric acid | Radical scavenging activity SH-SY5Y and U373-MG cells | Neuroprotective | ORAC (5.07 μmol TE/mg), DPPH (IC50 1393.83 μg/mL) ↑ cell survival, GSH/GSSG ↓ lipid peroxidation, ROS caspase-3 activation Avoid mitochondrial dysfunction and alterations in calcium homeostasis ↓ Pro-apoptotic signals Nrf2 pathway | [122] | |
| Cetraria islandica (L.) Ach | Methanol extract | U373 MG cells | Neuroprotective | ORAC (3.06 µmol TE/mg), DPPH (IC50 1183.55 µg/mL) ↑ Cell viability and GSH/GSSG ratio ↓ ROS generation and lipid peroxidation | [121] |
| Cetraria pinastri (Scop.) Gray. | Methanol extract | Gram-positive: Enterococcus fecalis, Staphylococcus aureus. Gram-negative: Escherichia coli, Klebsiella pneumoniae Micrococcus lysodeikticus, Pseudomonas aeruginosa | Antibacterial | Antimicrobial activity against all bacterial strains | [107] |
| Cetraria pinastri (Scop.) Gray. | Methanol extract | Alternaria alternate, Aspergillus flavus, A. niger, Candida albicans, Cladosporium cladosporioides, Paecilomyces variotii, Acremonium chrysogenum, Fusarium oxysporum, Penicillium verrucosum Trichoderma harsianum | Antifungal | Antifungal activity against all fungal species tested | [107] |
| Cetraria pinastri (Scop.) Gray. | Methanol extract | Thiocyanate method | Antioxidant | 48.79% inhibition of the oxidation of linoleic acid | [11] |
7. Conclusions and Future Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Gómez-Serranillos, M.; Fernández-Moriano, C.; González-Burgos, E.; Divakar, P.; Crespo, A. Parmeliaceae family: Phytochemistry, pharmacological potential and phylogenetic features. RSC Adv. 2014, 4, 59017–59047. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Liu, B.; Zhang, X.Y.; Zhou, Q.M.; Zhang, T.; Li, H.; Yu, Y.F.; Zhang, X.L.; Hao, X.Y.; Wang, M.; et al. Genome characteristics reveal the impact of lichenization on lichen-forming fungus Endocarpon pusillum Hedwig (Verrucariales, Ascomycota). BMC Genom. 2014, 15, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Printzen, C.; Fernández-Mendoza, F.; Muggia, L.; Berg, G.; Grube, M. Alphaproteobacterial communities in geographically distant populations of the lichen Cetraria aculeata. FEMS Microbiol. Ecol. 2012, 82, 316–325. [Google Scholar] [CrossRef] [Green Version]
- Klarenberg, I.J.; Keuschnig, C.; Warshan, D.; Jónsdóttir, I.S.; Vilhelmsson, O. The Total and Active Bacterial Community of the Chlorolichen Cetraria islandica and Its Response to Long-Term Warming in Sub-Arctic Tundra. Front. Microbiol. 2020, 11, 540404. [Google Scholar] [CrossRef] [PubMed]
- Sipman, H.J.M.; Aptroot, A. Where are the missing lichens? Mycol. Res. 2001, 105, 1433–1439. [Google Scholar] [CrossRef]
- Divakar, P.; Crespo, A.; Kraichak, E.; Leavitt, S.; Singh, G.; Schmitt, I.; Lumbsch, T. Using a temporal phylogenetic method to harmonize family- and genus-level classification in the largest clade of lichen-forming fungi. Fungal Divers. 2017, 84, 101–117. [Google Scholar] [CrossRef]
- Lücking, R.; Hodkinson, B.P.; Leavitt, S. The 2016 classification of lichenized fungi in the Ascomycota and Basidiomycota—Approaching one thousand genera. Bryologist 2016, 119, 361–416. [Google Scholar] [CrossRef]
- Fonnegra, R.; Jímenez, S. Plantas Medicinales Aprobadas en Colombia; Editorial Universidad de Antioquia: Medellín, Colombia, 2007; 368p. [Google Scholar]
- Thell, A.; Crespo, A.; Divakar, P.K.; Kärnefelt, I.; Leavitt, S.D.; Lumbsch, H.T.; Seaward, M.R.D. A review of the lichen family Parmeliaceae—history, phylogeny and current taxonomy. Nord. J. Bot. 2012, 30, 641–664. [Google Scholar] [CrossRef]
- Sharnoff, S. Use of Lichens by Wildlife in North America. Res. Explor. 1994, 10, 370–371. [Google Scholar]
- Peterson, J.; Schmoldt, D.; Peterson, D. Guidelines for Evaluating Air Pollution Impacts on Class 1 Wilderness Areas in the Pacific Northwest; Gen. Tech. Rep. PNW-GTR-299; U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station: Portland, OR, USA, 1992; 83p. [Google Scholar]
- EMA/HMPC/36866/2014. Assessment Report on Cetraria islandica (L.) Acharius s.l., Thallus. 2014. Available online: https://www.ema.europa.eu/en/documents/herbal-report/draft-assessment-report-cetraria-islandica-l-acharius-sl-thallus-first-version_en.pdf (accessed on 25 March 2022).
- Türk, A.O.; Yilmaz, M.; Kivanç, M.; Türk, H. The antimicrobial activity of extracts of the lichen Cetraria aculeata and its protolichesterinic acid constituent. Z. Naturforsch. C J. Biosci. 2003, 58, 850–854. [Google Scholar] [CrossRef]
- Ceker, S.; Orhan, F.; Sezen, S.; Gulluce, M.; Ozkan, H.; Aslan, A.; Agar, G. Anti-mutagenic and Anti-oxidant Potencies of Cetraria Aculeata (Schreb.) Fr., Cladonia Chlorophaea (Flörke ex Sommerf.) Spreng. and Cetrelia olivetorum (Nyl.) W.L. Culb. & C.F. Culb.). Iran. J. Pharm. Res. 2018, 17, 326–335. [Google Scholar] [PubMed]
- Grujičić, D.; Stošić, I.; Kosanić, M.; Stanojković, T.; Ranković, B.; Milošević-Djordjević, O. Evaluation of in vitro antioxidant, antimicrobial, genotoxic and anticancer activities of lichen Cetraria islandica. Cytotechnology 2014, 66, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Bakır, T.; Geyikoglu, F.; Çolak, S.; Türkez, H.; Aslan, A.; Bakır, M. The effects of Cetraria islandica and Pseudevernia furfuracea extracts in normal and diabetic rats. Toxicol. Ind. Health 2015, 31, 1304–1317. [Google Scholar] [CrossRef]
- Wichert, B. Medicine for Coughs and Other Disorders of the Respiratory Organs of Horses, and Feedstuff for Horses Produced Using the Same. Patent Number DE3229086, 4 September 1984. [Google Scholar]
- Etienne, L.P. Use of Extracts of Iceland Lichen and One of Its Constituents, Protolichesterinic Acid. Patent Number FR2756182, 31 December 1998. [Google Scholar]
- Cabrera, A.L.; Beguer, J.H. Preparation for Veterinary Use. Patent Number US20030068294, 4 October 2003. [Google Scholar]
- Reijonen, M.T. Cetraria islandica Based Wood Protection and Impregnation Product. Patent Number WO2008077997, 3 July 2008. [Google Scholar]
- Xu, M.; Heidmarsson, S.; Olafsdottir, E.S.; Buonfiglio, R.; Kogej, T.; Omarsdottir, S. Secondary metabolites from cetrarioid lichens: Chemotaxonomy, biological activities and pharmaceutical potential. Phytomedicine 2016, 23, 441–459. [Google Scholar] [CrossRef] [PubMed]
- Bisby, F.A. Characterization of Biodiversity; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar]
- Lücking, R. Stop the Abuse of Time! Strict Temporal Banding is not the Future of Rank-Based Classifications in Fungi (Including Lichens) and Other Organisms. Crit. Rev. Plant Sci. 2019, 38, 199–253. [Google Scholar] [CrossRef]
- Thell, A.; Stenroos, S.; Feuerer, T.; Kärnefelt, I.; Myllys, L.; Hyvönen, J. Phylogeny of cetrarioid lichens (Parmeliaceae) inferred from ITS and b-tubulin sequences, morphology, anatomy and secondary chemistry. Mycol. Prog. 2002, 1, 335–354. [Google Scholar] [CrossRef]
- Kraichak, E.; Crespo, A.; Divakar, P.; Leavitt, S.; Lumbsch, T. A temporal banding approach for consistent taxonomic ranking above the species level. Sci. Rep. 2017, 7, 2297. [Google Scholar] [CrossRef]
- Williams, D.; Schmitt, M.; Wheeler, Q. The Future of Phylogenetic Systematics: The Legacy of Willi Hennig; Cambridge University Press: Cambridge, UK, 2016. [Google Scholar]
- Divakar, P.K.; Crespo, A.; Wedin, M.; Leavitt, S.D.; Hawksworth, D.L.; Myllys, L.; McCune, B.; Randlane, T.; Bjerke, J.W.; Ohmura, Y.; et al. Evolution of complex symbiotic relationships in a morphologically derived family of lichen-forming fungi. New Phytol. 2015, 208, 1217–1226. [Google Scholar] [CrossRef] [Green Version]
- Acharius, E. Methodus Lichenum. 1803. Available online: https://www.biodiversitylibrary.org/bibliography/79411 (accessed on 1 April 2022).
- Eschweiler, F.G. Systema Lichenum, Genera Exhibens Rite Distincta, Pluribus Novis Adaucta; 1824. Available online: https://www.digitale-sammlungen.de/de/view/bsb10229581?page=5 (accessed on 1 April 2022).
- Thell, A.; Högnabba, F.; Elix, J.; Tassilo, F.; Kärnefelt, I.; Myllys, L.; Randlane, T.; Saag, A.; Stenroos, S.; Ahti, T.; et al. Phylogeny of the cetrarioid core (Parmeliaceae) based on five genetic markers. Lichenologist 2009, 41, 489–511. [Google Scholar] [CrossRef]
- Howe, R.H. The genus Cetraria as represented in the United states and Canada. Torreya 1915, 15, 213–230. [Google Scholar]
- Elix, J.A. Progress in the Generic Delimitation of Parmelia Sensu Lato Lichens (Ascomycotina: Parmeliaceae) and a Synoptic Key to the Parmeliaceae. Bryologist 1993, 96, 359–383. [Google Scholar] [CrossRef]
- Nash, T.H.; Ryan, B.D.; Gries, C.; Bungartz, F. Lichen Flora of the Greater Sonoran Desert Region; Lichens Unlimite: Tempe, AZ, USA, 2002; Volume 1. [Google Scholar]
- Randlane, T.; Saag, A. Cetrarioid lichens in Europe—An identification key for the species. In Central European Lichens—Diversity and Threat; Lackovičova, A., Guttova, A., Lisicka, E., Lizon, P., Eds.; Mycotaxon: Ithaca, NY, USA, 2006; pp. 75–84. [Google Scholar]
- Nadyeina, O.; Lutsak, T.; Blum, O.; Grakhov, V.; Scheidegger, C. Cetraria steppae Savicz is conspecific with Cetraria aculeata (Schreb.) Fr. according to morphology, secondary chemistry and ecology. Lichenologist 2013, 45, 841–856. [Google Scholar] [CrossRef] [Green Version]
- Kärnefelt, I.; Mattsson, J.-E.; Thell, A. The Lichen Genera Arctocetraria, Cetraria, and Cetrariella (Parmeliaceae) and Their Presumed Evolutionary Affinities. Bryologist 1993, 96, 394–404. [Google Scholar] [CrossRef]
- Thompson, J.N. Conserving Interaction Biodiversity. In The Ecological Basis of Conservation: Heterogeneity, Ecosystems, and Biodiversity; Pickett, S.T.A., Ostfeld, R.S., Shachak, M., Likens, G.E., Eds.; Springer: Boston, MA, USA, 1997; pp. 285–293. [Google Scholar]
- van den Boom, P.P.G.; Sipman, H.J.M. Cetraria obtusata comb. et stat. nov., an overlooked lichen species from the Central Alps. Lichenologist 1994, 26, 105–112. [Google Scholar] [CrossRef]
- Barreno, E.; Vázquez, V.M. Coelocaulon crespoae Barreno & Vázquez sp. nova (Lichenes): Notas sobre la flora liquénica de brezales españoles. Lazaroa 1981, 3, 235–246. [Google Scholar]
- Singh, C.P.; Bajpai, R.; Singh, R.; Upreti, D. Improving bioclimatic envelop modeling for Lichens through remote sensing based substratum correction: A study over Indian Himalaya. Cryptogam Biodivers. Assess. 2016, 1, 30–48. [Google Scholar] [CrossRef]
- Randlane, T.; Saag, A. Cetrarioid lichens in the southern hemisphere—An identification key and distribution patterns of the species. Bibl. Lichenol. 2007, 95, 489–499. [Google Scholar]
- Goffinet, B.; Thomson, J. American Arctic Lichens. 2. The Microlichens. Bryologist 2000, 102, 345. [Google Scholar] [CrossRef]
- Zhurbenko, M.; Laursen, G.; Walker, D. New and rare lichenicolous fungi and lichens from the North American Arctic. Mycotaxon 2005, 92, 201–212. [Google Scholar]
- Thompson, J. American Arctic Lichens: The Macrolichens; University of Wisconsin Press: Madison, WI, USA, 1984; Volume 1. [Google Scholar]
- Randlane, T.; Saag, A. Distribution patterns of some primary and secondary cetrarioid species. Symb. Bot. Ups. 2004, 34, 359–376. [Google Scholar]
- Fernández-Mendoza, F.; Printzen, C. Pleistocene expansion of the bipolar lichen Cetraria aculeata into the Southern hemisphere. Mol. Ecol. 2013, 22, 1961–1983. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Ortega, S.; Fernández-Mendoza, F.; Raggio, J.; Vivas, M.; Ascaso, C.; Sancho, L.; Printzen, C.; De los Ríos, A. Extreme phenotypic variation in Cetraria aculeata (lichenized Ascomycota): Adaptation or incidental modification? Ann. Bot. 2012, 109, 1133–1148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loppi, S. May the Diversity of Epiphytic Lichens Be Used in Environmental Forensics? Diversity 2019, 11, 36. [Google Scholar] [CrossRef] [Green Version]
- Vannini, A.; Tedesco, R.; Loppi, S.; Di Cecco, V.; Martino, L.; Nascimbene, J.; Dallo, F.; Barbante, C. Lichens as monitors of the atmospheric deposition of potentially toxic elements in high elevation Mediterranean ecosystems. Sci. Total Environ. 2021, 798, 149369. [Google Scholar] [CrossRef] [PubMed]
- Sancho, L.; Pintado, A.; Green, T. Antarctic Studies Show Lichens to be Excellent Biomonitors of Climate Change. Diversity 2019, 11, 42. [Google Scholar] [CrossRef] [Green Version]
- Sujetovienė, G.; Česynaitė, J. Assessment of air pollution at the indoor environment of a shooting range using lichens as biomonitors. J. Toxicol. Environ. Health Part A 2021, 84, 273–278. [Google Scholar] [CrossRef]
- Bajpai, R.; Shukla, V.; Raju, A.; Singh, C.P.; Upreti, D. A geostatistical approach to compare metal accumulation pattern by lichens in plain and mountainous regions of northern and central India. Environ. Earth Sci. 2022, 81, 203. [Google Scholar] [CrossRef]
- Loppi, S. Lichens as sentinels for air pollution at remote alpine areas (Italy). Environ. Sci. Pollut. Res. Int. 2014, 21, 2563–2571. [Google Scholar] [CrossRef]
- Bajpai, R.; Mishra, S.; Dwivedi, S.; Upreti, D.K. Change in atmospheric deposition during last half century and its impact on lichen community structure in Eastern Himalaya. Sci. Rep. 2016, 6, 30838. [Google Scholar] [CrossRef] [Green Version]
- Doğan, C.E.; Turhan, K.; Akcin, G.; Aslan, A. Biosorption of Au (III) and Cu (II) from Aqueous Solution by a Non-Living Cetraria islandica (L.) Ach. Ann. Chim. 2006, 96, 229–236. [Google Scholar] [CrossRef]
- Cuculovic, A.; Pavlovic, M.; Savovic, J.; Veselinović, D. Desorption of metals from Cetraria islandica (L.) Ach. Lichen using solutions simulating acid rain. Arch. Biol. Sci. 2014, 66, 273–284. [Google Scholar] [CrossRef] [Green Version]
- Nanda, S.A.; Haq, M.-u.; Singh, S.P.; Reshi, Z.A.; Rawal, R.S.; Kumar, D.; Bisht, K.; Upadhyay, S.; Upreti, D.K.; Pandey, A. Species richness and β-diversity patterns of macrolichens along elevation gradients across the Himalayan Arc. Sci. Rep. 2021, 11, 20155. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Verma, S.; Gupta, S.; Singh, A.; Pal, A.; Srivastava, S.K.; Srivastava, P.K.; Singh, S.C.; Darokar, M.P. Membrane-damaging potential of natural L-(-)-usnic acid in Staphylococcus aureus. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 3375–3383. [Google Scholar] [CrossRef] [PubMed]
- Minganti, V.; Drava, G.; Pellegrini, R.; Modenesi, P.; Malaspina, P.; Giordani, P. Temporal trends (1981-2007) of trace and rare earth elements in the lichen Cetraria islandica (L.) Ach. from Italian herbaria. Chemosphere 2014, 99, 180–185. [Google Scholar] [CrossRef]
- Cuculovic, A.; Veselinović, D.; Miljanic, S. Extraction of 137Cs from Cetraria islandica lichen with water. J. Serb. Chem. Soc. 2006, 71, 565–571. [Google Scholar] [CrossRef]
- Machart, P.; Hofmann, W.; Türk, R.; Steger, F. Ecological half-life of 137Cs in lichens in an alpine region. J. Environ. Radioact. 2007, 97, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Cercasov, V.; Pantelica, A.; Salagean, M.; Caniglia, G.; Scarlat, A. Comparative study of the suitability of three lichen species to trace-element air monitoring. Environ. Pollut. 2002, 119, 129–139. [Google Scholar] [CrossRef]
- EFSA. Compendium of botanicals reported to contain naturally occurring substances of possible concern for human health when used in food and food supplements. EFSA J. 2021, 10, 2663. [Google Scholar]
- C/2021/5831. Commission Regulation(EU) 2021/1317 of 9 August 2021 amending Regulation (EC) No 1881/2006 as Regards Maximum Levels of Lead in Certain Foodstuffs. Available online: https://eur-lex.europa.eu/eli/reg/2021/1317/oj (accessed on 23 May 2022).
- Giordani, P. Is the diversity of epiphytic lichens a reliable indicator of air pollution? A case study from Italy. Environ. Pollut. 2007, 146, 317–323. [Google Scholar] [CrossRef]
- Meli, P.; Holl, K.; Benayas, J.; Jones, H.; Jones, P.; Montoya, D.; Moreno Mateos, D. A global review of past land use, climate, and active vs. passive restoration effects on forest recovery. PLoS ONE 2017, 12, e0171368. [Google Scholar] [CrossRef]
- Insarov, G.; Schroeter, B. Lichen Monitoring and Climate Change. In Monitoring with Lichens—Monitoring Lichens; Nimis, P.L., Scheidegger, C., Wolseley, P.A., Eds.; Springer: Dordrecht, The Netherlands, 2002; pp. 183–201. [Google Scholar]
- Stapper, N.; John, V. Monitoring climate change with lichens as bioindicators. Pollut. Atmos. 2015, 226, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Aptroot, A.; Van Herk, K. Further evidence of the effects of global warming on lichens, particularly those with Trentepohlia phycobionts. Environ. Pollut. 2007, 146, 293–298. [Google Scholar] [CrossRef]
- Fernández-Mendoza, F.; Domaschke, S.; García, M.Á.; Jordan, P.; Martín, M.; Printzen, C. Population structure of mycobionts and photobionts of the widespread lichen Cetraria aculeata. Mol. Ecol. 2011, 20, 1208–1232. [Google Scholar] [CrossRef] [PubMed]
- Domaschke, S.; Fernández-Mendoza, F.; García, M.A.; Martín, M.; Printzen, C. Low genetic diversity in Antarctic populations of the lichen-forming ascomycete Cetraria aculeata and its photobiont. Polar Res. 2012, 31, 17353. [Google Scholar] [CrossRef]
- Ingólfsdóttir, K. Usnic acid. Phytochemistry 2002, 61, 729–736. [Google Scholar] [CrossRef]
- Richardson, D.H.S. Medicinal and other economic aspects of lichens. In GALUN, M. CRC Handbook of Lichenology; CRC Press: Boca Raton, FL, USA, 1988; Volume III, pp. 93–108. [Google Scholar]
- Ahmadjian, V.; Nilsson, S. Swedish Lichens. In Yearbook 1963; Goddard, H., Ed.; American Swedish Historical Foundation: Philadelphia, PA, USA, 1963; pp. 39–47. [Google Scholar]
- Baytop, T. Therapy with Medicinal Plants in Turkey (Past and Present); Istanbul University: Istanbul, Turkey, 1999; pp. 1–233. [Google Scholar]
- Dülger, B.; Gulcin, F.; Aslan, A. Cetraria islandica (L) Ach. Likeninin Antimikrobiyal Aktivitesi. Turk. J. Biol. 1998, 22, 111–118. [Google Scholar]
- Agelet, A.; Vallès, J. Studies on pharmaceutical ethnobotany in the region of Pallars (Pyrenees, Catalonia, Iberian Peninsula). Part III. Medicinal uses of non-vascular plants. J. Ethnopharmacol. 2003, 84, 229–234. [Google Scholar] [CrossRef]
- Gudjónsdóttir, G.A.; Ingólfsdóttir, K. Quantitative determination of protolichesterinic- and fumarprotocetraric acids in Cetraria islandica by high-performance liquid chromatography. J. Chromatogr. A 1997, 757, 303–306. [Google Scholar] [CrossRef]
- Xu, M.; Heidmarsson, S.; Thorsteinsdottir, M.; Kreuzer, M.; Hawkins, J.; Omarsdottir, S.; Olafsdottir, E.S. Authentication of Iceland Moss (Cetraria islandica) by UPLC-QToF-MS chemical profiling and DNA barcoding. Food Chem. 2018, 245, 989–996. [Google Scholar] [CrossRef]
- Airaksinen, M.M.; Peura, P.; Ala-Fossi-Salokangas, L.; Antere, S.; Lukkarinen, J.; Saikkonen, M.; Stenbäck, F. Toxicity of plant material used as emergency food during famines in Finland. J. Ethnopharmacol. 1986, 18, 273–296. [Google Scholar] [CrossRef]
- Illana-Esteban, C. Edible Lichens. Boletín de la Sociedad Micológica de Madrid 2009, 33, 273–282. [Google Scholar]
- FDA. Food Additive Status List. 2021. Available online: https://www.fda.gov/food/food-additives-petitions/food-additive-status-list (accessed on 5 May 2022).
- Ivanova, D.; Ivanov, D. Ethnobotanical use of lichens: Lichens for food review. Scr. Sci. Med. 2009, 41, 11. [Google Scholar] [CrossRef] [Green Version]
- Illana-Esteban, C. Líquenes usados en perfumería. Bol. Soc. Micol. Madr. 2016, 40, 217–223. [Google Scholar]
- Elix, J.A.; Stocker-Wörgötter, E. Biochemistry and secondary metabolites. In Lichen Biology, 2nd ed.; Nash, T.H., Ed.; Cambridge University Press: Cambridge, UK, 2008; pp. 104–133. [Google Scholar]
- Molnár, K.; Farkas, E. Current results on biological activities of lichen secondary metabolites: A review. Z. Naturforsch. C J. Biosci. 2010, 65, 157–173. [Google Scholar] [CrossRef]
- Ranković, B.; Kosanić, M. Lichens as a Potential Source of Bioactive Secondary Metabolites. In Lichen Secondary Metabolites Bioactive Properties and Pharmaceutical Potential; Ranković, B., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 1–26. [Google Scholar]
- Calcott, M.J.; Ackerley, D.F.; Knight, A.; Keyzers, R.A.; Owen, J.G. Secondary metabolism in the lichen symbiosis. Chem. Soc. Rev. 2018, 47, 1730–1760. [Google Scholar] [CrossRef]
- Huovinen, K.; Ahti, T. The composition and contents of aromatic lichen substances in Cladonia, section Unciales. Ann. Bot. Fenn. 1986, 23, 173–188. [Google Scholar]
- Freysdottir, J.; Omarsdottir, S.; Ingólfsdóttir, K.; Vikingsson, A.; Olafsdottir, E.S. In vitro and in vivo immunomodulating effects of traditionally prepared extract and purified compounds from Cetraria islandica. Int. Immunopharmacol. 2008, 8, 423–430. [Google Scholar] [CrossRef]
- Fernández-Moriano, C.; Divakar, P.K.; Crespo, A.; Gómez-Serranillos, M.P. In vitro neuroprotective potential of lichen metabolite fumarprotocetraric acid via intracellular redox modulation. Toxicol. Appl. Pharmacol. 2017, 316, 83–94. [Google Scholar] [CrossRef]
- Ureña-Vacas, I.; González-Burgos, E.; Divakar, P.K.; Gómez-Serranillos, M.P. Lichen Depsidones with Biological Interest. Planta Med. 2021. [Google Scholar] [CrossRef]
- González-Burgos, E.; Fernández-Moriano, C.; Gómez-Serranillos, M.P. Current knowledge on Parmelia genus: Ecological interest, phytochemistry, biological activities and therapeutic potential. Phytochemistry 2019, 165, 112051. [Google Scholar] [CrossRef]
- Sepahvand, A.; Studzińska-Sroka, E.; Ramak, P.; Karimian, V. Usnea sp.: Antimicrobial potential, bioactive compounds, ethnopharmacological uses and other pharmacological properties; a review article. J. Ethnopharmacol. 2021, 268, 113656. [Google Scholar] [CrossRef] [PubMed]
- Lutsak, T.; FernÁNdez-Mendoza, F.; Nadyeina, O.; ŞEnkardeŞLer, A.; Printzen, C. Testing the correlation between norstictic acid content and species evolution in the Cetraria aculeata group in Europe. Lichenologist 2017, 49, 39–56. [Google Scholar] [CrossRef]
- Krämer, P.; Wincierz, U.; Grübler, G.; Tschakert, J.; Voelter, W.; Mayer, H. Rational approach to fractionation, isolation, and characterization of polysaccharides from the lichen Cetraria islandica. Arzneimittelforschung 1995, 45, 726–731. [Google Scholar]
- Zacharski, D.M.; Esch, S.; König, S.; Mormann, M.; Brandt, S.; Ulrich-Merzenich, G.; Hensel, A. β-1,3/1,4-Glucan Lichenan from Cetraria islandica (L.) ACH. induces cellular differentiation of human keratinocytes. Fitoterapia 2018, 129, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.C.; Saldivar, R.K.; Liang, P.H.; Hsieh, Y.S.Y. Structures, Biosynthesis, and Physiological Functions of (1,3;1,4)-β-D-Glucans. Cells 2021, 10, 510. [Google Scholar] [CrossRef] [PubMed]
- Ingolfsdottir, K.; Jurcic, K.; Fischer, B.; Wagner, H. Immunologically active polysaccharide from Cetraria islandica. Planta Med. 1994, 60, 527–531. [Google Scholar] [CrossRef]
- Olafsdottir, E.S.; Omarsdottir, S.; Paulsen, B.S.; Jurcic, K.; Wagner, H. Rhamnopyranosylgalactofuranan, a new immunologically active polysaccharide from Thamnolia subuliformis. Phytomedicine 1999, 6, 273–279. [Google Scholar] [CrossRef]
- Ingólfsdóttir, K.; Jurcic, K.; Wagner, H. Immunomodulating polysaccharides from aqueous extracts of Cetraria islandica (Iceland moss). Phytomedicine 1998, 5, 333–339. [Google Scholar] [CrossRef]
- Schweiger, A.H.; Ullmann, G.M.; Nürk, N.M.; Triebel, D.; Schobert, R.; Rambold, G. Chemical properties of key metabolites determine the global distribution of lichens. Ecol. Lett. 2022, 25, 416–426. [Google Scholar] [CrossRef]
- Hauck, M. Susceptibility to acidic precipitation contributes to the decline of the terricolous lichens Cetraria aculeata and Cetraria islandica in central Europe. Environ. Pollut. 2008, 152, 731–735. [Google Scholar] [CrossRef]
- Hauck, M.; Huneck, S. The putative role of fumarprotocetraric acid in the manganese tolerance of the lichen Lecanora conizaeoides. Lichenologist 2007, 39, 301–304. [Google Scholar] [CrossRef]
- Rassabina, A.E.; Gurjanov, O.P.; Beckett, R.P.; Minibayeva, F.V. Melanin from the Lichens Cetraria islandica and Pseudevernia furfuracea: Structural Features and Physicochemical Properties. Biochemistry 2020, 85, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Nybakken, L.; Solhaug, K.A.; Bilger, W.; Gauslaa, Y. The lichens Xanthoria elegans and Cetraria islandica maintain a high protection against UV-B radiation in Arctic habitats. Oecologia 2004, 140, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Ranković, B.; Ranković, D.; Marić, D. Antioxidant and antimicrobial activity of some lichen species. Mikrobiologiia 2010, 79, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Ingolfsdottir, K.; Hjalmarsdottir, M.A.; Sigurdsson, A.; Gudjonsdottir, G.A.; Brynjolfsdottir, A.; Steingrimsson, O. In vitro susceptibility of Helicobacter pylori to protolichesterinic acid from the lichen Cetraria islandica. Antimicrob. Agents Chemother. 1997, 41, 215–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Igoli, J.O.; Gray, A.I.; Clements, C.J.; Kantheti, P.; Singla, R.K. Antitrypanosomal activity & docking studies of isolated constituents from the lichen Cetraria islandica: Possibly multifunctional scaffolds. Curr. Top. Med. Chem. 2014, 14, 1014–1021. [Google Scholar] [CrossRef] [PubMed]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Fernández-Moriano, C.; Gómez-Serranillos, M.P.; Crespo, A. Antioxidant potential of lichen species and their secondary metabolites. A systematic review. Pharm. Biol. 2016, 54, 1–17. [Google Scholar] [CrossRef]
- Tomovic, J.; Rancic, A.; Vasiljević, P.; Maskovic, P.; Zivanovic, S.; Manojlovic, N.; Sovrlić, M. Antioxidant activity of lichen Cetraria aculeata. Prax. Med. 2015, 44, 107–113. [Google Scholar] [CrossRef] [Green Version]
- Kotan, E.; Alpsoy, L.; Anar, M.; Aslan, A.; Agar, G. Protective role of methanol extract of Cetraria islandica (L.) against oxidative stress and genotoxic effects of AFB1 in human lymphocytes in vitro. Toxicol. Ind. Health 2011, 27, 599–605. [Google Scholar] [CrossRef]
- Gülçin, I.; Oktay, M.; Küfrevioğlu, O.I.; Aslan, A. Determination of antioxidant activity of lichen Cetraria islandica (L) Ach. J. Ethnopharmacol. 2002, 79, 325–329. [Google Scholar] [CrossRef]
- Stadnytska, N.; Fito, I.; Novikov, V.; Jasicka-Misiak, I.; Wieczorek, P. Effect of extraction solvent on total phenolic content, total flavonoid content and antioxidant activity of Cetraria islandica. Int. J. PharmTech Res. 2020, 13, 198–205. [Google Scholar] [CrossRef]
- Kosanić, M.; Ranković, B. Antioxidant and Antimicrobial Properties of Some Lichens and Their Constituents. J. Med. Food 2011, 14, 1624–1630. [Google Scholar] [CrossRef]
- Colak, S.; Geyikoglu, F.; Türkez, H.; Bakır, T.; Aslan, A. The ameliorative effect of Cetraria islandica against diabetes-induced genetic and oxidative damage in human blood. Pharm. Biol. 2013, 51, 1531–1537. [Google Scholar] [CrossRef] [PubMed]
- Colak, S.; Geyikoğlu, F.; Aslan, A.; Deniz, G.Y. Effects of lichen extracts on haematological parameters of rats with experimental insulin-dependent diabetes mellitus. Toxicol. Ind. Health 2014, 30, 878–887. [Google Scholar] [CrossRef]
- Deniz, G.Y.; Geyikoğlu, F.; Türkez, H.; Bakır, T.; Çolak, S.; Aslan, A. The biochemical and histological effects of lichens in normal and diabetic rats. Toxicol. Ind. Health 2016, 32, 601–613. [Google Scholar] [CrossRef]
- Çolak, S.; Geyikoğlu, F.; Bakır, T.; Türkez, H.; Aslan, A. Evaluating the toxic and beneficial effects of lichen extracts in normal and diabetic rats. Toxicol. Ind. Health 2016, 32, 1495–1504. [Google Scholar] [CrossRef]
- Fernández-Moriano, C.; Divakar, P.K.; Crespo, A.; Gómez-Serranillos, M.P. Neuroprotective activity and cytotoxic potential of two Parmeliaceae lichens: Identification of active compounds. Phytomedicine 2015, 22, 847–855. [Google Scholar] [CrossRef]
- Fernández-Moriano, C.; Divakar, P.K.; Crespo, A.; Gómez-Serranillos, M.P. Protective effects of lichen metabolites evernic and usnic acids against redox impairment-mediated cytotoxicity in central nervous system-like cells. Food. Chem. Toxicol. 2017, 105, 262–277. [Google Scholar] [CrossRef]
- Olafsdottir, E.S.; Ingolfsdottir, K.; Barsett, H.; Paulsen, B.S.; Jurcic, K.; Wagner, H. Immunologically active (1→3)-(1→4)-α-D-Glucan from Cetraria islandica. Phytomedicine 1999, 6, 33–39. [Google Scholar] [CrossRef]
- Güven, C.; Taskin, E.; Yumtutas, O.; Şener, L.; Ozay, Y.; Dal, F.; Ahbab, M.; Bozgeyik, I.; Albeniz, I.; Bagıs, H.; et al. The Anticancer Activity of Cetraria Islandica (L.) Ach in Breast Cancer Cells Through Crosstalk of Ampk-α1 and Erk1/2 Signalling. Turk. J. Agric.-Food Sci. Technol. 2018, 6, 783. [Google Scholar] [CrossRef]
- Ogmundsdóttir, H.M.; Zoëga, G.M.; Gissurarson, S.R.; Ingólfsdóttir, K. Anti-proliferative effects of lichen-derived inhibitors of 5-lipoxygenase on malignant cell-lines and mitogen-stimulated lymphocytes. J. Pharm. Pharmacol. 1998, 50, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Thorsteinsdottir, U.A.; Thorsteinsdottir, M.; Lambert, I.H. Protolichesterinic Acid, Isolated from the Lichen Cetraria islandica, Reduces LRRC8A Expression and Volume-Sensitive Release of Organic Osmolytes in Human Lung Epithelial Cancer Cells. Phytother. Res. 2016, 30, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Kristmundsdóttir, T.; Jónsdóttir, E.; Ogmundsdóttir, H.M.; Ingólfsdóttir, K. Solubilization of poorly soluble lichen metabolites for biological testing on cell lines. Eur. J. Pharm. Sci. 2005, 24, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Otaka, K.; Maoka, T.; Hidaka, K.; Ishijima, S.; Oda, M.; Ohnishi, M. Structure of beta-glucan oligomer from laminarin and its effect on human monocytes to inhibit the proliferation of U937 cells. Biosci. Biotechnol. Biochem. 2005, 69, 553–558. [Google Scholar] [CrossRef]
- Zeytinoglu, H.; Incesu, Z.; Tuylu, B.A.; Turk, A.O.; Barutca, B. Determination of genotoxic, antigenotoxic and cytotoxic potential of the extract from lichen Cetraria aculeata (Schreb.) Fr. in vitro. Phytother. Res. 2008, 22, 118–123. [Google Scholar] [CrossRef]
- Malaspina, P.; Catellani, E.; Burlando, B.; Brignole, D.; Cornara, L.; Bazzicalupo, M.; Candiani, S.; Obino, V.; De Feo, V.; Caputo, L.; et al. Depigmenting potential of lichen extracts evaluated by in vitro and in vivo tests. PeerJ 2020, 8, e9150. [Google Scholar] [CrossRef]


| Lichen Species | Distribution Pattern | Distribution Areas | References |
|---|---|---|---|
| Cetraria aculeata (Schreb.) Fr. | Cosmopolitan | Four continents and many oceanic islands. | [41] |
| Cetraria annae Oxner | Endemic | Baikal region, Russia | [30] |
| Cetraria australiensis W.A.Weber ex Kärnefelt | Endemic | South-eastern Australia | [41] |
| Cetraria crespoae (Barreno & Vázquez) Kärnefelt | Endemic | Western parts of the Iberian Peninsula and Italy | [29] |
| Cetraria ericetorum Opiz Sp ericetorum Sp reticulata Sp patagonica | Endemic (each subspecies separated geographically) | Europe and Asia North America Southern part of South America | [36,45] |
| Cetraria islandica (L.) Ach. Sp islandica Sp crispiformis Sp antartica | Bipolar Circumboreal Austral | High latitudes in northern and southern hemispheres Europe, Asia, North America Southern hemisphere | [41,45] |
| Cetraria kamczatica Savicz | Amphi-Beringian | Eastern Siberia and Alaska | [37] |
| Cetraria laevigata Rass. | Amphi-Beringian | North America (from Alaska through upper Canada) | [44,45] |
| Cetraria minuscula (Elenkin and Savicz) McCune | Amphi-Beringian | Eastern Siberia, Interior Alaska | [43] |
| Cetraria muricata (Ach.) Eckfeldt | Cosmopolitan | Four continents and many oceanic islands. | [41] |
| Cetraria nepalensis D.D. Awasthi | Endemic | Isolated localities at high elevations in the Great Himalayas | [45] |
| Cetraria nigricans Nyl. | Circumpolar | High Arctic (Alaska, Canada) Alpine sites in southern areas | [41,44] |
| Cetraria odontella (Ach.) Ach. | Circumboreal | Boreal regions of the northern hemisphere. | [41] |
| Cetraria obstusata (Schaer.) Van den Boom & Sipman | Endemic | Alpine areas of the Alps—Austria, Italy, Switzerland | [34] |
| Cetraria peruviana Kärnefelt & Thell | Endemic | Central part of South America | [41] |
| Cetraria rassadinae Makryi | Endemic | Northern Baikal region of central Siberia | [45] |
| Cetraria sepincola (Hoffm.) Ach. | Circumboreal | Boreal forest and in the tundras of the Arctic | [41,44] |
| Cetraria steppae (Savicz) Kärnefelt | Endemic | Semiarid Eurasian steppe biomes from Kazakhstan to Iran and Ukraine. | [35,45] |
| Lichen Species | Chemical Composition | References |
|---|---|---|
| Cetraria aculeata (Schreb.) Fr. | Lichesterinic acid, protolichesterinic acid | [34] |
| Cetraria annae Oxner | Major: usnic acid, isonephrosterinic acid Minor: lichesterinic acid, atranorin, squamatic acid Trace: protolichesterinic acid, nephrosterinic acid | [30] |
| Cetraria australiensis W.A.Weber ex Kärnefelt | Lichesterinic acid, protolichesterinic acid, ursolic acid. | [30] |
| Cetraria ericetorum Opiz | Lichesterinic protolichesterinic acid | [33] |
| Cetraria islandica (L.) Ach. | Fumarprotocetraric acid, protocetraric acid, protolichesterinic acid, usnic acid | [12] |
| Cetraria kamczatica Savicz | Protolichesterinic acid, rangiformic acid | [44] |
| Cetraria laevigata Rass. | Fumarprotocetraric acid. | [44] |
| Cetraria muricata (Ach.) Eckfeldt | Lichesterinic acid, protolichesterinic acid | [30] |
| Cetraria nigricans Nyl. | Protolichesterinic acid, rangiformic acid, secalonic acid | [34] |
| Cetraria odontella (Ach.) Ach. | Protolichesteric and rangiformic acids | |
| Cetraria obstusata (Schaer.) Van den Boom and Sipman | Lichesterinic acid, protolichesterinic acid, secalonic acid | [34] |
| Cetraria sepincola (Hoffm.) Ach. | Lichesterinic acid and protolichesterinic acid. | [34] |
| Cetraria steppae (Savicz) Kärnefelt | Usnic acid, lichesterinic acid, protolichesterinic acid, norstictic acid | [35] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez, M.; Ureña-Vacas, I.; González-Burgos, E.; Divakar, P.K.; Gómez-Serranillos, M.P. The Genus Cetraria s. str.—A Review of Its Botany, Phytochemistry, Traditional Uses and Pharmacology. Molecules 2022, 27, 4990. https://doi.org/10.3390/molecules27154990
Sánchez M, Ureña-Vacas I, González-Burgos E, Divakar PK, Gómez-Serranillos MP. The Genus Cetraria s. str.—A Review of Its Botany, Phytochemistry, Traditional Uses and Pharmacology. Molecules. 2022; 27(15):4990. https://doi.org/10.3390/molecules27154990
Chicago/Turabian StyleSánchez, Marta, Isabel Ureña-Vacas, Elena González-Burgos, Pradeep Kumar Divakar, and Maria Pilar Gómez-Serranillos. 2022. "The Genus Cetraria s. str.—A Review of Its Botany, Phytochemistry, Traditional Uses and Pharmacology" Molecules 27, no. 15: 4990. https://doi.org/10.3390/molecules27154990
APA StyleSánchez, M., Ureña-Vacas, I., González-Burgos, E., Divakar, P. K., & Gómez-Serranillos, M. P. (2022). The Genus Cetraria s. str.—A Review of Its Botany, Phytochemistry, Traditional Uses and Pharmacology. Molecules, 27(15), 4990. https://doi.org/10.3390/molecules27154990