Polyelectrolyte Precipitation: A New Green Chemistry Approach to Recover Value-Added Proteins from Different Sources in a Circular Economy Context
Abstract
:1. Introduction
2. Polyelectrolyte Definition and Characterization
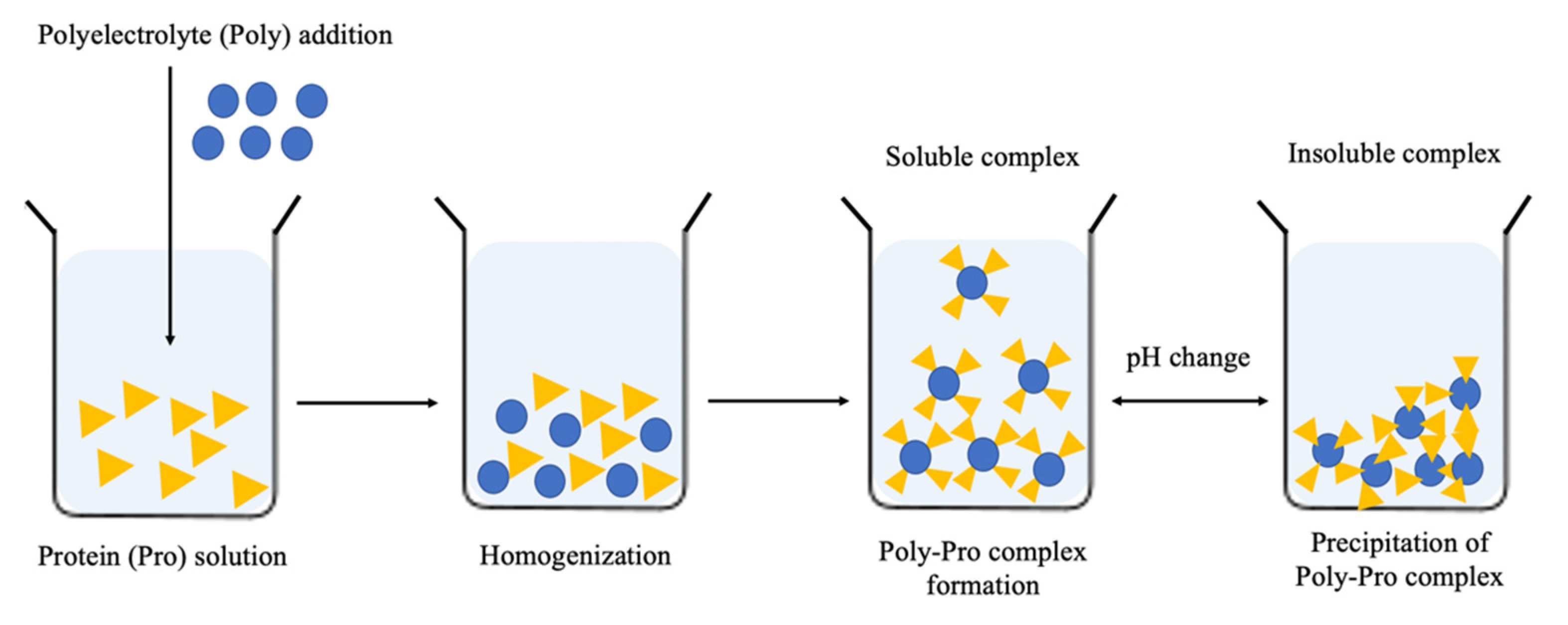
3. Polyelectrolyte Complex
4. Perspectives on Sustainable Protein Sources
5. Polyelectrolyte Precipitation vs. Traditional Extractive Methods
6. Innovation on Protein Recovery by Polyelectrolyte Precipitation—Case Studies
7. Future Perspectives on Proteins Recovery toward Circular Economy
8. Conclusions and Final Remarks
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Gianico, A.; Gallipoli, A.; Gazzola, G.; Pastore, C.; Tonanzi, B.; Braguglia, C.M. A Novel Cascade Biorefinery Approach to Transform Food Waste into Valuable Chemicals and Biogas through Thermal Pretreatment Integration. Bioresour. Technol. 2021, 338, 125517. [Google Scholar] [CrossRef] [PubMed]
- Tsegaye, B.; Jaiswal, S.; Jaiswal, A.K. Food Waste Biorefinery: Pathway towards Circular Bioeconomy. Foods 2021, 10, 1174. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Cabello, M.; Garcia, I.L.; Leiva-Candia, D.; Dorado, M.P. Valorization of Food Waste Based on Its Composition through the Concept of Biorefinery. Curr. Opin. Green Sustain. Chem. 2018, 14, 67–79. [Google Scholar] [CrossRef]
- Mak, T.M.W.; Xiong, X.; Tsang, D.C.W.; Yu, I.K.M.; Poon, C.S. Sustainable Food Waste Management towards Circular Bioeconomy: Policy Review, Limitations and Opportunities. Bioresour. Technol. 2020, 297, 122497. [Google Scholar] [CrossRef]
- Gómez-García, R.; Campos, D.A.; Aguilar, C.N.; Madureira, A.R.; Pintado, M. Valorisation of Food Agro-Industrial by-products: From the Past to the Present and Perspectives. J. Environ. Manag. 2021, 299, 113571. [Google Scholar] [CrossRef]
- Zuin, V.G.; Ramin, L.Z. Green and Sustainable Separation of Natural Products from Agro-Industrial Waste: Challenges, Potentialities, and Perspectives on Emerging Approaches. Top. Curr. Chem. 2018, 376, 3. [Google Scholar] [CrossRef]
- Woitovich Valetti, N.; Picó, G. A Friendly Method for Raphanus sativus L (Wild Radish) Peroxidase Purification by Polyelectrolyte Precipitation. Sep. Purif. Technol. 2013, 119, 1–6. [Google Scholar] [CrossRef]
- Martins, M.; Soares, B.P.; Santos, J.H.P.M.; Bharmoria, P.; Torres Acosta, M.A.; Dias, A.C.R.V.; Coutinho, J.A.P.; Ventura, S.P.M. Sustainable Strategy Based on Induced Precipitation for the Purification of Phycobiliproteins. ACS Sustain. Chem. Eng. 2021, 9, 3942–3954. [Google Scholar] [CrossRef]
- Woitovich Valetti, N.; Brassesco, M.E.; Picó, G.A. Polyelectrolytes–Protein Complexes: A Viable Platform in the Downstream Processes of Industrial Enzymes at Scaling up Level. J. Chem. Technol. Biotechnol. 2016, 91, 2921–2928. [Google Scholar] [CrossRef]
- Campos, D.A.; Coscueta, E.R.; Pintado, M.M. Green Precipitation with Polysaccharide as a Tool for Enzyme Recovery. In Green Chemistry and Applications; Taylor & Francis: Abingdon-on-Thames, UK, 2020; pp. 276–288. [Google Scholar] [CrossRef]
- Sun, X.; Xiao, J.X.; Huang, G.Q. Recovery of Lysozyme from Aqueous Solution by Polyelectrolyte Precipitation with Sodium Alginate. Food Hydrocoll. 2019, 90, 225–231. [Google Scholar] [CrossRef]
- Rutkaite, R.; Bendoraitiene, J.; Klimaviciute, R.; Zemaitaitis, A. Cationic starch nanoparticles based on polyelectrolyte complexes. Int. J. Biol. Macromol. 2012, 50, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Stuart, M.C.; de Vries, R.; Lyklema, H. Polyelectrolytes. In Soft Colloids; Academic Press: Cambridge, MA, USA, 2005; Volume 5, pp. 2.1–2.84. ISBN 1874-5679. [Google Scholar]
- Meka, V.S.; Sing, M.K.G.; Pichika, M.R.; Nali, S.R.; Kolapalli, V.R.M.; Kesharwani, P. A Comprehensive Review on Polyelectrolyte Complexes. Drug Discov. Today 2017, 22, 1697–1706. [Google Scholar] [CrossRef]
- Maciel, J.V.; Durigon, A.M.M.; Souza, M.M.; Quadrado, R.F.N.; Fajardo, A.R.; Dias, D. Polysaccharides Derived from Natural Sources Applied to the Development of Chemically Modified Electrodes for Environmental Applications: A Review. Trends Environ. Anal. Chem. 2019, 22, e00062. [Google Scholar] [CrossRef]
- Rinaudo, M. Polyelectrolytes Derived from Natural Polysaccharides. In Monomers, Polymers and Composites from Renewable Resources; Elsevier: Amsterdam, The Netherlands, 2008; pp. 495–516. [Google Scholar]
- Campos, D.A.; Valetti, N.W.; Oliveira, A.; Pastrana-Castro, L.M.; Teixeira, J.A.; Pintado, M.M.; Picó, G. Platform Design for Extraction and Isolation of Bromelain: Complex Formation and Precipitation with Carrageenan. Process Biochem. 2017, 54, 156–161. [Google Scholar] [CrossRef]
- Bueno, V.B.; Petri, D.F.S. Xanthan Hydrogel Films: Molecular Conformation, Charge Density and Protein Carriers. Carbohydr. Polym. 2014, 101, 897–904. [Google Scholar] [CrossRef]
- Basha, S.K.; Muzammil, M.S.; Dhandayuthabani, R.; Kumari, V.S. Polysaccharides as Excipient in Drug Delivery System. Mater. Today Proc. 2021, 36, 280–289. [Google Scholar] [CrossRef]
- Dranca, F.; Oroian, M. Extraction, Purification and Characterization of Pectin from Alternative Sources with Potential Technological Applications. Food Res. Int. 2018, 113, 327–350. [Google Scholar] [CrossRef]
- Maurstad, G.; Mørch, Y.A.; Bausch, A.R.; Stokke, B.T. Polyelectrolyte Layer Interpenetration and Swelling of Alginate–Chitosan Multilayers Studied by Dual Wavelength Reflection Interference Contrast Microscopy. Carbohydr. Polym. 2008, 71, 672–681. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and Biomedical Applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Saji, S.; Hebden, A.; Goswami, P.; Du, C. A Brief Review on the Development of Alginate Extraction Process and Its Sustainability. Sustainability 2022, 14, 5181. [Google Scholar] [CrossRef]
- Kabir, S.F.; Rahman, A.; Yeasmin, F.; Sultana, S.; Masud, R.A.; Kanak, N.A.; Haque, P. Occurrence, Distribution, and Structure of Natural Polysaccharides. In Radiation-Processed Polysaccharides; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–27. [Google Scholar]
- Campo, V.L.; Kawano, D.F.; da Silva, D.B., Jr.; Carvalho, I. Carrageenans: Biological Properties, Chemical Modifications and Structural Analysis—A Review. Carbohydr. Polym. 2009, 77, 167–180. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, Q. Recent Development of Chitosan-Based Polyelectrolyte Complexes with Natural Polysaccharides for Drug Delivery. Int. J. Biol. Macromol. 2014, 64, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Kean, T.; Thanou, M. Biodegradation, Biodistribution and Toxicity of Chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Pakizeh, M.; Moradi, A.; Ghassemi, T. Chemical Extraction and Modification of Chitin and Chitosan from Shrimp Shells. Eur. Polym. J. 2021, 159, 110709. [Google Scholar] [CrossRef]
- Wang, S.; Chao, C.; Cai, J.; Niu, B.; Copeland, L.; Wang, S. Starch–lipid and starch–lipid–protein complexes: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1056–1079. [Google Scholar] [CrossRef]
- Liu, S.; Liu, S. An Overview of Biological Basics. In Bioprocess Engineering, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 21–80. [Google Scholar]
- Makroo, H.A.; Naqash, S.; Saxena, J.; Sharma, S.; Majid, D.; Dar, B.N. Recovery and Characteristics of Starches from Unconventional Sources and Their Potential Applications: A Review. Appl. Food Res. 2021, 1, 100001. [Google Scholar] [CrossRef]
- Dakhara, S.L.; Anajwala, C.C. Polyelectrolyte Complex: A Pharmaceutical Review. Syst. Rev. Pharm. 2010, 1, 121–127. [Google Scholar] [CrossRef]
- Côlho, D.F.; Saturnino, T.P.; Fernandes, F.F.; Mazzola, P.G.; Silveira, E.; Tambourgi, E.B. Azocasein Substrate for Determination of Proteolytic Activity: Reexamining a Traditional Method Using Bromelain Samples. Biomed. Res. Int. 2016, 2016, 8409183. [Google Scholar] [CrossRef]
- Rusu, A.V.; Penedo, B.A.; Engelhardt, S.; Schwarze, A.K. EXCORNSEED EU Project: Separation, Fractionation and Isolation of Biologically Active Natural Substances from Corn Oil and Other Side Streams to Be Used in Food, Specialty Chemicals and Cosmetic Markets. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Food Sci. Technol. 2020, 77, 101. [Google Scholar] [CrossRef]
- Mangiagalli, M.; Brocca, S.; Orlando, M.; Lotti, M. The “Cold Revolution”. Present and Future Applications of Cold-Active Enzymes and Ice-Binding Proteins. New Biotechnol. 2020, 55, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Martin-Rios, C.; Hofmann, A.; Mackenzie, N. Sustainability-Oriented Innovations in Food Waste Management Technology. Sustainability 2021, 13, 210. [Google Scholar] [CrossRef]
- de Carvalho, N.M.; Madureira, A.R.; Pintado, M.E. The Potential of Insects as Food Sources—A Review. Crit. Rev. Food Sci. Nutr. 2019, 60, 3642–3652. [Google Scholar] [CrossRef]
- Bhatia, L.; Bachheti, R.K.; Garlapati, V.K.; Chandel, A.K. Third-Generation Biorefineries: A Sustainable Platform for Food, Clean Energy, and Nutraceuticals Production. Biomass Convers. Biorefinery 2020. [Google Scholar] [CrossRef]
- Floret, C.; Monnet, A.F.; Micard, V.; Walrand, S.; Michon, C. Replacement of Animal Proteins in Food: How to Take Advantage of Nutritional and Gelling Properties of Alternative Protein Sources. Crit. Rev. Food Sci. Nutr. 2021, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Toalá, J.E.; Hernández-Mendoza, A.; González-Córdova, A.F.; Vallejo-Cordoba, B.; Liceaga, A.M. Potential Role of Natural Bioactive Peptides for Development of Cosmeceutical Skin Products. Peptides 2019, 122, 170170. [Google Scholar] [CrossRef]
- Boukid, F. Plant-Based Meat Analogues: From Niche to Mainstream. Eur. Food Res. Technol. 2021, 247, 297–308. [Google Scholar] [CrossRef]
- Boukid, F. Chickpea (Cicer Arietinum L.) Protein as a Prospective Plant-Based Ingredient: A Review. Int. J. Food Sci. Technol. 2021, 56, 5435–5444. [Google Scholar] [CrossRef]
- Trigo, J.P.; Alexandre, E.M.C.; Saraiva, J.A.; Pintado, M.E. High Value-Added Compounds from Fruit and Vegetable by-Products—Characterization, Bioactivities, and Application in the Development of Novel Food Products. Crit. Rev. Food Sci. Nutr. 2019, 60, 1388–1416. [Google Scholar] [CrossRef]
- Magalhães, V.S.M.; Ferreira, L.M.D.F.; Silva, C. Using a Methodological Approach to Model Causes of Food Loss and Waste in Fruit and Vegetable Supply Chains. J. Clean. Prod. 2021, 283, 124574. [Google Scholar] [CrossRef]
- Camilleri, M.A. Sustainable Production and Consumption of Food. Mise-En-Place Circular Economy Policies and Waste Management Practices in Tourism Cities. Sustainability 2021, 13, 9986. [Google Scholar] [CrossRef]
- Esparza, I.; Jiménez-Moreno, N.; Bimbela, F.; Ancín-Azpilicueta, C.; Gandía, L.M. Fruit and Vegetable Waste Management: Conventional and Emerging Approaches. J. Environ. Manag. 2020, 265, 110510. [Google Scholar] [CrossRef] [PubMed]
- Campos, D.A.; Gómez-García, R.; Vilas-Boas, A.A.; Madureira, A.R.; Pintado, M.M. Management of Fruit Industrial By-products—a Case Study on Circular Economy Approach. Molecules 2020, 25, 320. [Google Scholar] [CrossRef] [PubMed]
- Pascoalino, L.A.; Reis, F.S.; Prieto, M.A.; Barreira, J.C.M.; Ferreira, I.C.F.R.; Barros, L. Valorization of Bio-Residues from the Processing of Main Portuguese Fruit Crops: From Discarded Waste to Health Promoting Compounds. Molecules 2021, 26, 2624. [Google Scholar] [CrossRef] [PubMed]
- Gençdağ, E.; Görgüç, A.; Yılmaz, F.M. Recent Advances in the Recovery Techniques of Plant-Based Proteins from Agro-Industrial By-Products. Food Rev. Int. 2021, 37, 447–468. [Google Scholar] [CrossRef]
- Galanakis, C.M. Recovery of High Added-Value Components from Food Wastes: Conventional, Emerging Technologies and Commercialized Applications. Trends Food Sci. Technol. 2012, 26, 68–87. [Google Scholar] [CrossRef]
- Rajagopalan, A.; Sukumaran, B.O. Three Phase Partitioning to Concentrate Milk Clotting Proteases from Wrightia Tinctoria R. Br and Its Characterization. Int. J. Biol. Macromol. 2018, 118, 279–288. [Google Scholar] [CrossRef]
- Sánchez-Trasviña, C.; González-Valdez, J.; Mayolo-Deloisa, K.; Rito-Palomares, M. Impact of Aqueous Two-Phase System Design Parameters upon the in Situ Refolding and Recovery of Invertase. J. Chem. Technol. Biotechnol. 2015, 90, 1765–1772. [Google Scholar] [CrossRef]
- Zhang, T.; Guo, X.; Meng, H.; Tang, X.; Ai, C.; Chen, H.; Lin, J.; Yu, S. Effects of Bovine Serum Albumin on the Ethanol Precipitation of Sugar Beet Pulp Pectins. Food Hydrocoll. 2020, 105, 105813. [Google Scholar] [CrossRef]
- Wojtkowiak, K.; Jezierska, A.; Panek, J.J. Interactions between Artificial Channel Protein, Water Molecules, and Ions Based on Theoretical Approaches. Symmetry 2022, 14, 691. [Google Scholar] [CrossRef]
- Woitovich Valetti, N.; Lombardi, J.; Boeris, V.; Picó, G. Precipitation of Chymotrypsin from Fresh Bovine Pancreas Using ι-Carrageenan. Process Biochem. 2012, 47, 2570–2574. [Google Scholar] [CrossRef]
- Lombardi, J.; Valetti, N.W.; Picó, G.; Boeris, V. Obtainment of a Highly Concentrated Pancreatic Serine Proteases Extract from Bovine Pancreas by Precipitation with Polyacrylate. Sep. Purif. Technol. 2013, 116, 170–174. [Google Scholar] [CrossRef]
- Valetti, N.W.; Boeris, V.; Picó, G. Characterization of chymotrypsin-iota-carrageenan complex in aqueous solution: A solubility and thermodynamical stability study. Int. J. Biol. Macromol. 2013, 52, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Boggione, M.J.; Becher, R.; Farruggia, B. Single Method of Purification for Endoglucanase from Aspergillus Niger by Polyelectrolyte Precipitation. Biocatal. Agric. Biotechnol. 2016, 7, 118–126. [Google Scholar] [CrossRef]
- Podestá, M.V.; Morilla, E.A.; Allasia, M.B.; Woitovich Valetti, N.; Tubio, G.; Boggione, M.J. An Eco-Friendly Method of Purification for Xylanase from Aspergillus Niger by Polyelectrolyte Precipitation. J. Polym. Environ. 2019, 27, 2895–2905. [Google Scholar] [CrossRef]
- Campos, D.A.; Coscueta, E.R.; Valetti, N.W.; Pastrana-Castro, L.M.; Teixeira, J.A.; Picó, G.A.; Pintado, M. M. Optimization of Bromelain Isolation from Pineapple Byproducts by Polysaccharide Complex Formation. Food Hydrocoll. 2019, 87, 792–804. [Google Scholar] [CrossRef]
- Estevez Pintado, M.M. Method for Extraction and/or Isolation of Bromelain from Pineapple. European Patent EP3252156A1, 28 July 2021. [Google Scholar]
- Gómez-García, R.; Campos, D.A.; Aguilar, C.N.; Madureira, A.R.; Pintado, M. Biological Protein Precipitation: A Green Process for the Extraction of Cucumisin from Melon (Cucumis melo L. Inodorus) by-Products. Food Hydrocoll. 2021, 116, 106650. [Google Scholar] [CrossRef]
- Fabian, C.B.; Huynh, L.H.; Ju, Y.H. Precipitation of Rice Bran Protein Using Carrageenan and Alginate. LWT-Food Sci. Technol. 2010, 43, 375–379. [Google Scholar] [CrossRef]
- Montellano Duran, N.; Galante, M.; Spelzini, D.; Boeris, V. The Effect of Carrageenan on the Acid-Induced Aggregation and Gelation Conditions of Quinoa Proteins. Food Res. Int. 2018, 107, 683–690. [Google Scholar] [CrossRef]
- Torres-León, C.; Ramírez-Guzman, N.; Londoño-Hernandez, L.; Martinez-Medina, G.A.; Díaz-Herrera, R.; Navarro-Macias, V.; Alvarez-Pérez, O.B.; Picazo, B.; Villarreal-Vázquez, M.; Ascacio-Valdes, J.; et al. Food Waste and Byproducts: An Opportunity to Minimize Malnutrition and Hunger in Developing Countries. Front. Sustain. Food Syst. 2018, 2, 52. [Google Scholar] [CrossRef]
- Ding, Q.; Khattak, S.I.; Ahmad, M. Towards Sustainable Production and Consumption: Assessing the Impact of Energy Productivity and Eco-Innovation on Consumption-Based Carbon Dioxide Emissions (CCO2) in G-7 Nations. Sustain. Prod. Consum. 2021, 27, 254–268. [Google Scholar] [CrossRef]
- Tidåker, P.; Karlsson Potter, H.; Carlsson, G.; Röös, E. Towards Sustainable Consumption of Legumes: How Origin, Processing and Transport Affect the Environmental Impact of Pulses. Sustain. Prod. Consum. 2021, 27, 496–508. [Google Scholar] [CrossRef]


| Polyelectrolyte | Target Protein | Source | Conditions | Recovery Yield | Purification Factor | Reference * |
|---|---|---|---|---|---|---|
| Alginate and Carrageenan | Protein | Rice bran | Alginate-to-protein ratio of 1:1 and Carrageenan-to-protein of 2:1; pH 3.5 | 93% and 95% protein recovery, respectively | - | [63] |
| Carrageenan | Chymotrypsin | Bovine pancreas | 0.06%, w/v; pH 4.5 | 60% enzymatic activity | 3-folds | [55] |
| Carrageenan | Standard Chymotrypsin | Sigma Aldrich | 0.005% w/v; pH 4.5 | - | - | [57] |
| Eudragit L 100 and Eudragit S 100 | Peroxidase | Raphanus sativus L. | 0.002% w/v; pH 4 | 50% and 45% enzymatic activity | 2-folds | [7] |
| Polyacrylate | Serine proteases | Bovine pancreas | 0.05% w/v; pH 4.5 | 33% protein recovery | 5-folds | [56] |
| Poly-vinyl sulfonate and Chitosan | Endoglucanase | Solid-state fermentation by Aspergillus niger | 1% w/w and 0.05% w/v, respectively; pH 5.3 | 40% enzymatic activity | 9- and 7-folds | [58] |
| Carrageenan | Standard Bromelain | Sigma Aldrich | 0.08% w/v; pH 5.1 | 85–90% enzymatic activity | - | [17] |
| Carrageenan | Protein | Chenopodium quinoa | 0.002–0.005% w/v; pH 2.9 | - | - | [64] |
| Chitosan and Carrageenan | Xylanase | Solid-state fermentation by Aspergillus niger | 0.05% w/v; pH 8.00 and 0.5% w/v; pH 7, respectively | 40% and 30% enzymatic activity | 6- and 9-folds | [59] |
| Sodium alginate | Lysozyme | Egg white | sodium alginate-to-protein ratio of 2:1 pH 3 | 97% protein recovery | - | [11] |
| Carrageenan | Bromelain | Ananas comosus Merr. peels and stem | 0.2–0.3% w/v; pH 5.1 | 80–90% enzymatic activity | - | [60] |
| Carrageenan | Cucumisin | Cucumis melo L. peels | 0.003% w/v; pH 3 | 90% proteolytic 60% milk-clotting activities. | 2- and 18-folds | [62] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-García, R.; Vilas-Boas, A.A.; Vilas-Boas, A.M.; Campos, D.A.; Pintado, M. Polyelectrolyte Precipitation: A New Green Chemistry Approach to Recover Value-Added Proteins from Different Sources in a Circular Economy Context. Molecules 2022, 27, 5115. https://doi.org/10.3390/molecules27165115
Gómez-García R, Vilas-Boas AA, Vilas-Boas AM, Campos DA, Pintado M. Polyelectrolyte Precipitation: A New Green Chemistry Approach to Recover Value-Added Proteins from Different Sources in a Circular Economy Context. Molecules. 2022; 27(16):5115. https://doi.org/10.3390/molecules27165115
Chicago/Turabian StyleGómez-García, Ricardo, Ana A. Vilas-Boas, Ana Martins Vilas-Boas, Débora A. Campos, and Manuela Pintado. 2022. "Polyelectrolyte Precipitation: A New Green Chemistry Approach to Recover Value-Added Proteins from Different Sources in a Circular Economy Context" Molecules 27, no. 16: 5115. https://doi.org/10.3390/molecules27165115










