Application of meso-CF3-Fluorophore BODIPY with Phenyl and Pyrazolyl Substituents for Lifetime Visualization of Lysosomes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of BODIPY 1
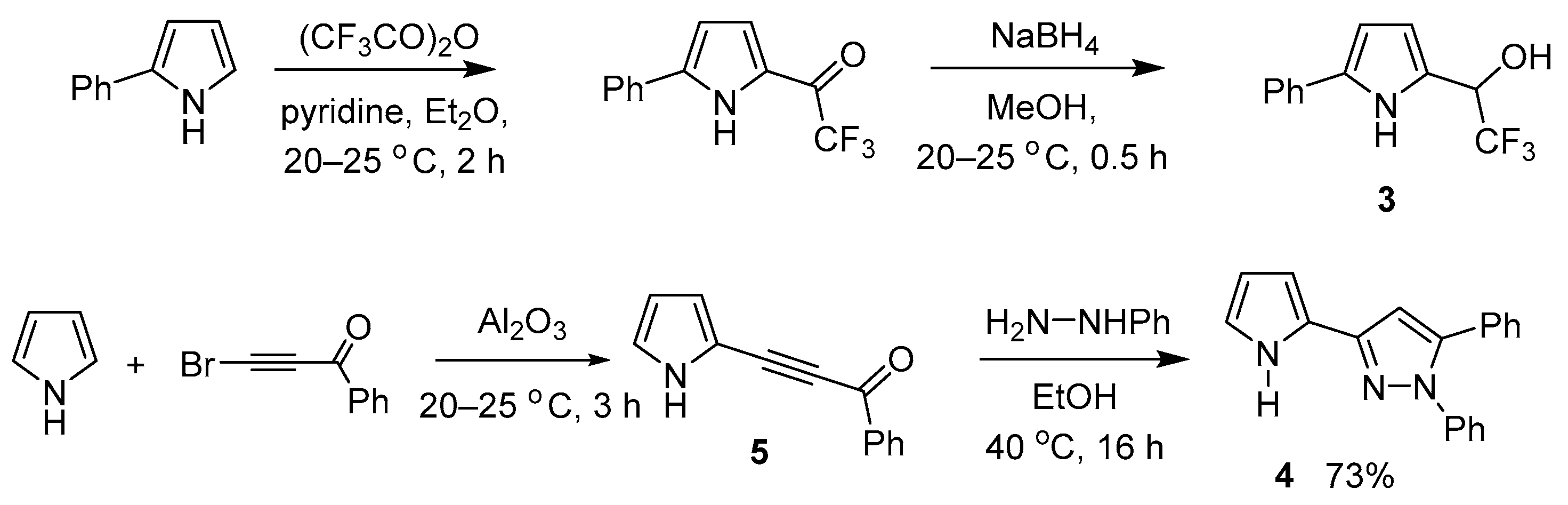
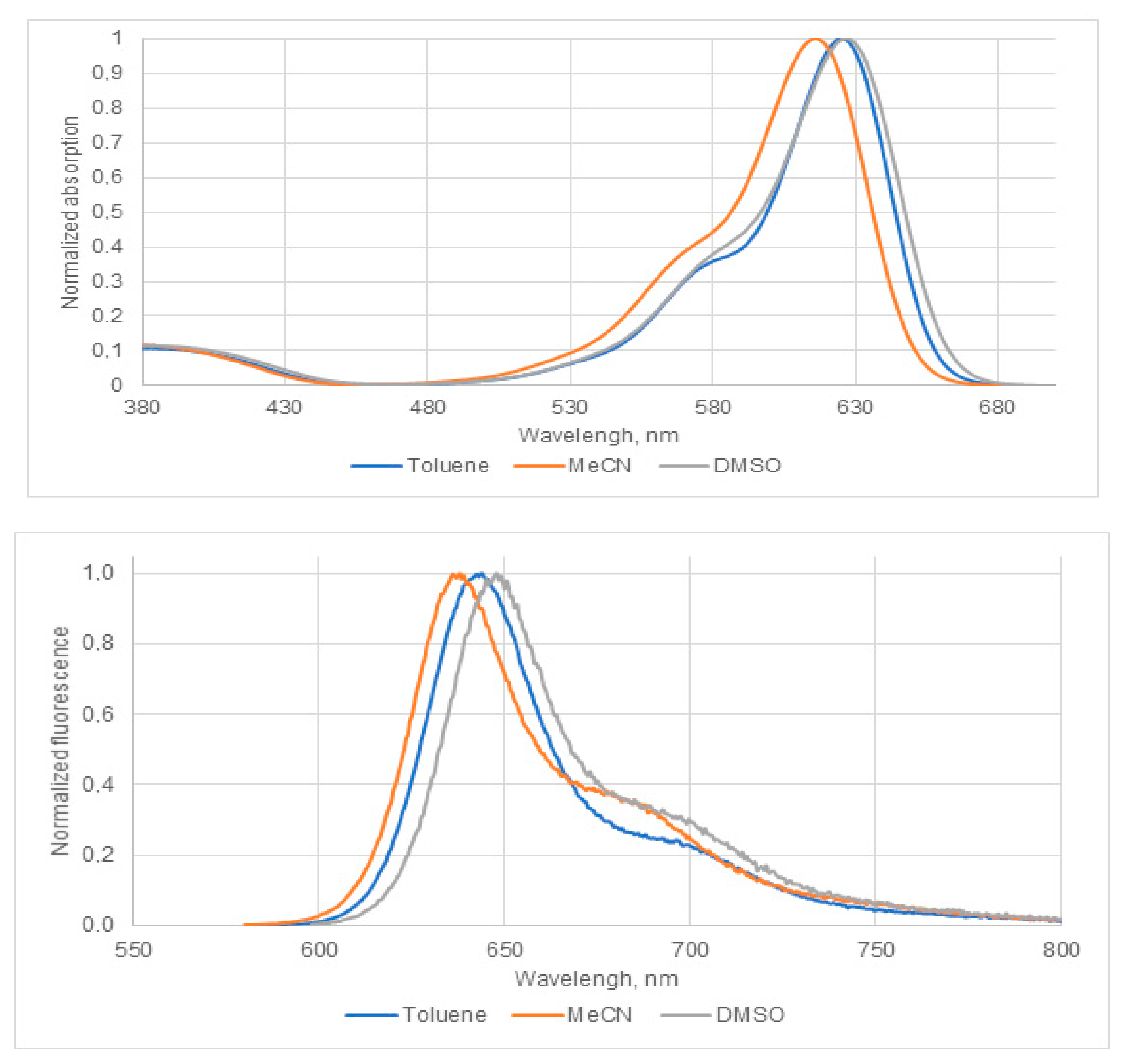
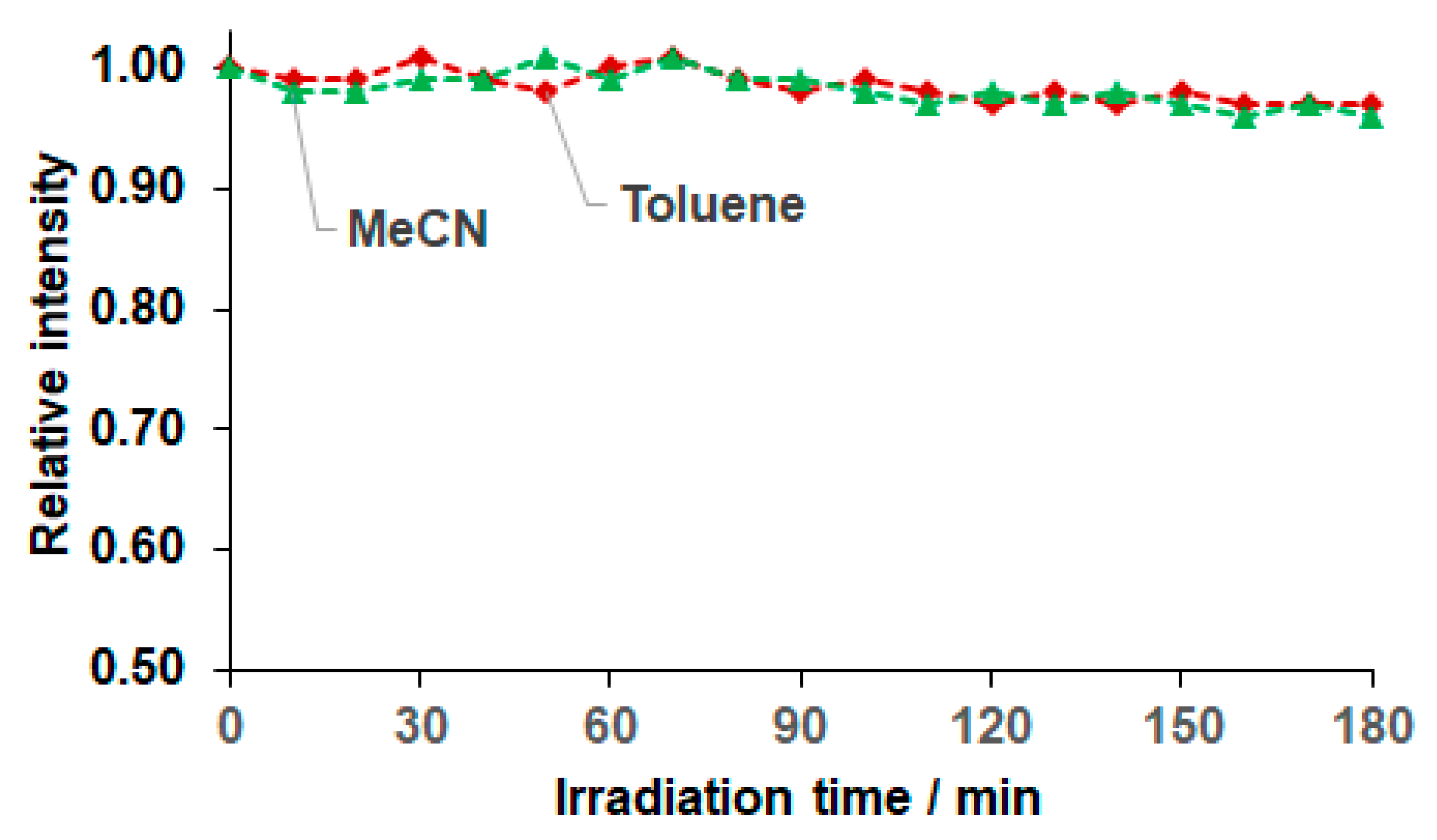
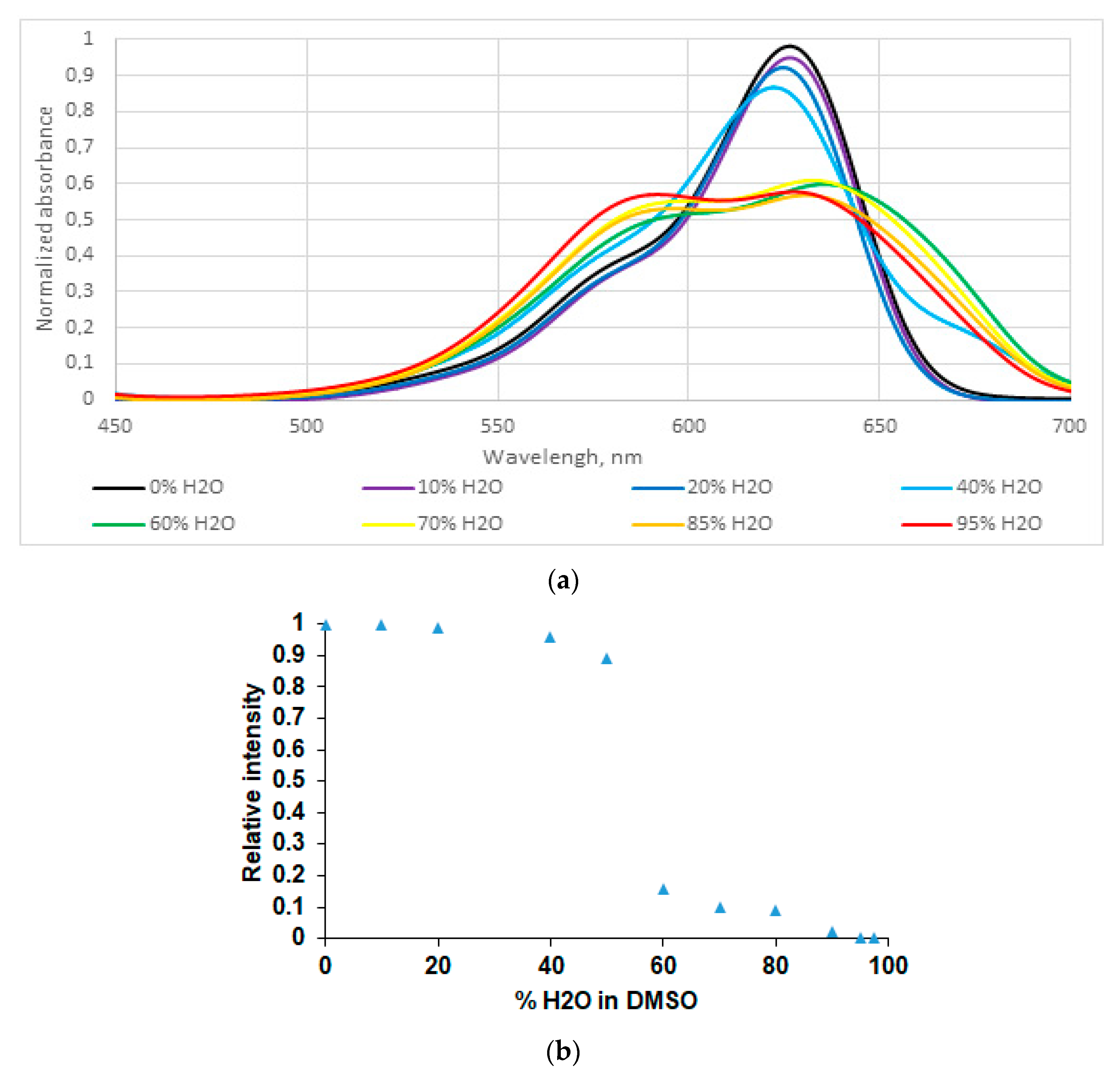
2.2. Spectroscopic and Photophysical Properties of BODIPY Dye
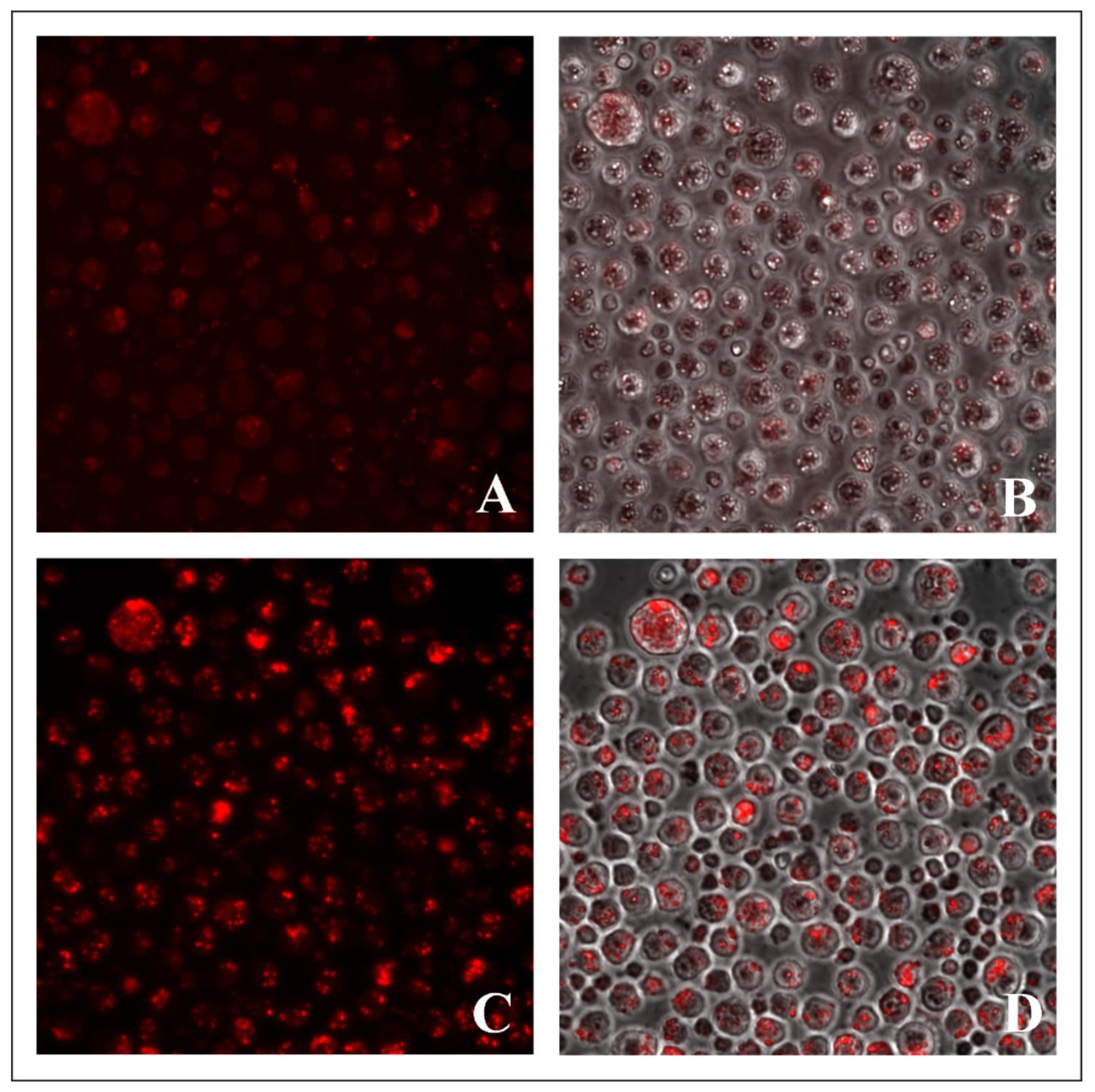
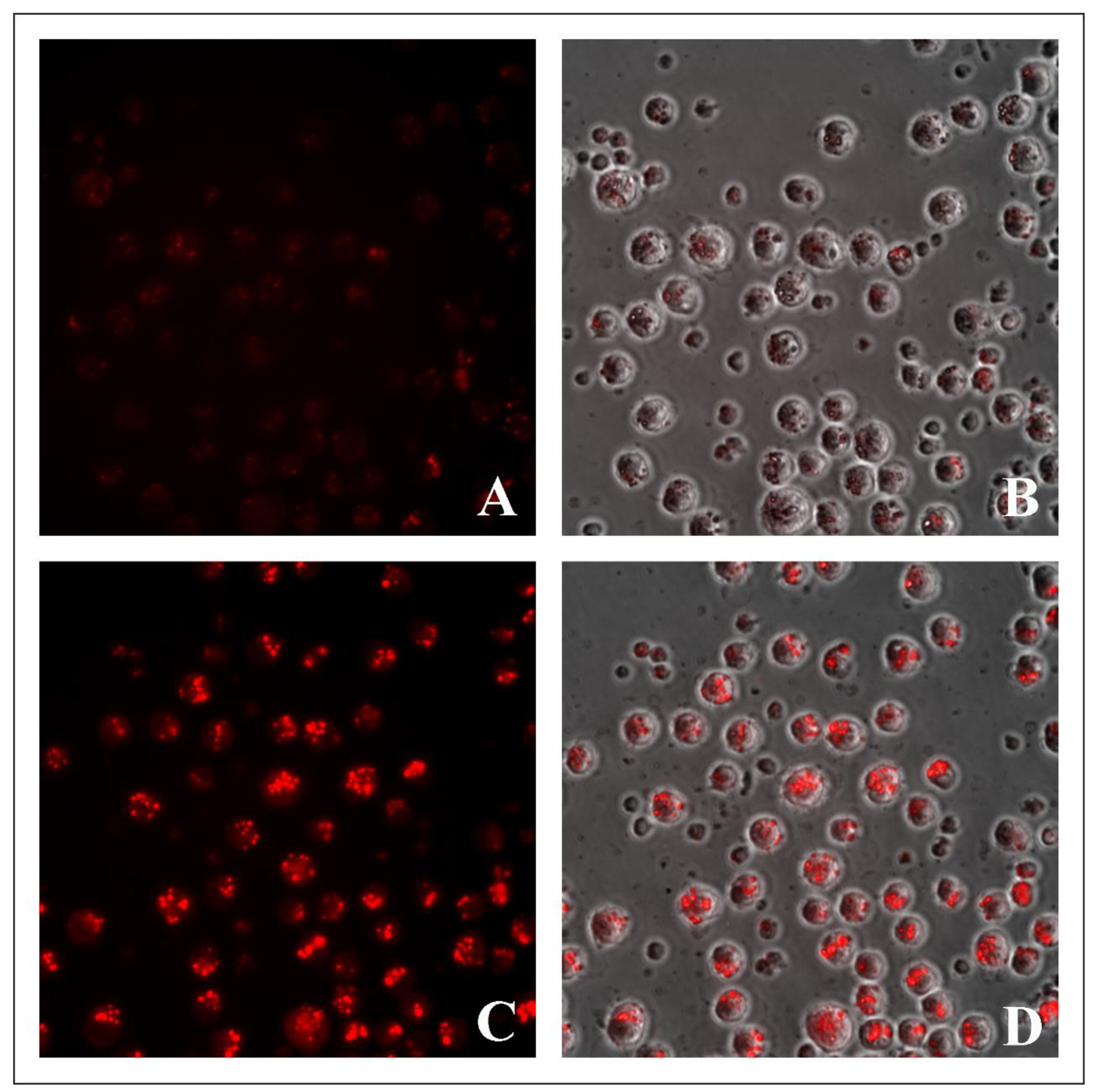
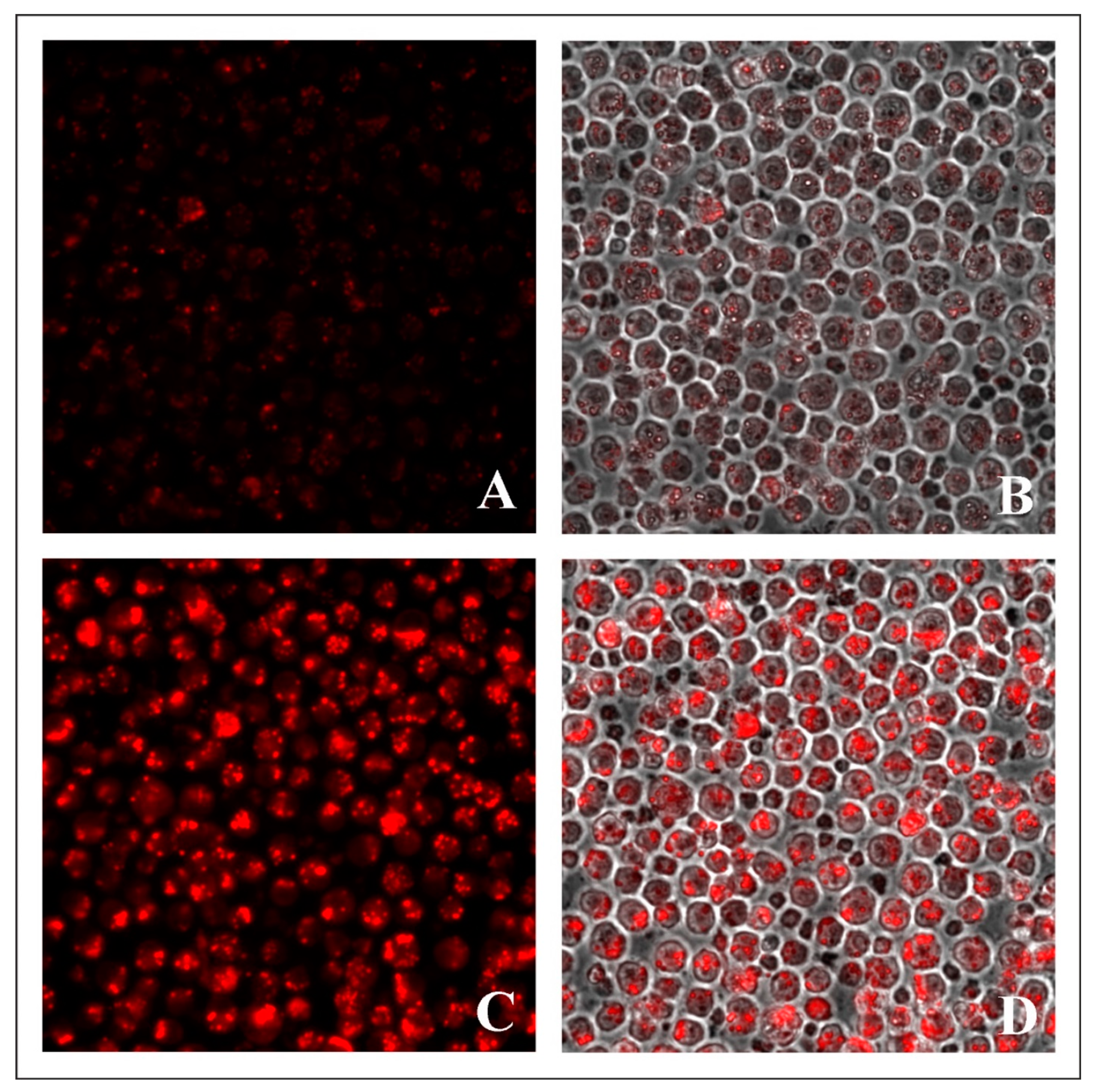
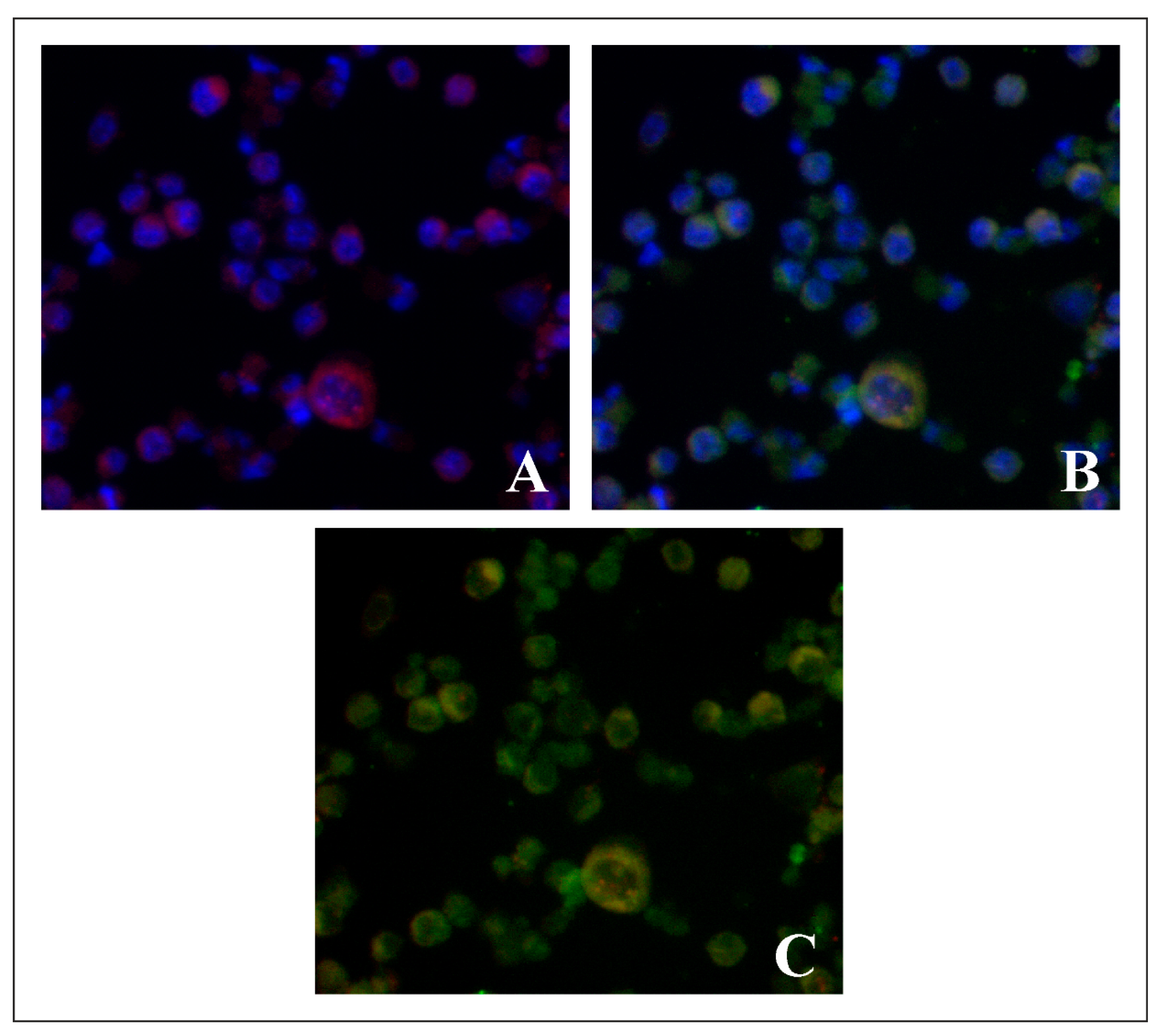
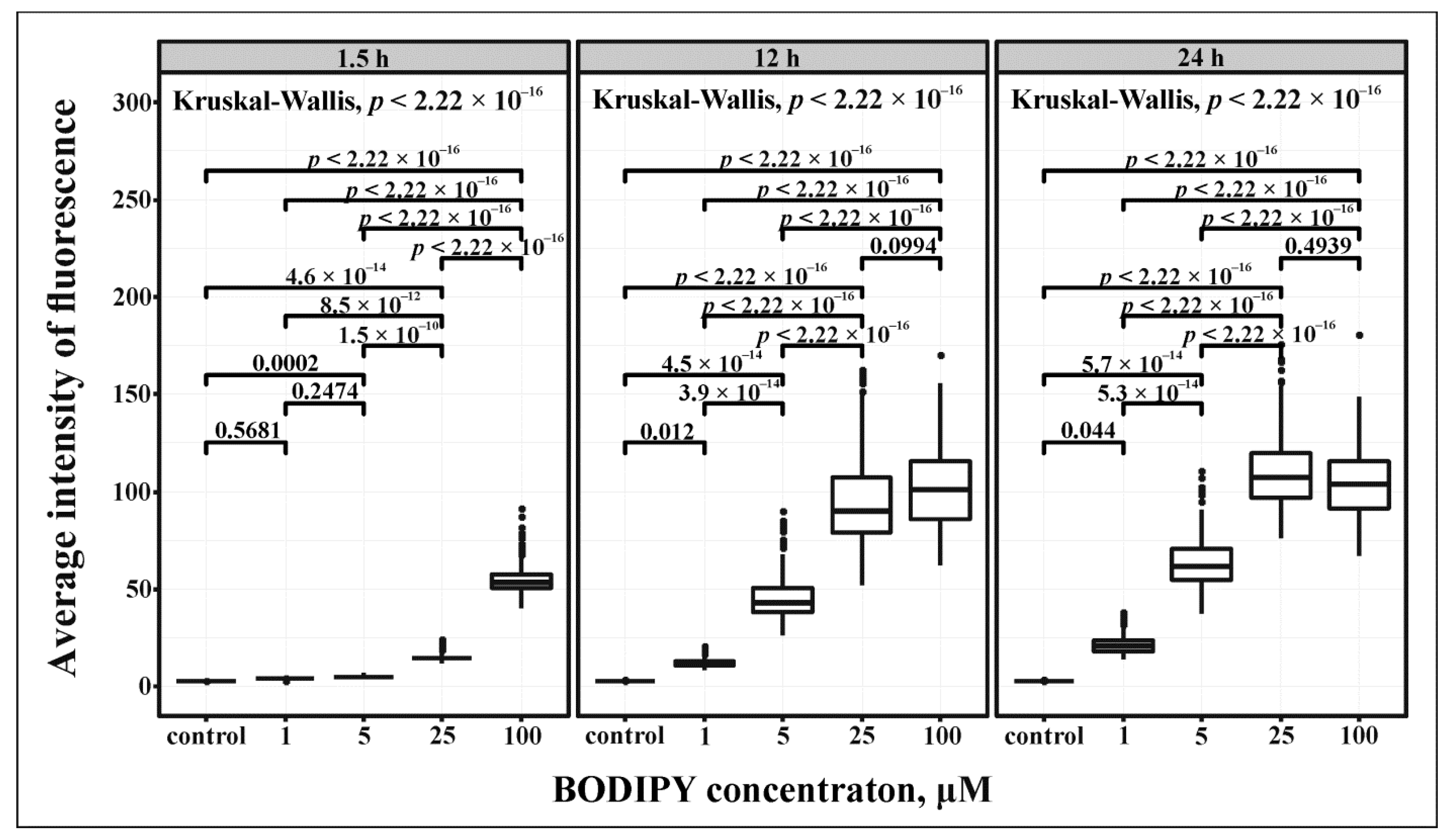
2.3. Evaluation of Lysosome-Specific Sensing
3. Materials and Methods
3.1. General Information
3.2. Synthesis of 1,5-Diphenyl-3-(1H-pyrrol-2-yl)-1H-pyrazole (4)
3.2.1. Synthesis of 1,5-Diphenyl-3-(5-(2,2,2-trifluoro-1-(5-phenyl-1H-pyrrol-2-yl)ethyl)-1H-pyrrol-2-yl)-1H-pyrazole (2)
3.2.2. Synthesis of 4,4-Difluoro-5-phenyl-3-(1,5-diphenyl-3-1H-pyrazolyl)-8-trifluoromethyl-4-bora-3a,4a-diaza-s-indacene (1)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Klfout, H.; Stewart, A.; Elkhalifa, M.; He, H. BODIPYs for Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2017, 9, 39873–39889. [Google Scholar] [CrossRef] [PubMed]
- Thumuganti, G.; Gupta, V.; Singh, S.P. New dithienosilole- and dithienogermole-based BODIPY for solar cell applications. New J. Chem. 2019, 43, 8735–8740. [Google Scholar] [CrossRef]
- Sharma, G.D.; Dahiya, H.; Singh, M.K.; Li, P.; Chayal, G.; Xu, H. Efficient Ternary Polymer Solar Cells Employing Well Matched Medium Band Gap and Narrow Band Gap Nonfullerene Acceptors. ACS Appl. Energy Mater. 2022, 5, 7813–7821. [Google Scholar] [CrossRef]
- Chapran, M.; Angioni, E.; Findlay, N.J.; Breig, B.; Cherpak, V.; Stakhira, P.; Tuttle, T.; Volyniuk, D.; Grazulevicius, J.V.; Nastishin, Y.A.; et al. An Ambipolar BODIPY Derivative for a White Exciplex OLED and Cholesteric Liquid Crystal Laser toward Multifunctional Devices. ACS Appl. Mater. Interfaces 2017, 9, 4750–4757. [Google Scholar] [CrossRef] [Green Version]
- Luo, G.-G.; Lu, H.; Zhang, X.-L.; Dai, J.-C.; Wu, J.-H.; Wu, J.-J. The relationship between the boron dipyrromethene (BODIPY) structure and the effectiveness of homogeneous and heterogeneous solar hydrogen-generating systems as well as DSSCs. Phys. Chem. Chem. Phys. 2015, 17, 9716–9729. [Google Scholar] [CrossRef]
- Shi, W.-J.; Lo, P.-C.; Singh, A.; Ledoux-Rak, I.; Ng, D.K.P. Synthesis and second-order nonlinear optical properties of push-pull BODIPY derivatives. Tetrahedron 2012, 68, 8712–8718. [Google Scholar] [CrossRef]
- Wang, H.-Q.; Ye, J.-T.; Zhang, Y.; Zhao, Y.-Y.; Qiu, Y.-Q. A thorough understanding of the nonlinear optical properties of BODIPY/carborane/diketopyrrolopyrrole hybrid chromophores: Module contribution, linear combination, one-/two-dimensional difference and carborane’s arrangement. J. Mater. Chem. C 2019, 7, 7531–7547. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, J.; Yu, X.; Ma, Y.; Huang, T.; Shen, X.; Qiu, H.; He, X.; Yin, S. A Cu2+-selective fluorescent chemosensor based on BODIPY with two pyridine ligands and logic gate. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 145, 25–32. [Google Scholar] [CrossRef]
- Qu, X.; Bian, Y.; Li, J.; Pan, Y.; Bai, Y. A red fluorescent BODIPY probe for iridium (III) ion and its application in living cells. R. Soc. Open Sci. 2019, 6, 181090. [Google Scholar] [CrossRef]
- Dorh, N.; Zhu, S.; Dhungana, K.B.; Pati, R.; Luo, F.-T.; Liu, H.; Tiwari, A. BODIPY-Based Fluorescent Probes for Sensing Protein Surface-Hydrophobicity. Sci. Rep. 2015, 5, 18337. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.-G.; Zhang, Y.; Shi, Y.-D.; Hao, H.-J.; Gong, B.; Lu, Z.-L. Fluorescent sensors based on [12] aneN3-modified BODIPY: Discrimination of different biological thiols in aqueous solution and living cells. Bioorg. Med. Chem. 2016, 24, 1550–1559. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, R.; Zhao, Y.; Tian, J.; Wang, S.; Gros, C.P.; Xu, H. Red/NIR neutral BODIPY-based fluorescent probes for lighting up mitochondria. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 248, 119199. [Google Scholar] [CrossRef] [PubMed]
- Kowada, T.; Maeda, H.; Kikuchi, K. BODIPY-based probes for the fluorescence imaging of biomolecules in living cells. Chem. Soc. Rev. 2015, 44, 4953–4972. [Google Scholar] [CrossRef]
- Kolemen, S.; Akkaya, E.U. Reaction-based BODIPY probes for selective bio-imaging. Coord. Chem. Rev. 2018, 354, 121–134. [Google Scholar] [CrossRef]
- Leblond, F.; Davis, S.C.; Valdés, P.A.; Pogue, B.W. Pre-clinical whole-body fluorescence imaging: Review of instruments, methods and applications. J. Photochem. Photobiol. B 2010, 98, 77–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shashkova, S.; Leake; Mark, C. Single-molecule fluorescence microscopy review: Shedding new light on old problems. Biosci. Rep. 2017, 37, BSR20170031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fröhlich, E. Cellular Screening Methods for the Study of Nanoparticle-Induced Lysosomal Damage. In Lysosomes-Associated Diseases and Methods to Study Their Function; Pooja Dhiman Sharma, IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Rut, W.; Groborz, K.; Zhang, L.; Sun, X.; Zmudzinski, M.; Pawlik, B.; Wang, X.; Jochmans, D.; Neyts, J.; Młynarski, W.; et al. SARS-CoV-2 Mpro inhibitors and activity-based probes for patient-sample imaging. Nat. Chem. Biol. 2020, 17, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Meng, X.; Yang, H.; Song, L.; Liu, S.; Xu, A.; Chen, Z.; Huang, W.; Zhao, Q. Lysosome-specific sensing and imaging of pH variations in vitro and in vivo utilizing a near-infrared boron complex. J. Mater. Chem. B 2019, 7, 3569–3575. [Google Scholar] [CrossRef]
- Marshall, M.V.; Rasmussen, J.C.; Tan, I.C.; Aldrich, M.B.; Adams, K.E.; Wang, X.; Fife, C.E.; Maus, E.A.; Smith, L.A.; Sevick-Muraca, E.M. Near-Infrared Fluorescence Imaging in Humans with Indocyanine Green: A Review and Update. Open Surg. Oncol. J. 2010, 2, 12–25. [Google Scholar] [CrossRef]
- Christensen, K.A.; Myers, J.T.; Swanson, J.A. pH-dependent regulation of lysosomal calcium in macrophages. J. Cell Sci. 2002, 115, 599–607. [Google Scholar] [CrossRef]
- Watts, C. The endosome–lysosome pathway and information generation in the immune system. Biochim. Et Biophys. Acta (BBA) Proteins Proteom. 2012, 1824, 14–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chikte, S.; Panchal, N.; Warnes, G. Use of LysoTracker dyes: A flow cytometric study of autophagy. Cytom. Part A 2014, 85, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.-L.; Xu, Z.; Yang, Y.; Xu, L. Small-molecule fluorescent probes for specific detection and imaging of chemical species inside lysosomes. Chem. Commun. 2019, 55, 6629–6671. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Han, X.; Xu, J. Lysosome as the Black Hole for Checkpoint Molecules. In Regulation of Cancer Immune Checkpoints: Molecular and Cellular Mechanisms and Therapy; Xu, J., Ed.; Springer: Singapore, 2020; pp. 325–346. [Google Scholar]
- Lawrence, R.E.; Zoncu, R. The lysosome as a cellular centre for signalling, metabolism and quality control. Nat. Cell Biol. 2019, 21, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Ballabio, A.; Bonifacino, J.S. Lysosomes as dynamic regulators of cell and organismal homeostasis. Nat. Rev. Mol. Cell Biol. 2020, 21, 101–118. [Google Scholar] [CrossRef]
- Platt, F.M.; Boland, B.; van der Spoel, A.C. Lysosomal storage disorders: The cellular impact of lysosomal dysfunction. J. Cell Biol. 2012, 199, 723–734. [Google Scholar] [CrossRef] [Green Version]
- He, L.-Q.; Lu, J.-H.; Yue, Z.-Y. Autophagy in ageing and ageing-associated diseases. Acta Pharmacol. Sin. 2013, 34, 605–611. [Google Scholar] [CrossRef] [Green Version]
- Fennelly, C.; Amaravadi, R.K. Lysosomal Biology in Cancer. In Lysosomes: Methods and Protocols; Öllinger, K., Appelqvist, H., Eds.; Springer: New York, NY, USA, 2017; pp. 293–308. [Google Scholar]
- Elliott, I.A.; Dann, A.M.; Xu, S.; Kim, S.S.; Abt, E.R.; Kim, W.; Poddar, S.; Moore, A.; Zhou, L.; Williams, J.L.; et al. Lysosome inhibition sensitizes pancreatic cancer to replication stress by aspartate depletion. Proc. Natl. Acad. Sci. USA 2019, 116, 6842–6847. [Google Scholar] [CrossRef] [Green Version]
- Rebecca, V.W.; Nicastri, M.C.; McLaughlin, N.; Fennelly, C.; McAfee, Q.; Ronghe, A.; Nofal, M.; Lim, C.-Y.; Witze, E.; Chude, C.I.; et al. A Unified Approach to Targeting the Lysosome’s Degradative and Growth Signaling Roles. Cancer Discov. 2017, 7, 1266–1283. [Google Scholar] [CrossRef] [Green Version]
- Bonam, S.R.; Wang, F.; Muller, S. Lysosomes as a therapeutic target. Nat. Rev. Drug Discov. 2019, 18, 923–948. [Google Scholar] [CrossRef] [Green Version]
- Johnson, I.D. Molecular Probes Handbook: A Guide to Fluorescent Probes and Labeling Technologies; Life Technologies Corporation: Carlsbad, CA, USA, 2010. [Google Scholar]
- Available online: https://www.blossombio.com/pdf/products/UG_ENZ-51034-K500.pdf (accessed on 12 May 2022).
- Freundt, E.C.; Czapiga, M.; Lenardo, M.J. Photoconversion of Lysotracker Red to a green fluorescent molecule. Cell Res. 2007, 17, 956–958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhitomirsky, B.; Farber, H.; Assaraf, Y.G. LysoTracker and MitoTracker Red are transport substrates of P-glycoprotein: Implications for anticancer drug design evading multidrug resistance. J. Cell. Mol. Med. 2018, 22, 2131–2141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, H.; Mack, J.; Yang, Y.; Shen, Z. Structural modification strategies for the rational design of red/NIR region BODIPYs. Chem. Soc. Rev. 2014, 43, 4778–4823. [Google Scholar] [CrossRef] [Green Version]
- Poirel, A.; De Nicola, A.; Ziessel, R. Oligothienyl-BODIPYs: Red and Near-Infrared Emitters. Org. Lett. 2012, 14, 5696–5699. [Google Scholar] [CrossRef]
- Awuah, S.G.; Polreis, J.; Biradar, V.; You, Y. Singlet Oxygen Generation by Novel NIR BODIPY Dyes. Org. Lett. 2011, 13, 3884–3887. [Google Scholar] [CrossRef]
- Awuah, S.G.; Das, S.K.; D’Souza, F.; You, Y. Thieno–Pyrrole-Fused BODIPY Intermediate as a Platform to Multifunctional NIR Agents. Chem. Asian J. 2013, 8, 3123–3132. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Awuah, S.; Watley, R. BODIPY Derivatives and Methods of Synthesis and Use Thereof. US 20150158888 A1, 5 December 2013. [Google Scholar]
- Watley, R.L.; Awuah, S.G.; Bio, M.; Cantu, R.; Gobeze, H.B.; Nesterov, V.N.; Das, S.K.; D’Souza, F.; You, Y. Dual Functioning Thieno-Pyrrole Fused BODIPY Dyes for NIR Optical Imaging and Photodynamic Therapy: Singlet Oxygen Generation without Heavy Halogen Atom Assistance. Chem. Asian J. 2015, 10, 335–1343. [Google Scholar] [CrossRef]
- Liu, L.; Fu, L.; Jing, T.; Ruan, Z.; Yan, L. pH-Triggered Polypeptides Nanoparticles for Efficient BODIPY Imaging-Guided Near Infrared Photodynamic Therapy. ACS Appl. Mater. Interfaces 2016, 8, 8980–8990. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; Chen, N.; Wu, Y.; Hao, E.; Wei, Y.; Mu, X.; Jiao, L. Synthesis, structure and properties of thiophene-fused BODIPYs and azaBODIPYs as near-infrared agents. New J. Chem. 2016, 40, 5966–5975. [Google Scholar] [CrossRef]
- Jiang, X.-D.; Fang, T.; Liu, X.; Xi, D. Synthesis of meso-CF3-Substituted BODIPY Compounds with Redshifted Absorption. Eur. J. Org. Chem. 2017, 2017, 5074–5079. [Google Scholar] [CrossRef]
- Li, L.; Nguyen, B.; Burgess, K. Functionalization of the 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene (BODIPY) core. Bioorg. Med. Chem. Lett. 2008, 18, 3112–3116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naim, M.; Alam, O.; Nawaz, F.; Alam, M.; Alam, P. Current status of pyrazole and its biological activities. J. Pharm. Bioallied Sci. 2016, 8, 2–17. [Google Scholar] [CrossRef] [PubMed]
- Karrouchi, K.; Radi, S.; Ramli, Y.; Taoufik, J.; Mabkhot, Y.N.; Al-aizari, F.A.; Ansar, M.H. Synthesis and Pharmacological Activities of Pyrazole Derivatives: A Review. Molecules 2018, 23, 134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, R.F.; Turones, L.C.; Cavalcante, K.V.N.; Rosa Júnior, I.A.; Xavier, C.H.; Rosseto, L.P.; Napolitano, H.B.; da Silva Castro, P.F.; Neto, M.L.F.; Galvão, G.M.; et al. Heterocyclic Compounds: Pharmacology of Pyrazole Analogs From Rational Structural Considerations. Front. Pharmacol. 2021, 12, 666725. [Google Scholar] [CrossRef]
- Bennani, F.E.; Doudach, L.; Cherrah, Y.; Ramli, Y.; Karrouchi, K.; Ansar, M.H.; Faouzi, M.E.A. Overview of recent developments of pyrazole derivatives as an anticancer agent in different cell line. Bioorg. Chem. 2020, 97, 103470. [Google Scholar] [CrossRef]
- Lanke, S.K.; Sekar, N. Pyrazole based solid state emissive NLOphores with TICT characteristics: Synthesis, DFT and TDDFT studies. Dyes Pigm. 2016, 126, 62–75. [Google Scholar] [CrossRef]
- Sobenina, L.N.; Vasil’tsov, A.M.; Petrova, O.V.; Petrushenko, K.B.; Ushakov, I.A.; Clavier, G.; Meallet-Renault, R.; Mikhaleva, A.I.; Trofimov, B.A. General Route to Symmetric and Asymmetric meso-CF3-3(5)-Aryl(hetaryl)- and 3,5-Diaryl(dihetaryl)-BODIPY Dyes. Org. Lett. 2011, 13, 2524–2527. [Google Scholar] [CrossRef]
- Trofimov, B.A.; Stepanova, Z.V.; Sobenina, L.N.; Mikhaleva, A.I.; Ushakov, I.A. Ethynylation of pyrroles with 1-acyl-2-bromoacetylenes on alumina: A formal ‘inverse Sonogashira coupling’. Tetrahedron Lett. 2004, 45, 6513–6516. [Google Scholar] [CrossRef]
- Trofimov, B.A.; Sobenina, L.N. Targets in Heterocyclic Systems; Attanasi, O.A., Spinelli, D., Eds.; Società Chimica Italiana: Roma, Italy, 2009; Volume 13, pp. 92–119. [Google Scholar]
- Sobenina, L.N.; Trofimov, B.A. Recent Strides in the Transition Metal-Free Cross-Coupling of Haloacetylenes with Electron-Rich Heterocycles in Solid Media. Molecules 2020, 25, 2490. [Google Scholar] [CrossRef]
- Sobenina, L.N.; Petrova, O.V.; Petrushenko, K.B.; Ushakov, I.A.; Mikhaleva, A.I.; Meallet-Renault, R.; Trofimov, B.A. Synthesis and Optical Properties of Difluorobora-s-diazaindacene Dyes with Trifluoromethyl meso-Substituents. Eur. J. Org. Chem. 2013, 2013, 4107–4118. [Google Scholar] [CrossRef]
- Adamoczky, A.; Nagy, T.; Fehér, P.P.; Pardi-Tóth, V.; Kuki, Á.; Nagy, L.; Zsuga, M.; Kéki, S. Isocyanonaphthol Derivatives: Excited-State Proton Transfer and Solvatochromic Properties. Int. J. Mol. Sci. 2022, 23, 7250. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhang, J.; Yu, Z.; Xie, Y.; He, H.; Qi, J.; Dong, X.; Lu, Y.; Zhao, W.; Wu, W. Environment-responsive aza-BODIPY dyes quenching in water as potential probes to visualize the in vivo fate of lipid-based nanocarriers. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1939–1948. [Google Scholar] [CrossRef] [PubMed]
- Descalzo, A.B.; Ashokkumar, P.; Shen, Z.; Rurack, K. On the Aggregation Behaviour and Spectroscopic Properties of Alkylated and Annelated Boron-Dipyrromethene (BODIPY) Dyes in Aqueous Solution. ChemPhotoChem 2020, 4, 120–131. [Google Scholar] [CrossRef] [Green Version]
- Umezawa, K.; Nakamura, Y.; Makino, H.; Citterio, D.; Suzuki, K. Bright, Color-Tunable Fluorescent Dyes in the Visible−Near-Infrared Region. J. Am. Chem. Soc. 2008, 130, 1550–1551. [Google Scholar] [CrossRef]
- Tomilin, D.N.; Petrushenko, K.B.; Sobenina, L.N.; Gotsko, M.D.; Ushakov, I.A.; Skitnevskaya, A.D.; Trofimov, A.B.; Trofimov, B.A. Synthesis and Optical Properties of meso-CF3-BODIPY with Acylethynyl Substituents in the 3-Position of the Indacene Core. Asian J. Org. Chem. 2016, 5, 1288–1294. [Google Scholar] [CrossRef]
- Petrushenko, K.B.; Petrushenko, I.K.; Petrova, O.V.; Sobenina, L.N.; Trofimov, B.A. Novel environment-sensitive 8-CF3-BODIPY dye with 4-(dimethylamino)phenyl group at the 3-position: Synthesis and optical properties. Dyes Pigm. 2017, 136, 488–495. [Google Scholar] [CrossRef]
- Petrushenko, K.B.; Petrushenko, I.K.; Petrova, O.V.; Sobenina, L.N.; Ushakov, I.A.; Trofimov, B.A. Environment-Responsive 8-CF3-BODIPY Dyes with Aniline Groups at the 3 Position: Synthesis, Optical Properties and RI-CC2 Calculations. Asian J. Org. Chem. 2017, 6, 852–861. [Google Scholar] [CrossRef]
- Tomilin, D.N.; Sobenina, L.N.; Petrushenko, K.B.; Ushakov, I.A.; Trofimov, B.A. Design of novel meso-CF3-BODIPY dyes with isoxazole substituents. Dyes Pigm. 2018, 152, 14–18. [Google Scholar] [CrossRef]
- Baruah, M.; Qin, W.; Flors, C.; Hofkens, J.; Vallée, R.A.L.; Beljonne, D.; Van der Auweraer, M.; De Borggraeve, W.M.; Boens, N. Solvent and pH Dependent Fluorescent Properties of a Dimethylaminostyryl Borondipyrromethene Dye in Solution. J. Phys. Chem. A 2006, 110, 5998–6009. [Google Scholar] [CrossRef]
- Tomilin, D.N.; Sagitova, E.F.; Petrushenko, K.B.; Sobenina, L.N.; Ushakov, I.A.; Yang, G.; Hu, R.; Trofimov, B.A. Asymmetric meso-CF3-BODIPY dyes based on cycloalkanopyrroles. Dyes Pigm. 2020, 176, 108228. [Google Scholar] [CrossRef]
- Wilke, S.; Krausze, J.; Büssow, K. Crystal structure of the conserved domain of the DC lysosomal associated membrane protein: Implications for the lysosomal glycocalyx. BMC Biol. 2012, 10, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alessandrini, F.; Pezzè, L.; Ciribilli, Y. LAMPs: Shedding light on cancer biology. Semin. Oncol. 2017, 44, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Han, X.; Qu, G.; Su, L.; Zhao, B.; Miao, J. A pH probe inhibits senescence in mesenchymal stem cells. Stem Cell Res. Ther. 2018, 9, 343. [Google Scholar] [CrossRef] [PubMed]
- Bhat, O.M.; Yuan, X.; Camus, S.; Salloum, F.N.; Li, P.-L. Abnormal Lysosomal Positioning and Small Extracellular Vesicle Secretion in Arterial Stiffening and Calcification of Mice Lacking Mucolipin 1 Gene. Int. J. Mol. Sci. 2020, 21, 1713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hilverling, A.; Szegö, E.M.; Dinter, E.; Cozma, D.; Saridaki, T.; Falkenburger, B.H. Maturing Autophagosomes are Transported Towards the Cell Periphery. Cell. Mol. Neurobiol. 2022, 42, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Sukhov, B.G.; Ganenko, T.V.; Pogodaeva, N.N.; Kuznetsov, S.V.; Silkin, I.I.; Losovsksya, E.A.; Shurygin, M.G.; Shurygina, I.A.; Trofimov, B.A. Agent with Antitumor Activity Based on Arabinogalactan Nanocomposites with Selenium and Methods for Prepariation of Such Nanobiocomposites. RU2015132794A, 5 August 2015. [Google Scholar]




| Solvent | λabs (Max), nm | ε (Max), M−1 cm−1 a | λem (Max), nm | ΔνSt, cm−1 | ΦF b |
|---|---|---|---|---|---|
| Toluene | 619 | 80,000 | 644 | 627 | 0.9 ± 0.1 |
| MeCN | 610 | 73,000 | 638 | 719 | 0.8 ± 0.1 |
| DMSO | 621 | 60,000 | 648 | 671 | 0.7 ± 0.1 |
| Transition | EV, eV | λ, nm | f | Main Configuration (%) | |
|---|---|---|---|---|---|
| S0 → S1 | 2.52 | 493 | 0.79 | H-L | (96) |
| →S2 | 3.82 | 324 | 0.06 | H-7-L | (55) |
| →S3 | 3.98 | 312 | 0.01 | H-1-L | (73) |
| S1 → S0 | 2.35 | 529 | 0.78 | H-L | (97) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trukhan, I.S.; Tomilin, D.N.; Dremina, N.N.; Sobenina, L.N.; Shurygin, M.G.; Petrushenko, K.B.; Petrushenko, I.K.; Trofimov, B.A.; Shurygina, I.A. Application of meso-CF3-Fluorophore BODIPY with Phenyl and Pyrazolyl Substituents for Lifetime Visualization of Lysosomes. Molecules 2022, 27, 5018. https://doi.org/10.3390/molecules27155018
Trukhan IS, Tomilin DN, Dremina NN, Sobenina LN, Shurygin MG, Petrushenko KB, Petrushenko IK, Trofimov BA, Shurygina IA. Application of meso-CF3-Fluorophore BODIPY with Phenyl and Pyrazolyl Substituents for Lifetime Visualization of Lysosomes. Molecules. 2022; 27(15):5018. https://doi.org/10.3390/molecules27155018
Chicago/Turabian StyleTrukhan, Irina S., Denis N. Tomilin, Nataliya N. Dremina, Lyubov N. Sobenina, Michael G. Shurygin, Konstantin B. Petrushenko, Igor K. Petrushenko, Boris A. Trofimov, and Irina A. Shurygina. 2022. "Application of meso-CF3-Fluorophore BODIPY with Phenyl and Pyrazolyl Substituents for Lifetime Visualization of Lysosomes" Molecules 27, no. 15: 5018. https://doi.org/10.3390/molecules27155018






