Pressurized Liquid Extraction as a Novel Technique for the Isolation of Laurus nobilis L. Leaf Polyphenols
Abstract
:1. Introduction
2. Results and Discussion
2.1. Pressurized Liquid Extraction (PLE) Optimization
2.2. Polyphenolic Characterization
2.3. Antioxidant Activity
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Material
3.3. Pressurized Liquid Extraction (PLE)
3.4. Total Phenolic Content (TPC)
3.5. UPLC-MS/MS Conditions
3.6. Antioxidant Activity
3.6.1. Oxygen Radical Absorbance Capacity (ORAC) Assay
3.6.2. DPPH Radical Scavenging Assay
3.6.3. Ferric Reducing Antioxidant Power (FRAP) Assay
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Batool, S.; Khera, R.A.; Hanif, M.A.; Ayub, M.A. Bay Leaf. Med. Plant. South Asia 2020, 5, 63–74. [Google Scholar] [CrossRef]
- Dias, M.I.; Barros, L.; Dueñas, M.; Alves, R.C.; Oliveira, M.B.P.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Nutritional and antioxidant contributions of Laurus nobilis L. leaves: Would be more suitable a wild or a cultivated sample? Food Chem. 2014, 156, 339–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzio, E.A.; Li, N.; Bauer, D.; Mendonca, P.; Taka, E.; Darb, M.; Thomas, L.; Williams, H.; Soliman, K.F.A. Natural product HTP screening for antibacterial (E.coli 0157:H7) and anti-inflammatory agents in (LPS from E. coli O111:B4) activated macrophages and microglial cells; focus on sepsis. BMC Complement. Altern. Med. 2016, 16, 467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houicher, A.; Hechachna, H.; Teldji, H.; Ozogul, F. In Vitro Study of the Antifungal Activity of Essential Oils Obtained from Mentha spicata, Thymus vulgaris, and Laurus nobilis. Recent Pat. Food. Nutr. Agric. 2016, 8, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Alejo-Armijo, A.; Altarejos, J.; Salido, S. Phytochemicals and biological activities of laurel tree (Laurus nobilis). Nat. Prod. Commun. 2017, 12, 743–757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinha, A.F.; Guido, L.F.; Costa, A.S.G.; Alves, R.C.; Oliveira, M.B.P.P. Monomeric and oligomeric flavan-3-ols and antioxidant activity of leaves from different Laurus sp. Food Funct. 2015, 6, 1944–1949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sang, S.; Hou, Z.; Liebert, J.D.; Yang, C.S. Redox Properties of Tea Polyphenols and Related Biological Activities. Antioxid. Redox Signal. 2005, 7, 1704–1714. [Google Scholar] [CrossRef]
- Routray, W.; Orsat, V. Preparative extraction and separation of phenolic compounds. In Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes.; Ramawat, G.K., Mérillon, J.-M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 2013–2045. ISBN 9783642221439. [Google Scholar]
- Dobroslavić, E.; Repajić, M.; Dragović-Uzelac, V.; Elez Garofulić, I. Isolation of Laurus nobilis Leaf Polyphenols: A Review on Current Techniques and Future Perspectives. Foods 2022, 11, 235. [Google Scholar] [CrossRef]
- Mustafa, A.; Turner, C. Pressurized liquid extraction as a green approach in food and herbal plants extraction: A review. Anal. Chim. Acta 2011, 703, 8–18. [Google Scholar] [CrossRef]
- López-Fernández, O.; Domínguez, R.; Pateiro, M.; Munekata, P.E.S.; Rocchetti, G.; Lorenzo, J.M. Determination of Polyphenols Using Liquid Chromatography–Tandem Mass Spectrometry Technique (LC–MS/MS): A Review. Antioxidants 2020, 9, 479. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, I.G.; Apetrei, C. Analytical methods used in determining antioxidant activity: A review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L. Oxygen radical absorbance capacity (ORAC): New horizons in relating dietary antioxidants/bioactives and health benefits. J. Funct. Foods 2015, 18, 797–810. [Google Scholar] [CrossRef]
- Lu, M.; Yuan, B.; Zeng, M.; Chen, J. Antioxidant capacity and major phenolic compounds of spices commonly consumed in China. Food Res. Int. 2011, 44, 530–536. [Google Scholar] [CrossRef]
- Dudonné, S.; Vitrac, X.; Coutière, P.; Woillez, M.; Mérillon, J.-M. Comparative Study of Antioxidant Properties and Total Phenolic Content of 30 Plant Extracts of Industrial Interest Using DPPH, ABTS, FRAP, SOD, and ORAC Assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef] [PubMed]
- Muñiz-Márquez, D.B.; Rodríguez, R.; Balagurusamy, N.; Carrillo, M.L.; Belmares, R.; Contreras, J.C.; Nevárez, G.V.; Aguilar, C.N. Phenolic content and antioxidant capacity of extracts of Laurus nobilis L., Coriandrum sativum L. and Amaranthus hybridus L. CYTA-J. Food 2014, 12, 271–276. [Google Scholar] [CrossRef] [Green Version]
- Dobroslavić, E.; Elez Garofulić, I.; Zorić, Z.; Pedisić, S.; Dragović-Uzelac, V. Polyphenolic Characterization and Antioxidant Capacity of Laurus nobilis L. Leaf Extracts Obtained by Green and Conventional Extraction Techniques. Processes 2021, 9, 1840. [Google Scholar] [CrossRef]
- Leyva-Jiménez, F.J.; Lozano-Sánchez, J.; Borrás-Linares, I.; Arráez-Román, D.; Segura-Carretero, A. Comparative study of conventional and pressurized liquid extraction for recovering bioactive compounds from Lippia citriodora leaves. Food Res. Int. 2018, 109, 213–222. [Google Scholar] [CrossRef]
- Pollini, L.; Cossignani, L.; Juan, C.; Mañes, J. Extraction of phenolic compounds from fresh apple pomace by different non-conventional techniques. Molecules 2021, 26, 4272. [Google Scholar] [CrossRef]
- Li, J.; Zhang, S.; Zhang, M.; Sun, B. Novel approach for extraction of grape skin antioxidants by accelerated solvent extraction: Box–Behnken design optimization. J. Food Sci. Technol. 2019, 56, 4879–4890. [Google Scholar] [CrossRef]
- Díaz-De-Cerio, E.; Arráez-Román, D.; Segura-Carretero, A.; Ferranti, P.; Nicoletti, R.; Perrotta, G.M.; Gómez-Caravaca, A.M. Establishment of pressurized-liquid extraction by response surface methodology approach coupled to HPLC-DAD-TOF-MS for the determination of phenolic compounds of myrtle leaves. Anal. Bioanal. Chem. 2018, 410, 3547–3557. [Google Scholar] [CrossRef] [PubMed]
- Haminiuk, C.W.I.; Plata-Oviedo, M.S.V.; de Mattos, G.; Carpes, S.T.; Branco, I.G. Extraction and quantification of phenolic acids and flavonols from Eugenia pyriformis using different solvents. J. Food Sci. Technol. 2014, 51, 2862–2866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saldana, M.D.A.; Ekaette, I.; Ramirez, C.S.V.; dos Reis Coimbra, J.S.; Cardozo-Filho, L. Pressurized fluid extraction of phytochemicals from fruits, vegetables, cereals, and herbs. Fruit Veg. Phytochem. Chem. Hum. Health Second Ed. 2017, 1, 721–748. [Google Scholar] [CrossRef]
- Oancea, A.-M.; Onofrei, C.; Turturică, M.; Bahrim, G.; Râpeanu, G.; Stănciuc, N. The kinetics of thermal degradation of polyphenolic compounds from elderberry (Sambucus nigra L.) extract. Food Sci. Technol. Int. 2018, 24, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.C.; He, Y.; Deng, X.; Yang, G.L.; Li, W.; Liang, J.; Tang, Q.L. Response surface modeling and optimization of accelerated solvent extraction of four lignans from fructus schisandrae. Molecules 2012, 17, 3618–3629. [Google Scholar] [CrossRef] [Green Version]
- Repajić, M.; Cegledi, E.; Kruk, V.; Pedisić, S.; Çinar, F.; Kovačević, D.B.; Žutić, I.; Dragović-Uzelac, V. Accelerated solvent extraction as a green tool for the recovery of polyphenols and pigments fromwild nettle leaves. Processes 2020, 8, 803. [Google Scholar] [CrossRef]
- Repajić, M.; Ekić, S.; Kruk, V.; Dragović Uzelac, V. Effect of accelerated solvent extraction conditions on the isolation of bioactive compounds from fennel (Foeniculum vulgare Mill.) seeds. Hrvat. Časopis Za Prehramben. Tehnol. Biotehnol. I Nutr. 2021, 15, 102–106. [Google Scholar] [CrossRef]
- Ahmad, R.; Ahmad, N.; Aljamea, A.; Abuthayn, S.; Aqeel, M. Evaluation of solvent and temperature effect on green accelerated solvent extraction (ASE) and UHPLC quantification of phenolics in fresh olive fruit (Olea europaea). Food Chem. 2021, 342, 128248. [Google Scholar] [CrossRef]
- Wibisono, R.; Zhang, J.; Saleh, Z.; Stevenson, D.E.; Joyce, N.I. Optimisation of accelerated solvent extraction for screening of the health benefits of plant food materials. Health (Irvine. Calif) 2009, 1, 220–230. [Google Scholar] [CrossRef] [Green Version]
- Hirondart, M.; Rombaut, N.; Fabiano-Tixier, A.S.; Bily, A.; Chemat, F. Comparison between pressurized liquid extraction and conventional Soxhlet extraction for rosemary antioxidants, yield, composition, and environmental footprint. Foods 2020, 9, 584. [Google Scholar] [CrossRef]
- Sandei, L.; Vadalà, R. Accelerated solvent extraction applied to tomato polyphenols assessment. Acta Hortic. 2013, 971, 225–234. [Google Scholar] [CrossRef]
- Setyaningsih, W.; Saputro, I.E.; Palma, M.; Barroso, C.G. Stability of 40 phenolic compounds during ultrasound-assisted extractions (UAE). AIP Conf. Proc. 2016, 1755, 080009. [Google Scholar] [CrossRef] [Green Version]
- De Paepe, D.; Valkenborg, D.; Coudijzer, K.; Noten, B.; Servaes, K.; De Loose, M.; Voorspoels, S.; Diels, L.; Van Droogenbroeck, B. Thermal degradation of cloudy apple juice phenolic constituents. Food Chem. 2014, 162, 176–185. [Google Scholar] [CrossRef]
- Volf, I.; Ignat, I.; Neamtu, M.; Popa, V. Thermal stability, antioxidant activity, and photo-oxidation of natural polyphenols. Chem. Pap. 2014, 68, 121–129. [Google Scholar] [CrossRef]
- Hostetler, G.L.; Riedl, K.M.; Schwartz, S.J. Effects of food formulation and thermal processing on flavones in celery and chamomile. Food Chem. 2013, 141, 1406–1411. [Google Scholar] [CrossRef] [Green Version]
- Khanal, R.C.; Howard, L.R.; Prior, R.L. Effect of heating on the stability of grape and blueberry pomace procyanidins and total anthocyanins. Food Res. Int. 2010, 43, 1464–1469. [Google Scholar] [CrossRef]
- El-Hamidi, M.; Zaher, F.A. Comparison Between Some Common Clays as Adsorbents of Carotenoids, Chlorophyll and Phenolic Compounds from Vegetable Oils. Am. J. Food Technol. 2016, 11, 92–99. [Google Scholar] [CrossRef]
- Zheng, W.; Yang, S.Y. Antioxidant Activity and Phenolic Compounds in Selected Herbs. J. Agric. Food Chem. 2001, 49, 5165–5170. [Google Scholar] [CrossRef]
- Kratchanova, M.; Denev, P.; Ciz, M.; Lojek, A.; Mihailov, A. Evaluation of antioxidant activity of medicinal plants containing polyphenol compounds. Comparison of two extraction systems. Acta Biochim. Pol. 2010, 57, 229–234. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.J.; Kim, G.H. Inhibitory effects of Tunisian plants extracts on oxidative stress and lipid accumulation in HepG2 cells. Korean J. Food Preserv. 2021, 28, 403–415. [Google Scholar] [CrossRef]
- da Silva, E.M.; Rogez, H.; Larondelle, Y. Optimization of extraction of phenolics from Inga edulis leaves using response surface methodology. Sep. Purif. Technol. 2007, 55, 381–387. [Google Scholar] [CrossRef]
- Vallverdú-Queralt, A.; Regueiro, J.; Martínez-Huélamo, M.; Rinaldi Alvarenga, J.F.; Leal, L.N.; Lamuela-Raventos, R.M. A comprehensive study on the phenolic profile of widely used culinary herbs and spices: Rosemary, thyme, oregano, cinnamon, cumin and bay. Food Chem. 2014, 154, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, M.; Halvorsen, B.; Holte, K.; Bohn, S.; Dragland, S.; Sampson, L.; Wiley, C.; Senoo, H.; Umezono, Y.; Sanada, C.; et al. The total antioxidant content of more than 3100 foods, beverages, spices, herbs and supplements used worldwide. Eur. Food Res. Technol. 2011, 233, 3–376. [Google Scholar] [CrossRef] [PubMed]
- Shan, B.; Cai, Y.Z.; Sun, M.; Corke, H. Antioxidant Capacity of 26 Spice Extracts and Characterization of Their Phenolic Constituents. J. Agric. Food Chem. 2005, 53, 7749–7759. [Google Scholar] [CrossRef]
- Ou, B.; Huang, D.; Hampsch-Woodill, M.; Flanagan, J.A.; Deemer, E.K. Analysis of Antioxidant Activities of Common Vegetables Employing Oxygen Radical Absorbance Capacity (ORAC) and Ferric Reducing Antioxidant Power (FRAP) Assays: A Comparative Study. J. Agric. Food Chem. 2002, 50, 3122–3128. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis: Changes in Official Methods of Analysis Made at the Annual Meeting. Supplement; Rockville, M.D., Ed.; Scientific Research: Washington, DC, USA, 1990; Volume 15. [Google Scholar]
- Shortle, E.; O’Grady, M.N.; Gilroy, D.; Furey, A.; Quinn, N.; Kerry, J.P. Influence of extraction technique on the anti-oxidative potential of hawthorn (Crataegus monogyna) extracts in bovine muscle homogenates. Meat Sci. 2014, 98, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Elez Garofulić, I.; Zorić, Z.; Pedisić, S.; Brnčić, M.; Dragović-Uzelac, V. UPLC-MS 2 Profiling of Blackthorn Flower Polyphenols Isolated by Ultrasound-Assisted Extraction. J. Food Sci. 2018, 83, 2782–2789. [Google Scholar] [CrossRef] [PubMed]
- Elez Garofulić, I.; Kruk, V.; Martić, A.; Martić, I.; Zorić, Z.; Pedisić, S.; Dragović, S.; Dragović-Uzelac, V. Evaluation of Polyphenolic Profile and Antioxidant Activity of Pistacia lentiscus L. Leaves and Fruit Extract Obtained by Optimized Microwave-Assisted Extraction. Foods 2020, 9, 1556. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
| Extraction Parameters | TPC mg GAE g−1 | |||
|---|---|---|---|---|
| % EtOH | Temperature (°C) | Extraction Cycles | Static Extraction Time (min) | |
| 50 | 90 | 1 | 5 | 34.10 ± 2.02 |
| 50 | 90 | 1 | 10 | 33.82 ± 1.87 |
| 50 | 90 | 2 | 5 | 35.38 ± 1.77 |
| 50 | 90 | 2 | 10 | 36.46 ± 0.81 |
| 50 | 90 | 3 | 5 | 35.39 ± 0.71 |
| 50 | 90 | 3 | 10 | 36.46 ± 0.81 |
| 50 | 120 | 1 | 5 | 36.63 ± 1.31 |
| 50 | 120 | 1 | 10 | 44.03 ± 2.02 |
| 50 | 120 | 2 | 5 | 38.93 ± 1.46 |
| 50 | 120 | 2 | 10 | 40.78 ± 1.56 |
| 50 | 120 | 3 | 5 | 39.85 ± 0.50 |
| 50 | 120 | 3 | 10 | 44.60 ± 1.11 |
| 50 | 150 | 1 | 5 | 46.34 ± 1.21 |
| 50 | 150 | 1 | 10 | 44.46 ± 1.82 |
| 50 | 150 | 2 | 5 | 46.09 ± 1.82 |
| 50 | 150 | 2 | 10 | 47.99 ± 1.11 |
| 50 | 150 | 3 | 5 | 45.82 ± 1.67 |
| 50 | 150 | 3 | 10 | 49.30 ± 1.01 |
| 70 | 90 | 1 | 5 | 31.87 ± 1.51 |
| 70 | 90 | 1 | 10 | 32.41 ± 1.87 |
| 70 | 90 | 2 | 5 | 33.25 ± 0.61 |
| 70 | 90 | 2 | 10 | 34.76 ± 1.56 |
| 70 | 90 | 3 | 5 | 35.39 ± 1.51 |
| 70 | 90 | 3 | 10 | 36.24 ± 2.72 |
| 70 | 120 | 1 | 5 | 35.33 ± 2.27 |
| 70 | 120 | 1 | 10 | 36.42 ± 2.57 |
| 70 | 120 | 2 | 5 | 38.19 ± 0.55 |
| 70 | 120 | 2 | 10 | 40.44 ± 1.51 |
| 70 | 120 | 3 | 5 | 37.49 ± 1.97 |
| 70 | 120 | 3 | 10 | 40.12 ± 2.02 |
| 70 | 150 | 1 | 5 | 43.39 ± 0.86 |
| 70 | 150 | 1 | 10 | 42.98 ± 1.92 |
| 70 | 150 | 2 | 5 | 42.46 ± 1.56 |
| 70 | 150 | 2 | 10 | 47.06 ± 1.46 |
| 70 | 150 | 3 | 5 | 40.56 ± 0.55 |
| 70 | 150 | 3 | 10 | 39.73 ± 1.36 |
| N | Source of Variation | TPC (mg GAE g−1) |
|---|---|---|
| % EtOH | p < 0.05 † | |
| 36 | 50% w/w | 40.91 ± 0.87 b |
| 36 | 70% w/w | 38.23 ± 0.72 a |
| T | p < 0.01 † | |
| 24 | 90 °C | 34.63 ± 0.39 a |
| 24 | 120 °C | 39.40 ± 0.62 b |
| 24 | 150 °C | 44.68 ± 0.62 c |
| Extraction cycles | p = 0.37 ‡ | |
| 24 | 1 | 38.48 ± 1.10 a |
| 24 | 2 | 40.15 ± 1.01 a |
| 24 | 3 | 40.08 ± 0.91 a |
| Static extraction time | p = 0.14 ‡ | |
| 36 | 5 min | 38.69 ± 0.76 a |
| 36 | 10 min | 40.45 ± 0.86 a |
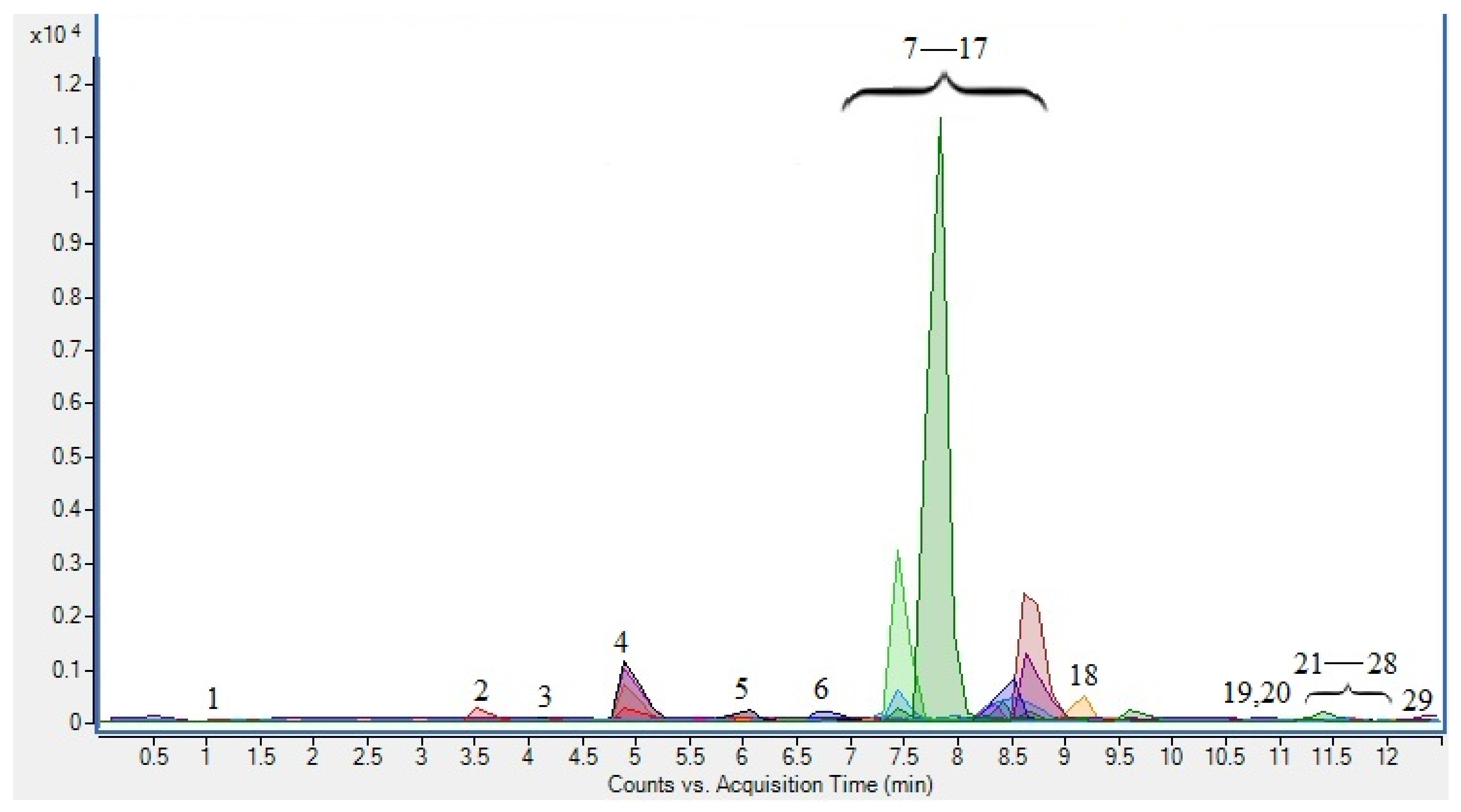
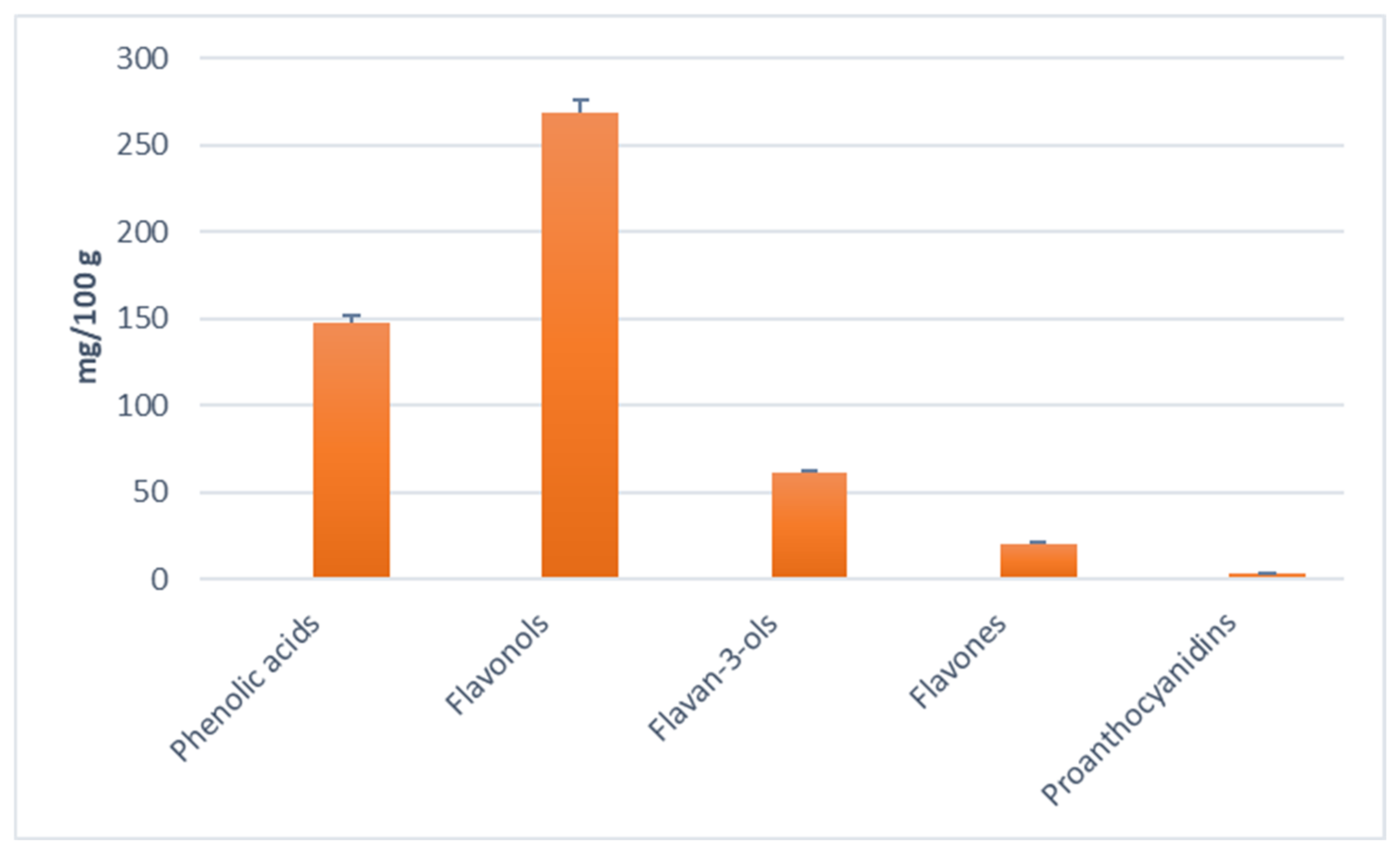
| Compound | Retention Time | Tentative Identification | Concentration mg/100 g |
|---|---|---|---|
| Phenolic acids | |||
| 1 | 1.008 | chlorogenic acid * | 0.46 ± 0.01 |
| 2 | 3.638 | protocatechuic acid * | 58.63 ± 1.66 |
| 3 | 4.259 | rosmarinic acid * | 0.99 ± 0.03 |
| 4 | 4.937 | p-coumaric acid * | 4.25 ± 0.12 |
| 5 | 5.961 | syringic acid * | 0.07 ± 0.00 |
| 8 | 7.917 | ferulic acid * | 1.01 ± 0.03 |
| 19 | 10.788 | caffeic acid * | 74.44 ± 2.11 |
| 20 | 10.802 | p-hydroxybenzoic acid | 2.83 ± 0.08 |
| 23 | 11.573 | gallic acid * | 0.28 ± 0.01 |
| 22 | 11.426 | 3.4-dihidrobenz-A-hexoside | 4.57 ± 0.13 |
| Flavones | |||
| 6 | 6.938 | luteolin-6-C-glucoside | 3.91 ± 0.11 |
| 13 | 8.678 | luteolin * | 7.15 ± 0.20 |
| 21 | 11.415 | apigenin * | 9.40 ± 0.27 |
| 27 | 11.998 | apigenin-6-C-(O-deoxyhexosyl)-hexoside | 0.27 ± 0.01 |
| Flavonols | |||
| 7 | 7.561 | rutin * | 97.31 ± 2.75 |
| 9 | 7.969 | quercetin-3-glucoside | 94.41 ± 2.67 |
| 10 | 8.349 | kaempferol-3-rutinoside | 6.00 ± 0.17 |
| 11 | 8.39 | quercetin-3-pentoside | 7.92 ± 0.22 |
| 12 | 8.64 | kaempferol-3-O-hexoside | 18.02 ± 0.51 |
| 14 | 8.747 | isorhamnetin-3-hexoside | 24.93 ± 0.71 |
| 15 | 8.791 | myricetin * | 2.25 ± 0.06 |
| 16 | 8.897 | quercetin-3-rhamnoside | 9.57 ± 0.27 |
| 18 | 9.178 | kaempferol-3-O-pentoside | 8.04 ± 0.23 |
| 29 | 12.299 | kaempferol-3-O-deoxyhexoside | 0.14 ± 0.00 |
| Flavan-3-ols | |||
| 17 | 9.014 | epigallocatechin gallate * | 0.15 ± 0.00 |
| 24 | 11.658 | catechin * | 31.35 ± 0.89 |
| 25 | 11.898 | epicatechin gallate * | 0.34 ± 0.01 |
| 28 | 12.055 | epicatechin | 29.20 ± 0.83 |
| Proanthocyanidins | |||
| 26 | 11.977 | procyanidin trimer | 3.89 ± 0.11 |
| Total phenols (mg 10−2 g−1) | - | - | 501.84 ± 2.27 |
| Assay | μmol TE g−1 |
|---|---|
| ORAC | 97.27 ± 2.01 |
| DPPH | 73.51 ± 0.22 |
| FRAP | 311.10 ± 5.67 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobroslavić, E.; Elez Garofulić, I.; Šeparović, J.; Zorić, Z.; Pedisić, S.; Dragović-Uzelac, V. Pressurized Liquid Extraction as a Novel Technique for the Isolation of Laurus nobilis L. Leaf Polyphenols. Molecules 2022, 27, 5099. https://doi.org/10.3390/molecules27165099
Dobroslavić E, Elez Garofulić I, Šeparović J, Zorić Z, Pedisić S, Dragović-Uzelac V. Pressurized Liquid Extraction as a Novel Technique for the Isolation of Laurus nobilis L. Leaf Polyphenols. Molecules. 2022; 27(16):5099. https://doi.org/10.3390/molecules27165099
Chicago/Turabian StyleDobroslavić, Erika, Ivona Elez Garofulić, Jelena Šeparović, Zoran Zorić, Sandra Pedisić, and Verica Dragović-Uzelac. 2022. "Pressurized Liquid Extraction as a Novel Technique for the Isolation of Laurus nobilis L. Leaf Polyphenols" Molecules 27, no. 16: 5099. https://doi.org/10.3390/molecules27165099
APA StyleDobroslavić, E., Elez Garofulić, I., Šeparović, J., Zorić, Z., Pedisić, S., & Dragović-Uzelac, V. (2022). Pressurized Liquid Extraction as a Novel Technique for the Isolation of Laurus nobilis L. Leaf Polyphenols. Molecules, 27(16), 5099. https://doi.org/10.3390/molecules27165099










