Polysaccharides as Carriers of Polyphenols: Comparison of Freeze-Drying and Spray-Drying as Encapsulation Techniques
Abstract
:1. Introduction
2. Polyphenols
3. Encapsulation
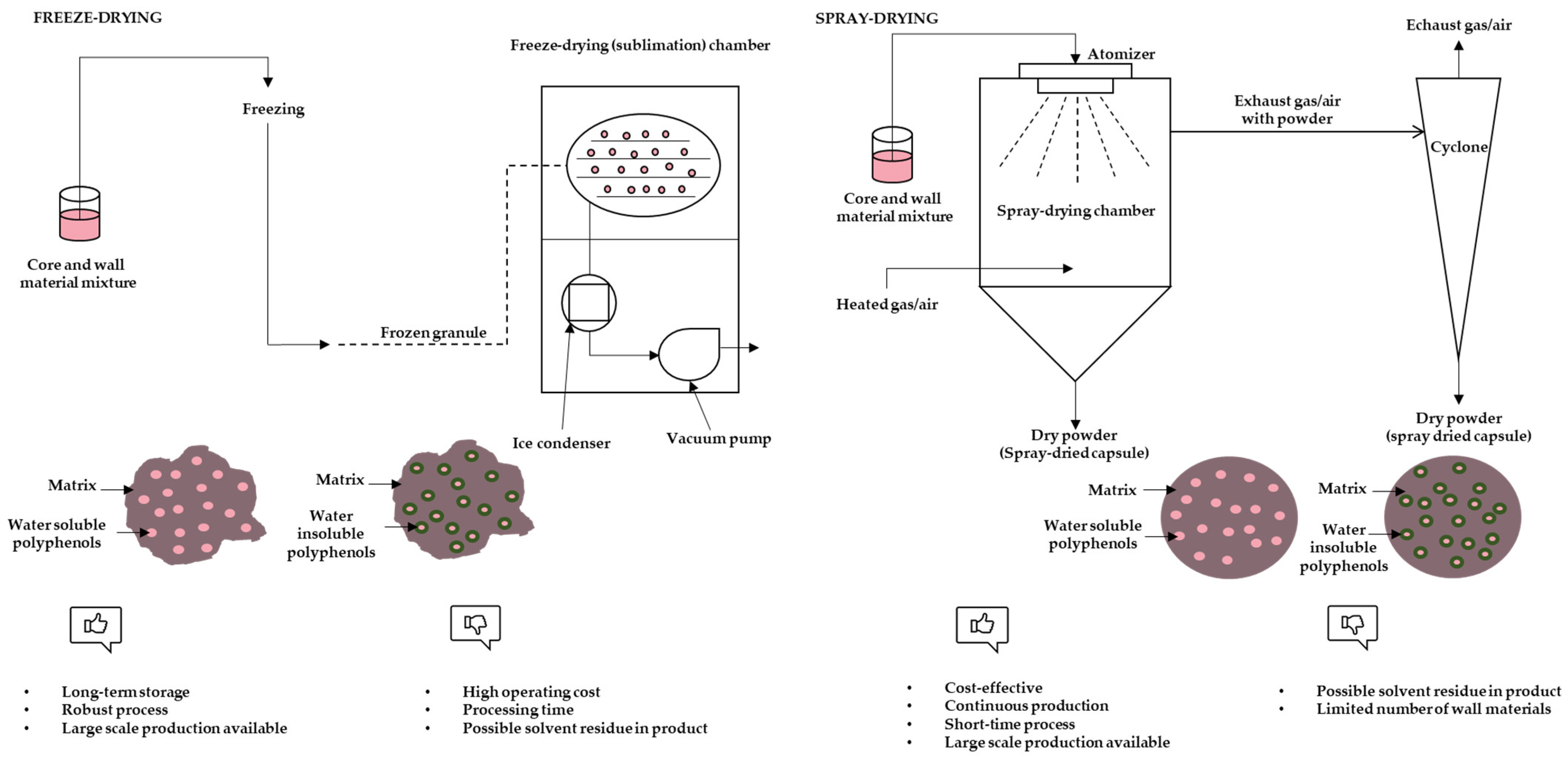
3.1. Freeze-Drying
3.2. Spray-Drying
3.3. Particle Morphology
3.4. Polyphenol Carriers
4. Application of Spray-Drying and Freeze-Drying for Encapsulation of Polyphenols
5. Application of Encapsulated Polyphenols in Food Products
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Quirόs-Sauceda, A.E.; Palafox-Carlos, H.; Sáyago-Ayerdi, S.G.; Ayala-Zavala, J.F.; Bello-Perez, L.A.; Álvarez-Parrilla, E.; de la Rosa, L.A.; Gonzáles-Cόrdova, A.F.; González-Aguilar, G.A. Dietary fiber and phenolic compunds as functional ingredients: Interaction and possible effect after ingestion. Food Funct. 2014, 5, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, L.F.; Ramirez, M.J.; Orrego, C.E.; Teixeira, J.A.; Mussatto, S.I. Encapsulation of antioxidant phenolic compounds extracted from spent coffee grounds by freeze-drying and spray-drying using different coating materials. Food Chem. 2017, 237, 623–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malapert, A.; Reboul, E.; Tourbin, M.; Dangles, O.; Thiéry, A.; Ziarelli, F.; Tomao, V. Characterization of hydroxytyrosol-β-cyclodextrin complexes in solution and in the solid state, a potential bioactive ingredient. LWT-Food Sci. Technol. 2019, 102, 317–323. [Google Scholar] [CrossRef] [Green Version]
- Velderrain-Rodríguez, G.R.; Palafox-Carlos, H.; Wall-Medrano, A.; Ayala-Zavala, J.F.; Chen, C.Y.O.; Robles-Sánchez, M.; Astiazaran-Garcia, H.; Alvarez-Parrilla, E.; González-Aguilar, G.A. Phenolic compounds: Their journey after intake. Food Funct. 2014, 5, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Selma, M.V.; Espin, J.C.; Tomás-Barberán, F.A. Interaction between phenolics andgut microbiota: Role in human health. J. Agric. Food Chem. 2009, 57, 6485–6501. [Google Scholar] [CrossRef]
- Zhu, F. Interactions between cell wall polysaccharides and polyphenols. Crit. Rev. Food Sci. Nutr. 2017, 58, 1808–1831. [Google Scholar] [CrossRef]
- Kardum, N.; Glibetic, M. Polyphenols and their interactionswith other dietary compounds: Implications for human health. Adv. Food Nutr. Res. 2018, 84, 103–144. [Google Scholar] [CrossRef]
- Sahiner, M.; Blake, D.A.; Fullerton, M.L.; Suner, S.S.; Sunol, A.K.; Sahiner, N. Enhancement of biocompatibility and carbohydrate absorption control potential of rosmarinic acid through crosslinking into microparticles. Int. J. Biol. Macromol. 2019, 137, 836–843. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [Green Version]
- Rahman, S.; Mathew, S.; Nair, P.; Ramadan, W.S.; Vazhappilly, C.G. Health benefits of cyanidin-3-glucoside as a potent modulator of Nrf2-mediated oxidative stress. Inflammopharmacology 2021, 29, 907–923. [Google Scholar] [CrossRef]
- Suner, S.S.; Sahiner, M.; Mohapatra, S.; Ayyala, R.S.; Bhethanabotla, V.R.; Sahiner, N. Degradable poly(catechin) nanoparticles as a versatile therapeutic agent. Int. J. Polym. Mater. Polym. Biomater. 2021, 71, 1–12. [Google Scholar] [CrossRef]
- Dludla, P.V.; Nkambule, B.B.; Jack, B.; Mkandla, Z.; Mutize, T.; Silvestri, S.; Orlando, P.; Tiano, L.; Louw, J.; Mazibuko-Mbeje, S.E. Inflammation and oxidative stress in an obese state and the protective effects of gallic acid. Nutrients 2019, 11, 23. [Google Scholar] [CrossRef] [Green Version]
- Ramírez, M.J.; Giraldo, G.I.; Orrego, C.E. Modeling and stability of polyphenol in spray-dried and freeze-dried fruit encapsulates. Powder Technol. 2015, 277, 89–96. [Google Scholar] [CrossRef]
- Cao, H.; Saroglu, O.; Karadag, A.; Diaconeasa, Z.; Zoccatelli, G.; Conte-Junior, C.A.; Gonzales-Aguilar, G.A.; Ou, J.; Bai, W.; Zamarioli, C.M.; et al. Available technologies on improving the stability of polyphenols in food processing. Food Front. 2021, 2, 109–139. [Google Scholar] [CrossRef]
- Wojdyło, A.; Lech, K.; Nowicka, P.; Hernandez, F.; Figiel, A.; Carbonell-Barrachina, A.A. Influence of Different Drying Techniques on Phenolic Compounds, Antioxidant Capacity and Colour of Ziziphus jujube Mill. Fruits. Molecules 2019, 24, 2361. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, Q.D.; Dang, T.T.; Nguyen, T.V.L.; Dung Nguyen, T.T.; Mguyen, N.N. Microencapsulation of roselle (Hibiscus sabdariffa L.) anthocyanins: Effects of different carriers on selected physicochemical properties and antioxidant activities of spray-dried and freeze-dried powder. Int. J. Food Prop. 2022, 25, 359–374. [Google Scholar] [CrossRef]
- Papoutsis, K.; Golding, J.; Vuong, Q.; Pristijono, P.; Stathopoulos, C.; Scarlett, C.; Bowyer, M. Encapsulation of Citrus By-Product Extracts by Spray-Drying and Freeze-Drying Using Combinations of Maltodextrin with Soybean Protein and ι-Carrageenan. Foods 2018, 7, 115. [Google Scholar] [CrossRef] [Green Version]
- Dobson, C.C.; Mottawea, W.; Rodrigue, A.; Buzati Pereira, L.B.; Hammami, R.; Power, A.K.; Bordenave, N. Impact of molecular interactions with phenolic compounds on food polysaccharides functionality. Adv. Food Nutr. Res. 2019, 90, 135–181. [Google Scholar] [CrossRef]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009, 26, 1001–10043. [Google Scholar] [CrossRef]
- Jakobek, L. Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chem. 2015, 175, 556–567. [Google Scholar] [CrossRef]
- Bellion, P.; Digles, J.; Will, F.; Dietrich, H.; Baum, M.; Eisenbrand, G.; Janzowski, C. Polyphenolic apple extracts: Effects of raw material and production method on antioxidant effectiveness and reduction of DNA damage in Caco-2 cells. J. Agric. Food Chem. 2010, 58, 6636–6642. [Google Scholar] [CrossRef]
- Hollman, P.C.H.; Cassidy, A.; Comte, B.; Heinonen, M.; Richelle, M.; Richling, E.; Richling, E.; Serafini, M.; Scalbert, A.; Sies, H.; et al. The biological relevance of direct antioxidant effects of polyphenols for cardiovascular health in humans is not established. J. Nutr. 2011, 141, 989S–1009S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Bourvellec, C.; Renard, C.M.G.C. Interactions between Polyphenols and Macromolecules: Quantification Methods and Mechanisms. Crit. Rev. Food Sci. Nutr. 2012, 52, 213–248. [Google Scholar] [CrossRef] [PubMed]
- Wilkowska, A.; Ambroziak, W.; Czyżowska, A.; Adamiec, J. Effect of Microencapsulation by Spray-drying and Freeze Drying Technique on the Antioxidant Properties of Blueberry (Vaccinium myrtillus) Juice Polyphenolic Compounds. Pol. J. Food Nutr. Sci. 2016, 66, 11–16. [Google Scholar] [CrossRef] [Green Version]
- Fang, Z.; Bhandari, B. Encapsulation of polyphenols—A review. Trends Food Sci. Technol. 2010, 21, 510–523. [Google Scholar] [CrossRef]
- Grgić, J.; Šelo, G.; Planinić, M.; Tišma, M.; Bucić-Kojić, A. Role of the Encapsulation in Bioavailability of Phenolic Compounds. Antioxidants 2020, 9, 923. [Google Scholar] [CrossRef]
- Saifullah, M.; Islam Shishir, M.R.; Ferdowsi, R.; Tanver Rahman, M.R.; Van Vuong, Q. Micro and nano encapsulation, retention and controlled release of flavor and aroma compounds: A critical review. Trends Food Sci. Technol. 2019, 86, 230–251. [Google Scholar] [CrossRef]
- Wu, G.; Hui, X.; Mu, J.; Brennan, M.A.; Brennan, C.S. Functionalization of whey protein isolate fortified with blackcurrant concentrate by spray-drying and freeze-drying strategies. Food Res. Int. 2021, 141, 110025. [Google Scholar] [CrossRef]
- Lesschaeve, I.; Noble, A.C. Polyphenols: Factors influencing their sensory properties and their effects on food and beverage preferences. Am. J. Clin. Nutr. 2005, 81, 330S–335S. [Google Scholar] [CrossRef] [Green Version]
- Bhatta, S.; StevanovicJanezic, T.; Ratti, C. Freeze-Drying of Plant-Based Foods. Foods 2020, 9, 87. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Li, P.; Kong, L.; Xu, B. Microencapsulation of curcumin by spray drying and freeze drying. LWT 2020, 132, 109892. [Google Scholar] [CrossRef]
- Demirci, S.; Khiev, D.; Can, M.; Sahiner, M.; Biswal, M.R.; Ayyala, R.S.; Sahiner, N. Chemically cross-linked poly(β-cyclodextrin) particles as promising drug delivery materials. Appl. Polym. Mater. 2021, 3, 6238–6251. [Google Scholar] [CrossRef]
- Sepelevs, I.; Stepanova, V.; Galoburda, R. Encapsulation of Gallic Acid with Acid-Modified Low Dextrose Equivalent Potato Starch Using Spray-and Freeze-Drying Techniques. Polish J. Food Nutr. Sci. 2018, 68, 273–280. [Google Scholar] [CrossRef] [Green Version]
- Shofian, N.M.; Hamid, A.A.; Osman, A.; Saari, N.; Anwar, F.; Dek, M.S.; Hairuddin, M.R. Effect of Freeze-Drying on the Antioxidant Compounds and Antioxidant Activity of Selected Tropical Fruits. Int. J. Mol. Sci. 2011, 12, 4678–4692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, J.J.Y.; Lim, Y.Y.; Siow, L.F.; Tan, J.B.L. Effects of drying on polyphenol oxidase and antioxidant activity of Morus alba leaves. J. Food Process. Preserv. 2015, 39, 2811–2819. [Google Scholar] [CrossRef]
- Pérez-Gregorio, M.R.; Regueiro, J.; González-Barreiro, C.; Rial-Otero, R.; Simal-Gándara, J. Changes in antioxidant flavonoids during freeze-drying of red onions and subsequent storage. Food Control 2011, 22, 1108–1113. [Google Scholar] [CrossRef]
- Robert, P.; Gorena, T.; Romero, N.; Sepulveda, E.; Chavez, J.; Saenz, C. Encapsulation of polyphenols and anthocyanins from pomegranate (Punica granatum) by spray-drying. Int. J. Food Sci. 2010, 45, 1386–1394. [Google Scholar] [CrossRef]
- Wu, G.; Hui, X.; Stipkovits, L.; Rachman, A.; Tu, J.; Brennan, M.A.; Brennan, C.S. Whey protein-blackcurrant concentrate particles obtained by spray-drying and freeze-drying for delivering structural and health benefits of cookies. Innov. Food Sci. Emerg. Technol. 2021, 68, 102606. [Google Scholar] [CrossRef]
- Ersus, S.; Yurdagel, U. Microencapsulation of anthocyanin pigments of black carrot (Daucus carota L.) by spray drier. J. Food Eng. 2007, 80, 805–812. [Google Scholar] [CrossRef]
- Gomes, W.F.; França, F.R.M.; Denadai, M.; Andrade, J.K.S.; da Silva Oliveira, E.M.; de Brito, E.S.; Rodrigues, S.; Narain, N. Effect of freeze- and spray-drying on physico-chemical characteristics, phenolic compounds and antioxidant activity of papaya pulp. J. Food Sci. Technol. 2018, 55, 2095–2102. [Google Scholar] [CrossRef]
- Saikia, S.; Mahnot, N.K.; Mahanta, C.L. Optimisation of phenolic extraction from Averrhoa carambola pomace by response surface methodology and its microencapsulation by spray and freeze drying. Food Chem. 2015, 171, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Lv, Y. Degradation kinetic of anthocyanins from rose (Rosa rugosa) as prepared by microencapsulation in freeze-drying and spray-drying. Int. J. Food Prop. 2019, 22, 2009–2021. [Google Scholar] [CrossRef] [Green Version]
- Hussain, S.A.; Hameed, A.; Nazir, Y.; Naz, T.; Wu, Y.; Suleria, H.; Song, Y. Microencapsulation and the Characterization of Polyherbal Formulation (PHF) Rich in Natural Polyphenolic Compounds. Nutrients 2018, 10, 843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Díaz-Bandera, D.; Villanueva-Carvajal, A.; Dublán-García, O.; Quintero-Salazar, B.; Dominguez-Lopez, A. Assessing release kinetics and dissolution of spray-dried Roselle (Hibiscus sabdariffa L.) extract encapsulated with different carrier agents. LWT-Food Sci. Technol. 2015, 64, 693–698. [Google Scholar] [CrossRef]
- Yibin, L.I.; Wu, L.; Weng, M.; Tang, B.; Lai, P.; Chen, J. Effect of different encapsulating agent combinations on physicochemical properties and stability of microcapsules loaded with phenolics of plum (Prunus salicina lindl.). Powder Technol. 2018, 340, 459–464. [Google Scholar] [CrossRef]
- Santiago, M.C.P.A.; Nogueira, R.I.; Paim, D.R.S.F.; Gouvêa, A.C.M.S.; Godoy, R.L.O.; Peixoto, F.M.; Pacheco, S.; Freitas, S.P. Effects of encapsulating agents on anthocyanin retention in pomegranate powder obtained by the spray-drying process. LWT 2016, 73, 551–556. [Google Scholar] [CrossRef]
- Ostroschi, L.C.; Brito de Souza, V.; Echalar-Barrientos, M.A.; Tulini, F.L.; Comunian, T.A.; Thomazini, M.; Baliero, C.C.; Roudaut, G.; Genovese, M.I.; Favaro-Trindade, C.S. Production of spray-dried proanthocyanidin-rich cinnamon (Cinnamomum zeylanicum) extract as a potential functional ingredient: Improvement of stability, sensory aspects and technological properties. Food Hydrocoll. 2018, 79, 343–351. [Google Scholar] [CrossRef]
- Pang, S.F.; Yusoff, M.M.; Gimbun, J. Assessment of phenolic compounds stability and retention during spray-drying of Orthosiphon stamineus extracts. Food Hydrocoll. 2014, 37, 159–165. [Google Scholar] [CrossRef] [Green Version]
- Sansone, F.; Mencherini, T.; Picerno, P.; d’Amore, M.; Aquino, R.P.; Lauro, M.R. Maltodextrin/pectin microparticles by spray-drying as carrier for nutraceutical extracts. J. Food Eng. 2011, 105, 468–476. [Google Scholar] [CrossRef]
- Nadeem, H.S.; Torun, M.; Özdemir, F. Spray-drying of the mountain tea (Sideritis stricta) water extract by using different hydrocolloid carriers. LWT-Food Sci. Technol. 2011, 44, 1626–1635. [Google Scholar] [CrossRef]
- Saénz, C.; Tapia, S.; Chávez, J.; Robert, P. Microencapsulation by spray-drying of bioactive compounds from cactus pear (Opuntia ficus-indica). Food Chem. 2009, 114, 616–622. [Google Scholar] [CrossRef]
- Tonon, R.V.; Brabet, C.; Hubinger, M.D. Anthocyanin stability and antioxidant activity of spray-dried açai (Euterpe oleracea Mart.) juice produced with different carrier agents. Int. Food Res. J. 2010, 43, 907–914. [Google Scholar] [CrossRef]
- Silva, P.I.; Stringheta, P.C.; Teófilo, R.F.; de Oliveira, I.R.N. Parameter optimization for spray-drying microencapsulation of jaboticaba (Myrciaria jaboticaba) peel extracts using simultaneous analysis of responses. J. Food Eng. 2013, 117, 538–544. [Google Scholar] [CrossRef] [Green Version]
- Bušić, A.; Komes, D.; Belšćak-Cvitanović, A.; VojvodićCebin, A.; Špoljarić, I.; Mršić, G.; Miao, S. The Potential of Combined Emulsification and Spray-drying Techniques for Encapsulation of Polyphenols from Rosemary (Rosmarinus officinalis L.) Leaves. Food Technol. Biotechnol. 2018, 56, 494–505. [Google Scholar] [CrossRef] [PubMed]
- Rocha, S.; Generalov, R.; Pereira, M.C.; Peres, I.; Juzenas, P.; Coelho, M.A. Epigallocatechin gallate-loaded polysaccharide nanoparticles for prostate cancer chemoprevention. Nanomed. J. 2011, 6, 79–87. [Google Scholar] [CrossRef]
- AniesraniDelfiya, D.S.; Thangavel, K.; Natarajan, N.; Kasthuri, R.; Kailappan, R. Microencapsulation of Turmeric Oleoresin by Spray-drying and In Vitro Release Studies of Microcapsules. J. Food Process Eng. 2015, 38, 37–48. [Google Scholar] [CrossRef]
- Akhavan Mahdavi, S.; Jafari, S.M.; Assadpoor, E.; Dehnad, D. Microencapsulation optimization of natural anthocyanins with maltodextrin, gum Arabic and gelatin. Int. J. Biol. Macromol. 2016, 85, 379–385. [Google Scholar] [CrossRef]
- Archaina, D.; Vasile, F.; Jiménez-Guzmán, J.; Alamilla-Beltrán, L.; Schebor, C. Physical and functional properties of roselle (Hibiscus sabdariffa L.) extract spray-dried with maltodextrin-gum arabic mixtures. J. Food Process. Preserv. 2019, 43, e14065. [Google Scholar] [CrossRef]
- Ferrari, C.C.; Germer, S.P.M.; Alvim, I.D.; Vissotto, F.Z.; de Aguirre, J.M. Influence of carrier agents on the physicochemical properties of blackberry powder produced by spray-drying. Int. J. Food Sci. 2012, 47, 1237–1245. [Google Scholar] [CrossRef]
- Pieczykolan, E.; Kurek, M.A. Use of guar gum, gum arabic, pectin, beta-glucan and inulin for microencapsulation of anthocyanins from chokeberry. Int. J. Biol. Macromol. 2019, 129, 665–671. [Google Scholar] [CrossRef]
- Farias-Cervantes, V.S.; Chávez-Rodríguez, A.; García-Salcedo, P.A.; García-López, P.M.; Casas-Solís, J.; Andrade-González, I. Antimicrobial effect and in vitro release of anthocyanins from berries and Roselle obtained via microencapsulation by spray-drying. J. Food Process. Preserv. 2018, 42, e13713. [Google Scholar] [CrossRef]
- Sanchez, V.; Baeza, R.; Galmarini, M.V.; Zamora, M.C.; Chirife, J. Freeze-Drying Encapsulation of Red Wine Polyphenols in an Amorphous Matrix of Maltodextrin. Food. Bioproc. Tech. 2011, 6, 1350–1354. [Google Scholar] [CrossRef]
- Ravichai, K.; Muangrat, R. Effect of different coating materials on freeze-drying encapsulation of bioactive compounds from fermented tea leaf wastewater. J. Food Process. Preserv. 2019, 43, e14145. [Google Scholar] [CrossRef]
- Muangrat, R.; Ravichai, K.; Jirarattanarangsri, W. Encapsulation of polyphenols from fermented wastewater of Miang processing by freeze drying using a maltodextrin/gum Arabic mixture as coating material. J. Food Process. Preserv. 2019, 43, e13908. [Google Scholar] [CrossRef]
- Laine, P.; Kylli, P.; Heinonen, M.; Jouppila, K. Storage Stability of Microencapsulated Cloudberry (Rubus chamaemorus) Phenolics. J. Agric. Food Chem. 2008, 56, 11251–11261. [Google Scholar] [CrossRef] [PubMed]
- Elsebaie, E.M.; Essa, R.Y. Microencapsulation of red onion peel polyphenols fractions by freeze drying technicality and its application in cake. J. Food Process. Preserv. 2018, 42, e13654. [Google Scholar] [CrossRef]
- Milea, Ș.A.; Aprodu, I.; Vasile, A.M.; Barbu, V.; Râpeanu, G.; Bahrim, G.E.; Stănciuc, N. Widen the functionality of flavonoids from yellow onion skins through extraction and microencapsulation in whey proteins hydrolysates and different polymers. J. Food Eng. 2019, 251, 29–35. [Google Scholar] [CrossRef]
- Ben Sassi, C.; Marcet, I.; Rendueles, M.; Díaz, M.; Fattouch, S. Egg yolk protein as a novel wall material used together with gum Arabic to encapsulate polyphenols extracted from Phoenix dactylifera L pits. LWT 2020, 131, 109778. [Google Scholar] [CrossRef]
- Pudziuvelyte, L.; Marksa, M.; Sosnowska, K.; Winnicka, K.; Morkuniene, R.; Bernatoniene, J. Freeze-Drying Technique for Microencapsulation of Elsholtziaciliata Ethanolic Extract Using Different Coating Materials. Molecules 2020, 25, 2237. [Google Scholar] [CrossRef]
- Buljeta, I.; Nosić, M.; Pichler, A.; Ivić, I.; Šimunović, J.; Kopjar, M. Apple Fibers as Carriers of Blackberry Juice Polyphenols: Development of Natural Functional Food Additives. Molecules 2022, 27, 3029. [Google Scholar] [CrossRef]
- Buljeta, I.; Pichler, A.; Šimunović, J.; Kopjar, M. Polyphenols and Antioxidant Activity of Citrus Fiber/Blackberry Juice Complexes. Molecules 2021, 26, 4400. [Google Scholar] [CrossRef] [PubMed]
- Vukoja, J.; Buljeta, I.; Pichler, A.; Šimunović, J.; Kopjar, M. Formulation and Stability of Cellulose-Based Delivery Systems of Raspberry Phenolics. Processes 2021, 9, 90. [Google Scholar] [CrossRef]
- Kuck, L.S.; Noreña, C.P.Z. Microencapsulation of grape (Vitis labrusca var. Bordo) skin phenolic extract using gum Arabic, polydextrose, and partially hydrolyzed guar gum as encapsulating agents. Food Chem. 2016, 194, 569–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rezende, Y.R.R.S.; Nogueira, J.P.; Narain, N. Microencapsulation of extracts of bioactive compounds obtained from acerola (Malpighia emarginata DC) pulp and residue by spray and freeze drying: Chemical, morphological and chemometric characterization. Food Chem. 2018, 254, 281–291. [Google Scholar] [CrossRef]
- Dadi, D.W.; Emire, S.A.; Hagos, A.D.; Eun, J.B. Physical and Functional Properties, Digestibility, and Storage Stability of Spray- and Freeze-Dried Microencapsulated Bioactive Products from Moringa stenopetala Leaves Extract. Ind. Crops. Prod. 2020, 156, 112891. [Google Scholar] [CrossRef]
- Michalska, A.; Wojdyło, A.; Honke, J.; Ciska, E.; Andlauer, W. Drying-induced physico-chemical changes in cranberry products. Food Chem. 2018, 240, 448–455. [Google Scholar] [CrossRef] [Green Version]
- El-Messery, T.M.; El-Said, M.M.; Demircan, E.; Ozçelik, B. Microencapsulation of natural polyphenolic compounds extracted from apple peel and its application in yoghurt. Acta Sci. Pol. Technol. Aliment. 2019, 18, 25–34. [Google Scholar] [CrossRef]
- Delfanian, M.; Ali Sahari, M. Improving functionality, bioavailability, nutraceutical and sensory attributes of fortified foods using phenolics-loaded nanocarriers as natural ingredients. Food Res. Int. 2020, 137, 109555. [Google Scholar] [CrossRef]
- Francisco, C.R.L.; Heleno, S.A.; Fernandes, I.P.M.; Barreira, J.C.M.; Calhelha, R.C.; Barros, L.; Barreiro, M.F. Functionalization of yogurts with Agaricusbisporus extracts encapsulated in spray-dried maltodextrin crosslinked with citric acid. Food Chem. 2018, 245, 845–853. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, D.; Bhattacharjee, P. Comparative evaluation of the antioxidant efficacy of encapsulated and un-encapsulated eugenol-rich clove extracts in soybean oil: Shelf-life and frying stability of soybean oil. J. Food Eng. 2013, 117, 545–550. [Google Scholar] [CrossRef]
- Ezhilarasi, P.N.; Indrani, D.; Jena, B.S.; Anandharamakrishnan, C. Microencapsulation of Garciniafruit extract by spray drying and its effect on bread quality. J. Sci. Food Agric. 2013, 94, 1116–1123. [Google Scholar] [CrossRef] [PubMed]
- Pasrija, D.; Ezhilarasi, P.N.; Indrani, D.; Anandharamakrishnan, C. Microencapsulation of green tea polyphenols and its effect on incorporated bread quality. LWT 2015, 64, 289–296. [Google Scholar] [CrossRef]
- Stoica, F.; Condurache, N.N.; Horincar, G.; Constantin, O.E.; Turturicặ, M.; Stặnciuc, N.; Aprodu, I.; Croitoru, C.; Râpeanu, G. Value-Added Crackers Enriched with Red Onion Skin Anthocyanins Entrapped in Different Combinations of Wall Materials. Antioxidants 2022, 11, 1048. [Google Scholar] [CrossRef] [PubMed]
- Papillo, V.A.; Locatelli, M.; Travaglia, F.; Bordiga, M.; Garino, C.; Arlorio, M.; Coïsson, J.D. Spray-dried polyphenolic extract from Italian black rice (Oryza sativa L., var. Artemide) as new ingredient for bakery products. Food Chem. 2018, 269, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Papillo, V.A.; Locatelli, M.; Travaglia, F.; Bordiga, M.; Garino, C.; Coïsson, J.D.; Arlorio, M. Cocoa hulls polyphenols stabilized by microencapsulation as functional ingredient for bakery applications. Food Res. Int. 2018, 115, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Luca, A.; Cilek, B.; Hasirci, V.; Sahin, S.; Sumnu, G. Storage and Baking Stability of Encapsulated Sour Cherry Phenolic Compounds Prepared from Micro- and Nano-Suspensions. Food Bioprocess Technol. 2014, 7, 204–211. [Google Scholar] [CrossRef]
- Dean, L.L.; Klevorn, C.M.; Hess, B.J. Minimizing the Negative Flavor Attributes and Evaluating Consumer Acceptance of Chocolate Fortified with Peanut Skin Extracts. J. Food Sci. 2016, 81, S2824–S2830. [Google Scholar] [CrossRef]
- Mahdavi, S.A.; Jafari, S.M.; Assadpour, E.; Ghorbani, M. Storage stability of encapsulated barberry’s anthocyanin and its application in jelly formulation. J. Food Eng. 2016, 181, 59–66. [Google Scholar] [CrossRef]

| Bioactive Material | Reference | |
|---|---|---|
| Roselle (Hibiscus sabdariffa L.) extract | [44] | |
| Carrier | Maltodextrin, gelatin, pectin, carboxymethyl cellulose, carrageenan, gum Arabic and whey protein | |
| Conditions | 0.5 mm—nozzle diameter; 400 kPa—compressor air pressure; 7.5 mL/min—feed flow rate; 155 °C—inlet temperature; 55 °C—outlet temperature; 56 m3/h—air flow rate | |
| Observations | Pectin can be a suitable carrier of roselle polyphenols because it retained the highest amount of polyphenols (98.20 mg/100 mg) | |
| Plum (Prunus salicina Lindl.) polyphenols | [45] | |
| Carrier | Maltodextrin, gum Arabic, gelatin, chitosan and β-cyclodextrin | |
| Conditions | 150 °C—inlet temperature; 85 °C—outlet temperature; 850 mL/h—feed flow rate; 90 MPa—atomization pressure; 1.15 m3/min—blower rate; 0.7 mm—nozzle diameter | |
| Observations | Powders obtained with maltodextrin/chitosan possessed the highest stability as well as retention of total polyphenols (94%) after storage (60 days at 25 °C) | |
| Pomegranate juice anthocyanins | [46] | |
| Carrier | Gum Arabic, modified starch from waxy maize and maltodextrin | |
| Conditions | 1 mm—nozzle diameter; 1 kg/h—mass flow rate; 162–170 °C—inlet temperatures; 89–93 °C—outlet temperatures; 500 m3/h—air flow rate | |
| Observations | By using gum Arabic:modified starch from waxy maize (1:1) mixture, up to 70% of total monomeric anthocyanins were retained | |
| Cinnamon (Cinnamomum zeylanicum) proanthocyanidins | [47] | |
| Carrier | Maltodextrin | |
| Conditions | 1.2 mm—nozzle diameter; 130 °C and 160 °C—drying temperatures; 40 mL/min—feed flow rate | |
| Observations | The highest retention of proanthocyanidins (100%) was obtained in the sample with 20% of maltodextrin and dried at 160 °C; during 90 days of storage, maltodextrin contributed to stability of proanthocyanidins | |
| Polyphenols of Orthosiphon stamineus leaves | [48] | |
| Carrier | Maltodextrin and whey protein isolate | |
| Conditions | 0.5 mm—atomizer; 180 °C—inlet temperature; 407 mL/h—feed flow rate | |
| Observations | By using 5.33 wt.% of maltodextrin, the highest retention of sinensetin (82.24%), rosmarinic acid (82.67%) and eupatorine (80.19%) was obtained | |
| Fadogia ancylantha, Tussilago farfara and Melissa officinalis extracts | [49] | |
| Carrier | Maltodextrin and apple pectin | |
| Conditions | 120 °C—inlet temperature; 69–71 °C—outlet temperature; 5 mL/min—feed flow rate; 0.5 mm—nozzle diameter; 500 L/h—drying air flow; 6 bar—air pressure; 100%—aspiration | |
| Observations | By using 10:1 maltodextrin:pectin ratio, 3% w/v of raw dried extract was encapsulated; loading efficiency values were very high: 80.1%, 91.5% and 97.2% for Tussilago, Melissa and Fadogia polyphenols, respectively | |
| Black carrot (Daucus carota L.) | [39] | |
| Carrier | Maltodextrins (DE 10, DE 28–31 and DE 20–23) | |
| Conditions | 160 °C, 180 °C and 200 °C—inlet temperatures; 107 °C, 118 °C and 131 °C—outlet temperatures; 5 mL/min—feed flow rate | |
| Observations | Powders with maltodextrin DE 20–23 contained the highest anthocyanin concentration (630 mg anthocyanin/100 g dry matter of powder) | |
| Mountain tea (Sideritis stricta) extract | [50] | |
| Carrier | β-cyclodextrin, maltodextrin and gum Arabic | |
| Conditions | 145 °C, 155 °C and 165 °C—inlet temperatures; 75 °C—outlet temperature; 500 L/h—flow rate; 70%—aspiration rate; 240–640 mL/h—feed rate | |
| Observations | By increasing inlet air temperature from 145 °C to 155 °C, a 4% increase in total polyphenols content occurred, but a further increase in inlet temperature had a negative effect; an increase in the concentration of carriers resulted in a decrease in total polyphenols content (18.96 g/100 g—without carrier, 7.61 g/100 g—for 3 g/100 g of carrier, and 5.43 g/100 g—for 5 g/100 g of carrier) | |
| Cactus pear (Opuntia ficus-indica) | [51] | |
| Carrier | Maltodextrin and inulin | |
| Conditions | 140–160 °C and 120–160 °C—inlet temperatures for maltodextrin and inulin, respectively; 600 L/h—air flow; 10 mL/min—feed rate; 20 psi—atomization pressure | |
| Observations | Optimal conditions for encapsulation with maltodextrin were 3:1 ratio of core/coating material and 140 °C inlet temperature while for inulin the optimal ratio was 3:1 for encapsulation of pulp extract and 5:1 for encapsulation of ethanolic extract and 120 °C inlet temperature; concentration of polyphenols after encapsulation ranged as follows: cactus ethanolic extract + inulin (2410 mg GAE/g powder) > cactus pulp + maltodextrin (2135 mg GAE/g powder) > cactus pulp + inulin (2028 mg GAE/g powder) > cactus ethanolic extract + maltodextrin (1812 mg GAE/g powder) | |
| Açai (Euterpe oleracea Mart.) juice | [52] | |
| Carrier | Maltodextrin (DE 10 and DE 20), gum Arabic, and tapioca starch | |
| Conditions | 1.5 mm—nozzle diameter; 0.06 MPa—compressor air pressure; 15 g/min—feed flow rate; 140 °C—inlet temperature; 78 °C—outlet temperature; 73 m3/h—air flow rate | |
| Observations | Powders obtained with tapioca starch possessed the lowest anthocyanin content (3247.15 mg/100 g) while between the two types of maltodextrin (for DE 10 3436.85 mg/100 g and for DE 20 3402.30 mg/100 g) and gum Arabic (3415.96 mg/100 g) no statistical difference was determined in anthocyanin content | |
| Jaboticaba (Myrciaria jaboticaba) peel extracts | [53] | |
| Carrier | Maltodextrin, gum Arabic, and modified starch from waxy maize | |
| Conditions | 140 °C, 160 °C and 180 °C—inlet temperatures; 360 mL/h—feed flow rate; 20 mbar—vacuum; 28 m3/h—aspiration | |
| Observations | The highest anthocyanins retention was obtained at 160 °C air drying temperature using maltodextrin (99.02%) and gum Arabic/maltodextrin (100%) as carriers; a combination of maltodextrin with starch resulted in the lowest anthocyanins retention (around 80%) regardless of air drying temperatures | |
| Pomegranate (Punica granatum) polyphenols and anthocyanins of juice and ethanolic extracts | [37] | |
| Carrier | Maltodextrin and soybean protein isolates | |
| Conditions | 140–160 °C and 100–140 °C—inlet temperatures for maltodextrin and soybean protein isolates, respectively; 600 L/h—air flow; 10 mL/min—feed rate; 20 psi—atomization pressure | |
| Observations | Encapsulation efficiency of polyphenols was better in the soybean protein isolates matrix (76.2% for pomegranate juice and 82.9% for pomegranate ethanolic extract) than for maltodextrin (53.3% for pomegranate juice and 71.0% for pomegranate ethanolic extract); the highest encapsulation efficiency of anthocyanins from pomegranate ethanolic extract was in the soybean protein isolates matrix (100%) while the highest efficiency of pomegranate juice anthocyanins was in the maltodextrin matrix (86.6%) | |
| Rosemary (Rosmarinus officinalis L.) leaves polyphenols | [54] | |
| Carrier | Maltodextrins (MDE 10 and 21), whey protein isolates and polyglycerol polyricinoleate | |
| Conditions | 175 °C—inlet temperature; 90 °C—outlet temperature; 600 L/h—drying air flow; 18%—feed flow rate; 700 kPa compressor air pressure | |
| Observations | Higher encapsulation efficiency (around 42%) of total polyphenols was obtained when higher amounts of protein (4%) were used, pointing to the important impact of protein in that target delivery system; encapsulation efficiency with 2% of whey protein isolates was around 28%; type of maltodextrin showed no significant impact on encapsulation efficiency | |
| Epigallocatechin-3-gallate (EGCG) | [55] | |
| Carrier | Gum Arabic and maltodextrin | |
| Conditions | 160 °C—inlet temperature; 60 °C—outlet temperature | |
| Observations | Concentration of EGCG at surface was 8% and loading efficiency (EGCG in the inner space) was 85% | |
| Turmeric oleoresin (curcumin) | [56] | |
| Carrier | Maltodextrin and gum Arabic | |
| Conditions | 5 mL/min—feed flow rate; 150 °C, 175 °C and 200 °C—inlet air temperatures; 90 °C—outlet air temperature | |
| Observations | Gum Arabic used as a carrier at the inlet air temperature of 175 °C showed the highest encapsulation efficiency (71.74%) and total curcumin content (3.41 g/100 g) | |
| Barberry (Berberis vulgaris) extract | [57] | |
| Carrier | Gum Arabic, gelatin and maltodextrin | |
| Conditions | 800 mL/h—flow rate; 150 °C—inlet temperature; 100—outlet temperature | |
| Observations | Samples produced with gum Arabic and maltodextrin (core/wall material ratio 25%) in combination possessed the highest microencapsulation efficiency (96.22%) | |
| Roselle (Hibiscus sabdariffa L.) extract | [58] | |
| Carrier | Maltodextrin and gum Arabic | |
| Conditions | 180 °C—inlet air temperature; 80 °C—outlet air temperature; 12 mL/min—feed flow rate; 0.8 bar—atomizing-air pressure | |
| Observations | In sample where maltodextrin:gum Arabic ratio was 70:30, improved retention of polyphenols (465.80 mg GAE/100 g), anthocyanins (171.21 mg cyanidn-3-glucoside/100 g) and antioxidant activity (3.81 mmol Trolox/kg) was achieved | |
| Blackberry pulp | [59] | |
| Carrier | Maltodextrin and gum Arabic | |
| Conditions | 0.49 kg/h—flow rate; 145 °C—inlet temperature; 75–80 °C outlet temperature; 0.36 m3/h—drying air flow rate; 35 m3/h—aspirator flow rate | |
| Observations | Samples with maltodextrin and a combination of both carriers possessed higher anthocyanin retention (around 85%) than gum Arabic (around 78%) | |
| Chokeberry anthocyanins | [60] | |
| Carrier | Maltodextrin, guar gum, gum Arabic, inulin, pectin and β-glucan | |
| Conditions | 140 °C—drying temperature; 25%—pump flow; 600 L/h—air flow | |
| Observations | Capsules with β-glucan had the highest content of anthocyanins (first day and after 7 days of storage) while the ones with gum Arabic had the lowest content; the following encapsulation efficiency was observed: maltodextrin + gum Arabic—78.61%, maltodextrin + inulin—88.37%, maltodextrin + β-glucan—92.78%, maltodextrin + pectin—91.85% and maltodextrin + guar gum—92.98% | |
| Berries and roselle anthocyanins | [61] | |
| Carrier | Gum Arabic, maltodextrin, whey protein isolate and agave fructans | |
| Conditions | 180 °C—inlet temperature; 80 °C—outlet temperature; 13%—feed rate; 94%—air flow | |
| Observations | Retention of anthocyanins was 10.71–86.09%; retention of total polyphenols ranged from 34.71–100%; whey protein isolate was the best carrier agent for anthocyanins and phenolic compounds | |
| Bioactive Material | Reference | |
|---|---|---|
| Red wine polyphenols | [62] | |
| Carrier | Maltodextrin 20% DE 10 | |
| Conditions | Freezing plate and condenser at −40 °C, vacuum below 200 µm Hg; process duration—40 h | |
| Observations | The freeze-drying process resulted with 97% polyphenol retention; there was no significant changes in polyphenols during 15 days of storage at 38 °C | |
| Fermented Miang wastewater bioactive compounds | [63] | |
| Carrier | Maltodextrin, gum Arabic and modified starch | |
| Conditions | Samples were frozen at −18 °C for 24 h and then freeze-dried at −45 °C under a pressure of 0.133 mbar for 72 h | |
| Observations | Between used carriers, there were no statistical difference in total polyphenols content but in surface polyphenols content, differences were observed: by using gum Arabic in 10:1 core:coating material ratio (% w/w), the lowest concentration of surface polyphenols was observed with the highest encapsulation efficiency (98.05%); encapsulation efficiency for maltodextrin and modified starch was 89.07% and 81.58%, respectively | |
| Polyphenols of wastewater from Miang (fermented tea leaf) production | [64] | |
| Carrier | Maltodextrin and gum Arabic | |
| Conditions | Samples were frozen at −18 °C for 24 h and then freeze-dried under a pressure of 0.133 mbar for 72 h | |
| Observations | The weight ratio of maltodextrin:gum Arabic mixture to concentrated fermented Miang water of 1:10 was the best for polyphenols encapsulation with 99.4% efficiency | |
| Cloudberry (Rubus chamaemorus) polyphenols | [65] | |
| Carrier | Maltodextrins DE5–8 and DE18.5 | |
| Conditions | Pressure < 0.1 mbar and duration of 48 h | |
| Observations | Maltodextrin DE 5–8 resulted in higher encapsulation efficiency for all polyphenols, especially for ellagitannins (99%), proanthocyanidins (94%) and flavonols (90%); the highest encapsulation efficiency values of maltodextrin DE 18.8 were for hydroxycinnamic acids and hydroxybenzoic acids (69% and 68%, respectively) | |
| Red onion peel polyphenols | [66] | |
| Carrier | Maltodextrin and soybean protein isolate | |
| Conditions | Not defined | |
| Observations | The combination of maltodextrin with soybean protein isolate showed higher encapsulation efficiency (94.30%) than for the carriers individually (maltodextrin 91.5% and soybean protein isolate 89.83%) | |
| Yellow onions skins flavonoids extract | [67] | |
| Carrier | Maltodextrin, pectin and whey proteins hydrolysates | |
| Conditions | Samples were frozen at −70 °C and then freeze-dried at −42 °C under a pressure of 0.1 mbar for 48 h | |
| Observations | Maltodextrin:pectin:whey proteins hydrolysates in a ratio of 2:1:0.4 resulted in the highest flavonoid encapsulation (66.46%) | |
| Phoenix dactylifera L pit polyphenols | [68] | |
| Carrier | Gum Arabic and egg yolk protein | |
| Conditions | Samples were frozen at −80 °C for 12 h and then freeze-dried for 48 h | |
| Observations | Microparticles with a higher amount of egg yolk protein showed the highest encapsulation efficiency (99.75%); the lowest encapsulation efficiency was observed when gum Arabic and gum Arabic:egg yolk protein (3:1) were used (44.06% and 43.04%, respectively) | |
| Elsholtzia ciliate ethanolic extract | [69] | |
| Carrier | Gum Arabic, maltodextrin, beta-maltodextrin, resistant-maltodextrin, skim milk and sodium caseinate | |
| Conditions | Samples were frozen at −80 °C for 24 h and then freeze-dried at −50 °C at 0.05 mbar for 24 h | |
| Observations | The highest encapsulation efficiency of total polyphenols content were observed with sodium caseinate (83.02%) while the lowest were with maltodextrin (21.17%) | |
| Blackberry juice polyphenols | [70] | |
| Carrier | Apple fibers | |
| Conditions | Samples were frozen at −18 °C for 24 h and then freeze-dried under the following conditions: −55 °C—freezing temperature; −35–0 °C—temperature of sublimation; 0.220 mbar—vacuum level; 0–21 °C—isothermal desorption temperatures; 12 h—process duration | |
| Observations | Different amounts of apple fibers (1%, 2%, 4%, 6%, 8%, and 10%) were used for polyphenol encapsulation, and results showed the best adsorption of total polyphenols when 1% (1.82 g GAE/100 g) and 2% (1.79 g GAE/100 g) of fiber was used | |
| Blackberry juice polyphenols | [71] | |
| Carrier | Citrus fibers | |
| Conditions | Samples were frozen at −18 °C for 24 h and then freeze-dried under the following conditions: −55 °C—freezing temperature; −35–0 °C—temperature of sublimation; 0.220 mbar—vacuum level; 0–21 °C—isothermal desorption temperatures; 12 h—process duration | |
| Observations | By increasing the amount of fiber above 1%, a decrease in the concentration of adsorbed polyphenols occurred; complexes with higher amounts of fiber (2% and 4%) had higher retention levels of polyphenols after eight months’ storage (70% and 79%, respectively) | |
| Raspberry juice polyphenols | [72] | |
| Carrier | Cellulose | |
| Conditions | Samples were frozen at −18 °C for 24 h and then freeze-dried under the following conditions: −55 °C—freezing temperature; −35–0 °C—temperature of sublimation; 0.220 mbar—vacuum level; 0–21 °C—isothermal desorption temperatures; 12 h—process duration | |
| Observations | The complex with 2.5% of cellulose resulted in the highest concentration of polyphenols (2.43 g/kg for 15 min of complexation and 1.96 g/kg for 60 min of complexation); higher amounts of the carrier (5%, 7.5% and 10%) negatively affected polyphenols adsorption; the highest retention of polyphenols during storage was observed in powders with 5% and 7.5% of cellulose (from 90–100%) | |
| Bioactive Material | Reference | |
|---|---|---|
| Roselle (Hibiscus sabdariffa L.) anthocyanins | [16] | |
| Carrier | Maltodextrin, gum Arabic, inulin and konjac | |
| Conditions | SD: 500 mL/h—flow rate; 150 °C—inlet temperature; 91 °C—outlet temperature FD: Not defined | |
| Observations | The freeze-dried sample with 100% konjac had the highest antioxidant content but with low encapsulation efficiency of 43.6% (anthocyanins located on the surface); a mixture of maltodextrin and gum Arabic provided powders with high antioxidant content and efficiency for both methods of drying (around 95%) | |
| Grape (Vitis labrusca var. Bordo) skin phenolic extract | [73] | |
| Carrier | Gum Arabic, polydextrose, and partially hydrolyzed guar gum | |
| Conditions | SD: 0.60 L/h—flow rate; 140 °C—drying air temperature; 3.5 kg/cm2—air pressure; 40.5 L/h—air flow rate FD: Dispersions were frozen at −68 °C for 24 h and then freeze-dried at −57 °C for 48 h at vacuum pressure of less than 20 µm Hg | |
| Observations | Between these drying methods, there was no difference in polyphenol retention; the spray-drying method using 10% gum Arabic had the highest retention of phenolic compounds (25.03 mg GAE/g) as well as freeze-drying with 5% gum Arabic and 5% of polydextrose (24.57 mg GAE/g) | |
| Coffee grounds polyphenols extract | [2] | |
| Carrier | Maltodextrin and gum Arabic | |
| Conditions | SD: 108 mL/h—flow rate; 100 °C—air inlet temperature; 75% (28 m3/h)—aspiration FD: The samples were previously frozen and then freeze-dried at −60 °C at 0.05 bar for 48 h | |
| Observations | In spray-drying, using maltodextrin as wall material achieved the best encapsulation of flavonoids (around 52%) while a combination of maltodextrin and gum Arabic was the best for encapsulation of total phenolic compounds (around 65%); In freeze-drying, 100% maltodextrin as wall material was the best for encapsulation of total polyphenols and flavonoids (62% and 73%, respectively) | |
| Model fruit juice (0.1% citrus pectin, 10% sucrose and 0.5% gallic acid) | [13] | |
| Carrier | Maltodextrin and gum Arabic | |
| Conditions | SD: 72–144 mL/h—flow rate; 80–120 °C—inlet temperature; 600 L/h—nozzle air flow rate; 75% (28 m3/h)—aspiration FD: 300–500mTorr—chamber pressure; 0.3–0.7 °C—freezing rate; 16 ± 0.5 h—process duration | |
| Observations | The higher concentrations of gallic acid in freeze-dried samples were achieved with close to 100% gum Arabic and encapsulant concentration of 10–20% and with maltodextrin concentration of 80–100%; for spray-dried samples the best conditions also included 10–20% encapsulant concentration and maltodextrin:gum Arabic ratio of 50–80% | |
| Lemon by-product aqueous extract | [17] | |
| Carrier | Maltodextrin, soybean protein and ȷ-carrageenan | |
| Conditions | SD: 125 °C—inlet temperature; 55 °C—maximum outlet temperature; 601 L/h—atomization air flow rate; 4 mL/min—liquid feed pump rate; 38 m3/h—main drying air flow rate; 70 °C—feed solution temperature; 70 mL—feed solution FD: Using liquid nitrogen, samples were initially frozen and then freeze-dried (48 h) | |
| Observations | Freeze-dried samples obtained with a combination of maltodextrin and soybean protein achieved the highest encapsulation productivity of total polyphenols content and total flavonoids content (74%); in spray-drying, the best encapsulation productivity for total polyphenols content (67%) was achieved with the same combination of wall materials as for freeze-drying, while for encapsulation productivity of total flavonoids content (58%), no statistical difference was observed between wall materials | |
| Acerola (Malpighia emarginata DC) pulp and residue | [74] | |
| Carrier | Gum Arabic and maltodextrin mixture | |
| Conditions | SD: 1 mm—feed nozzle diameter; 4 m3/min—drying air flow rate; 0.36 L/h—feed rate; 30 L/min—compressed air flow; 3.5 kgf/cm2—air pressure; 170 °C—inlet temperature; 82 °C—outlet temperature FD: Samples were previously frozen at −18 °C for 48 h and then freeze-dried at −58.8 °C for 48 h at 0.42 a mbar vacuum | |
| Observations | Microencapsulation efficiency for total polyphenols content of freeze-dried samples of both pulp and residue was higher (around 68%) than for spray-dried samples; for microencapsulation efficiency of total flavonoids content, the best results (around 59%) were obtained for freeze-dried acerola residue | |
| Star fruit (Averrhoa carambola) pomace polyphenols | [41] | |
| Carrier | Maltodextrin | |
| Conditions | SD: 185 °C—inlet temperature; 88 °C—outlet temperature; 6 mL/min—feed rate; 0.1 mm—nozzle size FD: Frozen samples (−40 °C) were freeze-dried at −55 °C for 24 h | |
| Observations | Freeze-dried encapsulates had a higher encapsulation efficiency (78–97%) than spray-dried ones (63–79%); an increase in maltodextrin concentration led to an increase in core polyphenols content | |
| Gallic acid | [33] | |
| Carrier | Acid-hydrolyzed low dextrose equivalent potato starch | |
| Conditions | SD: 160 °C—inlet temperature; 75 °C—outlet temperature FD: Not defined | |
| Observations | Encapsulation efficiency for freeze-dried samples ranged from 70–84% and for spray-dried from 65–79% without statistically significant differences between methods | |
| Hydroxytyrosol | [3] | |
| Carrier | β-cyclodextrin | |
| Conditions | SD: 100 °C—gas inlet temperature; 100 L/min—drying gas (air) flow rate; 0.5 mL/min—feed rate; 35 mbar—inside pressure; 100%—spray rate FD: Frozen samples were slowly dried at −50 °C under 0.06 mbar | |
| Observations | Spray-dried particles had a spherical and smooth surface with encapsulation efficiency of 84.4%; freeze-dried particles had an irregular shape and encapsulation efficiency was 89.6% | |
| Papaya pulp | [40] | |
| Carrier | Maltodextrin | |
| Conditions | SD: 150 °C—inlet temperature; 4 m3/min—air flow; 3 kgf/cm2—air pressure; 0.4 L/h—feed flow FD: −62 °C—processing temperature; 6.11 mbar—vacuum degree; 48 h—process duration | |
| Observations | Freeze-dried products possessed lower retention of vanillic, ferulic, and caffeic acids (15.85 ng/g, under detection limit, and under detection limit, respectively) than spray-dried (30.73 ng/g, 11.26 ng/g, and 9.45 ng/g, respectively) | |
| Moringa stenopetala leaves extract | [75] | |
| Carrier | Maltodextrin and high methoxyl pectin | |
| Conditions | SD: 0.5 mm—nozzle diameter; 485 mL/h—flow rate; 140 °C—inlet temperature; 78–81 °C—outlet temperature FD: Samples were frozen at −70 °C for 2 h and then freeze-dried for 72 h | |
| Observations | Total polyphenols content and total flavonoid content of freeze-dried samples were higher than in spray-dried ones, but encapsulation efficiency and storage stability were better in spray-dried samples; encapsulation efficiency for spray-dried powders with maltodextrin and maltodextrin/high methoxyl pectin was 83.52% and 87.93%, while for freeze-dried ones, it was 71.44% and 82.12%, respectively | |
| Cranberry juice | [76] | |
| Carrier | Maltodextrin | |
| Conditions | SD: 50%—pump capacity; 35 m3/h—air flow FD: 0.03 mbar | |
| Observations | Powders obtained with sugar free cranberry juice and without maltodextrin had almost five times higher contents of polyphenols (6423 mg/kg—FD and 6433 mg/kg—SD) than powders with 15% maltodextrin and cranberry juice (961 mg/kg—FD and 807 mg/kg—SD); in samples with maltodextrin, p-coumaroyl-hexose concentration was higher when spray-drying was applied (180 mg/kg); considering anthocyanins, spray- and freeze-drying equally affected their retention | |
| Rose (Rosa rugosa) anthocyanins | [42] | |
| Carrier | Gum Arabic and maltodextrin | |
| Conditions | SD: 170 °C—inlet temperature; 5 m3/min—drying airflow rate; 0.36 L/h—feed rate FD: −52 °C—processing temperature; 0.45 mbar—vacuum degree; 48 h—process duration | |
| Observations | The retention rate of total polyphenols content and anthocyanin content was 86% and 75.85% for spray-dried powder, and 91.44% and 95.12% for freeze-dried powder, respectively | |
| Lowbush Vaccinium myrtillus blueberry fruit juice | [24] | |
| Carrier | Hydroxypropyl-β-cyclodextrin and maltodextrin | |
| Conditions | SD: 140 °C—inlet temperature; 70 °C—outlet temperature; 75%—air flow rate; 0.7 mm—nozzle diameter FD: Slowly freezing at −50 °C; pre-drying at 0.42 mbar and 30 °C; secondary drying—reducing pressure to 0.05 mbar and increasing temperature to 40 °C | |
| Observations | Spray-dried microparticles with maltodextrin and hydroxypropyl-β-cyclodextrin microcapsules had higher total polyphenols content and total anthocyanins content (around 1.65 g/100 g and 1.3 g/100 g, respectively) than freeze-dried microparticles with β-cyclodextrin (around 1.45 g/100 g and 1.1 g/100 g, respectively); total losses of anthocyanins and total polyphenols during drying were lower in the freeze-drying process | |
| Apple peel polyphenols | [77] | |
| Carrier | Maltodextrin, gum Arabic, and whey protein concentrate | |
| Conditions | SD: 150 °C—inlet temperature; 50 °C—outlet temperature FD: Samples were frozen at −20 °C for 24 h and then freeze-dried at −45 °C under a pressure of less than 0.12 mbar for more than 48 h | |
| Observations | Freeze-dried samples homogenized by ultrasonication possessed higher encapsulation efficiency values for phenolic content (83.69%), flavonoid content (85.47%), and antioxidant activity (86.85%) compared to the spray-dried samples previously homogenized by ultra turrax (83.58%, 48.31% and 80.21%, respectively) | |
| Food Product | Source of Polyphenols | Wall Material | Encapsulation Technique | Major Findings | Reference |
|---|---|---|---|---|---|
| Biscuit | Italian black rice polyphenols | Maltodextrin Gum Arabic | Spray-drying Freeze-drying | Spray-dried encapsulates, added to biscuits, were the most stable during storage and were partially protected during the baking; enriched biscuits showed a higher content of polyphenols, anthocyanins and antioxidant activity than control biscuits | [84] |
| Biscuit | Cocoa hulls polyphenols | Maltodextrin Gum Arabic | Spray-drying | By using powder with maltodextrin, the most stable sample with unaffected total polyphenols content after baking was obtained | [85] |
| Cake | Sour cherry polyphenols | Maltodextrin Gum Arabic | Freeze-drying | The incorporation of encapsulated polyphenols didn’t impair the sensory or quality properties of cakes; a positive effect on hygroscopicity, baking stability, storage and digestibility was observed | [86] |
| Chocolate | Peanut skins polyphenols | Maltodextrin | Spray-drying | Antioxidant activity increased after the addition of encapsulates; with 9% of additives antioxidant activity was similar to dark chocolate while flavor was similar to milk chocolate | [87] |
| Jelly | Barberry polyphenols | Maltodextrin Gum Arabic Gelatin | Spray-drying | Gum Arabic/maltodextrin was the best wall material; jelly with 7% of powder showed better consumer acceptability than commercial jelly; antioxidant activity was increased | [88] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buljeta, I.; Pichler, A.; Šimunović, J.; Kopjar, M. Polysaccharides as Carriers of Polyphenols: Comparison of Freeze-Drying and Spray-Drying as Encapsulation Techniques. Molecules 2022, 27, 5069. https://doi.org/10.3390/molecules27165069
Buljeta I, Pichler A, Šimunović J, Kopjar M. Polysaccharides as Carriers of Polyphenols: Comparison of Freeze-Drying and Spray-Drying as Encapsulation Techniques. Molecules. 2022; 27(16):5069. https://doi.org/10.3390/molecules27165069
Chicago/Turabian StyleBuljeta, Ivana, Anita Pichler, Josip Šimunović, and Mirela Kopjar. 2022. "Polysaccharides as Carriers of Polyphenols: Comparison of Freeze-Drying and Spray-Drying as Encapsulation Techniques" Molecules 27, no. 16: 5069. https://doi.org/10.3390/molecules27165069
APA StyleBuljeta, I., Pichler, A., Šimunović, J., & Kopjar, M. (2022). Polysaccharides as Carriers of Polyphenols: Comparison of Freeze-Drying and Spray-Drying as Encapsulation Techniques. Molecules, 27(16), 5069. https://doi.org/10.3390/molecules27165069







