Natural Polyphenols as SERCA Activators: Role in the Endoplasmic Reticulum Stress-Related Diseases
Abstract
:1. Introduction
2. Intracellular Ca2+ Regulation: The Role of SERCA
3. Polyphenol–SERCA Interactions
4. Pharmacological Activation of SERCA by Polyphenols
4.1. Resveratrol
4.2. 6-Gingerol
4.3. Ellagic Acid
4.4. Luteolin
4.5. Other Polyphenols
5. Concluding Remarks
- (i)
- Upregulation of SERCA expression, specifically via AMPK/SIRT activation;
- (ii)
- Increase in SERCA activity and stability;
- (iii)
- Relieving SERCA2 from the SERCA–PLB complex;
- (iv)
- Enhancing RyR1 and RyR2 activity/expression,
- (v)
- Affecting Ca2+-dependent channels, such as L-type and T-type VGCCs, Ca2+-activated K+ channels, or SOCs.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Wang, R.; Wang, M.; He, S.; Sun, G.; Sun, X. Targeting Calcium Homeostasis in Myocardial Ischemia/Reperfusion Injury: An Overview of Regulatory Mechanisms and Therapeutic Reagents. Front. Pharmacol. 2020, 11, 872. [Google Scholar] [CrossRef]
- Hamilton, S.; Terentyev, D. Proarrhythmic Remodeling of Calcium Homeostasis in Cardiac Disease; Implications for Diabetes and Obesity. Front. Physiol. 2018, 9, 1517. [Google Scholar] [CrossRef] [PubMed]
- Bergantin, L.B. Diabetes and Cancer: Debating the Link through Ca2+/CAMP Signalling. Cancer Lett. 2019, 448, 128–131. [Google Scholar] [CrossRef]
- Eshima, H.; Poole, D.C.; Kano, Y. In Vivo Calcium Regulation in Diabetic Skeletal Muscle. Cell Calcium 2014, 56, 381–389. [Google Scholar] [CrossRef]
- Cui, C.; Merritt, R.; Fu, L.; Pan, Z. Targeting Calcium Signaling in Cancer Therapy. Acta Pharm. Sin. B 2017, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Ge, M.; Zhang, J.; Chen, S.; Huang, Y.; Chen, W.; He, L.; Zhang, Y. Role of Calcium Homeostasis in Alzheimer’s Disease. Neuropsychiatr. Dis. Treat. 2022, 18, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Kim, S.Y.; Canbakis Cecen, F.S.; Cho, Y.; Kwon, S.K. Dysfunction of Mitochondrial Ca 2+ Regulatory Machineries in Brain Aging and Neurodegenerative Diseases. Front. Cell Dev. Biol. 2020, 8, 599792. [Google Scholar] [CrossRef] [PubMed]
- Missiaen, L.; Robberecht, W.; Van Den Bosch, L.; Callewaert, G.; Parys, J.B.; Wuytack, F.; Raeymaekers, L.; Nilius, B.; Eggermont, J.; De Smedt, H. Abnormal Intracellular Ca2+ Homeostasis and Disease. Cell Calcium 2000, 28, 1–21. [Google Scholar] [CrossRef]
- Feno, S.; Butera, G.; Reane, D.V.; Rizzuto, R.; Raffaello, A. Crosstalk between Calcium and ROS in Pathophysiological Conditions. Oxid. Med. Cell. Longev. 2019, 2019, 9324018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stutzmann, G.E.; Mattson, M.P. Endoplasmic Reticulum Ca2+ Handling in Excitable Cells in Health and Disease. Pharmacol. Rev. 2011, 63, 700–727. [Google Scholar] [CrossRef] [Green Version]
- Toyoshima, C. How Ca2+-ATPase Pumps Ions across the Sarcoplasmic Reticulum Membrane. Biochim. Biophys. Acta 2009, 1793, 941–946. [Google Scholar] [CrossRef] [Green Version]
- Periasamy, M.; Kalyanasundaram, A. SERCA Pump Isoforms: Their Role in Calcium Transport and Disease. Muscle Nerve 2007, 35, 430–442. [Google Scholar] [CrossRef]
- Lipskaia, L.; Keuylian, Z.; Blirando, K.; Mougenot, N.; Jacquet, A.; Rouxel, C.; Sghairi, H.; Elaib, Z.; Blaise, R.; Adnot, S.; et al. Expression of Sarco (Endo) Plasmic Reticulum Calcium ATPase (SERCA) System in Normal Mouse Cardiovascular Tissues, Heart Failure and Atherosclerosis. Biochim. Biophys. Acta 2014, 1843, 2705–2718. [Google Scholar] [CrossRef]
- Chemaly, E.R.; Bobe, R.; Adnot, S.; Hajjar, R.J.; Lipskaia, L. Sarco (Endo) Plasmic Reticulum Calcium Atpases (SERCA) Isoforms in the Normal and Diseased Cardiac, Vascular and Skeletal Muscle. J. Cardiovasc. Dis. Diagn. 2013, 1, 1000113. [Google Scholar] [CrossRef]
- Brown, M.K.; Naidoo, N. The endoplasmic reticulum stress response in aging and age-related diseases. Front. Physiol. 2012, 3, 263. [Google Scholar] [CrossRef] [Green Version]
- Chong, W.C.; Shastri, M.D.; Eri, R. Endoplasmic Reticulum Stress and Oxidative Stress: A Vicious Nexus Implicated in Bowel Disease Pathophysiology. Int. J. Mol. Sci. 2017, 18, 771. [Google Scholar] [CrossRef]
- Hetz, C.; Zhang, K.; Kaufman, R.J. Mechanisms, Regulation and Functions of the Unfolded Protein Response. Nat. Rev. Mol. Cell Biol. 2020, 21, 421–438. [Google Scholar] [CrossRef]
- Liu, H.; Yang, J.; Li, L.; Shi, W.; Yuan, X.; Wu, L. The Natural Occurring Compounds Targeting Endoplasmic Reticulum Stress. Evid.-Based Complement. Alternat. Med. 2016, 2016, 7831282. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.H.; Walter, P.; Yen, T.S.B. Endoplasmic Reticulum Stress in Disease Pathogenesis. Annu. Rev. Pathol. 2008, 3, 399–425. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, M.; Imaizumi, K.; Saito, A.; Kanemoto, S.; Asada, R.; Matsuhisa, K.; Ohtake, Y. ER Stress and Disease: Toward Prevention and Treatment. Biol. Pharm. Bull. 2017, 40, 1337–1343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.W.; Zhou, Y.; Lee, J.; Lee, J.; Ozcan, U. Sarco(Endo)Plasmic Reticulum Ca2+-ATPase 2b Is a Major Regulator of Endoplasmic Reticulum Stress and Glucose Homeostasis in Obesity. Proc. Natl. Acad. Sci. USA 2010, 107, 19320–19325. [Google Scholar] [CrossRef] [Green Version]
- Jessup, M.; Greenberg, B.; Mancini, D.; Cappola, T.; Pauly, D.F.; Jaski, B.; Yaroshinsky, A.; Zsebo, K.M.; Dittrich, H.; Hajjar, R.J. Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease (CUPID): A Phase 2 Trial of Intracoronary Gene Therapy of Sarcoplasmic Reticulum Ca2+-ATPase in Patients with Advanced Heart Failure. Circulation 2011, 124, 304–313. [Google Scholar] [CrossRef] [Green Version]
- MacLennan, D.H.; Asahi, M.; Tupling, A.R. The Regulation of SERCA-Type Pumps by Phospholamban and Sarcolipin. Ann. N. Y. Acad. Sci. 2003, 986, 472–480. [Google Scholar] [CrossRef]
- Fajardo, V.A.; Bombardier, E.; Vigna, C.; Devji, T.; Bloemberg, D.; Gamu, D.; Gramolini, A.O.; Quadrilatero, J.; Tupling, A.R. Co-Expression of SERCA Isoforms, Phospholamban and Sarcolipin in Human Skeletal Muscle Fibers. PLoS ONE 2013, 8, e84304. [Google Scholar] [CrossRef] [Green Version]
- Nelson, B.R.; Makarewich, C.A.; Anderson, D.M.; Winders, B.R.; Troupes, C.D.; Wu, F.; Reese, A.L.; McAnally, J.R.; Chen, X.; Kavalali, E.T.; et al. Muscle Physiology: A Peptide Encoded by a Transcript Annotated as Long Noncoding RNA Enhances SERCA Activity in Muscle. Science 2016, 351, 271–275. [Google Scholar] [CrossRef] [Green Version]
- Fisher, M.E.; Bovo, E.; Aguayo-Ortiz, R.; Cho, E.E.; Pribadi, M.P.; Dalton, M.P.; Rathod, N.; Lemieux, M.J.; Espinoza-Fonseca, L.M.; Robia, S.L.; et al. Dwarf Open Reading Frame (Dworf) Is a Direct Activator of the Sarcoplasmic Reticulum Calcium Pump Serca. eLife 2021, 10, e65545. [Google Scholar] [CrossRef]
- Lemos, F.O.; Bultynck, G.; Parys, J.B. A Comprehensive Overview of the Complex World of the Endo- and Sarcoplasmic Reticulum Ca2+-Leak Channels. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 119020. [Google Scholar] [CrossRef]
- Chemaly, E.R.; Troncone, L.; Lebeche, D. SERCA Control of Cell Death and Survival. Cell Calcium 2018, 69, 46–61. [Google Scholar] [CrossRef]
- Vervliet, T.; Parys, J.B.; Bultynck, G. Bcl-2 Proteins and Calcium Signaling: Complexity beneath the Surface. Nat. Publ. Gr. 2016, 35, 5079–5092. [Google Scholar] [CrossRef] [Green Version]
- Rahate, K.; Bhatt, L.K.; Prabhavalkar, K.S. SERCA Stimulation: A Potential Approach in Therapeutics. Chem. Biol. Drug Des. 2020, 95, 5–15. [Google Scholar] [CrossRef]
- Bagur, R.; Hajnóczky, G. Intracellular Ca2+ Sensing: Its Role in Calcium Homeostasis and Signaling. Mol. Cell 2017, 66, 780–788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Cao, R.; Zhong, W. Host Calcium Channels and Pumps in Viral Infections. Cells 2020, 9, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooper, D.; Dimri, M. Biochemistry, Calcium Channels; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Gilon, P.; Chae, H.Y.; Rutter, G.A.; Ravier, M.A. Calcium signaling in pancreatic β-cells in health and in Type 2 diabetes. Cell Calcium 2014, 56, 340–361. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Arora, S.; Saurav, S.; Motiani, R.K. Pathophysiological significance of calcium signaling at Mitochondria-Associated Endoplasmic Reticulum Membranes (MAMs). Curr. Opin. Physiol. 2020, 17, 234–242. [Google Scholar] [CrossRef]
- Hovnanian, A. SERCA pumps and human diseases. Subcell Biochem. 2007, 45, 337–363. [Google Scholar] [CrossRef] [PubMed]
- Nakka, K.; Ghigna, C.; Gabellini, D.; Dilworth, F.J. Diversification of the muscle proteome through alternative splicing. Skelet Muscle 2018, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Fredersdorf, S.; Thumann, C.; Zimmermann, W.H.; Vetter, R.; Graf, T.; Luchner, A.; Riegger, G.A.; Schunkert, H.; Eschenhagen, T.; Weil, J. Increased myocardial SERCA expression in early type 2 diabetes mellitus is insulin dependent: In vivo and in vitro data. Cardiovasc. Diabetol. 2012, 11, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mekahli, D.; Bultynck, G.; Parys, J.B.; de Smedt, H.; Missiaen, L. Endoplasmic-Reticulum Calcium Depletion and Disease. Cold Spring Harb. Perspect. Biol. 2011, 3, a004317. [Google Scholar] [CrossRef] [PubMed]
- Britzolaki, A.; Saurine, J.; Klocke, B.; Pitychoutis, P.M. A Role for SERCA Pumps in the Neurobiology of Neuropsychiatric and Neurodegenerative Disorders. Adv. Exp. Med. Biol. 2020, 1131, 131–161. [Google Scholar] [CrossRef]
- Varghese, E.; Samuel, S.M.; Sadiq, Z.; Kubatka, P.; Liskova, A.; Benacka, J.; Pazinka, P.; Kruzliak, P.; Büsselberg, D. Anti-Cancer Agents in Proliferation and Cell Death: The Calcium Connection. Int. J. Mol. Sci. 2019, 20, 3017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Britzolaki, A.; Saurine, J.; Flaherty, E.; Thelen, C.; Pitychoutis, P.M. The SERCA2: A Gatekeeper of Neuronal Calcium Homeostasis in the Brain. Cell Mol. Neurobiol. 2018, 38, 981–994. [Google Scholar] [CrossRef]
- Dally, S.; Corvazier, E.; Bredoux, R.; Bobe, R.; Enouf, J. Multiple and diverse coexpression, location, and regulation of additional SERCA2 and SERCA3 isoforms in nonfailing and failing human heart. J. Mol. Cell Cardiol. 2010, 48, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Manjarrés, I.M.; Rodríguez-García, A.; Alonso, M.T.; García-Sancho, J. The sarco/endoplasmic reticulum Ca(2+) ATPase (SERCA) is the third element in capacitative calcium entry. Cell Calcium 2010, 47, 412–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oláh, T.; Fodor, J.; Ruzsnavszky, O.; Vincze, J.; Berbey, C.; Allard, B.; Csernoch, L. Overexpression of transient receptor potential canonical type 1 (TRPC1) alters both store operated calcium entry and depolarization-evoked calcium signals in C2C12 cells. Cell Calcium 2011, 49, 415–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.J.; Hyun, C.; Woo, J.S.; Park, C.S.; Kim, D.H.; Lee, E.H. Stromal interaction molecule 1 (STIM1) regulates sarcoplasmic/endoplasmic reticulum Ca2+-ATPase 1a (SERCA1a) in skeletal muscle. Pflug. Arch. Eur. J. Phys. 2014, 466, 987–1001. [Google Scholar] [CrossRef] [PubMed]
- Redondo, P.C.; Salido, G.M.; Pariente, J.A.; Sage, S.O.; Rosado, J.A. SERCA2b and 3 play a regulatory role in store-operated calcium entry in human platelets. Cell Signal 2008, 20, 337–346. [Google Scholar] [CrossRef]
- López, J.J.; Jardín, I.; Bobe, R.; Pariente, J.A.; Enouf, J.; Salido, G.M.; Rosado, J.A. STIM1 regulates acidic Ca2+ store refilling by interaction with SERCA3 in human platelets. Biochem. Pharmacol. 2008, 75, 2157–2164. [Google Scholar] [CrossRef]
- Hussein, R.A.; El-Anssary, A.A. Plants Secondary Metabolites: The Key Drivers of the Pharmacological Actions of Medicinal Plants. In Herbal Medicine, 1st ed.; Builders, P.F., Ed.; IntechOpen: London, UK, 2018; pp. 11–31. [Google Scholar] [CrossRef] [Green Version]
- Ganesan, K.; Xu, B. A Critical Review on Polyphenols and Health Benefits of Black Soybeans. Nutrients 2017, 9, 455. [Google Scholar] [CrossRef] [Green Version]
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [Green Version]
- Hussain, S.A.; Sulaiman, A.A.; Alhaddad, H.; Alhadidi, Q. Natural Polyphenols: Influence on Membrane Transporters. J. Intercult. Ethnopharmacol. 2016, 5, 97–104. [Google Scholar] [CrossRef]
- Lacroix, S.; Klicic Badoux, J.; Scott-Boyer, M.P.; Parolo, S.; Matone, A.; Priami, C.; Morine, M.J.; Kaput, J.; Moco, S. A Computationally Driven Analysis of the Polyphenol-Protein Interactome. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez, A.I.; Real, R.; Pérez, M.; Mendoza, G.; Prieto, J.G.; Merino, G. Modulation of the Activity of ABC Transporters (P-Glycoprotein, MRP2, BCRP) by Flavonoids and Drug Response. J. Pharm. Sci. 2010, 99, 598–617. [Google Scholar] [CrossRef] [PubMed]
- De Meis, L.; Vianna, A.L. Energy Interconversion by the Ca2+-Dependent ATPase of the Sarcoplasmic Reticulum. Annu. Rev. Biochem. 1979, 48, 275–292. [Google Scholar] [CrossRef]
- Rinaldi, D.E.; Ontiveros, M.Q.; Saffioti, N.A.; Vigil, M.A.; Mangialavori, I.C.; Rossi, R.C.; Rossi, J.P.; Espelt, M.V.; Ferreira-Gomes, M.S. Epigallocatechin 3-Gallate Inhibits the Plasma Membrane Ca2+-ATPase: Effects on Calcium Homeostasis. Heliyon 2021, 7, 1–12. [Google Scholar] [CrossRef]
- Ontiveros, M.; Rinaldi, D.; Marder, M.; Espelt, M.V.; Mangialavori, I.; Vigil, M.; Rossi, J.P.; Ferreira-Gomes, M. Natural Flavonoids Inhibit the Plasma Membrane Ca2+-ATPase. Biochem. Pharmacol. 2019, 166, 149446037. [Google Scholar] [CrossRef] [PubMed]
- Ogunbayo, O.A.; Michelangeli, F. Related Flavonoids Cause Cooperative Inhibition of the Sarcoplasmic Reticulum Ca2+ ATPase by Multimode Mechanisms. FEBS J. 2014, 281, 766–777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soler, F.; Asensio, M.C.; Fernández-Belda, F. Inhibition of the Intracellular Ca2+ Transporter SERCA (Sarco-Endoplasmic Reticulum Ca2+-ATPase) by the Natural Polyphenol Epigallocatechin-3-Gallate. J. Bioenerg. Biomembr. 2012, 44, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Bilmen, J.G.; Khan, S.Z.; Javed, M.U.H.; Michelangeli, F. Inhibition of the SERCA Ca2+ Pumps by Curcumin. Eur. J. Biochem. 2001, 268, 6318–6327. [Google Scholar] [CrossRef] [PubMed]
- Wictome, M.; Michelangeli, F.; Lee, A.G.; East, J.M. The Inhibitors Thapsigargin and 2,5-Di(Tert-Butyl)-1,4-Benzohydroquinone Favour the E2 Form of the Ca2+, Mg2+-ATPase. FEBS Lett. 1992, 304, 109–113. [Google Scholar] [CrossRef] [Green Version]
- Lytton, J.; Westlin, M.; Hanley, M.R. Thapsigargin inhibits the sarcoplasmic or endoplasmic reticulum Ca-ATPase family of calcium pumps. J. Biol. Chem. 1991, 266, 17067–17071. [Google Scholar] [CrossRef]
- Yen, C.-L.; Liao, Y.-C.; Chen, R.-F.; Huang, Y.-F.; Chung, W.-C.; Lo, P.-C.; Chang, C.-F.; Wu, P.-C.; Shieh, D.-B.; Jiang, S.-T.; et al. Targeted Delivery of Curcumin Rescues Endoplasmic Reticulum–Retained Mutant NOX2 Protein and Avoids Leukocyte Apoptosis. J. Immunol. 2019, 202, 3394–3403. [Google Scholar] [CrossRef]
- Ogunbayo, O.A.; Harris, R.M.; Waring, R.H.; Kirk, C.J.; Michelangeli, F. Inhibition of the Sarcoplasmic/Endoplasmic Reticulum Ca2+-ATPase by Flavonoids: A Quantitative Structure-Activity Relationship Study. IUBMB Life 2008, 60, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Shoshan, V.; MacLennan, D.H. Quercetin Interaction with the (Ca2+ + Mg2+)-ATPase of Sarcoplasmic Reticulum. J. Biol. Chem. 1981, 256, 887–892. [Google Scholar] [CrossRef]
- Viskupicova, J.; Majekova, M.; Horakova, L. Inhibition of the Sarco/Endoplasmic Reticulum Ca2+-ATPase (SERCA1) by Rutin Derivatives. J. Muscle Res. Cell Motil. 2015, 36, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Blaškovič, D.; Zižková, P.; Držík, F.; Viskupičová, J.; Veverka, M.; Horáková, L. Modulation of Rabbit Muscle Sarcoplasmic Reticulum Ca(2+)-ATPase Activity by Novel Quercetin Derivatives. Interdiscip. Toxicol. 2013, 6, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Blaskovic, D.; Drzik, F.; Viskupicova, J.; Zizkova, P.; Veverka, M.; Horakova, L. Effects of Novel Quercetin Derivatives on Sarco/Endoplasmic Reticulum Ca2+-ATPase Activity. Neuro Endocrinol. Lett. 2012, 33, 190–197. [Google Scholar]
- Heger, V.; Benesova, B.; Viskupicova, J.; Majekova, M.; Zoofishan, Z.; Hunyadi, A.; Horakova, L. Phenolic Compounds from Morus Nigra Regulate Viability and Apoptosis of Pancreatic β-Cells Possibly via SERCA Activity. ACS Med. Chem. Lett. 2020, 11, 1006–1013. [Google Scholar] [CrossRef]
- García-Casas, P.; Arias-del-Val, J.; Alvarez-Illera, P.; Fonteriz, R.I.; Montero, M.; Alvarez, J. Inhibition of Sarco-Endoplasmic Reticulum Ca2+ ATPase Extends the Lifespan in C. Elegans Worms. Front. Pharmacol. 2018, 9, 669. [Google Scholar] [CrossRef] [Green Version]
- Tabeshpour, J.; Banaeeyeh, S.; Eisvand, F.; Sathyapalan, T.; Hashemzaei, M.; Sahebkar, A. Effects of Curcumin on Ion Channels and Pumps: A Review. IUBMB Life 2019, 71, 812–820. [Google Scholar] [CrossRef]
- Horáková, L. Flavonoids in Prevention of Diseases with Respect to Modulation of Ca-Pump Function. Interdiscip. Toxicol. 2011, 4, 114–124. [Google Scholar] [CrossRef] [Green Version]
- Gruber, S.J.; Cornea, R.L.; Li, J.; Peterson, K.C.; Schaaf, T.M.; Gillispie, G.D.; Dahl, R.; Zsebo, K.M.; Robia, S.L.; Thomas, D.D. Discovery of Enzyme Modulators via High-Throughput Time-Resolved FRET in Living Cells. J. Biomol. Screen. 2014, 19, 215–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cornea, R.L.; Gruber, S.J.; Lockamy, E.L.; Muretta, J.M.; Jin, D.; Chen, J.; Dahl, R.; Bartfai, T.; Zsebo, K.M.; Gillispie, G.D.; et al. High-Throughput FRET Assay Yields Allosteric SERCA Activators. J. Biomol. Screen. 2013, 18, 97–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Britzolaki, A.; Cronin, C.C.; Flaherty, P.R.; Rufo, R.L.; Pitychoutis, P.M. Chronic but Not Acute Pharmacological Activation of SERCA Induces Behavioral and Neurochemical Effects in Male and Female Mice. Behav. Brain Res. 2021, 399, 1–10. [Google Scholar] [CrossRef]
- Nogami, K.; Maruyama, Y.; Sakai-Takemura, F.; Motohashi, N.; Elhussieny, A.; Imamura, M.; Miyashita, S.; Ogawa, M.; Noguchi, S.; Tamura, Y.; et al. Pharmacological Activation of SERCA Ameliorates Dystrophic Phenotypes in Dystrophin-Deficient Mdx Mice. Hum. Mol. Genet. 2021, 30, 1006–1019. [Google Scholar] [CrossRef]
- Kang, S.; Dahl, R.; Hsieh, W.; Shin, A.; Zsebo, K.M.; Buettner, C.; Hajjar, R.J.; Lebeche, D. Small Molecular Allosteric Activator of the Sarco/Endoplasmic Reticulum Ca2+-ATPase (SERCA) Attenuates Diabetes and Metabolic Disorders. J. Biol. Chem. 2016, 291, 5185–5198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tadini-Buoninsegni, F.; Smeazzetto, S.; Gualdani, R.; Moncelli, M.R. Drug Interactions with the Ca2+-ATPase from Sarco(Endo)Plasmic Reticulum (SERCA). Front. Mol. Biosci. 2018, 5, 36. [Google Scholar] [CrossRef]
- Zhao, F.; Fu, L.; Yang, W.; Dong, Y.; Yang, J.; Sun, S.; Hou, Y. Cardioprotective Effects of Baicalein on Heart Failure via Modulation of Ca2 + Handling Proteins in Vivo and in Vitro. Life Sci. 2016, 145, 213–223. [Google Scholar] [CrossRef]
- Kumar, M.; Kasala, E.R.; Bodduluru, L.N.; Dahiya, V.; Lahkar, M. Baicalein Protects Isoproterenol Induced Myocardial Ischemic Injury in Male Wistar Rats by Mitigating Oxidative Stress and Inflammation. Inflamm. Res. 2016, 65, 613–622. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, L.; Gao, P.; Liu, D.; Zhu, Z. Caffeic Acid Ameliorates Angiotensin II-Induced Increase In Blood Pressure By Activating Vascular Sarco-/Endoplasmic Reticulum Ca-ATPase2a-MedSci.Cn. J. Hypertens. 2021, 39, E248–E249. [Google Scholar] [CrossRef]
- Namekata, I.; Hamaguchi, S.; Wakasugi, Y.; Ohhara, M.; Hirota, Y.; Tanaka, H. Ellagic Acid and Gingerol, Activators of the Sarco-Endoplasmic Reticulum Ca2+-ATPase, Ameliorate Diabetes Mellitus-Induced Diastolic Dysfunction in Isolated Murine Ventricular Myocardia. Eur. J. Pharmacol. 2013, 706, 48–55. [Google Scholar] [CrossRef]
- Coll, K.E.; Johnson, R.G.; McKenna, E. Relationship between Phospholamban and Nucleotide Activation of Cardiac Sarcoplasmic Reticulum Ca2+ Adenosinetriphosphatase. Biochemistry 1999, 38, 2444–2451. [Google Scholar] [CrossRef]
- Antipenko, A.Y.; Spielman, A.I.; Kirchberger, M. Interactions of 6-Gingerol and Ellagic Acid with the Cardiac Sarcoplasmic Reticulum Ca2+-ATPase. J. Pharmacol. Exp. Ther. 1999, 290, 227–234. [Google Scholar] [PubMed]
- Feng, W.; Cherednichenko, G.; Ward, C.W.; Padilla, I.T.; Cabrales, E.; Lopez, J.R.; Eltit, J.M.; Allen, P.D.; Pessah, I.N. Green Tea Catechins Are Potent Sensitizers of Ryanodine Receptor Type 1 (RyR1). Biochem. Pharmacol. 2010, 80, 512–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kargacin, M.E.; Emmett, T.L.; Kargacin, G.J. Epigallocatechin-3-Gallate Has Dual, Independent Effects on the Cardiac Sarcoplasmic Reticulum/Endoplasmic Reticulum Ca 2+ ATPase. J. Muscle Res. Cell Motil. 2011, 32, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Hwang, H.S.; Kryshtal, D.O.; Yang, T.; Padilla, I.T.; Tiwary, A.K.; Puschner, B.; Pessah, I.N.; Knollmann, B.C. Coordinated Regulation of Murine Cardiomyocyte Contractility by Nanomolar (-)-Epigallocatechin-3-Gallate, the Major Green Tea Catechin. Mol. Pharmacol. 2012, 82, 993–1000. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, M.; Shoji, N.; Ohizumi, Y. Gingerol, a Novel Cardiotonic Agent, Activates the Ca2+-Pumping ATPase in Skeletal and Cardiac Sarcoplasmic Reticulum. Biochim. Biophys. Acta Biomembr. 1987, 903, 96–102. [Google Scholar] [CrossRef]
- Zhang, C.; Bose, D.D.; Thomas, D.W. Paradoxical Effects of Sarco/Endoplasmic Reticulum Ca2+-ATPase (SERCA) Activator Gingerol on NG115-401L Neuronal Cells: Failure to Augment ER Ca2+ Uptake and Protect against ER Stress-Induced Cell Death. Eur. J. Pharmacol. 2015, 762, 165–173. [Google Scholar] [CrossRef]
- Du, Y.; Liu, P.; Xu, T.; Pan, D.; Zhu, H.; Zhai, N.; Zhang, Y.; Li, D. Luteolin Modulates SERCA2a Leading to Attenuation of Myocardial Ischemia/Reperfusion Injury via Sumoylation at Lysine 585 in Mice. Cell. Physiol. Biochem. 2018, 45, 883–898. [Google Scholar] [CrossRef] [Green Version]
- Hu, W.; Xu, T.; Wu, P.; Pan, D.; Chen, J.; Chen, J.; Zhang, B.; Zhu, H.; Li, D. Luteolin Improves Cardiac Dysfunction in Heart Failure Rats by Regulating Sarcoplasmic Reticulum Ca2+-ATPase 2a. Sci. Rep. 2017, 7, 41017. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Zhang, C.; Zhu, H.; Wang, S.; Zhou, Y.; Zhao, J.; Xia, Y.; Li, D. Luteolin Modulates SERCA2a via Sp1 Upregulation to Attenuate Myocardial Ischemia/Reperfusion Injury in Mice. Sci. Rep. 2020, 10, 15407. [Google Scholar] [CrossRef]
- Zhu, S.; Xu, T.; Luo, Y.; Zhang, Y.; Xuan, H.; Ma, Y.; Pan, D.; Li, D.; Zhu, H. Luteolin Enhances Sarcoplasmic Reticulum Ca2+-ATPase Activity through P38 MAPK Signaling Thus Improving Rat Cardiac Function after Ischemia/Reperfusion. Cell. Physiol. Biochem. 2017, 41, 999–1010. [Google Scholar] [CrossRef] [PubMed]
- Nai, C.; Xuan, H.; Zhang, Y.; Shen, M.; Xu, T.; Pan, D.; Zhang, C.; Zhang, Y.; Li, D. Luteolin Exerts Cardioprotective Effects through Improving Sarcoplasmic Reticulum Ca(2+)-ATPase Activity in Rats during Ischemia/Reperfusion In Vivo. Evid.-Based Complement. Alternat. Med. 2015, 2015, 685998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karunakaran, U.; Elumalai, S.; Moon, J.S.; Jeon, J.H.; Kim, N.D.; Park, K.G.; Won, K.C.; Leem, J.; Lee, I.K. Myricetin Protects against High Glucose-Induced β-Cell Apoptosis by Attenuating Endoplasmic Reticulum Stress via Inactivation of Cyclin-Dependent Kinase 5. Diabetes Metab. J. 2019, 43, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Yao, H.; Caito, S.; Hwang, J.W.; Arunachalam, G.; Rahman, I. Regulation of SIRT1 in Cellular Functions: Role of Polyphenols. Arch. Biochem. Biophys. 2010, 501, 79–90. [Google Scholar] [CrossRef] [Green Version]
- Sulaiman, M.; Matta, M.J.; Sunderesan, N.R.; Gupta, M.P.; Periasamy, M.; Gupta, M. Resveratrol, an Activator of SIRT1, Upregulates Sarcoplasmic Calcium ATPase and Improves Cardiac Function in Diabetic Cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H833–H843. [Google Scholar] [CrossRef] [Green Version]
- Dong, Q.; Wu, Z.; Li, X.; Yan, J.; Zhao, L.; Yang, C. Resveratrol ameliorates cardiac dysfunction induced by pressure overload in rats via structural protection and modulation of Ca2+ cycling proteins. J. Transl. Med. 2014, 12, 323. [Google Scholar] [CrossRef] [Green Version]
- Javidanpour, S.; Dianat, M.; Badavi, M.; Mard, S.A. The Cardioprotective Effect of Rosmarinic Acid on Acute Myocardial Infarction and Genes Involved in Ca2+ Homeostasis. Free Radic. Res. 2017, 51, 911–923. [Google Scholar] [CrossRef] [PubMed]
- Viskupicova, J.; Strosova, M.K.; Zizkova, P.; Majekova, M.; Horakova, L. Rutin Stimulates Sarcoplasmic Reticulum Ca2+-ATPase Activity (SERCA1) and Protects SERCA1 from Peroxynitrite Mediated Injury. Mol. Cell. Biochem. 2015, 402, 51–62. [Google Scholar] [CrossRef]
- Chiesi, M.; Schwaller, R. Reversal of Phospholamban-Induced Inhibition of Cardiac Sarcoplasmic Reticulum Ca2+-ATPase by Tannin. Biochem. Biophys. Res. Commun. 1994, 202, 1668–1673. [Google Scholar] [CrossRef]
- Singh, A.P.; Singh, R.; Verma, S.S.; Rai, V.; Kaschula, C.H.; Maiti, P.; Gupta, S.C. Health Benefits of Resveratrol: Evidence from Clinical Studies. Med. Res. Rev. 2019, 39, 1851–1891. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A Double-Edged Sword in Health Benefits. Biomedicine 2018, 6, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berman, A.Y.; Motechin, R.A.; Wiesenfeld, M.Y.; Holz, M.K. The Therapeutic Potential of Resveratrol: A Review of Clinical Trials. npj Precis. Oncol. 2017, 1, 35. [Google Scholar] [CrossRef] [Green Version]
- McCalley, A.E.; Kaja, S.; Payne, A.J.; Koulen, P. Resveratrol and Calcium Signaling: Molecular Mechanisms and Clinical Relevance. Molecules 2014, 19, 7327–7340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almeida, L.; Vaz-da-Silva, M.; Falcão, A.; Soares, E.; Costa, R.; Loureiro, A.I.; Fernandes-Lopes, C.; Rocha, J.F.; Nunes, T.; Wright, L.; et al. Pharmacokinetic and Safety Profile of Trans-Resveratrol in a Rising Multiple-Dose Study in Healthy Volunteers. Mol. Nutr. Food Res. 2009, 53, S7–S15. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.S.; Cantó, C. The Molecular Targets of Resveratrol. Biochim. Biophys. Acta Mol. Basis Dis. 2015, 1852, 1114–1123. [Google Scholar] [CrossRef] [Green Version]
- Campos-Toimil, M.; Elíes, J.; Álvarez, E.; Verde, I.; Orallo, F. Effects of Trans- and Cis-Resveratrol on Ca2+ Handling in A7r5 Vascular Myocytes. Eur. J. Pharmacol. 2007, 577, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Borra, M.T.; Smith, B.C.; Denu, J.M. Mechanism of Human SIRT1 Activation by Resveratrol. J. Biol. Chem. 2005, 280, 17187–17195. [Google Scholar] [CrossRef] [Green Version]
- Iside, C.; Scafuro, M.; Nebbioso, A.; Altucci, L. SIRT1 Activation by Natural Phytochemicals: An Overview. Front. Pharmacol. 2020, 11, 1225. [Google Scholar] [CrossRef]
- Öztürk, E.; Arslan, A.K.K.; Yerer, M.B.; Bishayee, A. Resveratrol and Diabetes: A Critical Review of Clinical Studies. Biomed. Pharmacother. 2017, 95, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Bartoli-Leonard, F.; Wilkinson, F.L.; Schiro, A.; Inglott, F.S.; Alexander, M.Y.; Weston, R. Suppression of SIRT1 in Diabetic Conditions Induces Osteogenic Differentiation of Human Vascular Smooth Muscle Cells via RUNX2 Signalling. Sci. Rep. 2019, 9, 878. [Google Scholar] [CrossRef]
- Bernal-Ramírez, J.; Silva-Platas, C.; Jerjes-Sánchez, C.; Ramos-González, M.R.; Vázquez-Garza, E.; Chapoy-Villanueva, H.; Ramírez-Rivera, A.; Zarain-Herzberg, Á.; Garciá, N.; Garciá-Rivas, G. Resveratrol Prevents Right Ventricle Dysfunction, Calcium Mishandling, and Energetic Failure via SIRT3 Stimulation in Pulmonary Arterial Hypertension. Oxid. Med. Cell. Longev. 2021, 2021, 9912434. [Google Scholar] [CrossRef] [PubMed]
- Bagul, P.K.; Katare, P.B.; Bugga, P.; Dinda, A.K.; Banerjee, S.K. SIRT-3 Modulation by Resveratrol Improves Mitochondrial Oxidative Phosphorylation in Diabetic Heart through Deacetylation of TFAM. Cells 2018, 7, 235. [Google Scholar] [CrossRef] [Green Version]
- Gueguen, N.; Desquiret-Dumas, V.; Leman, G.; Chupin, S.; Baron, S.; Nivet-Antoine, V.; Vessières, E.; Ayer, A.; Henrion, D.; Lenaers, G.; et al. Resveratrol Directly Binds to Mitochondrial Complex I and Increases Oxidative Stress in Brain Mitochondria of Aged Mice. PLoS ONE 2015, 10, e0144290. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.; Ramirez, V.D. Inhibition of Mitochondrial Proton F0F1-ATPase/ATP Synthase by Polyphenolic Phytochemicals. Br. J. Pharmacol. 2000, 130, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Madreiter-Sokolowski, C.T.; Sokolowski, A.A.; Graier, W.F. Dosis Facit Sanitatem—Concentration-Dependent Effects of Resveratrol on Mitochondria. Nutrients 2017, 9, 1117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desquiret-Dumas, V.; Gueguen, N.; Leman, G.; Baron, S.; Nivet-Antoine, V.; Chupin, S.; Chevrollier, A.; Vessières, E.; Ayer, A.; Ferré, M.; et al. Resveratrol Induces a Mitochondrial Complex I-Dependent Increase in NADH Oxidation Responsible for Sirtuin Activation in Liver Cells. J. Biol. Chem. 2013, 288, 36662–36675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lejri, I.; Agapouda, A.; Grimm, A.; Eckert, A. Mitochondria- And Oxidative Stress-Targeting Substances in Cognitive Decline-Related Disorders- And Molecular Mechanisms to Clinical Evidence. Oxid. Med. Cell. Longev. 2019, 2019, 9695412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luyten, T.; Welkenhuyzen, K.; Roest, G.; Kania, E.; Wang, L.; Bittremieux, M.; Yule, D.I.; Parys, J.B.; Bultynck, G. Resveratrol-Induced Autophagy Is Dependent on IP3Rs and on Cytosolic Ca2 +. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 947–956. [Google Scholar] [CrossRef]
- Lu, T.; Zhou, D.; Gao, P.; Si, L.; Xu, Q. Resveratrol Attenuates High Glucose-Induced Endothelial Cell Apoptosis via Mediation of Store-Operated Calcium Entry. Mol. Cell. Biochem. 2018, 442, 73–80. [Google Scholar] [CrossRef]
- Lu, J.; Yang, J.; Zheng, Y.; Fang, S.; Chen, X.; Lu, J.; Yang, J.; Zheng, Y.; Fang, S.; Chen, X. Resveratrol Reduces Store-Operated Ca2+ Entry and Enhances the Apoptosis of Fibroblast-like Synoviocytes in Adjuvant Arthritis Rats Model via Targeting ORAI1-STIM1 Complex. Biol. Res. 2019, 52, 45. [Google Scholar] [CrossRef]
- Chaudhari, S.; Ma, R. Store-Operated Calcium Entry and Diabetic Complications. Exp. Biol. Med. 2016, 241, 343–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madreiter-Sokolowski, C.T.; Gottschalk, B.; Parichatikanond, W.; Eroglu, E.; Klec, C.; Waldeck-Weiermair, M.; Malli, R.; Graier, W.F. Resveratrol Specifically Kills Cancer Cells by a Devastating Increase in the Ca2+ Coupling Between the Greatly Tethered Endoplasmic Reticulum and Mitochondria. Cell. Physiol. Biochem. 2016, 39, 1404–1420. [Google Scholar] [CrossRef] [PubMed]
- Kerkhofs, M.; Bittremieux, M.; Morciano, G.; Giorgi, C.; Pinton, P.; Parys, J.B.; Bultynck, G. Emerging Molecular Mechanisms in Chemotherapy: Ca2+ Signaling at the Mitochondria-Associated Endoplasmic Reticulum Membranes. Cell Death Dis. 2018, 9, 334. [Google Scholar] [CrossRef]
- Doghman-Bouguerra, M.; Lalli, E. ER-Mitochondria Interactions: Both Strength and Weakness within Cancer Cells. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 650–662. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo-Torres, E.; Hernández-Oliveras, A.; Meneses-Morales, I.; Rodríguez, G.; Fuentes-García, G.; Zarain-Herzberg, Á. Resveratrol Up-Regulates ATP2A3 Gene Expression in Breast Cancer Cell Lines through Epigenetic Mechanisms. Int. J. Biochem. Cell Biol. 2019, 113, 37–47. [Google Scholar] [CrossRef]
- Izquierdo-Torres, E.; Rodríguez, G.; Meneses-Morales, I.; Zarain-Herzberg, A. ATP2A3 gene as an important player for resveratrol anticancer activity in breast cancer cells. Mol. Carcinog. 2017, 56, 1703–1711. [Google Scholar] [CrossRef]
- Akter, R.; Rahman, M.H.; Kaushik, D.; Mittal, V.; Uivarosan, D.; Nechifor, A.C.; Behl, T.; Karthika, C.; Stoicescu, M.; Munteanu, M.A.; et al. Chemo-Preventive Action of Resveratrol: Suppression of P53-A Molecular Targeting Approach. Molecules 2021, 26, 5325. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.X.; Yao, X.J.; Xu, S.W.; Wong, V.K.W.; He, J.X.; Ding, J.; Xue, W.W.; Mujtaba, T.; Michelangeli, F.; Huang, M.; et al. (Z)3,4,5,4′-Trans-Tetramethoxystilbene, a New Analogue of Resveratrol, Inhibits Gefitinb-Resistant Non-Small Cell Lung Cancer via Selectively Elevating Intracellular Calcium Level. Sci. Rep. 2015, 5, 16348. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Zhang, C.; Yang, G.; Yang, Y. Biological Properties of 6-Gingerol: A Brief Review. Nat. Prod. Commun. 2014, 9, 1027–1030. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Zhao, R.; Wang, D.; Wang, L.; Zhang, Q.; Wei, S.; Lu, F.; Peng, W.; Wu, C. Ginger (Zingiber Officinale Rosc.) and Its Bioactive Components Are Potential Resources for Health Beneficial Agents. Phytother. Res. 2021, 35, 711–742. [Google Scholar] [CrossRef]
- Li, X.H.; McGrath, K.C.Y.; Tran, V.H.; Li, Y.M.; Duke, C.C.; Roufogalis, B.D.; Heather, A.K. Attenuation of Proinflammatory Responses by S -[6]-Gingerol via Inhibition of ROS/NF-Kappa B/COX2 Activation in HuH7 Cells. Evid.-Based Complement. Altern. Med. 2013, 2013, 146142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, T.Y.; Lee, K.C.; Chen, S.Y.; Chang, H.H. 6-Gingerol Inhibits ROS and INOS through the Suppression of PKC-Alpha and NF-KappaB Pathways in Lipopolysaccharide-Stimulated Mouse Macrophages. Biochem. Biophys. Res. Commun. 2009, 382, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Roudsari, N.M.; Lashgari, N.A.; Momtaz, S.; Roufogalis, B.; Abdolghaffari, A.H.; Sahebkar, A. Ginger: A Complementary Approach for Management of Cardiovascular Diseases. BioFactors 2021, 47, 933–951. [Google Scholar] [CrossRef] [PubMed]
- Arcusa, R.; Villaño, D.; Marhuenda, J.; Cano, M.; Cerdà, B.; Zafrilla, P. Potential Role of Ginger (Zingiber Officinale Roscoe) in the Prevention of Neurodegenerative Diseases. Front. Nutr. 2022, 9, 809621. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Ishida, Y.; Shoji, N.; Ohizumi, Y. Cardiotonic Action of [8]-Gingerol, an Activator of the Ca++-Pumping Adenosine Triphosphatase of Sarcoplasmic Reticulum, in Guinea Pig Atrial Muscle. J. Pharmacol. Exp. Ther. 1988, 246, 667–673. [Google Scholar] [PubMed]
- MacLennan, D.H.; Kranias, E.G. Phospholamban: A Crucial Regulator of Cardiac Contractility. Nat. Rev. Mol. Cell Biol. 2003, 4, 566–577. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.Q.; Guo, Z.; Liu, F.Y.; Hasan, S.G.; Yang, D.; Tang, N.; An, P.; Wang, M.Y.; Wu, H.M.; Yang, Z.; et al. 6-Gingerol Protects against Cardiac Remodeling by Inhibiting the P38 Mitogen-Activated Protein Kinase Pathway. Acta Pharmacol. Sin. 2021, 42, 1575–1586. [Google Scholar] [CrossRef]
- Yokota, T.; Wang, Y. P38 MAP Kinases in Heart. Gene 2016, 575, 369–376. [Google Scholar] [CrossRef] [Green Version]
- Schepici, G.; Contestabile, V.; Valeri, A.; Mazzon, E. Ginger, a Possible Candidate for the Treatment of Dementias? Molecules 2021, 26, 5700. [Google Scholar] [CrossRef]
- Cai, Z.X.; Tang, X.D.; Wang, F.Y.; Duan, Z.J.; Li, Y.C.; Qiu, J.J.; Guo, H.S. Effect of Gingerol on Colonic Motility via Inhibition of Calcium Channel Currents in Rats. World J. Gastroenterol. 2015, 21, 13466–13472. [Google Scholar] [CrossRef]
- Lee, G.H.; Peng, C.; Jeong, S.Y.; Park, S.A.; Lee, H.Y.; Hoang, T.H.; Kim, J.; Chae, H.J. Ginger Extract Controls MTOR-SREBP1-ER Stress-Mitochondria Dysfunction through AMPK Activation in Obesity Model. J. Funct. Foods 2021, 87, 1–9. [Google Scholar] [CrossRef]
- Ohizumi, Y.; Sasaki, S.; Shibusawa, K.; Ishikawa, K.; Ikemoto, F. Stimulation of Sarcoplasmic Reticulum Ca2+-ATPase by Gingerol Analogues. Biol. Pharm. Bull. 1996, 19, 1377–1379. [Google Scholar] [CrossRef] [Green Version]
- Berrebi-Bertrand, I.; Lahouratate, P.; Lahouratate, V.; Camelin, J.C.; Guibert, J.; Bril, A. Mechanism of Action of Sarcoplasmic Reticulum Calcium-Uptake Activators. Eur. J. Biochem. 1997, 247, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Kang, I.; Buckner, T.; Shay, N.F.; Gu, L.; Chung, S. Improvements in Metabolic Health with Consumption of Ellagic Acid and Subsequent Conversion into Urolithins: Evidence and Mechanisms. Adv. Nutr. 2016, 7, 961–972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.T.L.; Yao, Q.; Soares, T.A.; Squier, T.C.; Bigelow, D.J. Phospholamban Modulates the Functional Coupling between Nucleotide Domains in Ca-ATPase Oligomeric Complexes in Cardiac Sarcoplasmic Reticulum. Biochemistry 2009, 48, 2411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zarain-Herzberg, A.; García-Rivas, G.; Estrada-Avilés, R. Regulation of SERCA Pumps Expression in Diabetes. Cell Calcium 2014, 56, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.T.; Qin, X.; Kaul, S.; Barrientos, G.; Zou, Z.; Mathias, C.B.; Thomas, D.; Bose, D.D. The Polyphenol Ellagic Acid Exerts Anti-Inflammatory Actions via Disruption of Store-Operated Calcium Entry (SOCE) Pathway Activators and Coupling Mediators. Eur. J. Pharmacol. 2020, 875, 173036. [Google Scholar] [CrossRef]
- Hsu, S.S.; Chou, C.T.; Liao, W.C.; Shieh, P.; Kuo, D.H.; Kuo, C.C.; Jan, C.R.; Liang, W.Z. The Effect of Gallic Acid on Cytotoxicity, Ca2+ Homeostasis and ROS Production in DBTRG-05MG Human Glioblastoma Cells and CTX TNA2 Rat Astrocytes. Chem. Biol. Interact. 2016, 252, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Lazaro, M. Distribution and Biological Activities of the Flavonoid Luteolin. Mini Rev. Med. Chem. 2009, 9, 31–59. [Google Scholar] [CrossRef]
- Marniemi, J.; Alanen, E.; Impivaara, O.; Seppänen, R.; Hakala, P.; Rajala, T.; Rönnemaa, T. Dietary and Serum Vitamins and Minerals as Predictors of Myocardial Infarction and Stroke in Elderly Subjects. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 188–197. [Google Scholar] [CrossRef]
- Kho, C.; Lee, A.; Jeong, D.; Oh, J.G.; Chaanine, A.H.; Kizana, E.; Park, W.J.; Hajjar, R.J. SUMO1-Dependent Modulation of SERCA2a in Heart Failure. Nature 2011, 477, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Shang, P.; Li, D. Luteolin: A Flavonoid That Has Multiple Cardio-Protective Effects and Its Molecular Mechanisms. Front. Pharmacol. 2017, 8, 692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, R.J.; Spencer, J.P.; Rice-Evans, C. Flavonoids: Antioxidants or Signalling Molecules? Free Radic. Biol. Med. 2004, 36, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Hendrich, A.B. Flavonoid-Membrane Interactions: Possible Consequences for Biological Effects of Some Polyphenolic Compounds. Acta Pharmacol. Sin. 2006, 27, 27–40. [Google Scholar] [CrossRef] [Green Version]
- Song, X.; Tan, L.; Wang, M.; Ren, C.; Guo, C.; Yang, B.; Ren, Y.; Cao, Z.; Li, Y.; Pei, J. Myricetin: A Review of the Most Recent Research. Biomed. Pharmacother. 2021, 134, 111017. [Google Scholar] [CrossRef]
- Shimmyo, Y.; Kihara, T.; Akaike, A.; Niidome, T.; Sugimoto, H. Three Distinct Neuroprotective Functions of Myricetin against Glutamate-Induced Neuronal Cell Death: Involvement of Direct Inhibition of Caspase-3. J. Neurosci. Res. 2008, 86, 1836–1845. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.G.; Liu, T.W. Myricetin Facilitates Potassium Currents and Inhibits Neuronal Activity of PVN Neurons. Neurochem. Res. 2012, 37, 1450–1456. [Google Scholar] [CrossRef]
- Gan, C.; Liu, F.; Jiang, S.; Cao, S.; Dong, N. Effects of Five Flavonols on [Ca2+]i in Cardiomyocytes of Rats. Chin. J. Endem. 2007, 26, 624–626. [Google Scholar]
- Fusi, F.; Saponara, S.; Frosini, M.; Gorelli, B.; Sgaragli, G. L-Type Ca2+ Channels Activation and Contraction Elicited by Myricetin on Vascular Smooth Muscles. Naunyn Schmiedebergs Arch. Pharmacol. 2003, 368, 470–478. [Google Scholar] [CrossRef]
- Fusi, F.; Sgaragli, G.; Saponara, S. Mechanism of Myricetin Stimulation of Vascular L-Type Ca2+ Current. J. Pharmacol. Exp. Ther. 2005, 313, 790–797. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.H.; Ma, Z.G.; Rowlands, D.K.; Gou, Y.L.; Fok, K.L.; Wong, H.Y.; Yu, M.K.; Tsang, L.L.; Mu, L.; Chen, L.; et al. Flavonoid Myricetin Modulates GABAA Receptor Activity through Activation of Ca2+ Channels and CaMK-II Pathway. Evid.-Based Complement. Altern. Med. 2012, 2012, 758097. [Google Scholar] [CrossRef] [Green Version]
- Jiang, F. Cellular and Molecular Mechanisms of Polyphenol-Induced Beneficial Effects on Cardiac Remodeling. In Polyphenols: Prevention and Treatment of Human Disease, 2nd ed.; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: Cambridge, MA, USA, 2018; Volume 21, pp. 77–88. [Google Scholar] [CrossRef]
- Bao-An, C.; Ravich; Senthilkumar, R.; Rong, F.; Guo, Q. Long Cardioprotective Potential of Baicalein: A Short Review of In Vitro and In Vivo Studies. Pharm. Anal. Acta 2014, 5, 280. [Google Scholar] [CrossRef]
- Chandrashekar, N.; Pandi, A. Baicalein: A Review on Its Anti-Cancer Effects and Mechanisms in Lung Carcinoma. J. Food Biochem. 2022, e14230. [Google Scholar] [CrossRef]
- Deniz, F.S.Ş.; Eren, G.; Orhan, I.E. Flavonoids as Sirtuin Modulators. Curr. Top. Med. Chem. 2022; 22, in press. [Google Scholar] [CrossRef]
- Bauer, I.; Grozio, A.; Lasiglie, D.; Basile, G.; Sturla, L.; Magnone, M.; Sociali, G.; Soncini, D.; Caffa, I.; Poggi, A.; et al. The NAD+-Dependent Histone Deacetylase SIRT6 Promotes Cytokine Production and Migration in Pancreatic Cancer Cells by Regulating Ca2+ Responses. J. Biol. Chem. 2012, 287, 40924–40937. [Google Scholar] [CrossRef] [Green Version]
- Xiong, X.; Wang, G.; Tao, R.; Wu, P.; Kono, T.; Li, K.; Ding, W.X.; Tong, X.; Tersey, S.A.; Harris, R.A.; et al. Sirtuin 6 Regulates Glucose-Stimulated Insulin Secretion in Mouse Pancreatic Beta Cells. Diabetologia 2016, 59, 151–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandramowlishwaran, P.; Vijay, A.; Abraham, D.; Li, G.; Mwangi, S.M.; Srinivasan, S. Role of Sirtuins in Modulating Neurodegeneration of the Enteric Nervous System and Central Nervous System. Front. Neurosci. 2020, 14, 614331. [Google Scholar] [CrossRef]
- Akter, R.; Afrose, A.; Rahman, M.R.; Chowdhury, R.; Nirzhor, S.S.R.; Khan, R.I.; Kabir, M.T. A Comprehensive Analysis into the Therapeutic Application of Natural Products as Sirt6 Modulators in Alzheimer’s Disease, Aging, Cancer, Inflammation, and Diabetes. Int. J. Mol. Sci. 2021, 22, 4180. [Google Scholar] [CrossRef] [PubMed]
- Peredo-Escárcega, A.E.; Guarner-Lans, V.; Pérez-Torres, I.; Ortega-Ocampo, S.; Carreón-Torres, E.; Castrejón-Tellez, V.; Díaz-Díaz, E.; Rubio-Ruiz, M.E. The Combination of Resveratrol and Quercetin Attenuates Metabolic Syndrome in Rats by Modifying the Serum Fatty Acid Composition and by Upregulating SIRT 1 and SIRT 2 Expression in White Adipose Tissue. Evid.-Based Complement. Alternat. Med. 2015, 2015, 474032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.S.; Quon, M.J.; Kim, J.A. New Insights into the Mechanisms of Polyphenols beyond Antioxidant Properties; Lessons from the Green Tea Polyphenol, Epigallocatechin 3-Gallate. Redox Biol. 2014, 2, 187–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Al-Maghout, T.; Bissinger, R.; Zeng, N.; Pelzl, L.; Salker, M.S.; Cheng, A.; Singh, Y.; Lang, F. Epigallocatechin-3-Gallate (EGCG) up-Regulates MiR-15b Expression Thus Attenuating Store Operated Calcium Entry (SOCE) into Murine CD4+ T Cells and Human Leukaemic T Cell Lymphoblasts. Oncotarget 2017, 8, 89500–89514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awuah Boadi, E.; Shin, S.; Bandyopadhyay, B.C. Tannic Acid Attenuates Vascular Calcification-Induced Proximal Tubular Cells Damage through Paracrine Signaling. Biomed. Pharmacother. 2021, 140, 111762. [Google Scholar] [CrossRef]
- Yazawa, K.; Kihara, T.; Shen, H.; Shimmyo, Y.; Niidome, T.; Sugimoto, H. Distinct Mechanisms Underlie Distinct Polyphenol-Induced Neuroprotection. FEBS Lett. 2006, 580, 6623–6628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maiuolo, J.; Carresi, C.; Gliozzi, M.; Musolino, V.; Scarano, F.; Coppoletta, A.R.; Guarnieri, L.; Nucera, S.; Scicchitano, M.; Bosco, F.; et al. Effects of Bergamot Polyphenols on Mitochondrial Dysfunction and Sarcoplasmic Reticulum Stress in Diabetic Cardiomyopathy. Nutrients 2021, 13, 2476. [Google Scholar] [CrossRef] [PubMed]
- Kang, P.; Han, S.H.; Moon, H.K.; Lee, J.M.; Kim, H.K.; Min, S.S.; Seol, G.H. Citrus Bergamia Risso Elevates Intracellular Ca2+ in Human Vascular Endothelial Cells Due to Release of Ca2+ from Primary Intracellular Stores. Evid.-Based Complement. Altern. Med. 2013, 2013, 759615. [Google Scholar] [CrossRef] [Green Version]


| SERCA Isoform | Tissue Distribution | Disease/Complication | SERCA Activity/Expression | Reference |
|---|---|---|---|---|
| SERCA1a | Adult fast-twitch skeletal muscle | Brody’s disease | ↓/↓ | [12,36] |
| SERCA1b | Fetal fast-twitch skeletal muscle | Myotonic dystrophy type 1 | ↓/↑ | [12,37] |
| SERCA2a | Slow twitch skeletal muscle, cardiac muscle, smooth muscle cells | Heart failure Cardiac hypertrophy Diabetic cardiomyopathy Vascular complications Early type 2 diabetes | ↓/↓ -/↓ ↓/↓ ↓/↓ -/↑ | [12,13,38,39] |
| SERCA2b | All tissues (muscle and non-muscle cells) | Darier’s disease Type 1 and 2 diabetes Cancer Neurodegenerative diseases | ↓/↓ ↓/↓ -/↓ ↓/↓↑ | [12,36,40,41,42] |
| SERCA2c | Epithelial, mesenchymal, and hematopoietic cells; monocytes | Cardiomyopathy | -/↑ | [28,36,43] |
| SERCA2d | Skeletal muscle | Myotonic dystrophy type 1 | -/↓ | [37] |
| SERCA3a-f | Non-muscle tissues | Type 2 diabetes Type 1 diabetes Cardiomyopathy Cancer | -/↓ -/SERCA3b↑ -/SERCA3f↑ -/↑↓ | [39,41,43] |
| Compound (MW) | Structure | Mode of Action Related to SERCA | Study Model | Health Implications | Ref. |
|---|---|---|---|---|---|
| Baicalein; BAI (270.24) | 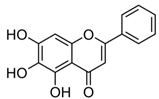 | Upregulation of SERCA2 and RyR2, downregulation of CAMKII | Rats, H9C2 myocardial cells | Cardioprotection, alleviation of heart failure | [79,80] |
| Caffeic acid; CA (180.16) |  | Activation of SERCA2a by direct binding | Wild-type mice | Improved vasoconstriction, lowered blood pressure | [81] |
| Ellagic acid; EA (302.197) | 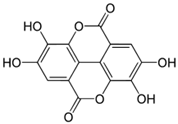 | Activation of SERCA2 via removing PLB’s inhibition of SERCA | Myocardium from diabetic mice, cardiac SR vesicles | Amelioration of diastolic dysfunction, mediating cardiac contractile responses | [82,83,84] |
| (-)-Epigallo- catechin-3-gallate; EGCG (458.37) | 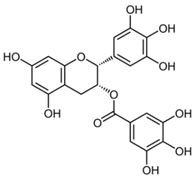 | Enhancing RyR1 and RyR2 activity, and affecting SERCA via the interaction with PLB | Skeletal myotubes/myofibers, murine myocytes, cardiac SR vesicles | Improved contractility and muscle function, positive inotropic effects | [85,86,87] |
| 6-Gingerol; GIN (294.38) | 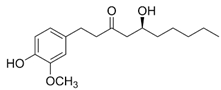 | Direct SERCA1 and SERCA2 activation | Myocardium from diabetic mice, cardiac and skeletal SR vesicles, NG115-401L neuronal cells | Amelioration of diastolic dysfunction, mediating cardiac contractile responses | [82,84,88,89] |
| Luteolin; LUT (286.24) | 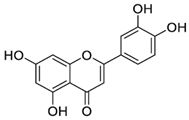 | Improvement of SERCA2a expression, activity and stability, partially via SUMO1 and Sp1; increasing SERCA2a activity via suppression of p38 MAPK and activation of PI3K/Akt pathways | Cardiac HL-1 cells, C57BL/6J mice, cardiomyocytes, intact heart, ischemia–reperfusion rat model | Attenuation of myocardial Ischemia–reperfusion injury, improved systolic/diastolic function, amelioration of myocardium fibrosis and heart failure | [90,91,92,93,94] |
| Myricetin; MYR (318.23) | 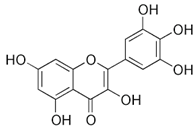 | Upregulation of SERCA2b expression, partially via PDX1 | INS-1 cells, isolated rat islets | Protection of beta cells from apoptosis, attenuation of type 2 DM | [95] |
| Resveratrol; RSV (228.24) |  | Upregulation of SERCA via SIRT1 activation | Mouse model of type 1 DM, Sprague–Dawley rats | Improvement of cardiac function in diabetes, prevention of cardiac hypertrophy | [96,97,98] |
| Rosmarinic acid; RA (360.32) | 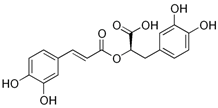 | Upregulation of the expression of SERCA2 and RyR2 | Sprague Dawley rats, isolated hearts | Cardioprotective effects against myocardial infarction and arrhythmia | [99] |
| Rutin; RUT (610.52) | 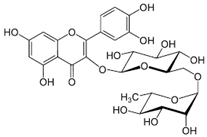 | Stimulation of SERCA1 activity by direct binding | Skeletal SR vesicles | Potential significance in cardiovascular and skeletal muscle diseases | [100] |
| Tannic acid; TA (1701.19) | 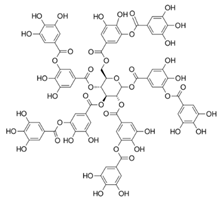 | Activation of SERCA2 through relieving the inhibitory effect of PLB on SERCA | Cardiac SR vesicles | Pharmacological intervention in impaired cardiac contractility and function | [83,101] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viskupicova, J.; Rezbarikova, P. Natural Polyphenols as SERCA Activators: Role in the Endoplasmic Reticulum Stress-Related Diseases. Molecules 2022, 27, 5095. https://doi.org/10.3390/molecules27165095
Viskupicova J, Rezbarikova P. Natural Polyphenols as SERCA Activators: Role in the Endoplasmic Reticulum Stress-Related Diseases. Molecules. 2022; 27(16):5095. https://doi.org/10.3390/molecules27165095
Chicago/Turabian StyleViskupicova, Jana, and Petronela Rezbarikova. 2022. "Natural Polyphenols as SERCA Activators: Role in the Endoplasmic Reticulum Stress-Related Diseases" Molecules 27, no. 16: 5095. https://doi.org/10.3390/molecules27165095






