Fresh and Stored Sugar Beet Roots as a Source of Various Types of Mono- and Oligosaccharides
Abstract
1. Introduction
2. Results and Discussion
2.1. Free Carbohydrates (Mono- and Oligosaccharides) in Sugar Beet Roots
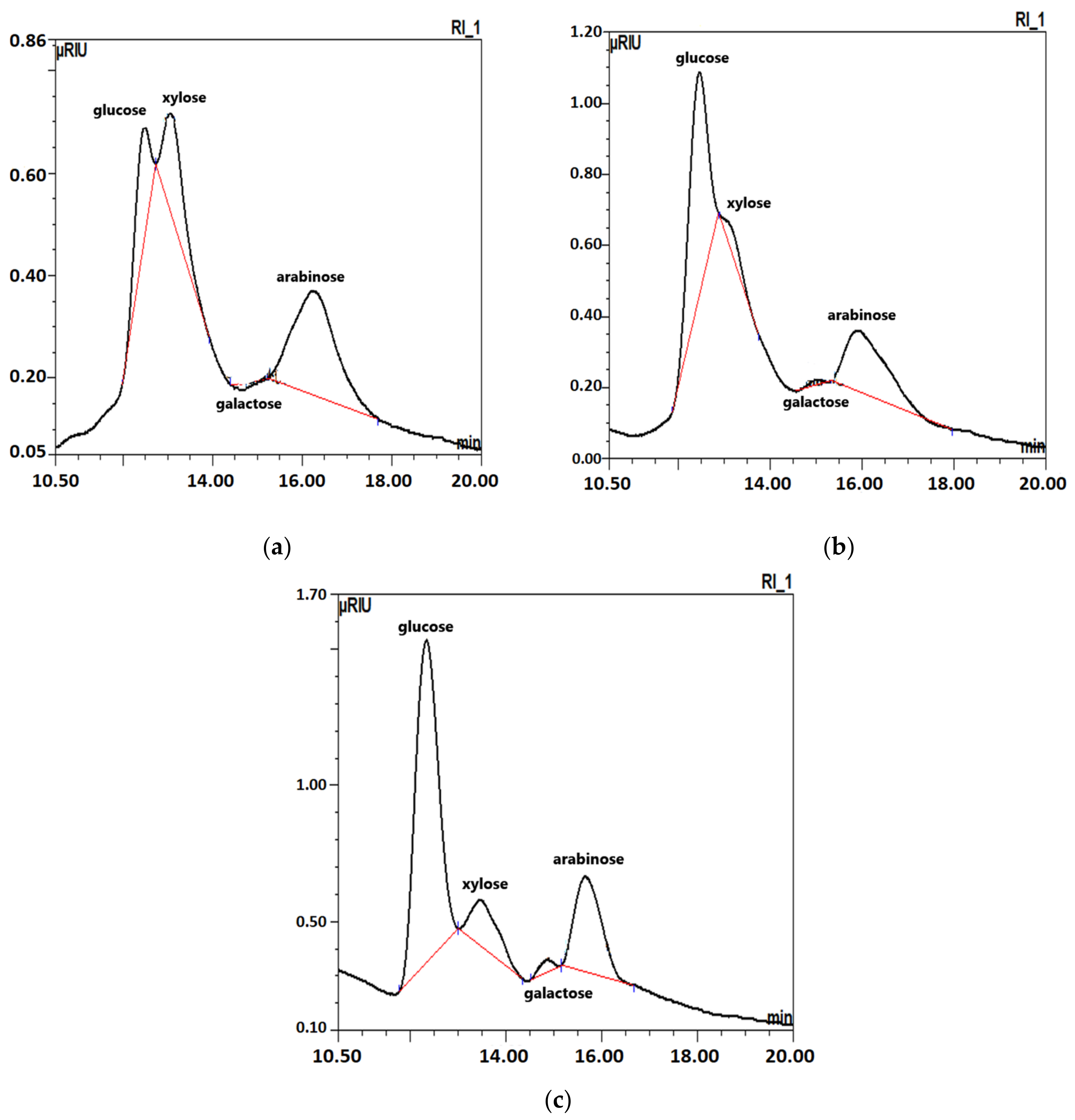
2.2. Carbohydrates after Hydrolysis of Complex Carbohydrates (Oligosaccharides) Found in Sugar Beet Roots
2.3. Study of the Molecular Mass Profile of Carbohydrate Compounds Present in Sugar Beet Roots
3. Materials and Methods
3.1. Sugar Beet Roots
- Fresh roots directly from the field;
- Roots stored in the mounds (beet clamps) for three months (from September to December). The hills were not covered;
- Roots stored in the mounds (beet clamps) for three months (from September to December). To prevent rain, the mounds were covered with an airy and waterproof fabric.
3.2. Determination of Free Carbohydrates in Sugar Beet Roots
3.3. Determination of Sugar Beet Roots Carbohydrates after Hydrolysis
3.4. Determination of the Molecular Mass of Carbohydrates
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Maier, K.; Baron, O.; Bruhns, J. Sugar Economy Europe; Verlag Dr. Albert Bartens: Berlin, Germany, 2014; pp. 50–53. [Google Scholar]
- Brar, N.S.; Dhillon, B.S.; Saini, K.S.; Sharma, P.K. Agronomy of sugar beet cultivation-A review. Agric. Rev. 2015, 36, 184–197. [Google Scholar] [CrossRef]
- Al-Jbawi, E.; Al Geddawi, S.; Gaidaa, A. Quality Changes in Sugar Beet (Beta vulgaris L.) Roots During Storage Period in Piles. Int. J. Environ. 2015, 4, 77–85. [Google Scholar] [CrossRef][Green Version]
- Van der Poel, P.W.; Schiweck, H.; Schwartz, T. Sugar Technology. Beet and Cane Sugar Manufacture; Dr. Albert Bartens KG: Berlin, Germany, 1998. [Google Scholar]
- McGrath, J.M.; Fugate, K.K. Chapter: Analysis of Sucrose from Sugar Beet. In Dietary Sugars: Chemistry, Analysis, Function and Effects; The Royal Society of Chemistry Publishing: London, UK, 2012; pp. 526–545. [Google Scholar] [CrossRef]
- Fasahat, P.; Aghaeezadeh, M.; Jabbari, L.; Hemayati, S.S.; Townson, P. Sucrose Accumulation in Sugar Beet: From Fodder Beet Selection to Genomic Selection. Sugar Tech 2018, 20, 635–644. [Google Scholar] [CrossRef]
- Hoffmann, C.M.; Kenter, C. Yield potential of Sugar beet—Have we hit the ceiling? Front. Plant Sci. 2018, 9, 289. [Google Scholar] [CrossRef]
- Micard, V.; Renard, C.M.G.C.; Thibault, J.F. Enzymatic saccharification of sugar-beet pulp. Enzym. Microb. Technol. 1996, 19, 162–170. [Google Scholar] [CrossRef]
- Bertin, C.; Rouau, X.; Thibault, J.-F. Structure and properties of sugar beet fibres. J. Sci. Food Agric. 1988, 44, 15–29. [Google Scholar] [CrossRef]
- Micard, V.; Renard, C.M.G.C.; Thibault, J.F. Studies on enzymic release of ferulic acid from sugar-beet pulp. LWT Food Sci. Technol. 1994, 27, 59–66. [Google Scholar] [CrossRef]
- Dinand, E.; Chanzy, H.; Vignon, R.M. Suspensions of cellulose microfibrils from sugar beet pulp. Food Hydrocolloid. 1999, 13, 275–283. [Google Scholar] [CrossRef]
- Baryga, A.; Połeć, B.; Sumińska, T.; Kowalska, M. Influence of macromolecular chemical compounds on the sugar production process. In IBPRS Sugar Plant; Report on Work BST-123; Institute of Agriculture and Food Biotechnology: Leszno, Poland, 2020. [Google Scholar]
- Baryga, A. Studies on the Technological Value of Sugar Beet and the Quality of Sugar in the Aspect of Use in the Cultivation of Digestate from Biogas Plants; Publisher UWM: Olsztyn, Poland, 2019. [Google Scholar]
- Vacari, G.; Mantovani, G.; Campi, A. Beet technological value under Mediterranean storage conditions. Food Chem. 1991, 40, 241–249. [Google Scholar] [CrossRef]
- Renard, C.M.G.C.; Thibault, J.-F. Structure and properties of apple and sugar-beet pectins extracted by chelating agents. Carbohyd. Res. 1993, 244, 99–114. [Google Scholar] [CrossRef]
- Osterveld, A. Pectic Substances from Sugar Beet Pulp: Structural Features, Enzymatic Modification and Gel Formation. Ph.D. Thesis, Wageningen University & Research, Wageningen, The Netherlands, 1997. [Google Scholar]
- Michel, F.; Thibault, J.-F.; Mercier, C.; Heitz, F. Extraction and characterization of pectins from sugar beet pulp. J. Food Sci. 1985, 50, 1499–1500. [Google Scholar] [CrossRef]
- Wen, L.F.; Chang, K.C.; Brown, G.; Gallaher, D.D. Isolation and characterization of hemicellulose and cellulose from sugar beet pulp. J. Food Sci. 1998, 53, 826–829. [Google Scholar] [CrossRef]
- Renard, C.M.C.G.; Jarvis, M.J. A Cross-Polarization, Magic-Angle Spinning, 13C-Nuclear-Magnetic-Resonance study of polysaccharides in sugar beet cell walls. Plant Physiol. 1999, 119, 1315–1322. [Google Scholar] [CrossRef]
- Kubik, C.; Galas, E.; Sikora, B. Determination of dextrans in raw juices by the Haze-enzymatic method. Int. Sugar J. 1994, 96, 376–426. [Google Scholar]
- Fares, K.; Renard, C.M.G.C.; R’zina, Q.; Thibault, J.-F. Extraction and composition of pectins and hemicelluloses of cell walls of sugar beet roots grown in Morocco. Int. J. Food Sci. Technol. 2001, 36, 35–46. [Google Scholar] [CrossRef]
- Benhamou, R.W.; Foster, D.P.; Ward, K.; Wheelhouse, L.; Sloan, L.; Tame, C.J.; Bučar, D.-K.; Lye, G.J.; Hailes, H.C.; Sheppard, T.D. Functionalised tetrahydrofuran fragments from carbohydrates or sugar beet pulp biomass. Green Chem. 2019, 21, 2035–2042. [Google Scholar] [CrossRef]
- Frieder, W.L.; Siegfried, P. Carbohydrates as green raw materials for the chemical industry. C. R. Chim. 2004, 7, 65–90. [Google Scholar] [CrossRef]
- Cárdenas-Fernández, M.; Bawn, M.; Hamley-Bennett, C.; Bharat, P.K.V.; Subrizi, F.; Suhaili, N.; Ward, D.P.; Bourdin, S.; Dalby, P.A.; Hailes, H.C.; et al. An integrated biorefinery concept for conversion of sugar beet pulp into value-added chemicals and pharmaceutical intermediates. Faraday Discuss. 2017, 202, 415–431. [Google Scholar] [CrossRef]
- Leijdekkers, A.G.M.; Bink, J.P.M.; Geutjes, S.; Schols, H.A.; Gruppen, H. Enzymatic saccharification of sugar beet pulp for the production of galacturonic acid and arabinose; a study on the impact of the formation of recalcitrant oligosaccharides. Bioresour. Technol. 2013, 128, 518–525. [Google Scholar] [CrossRef]
- Narisetty, V.; Narisetty, S.; Jacob, S.; Kumar, D.; Leeke, G.A.; Chandel, A.K.; Singh, V.; Srivastava, V.C.; Kumar, V. Biological production and recovery of 2,3-butanediol using arabinose from sugar beet pulp by Enterobacter ludwigii. Renew. Energy 2022, 191, 394–404. [Google Scholar] [CrossRef]
- Berlowska, J.; Binczarski, M.; Dziugan, P.; Wilkowska, A. Sugar beet pulp as a source of valuable biotechnological products. In Advances in Biotechnology for Food Industry; Academic Press: London, UK, 2018; pp. 359–392. [Google Scholar] [CrossRef]
- Alexandri, M.; Schneider, R.; Papapostolou, H.; Ladakis, D.; Koutinas, A.; Venus, J. Restructuring the conventional sugar beet industry into a novel biorefinery: Fractionation and bioconversion of sugar beet pulp into succinic acid and value-added coproducts. ACS Sustain. Chem. Eng. 2017, 7, 6569–6579. [Google Scholar] [CrossRef]
- Thibault, J.-F. Characterization and oxidative crosslinking of sugar beet pectins extracted from cossettes and pulps under different conditions. Carbohyd. Polym. 1988, 8, 209–223. [Google Scholar] [CrossRef]
- Rombouts, F.M.; Thibault, J.F. Feruloylated pectic substances from sugar beet pulp. Carbohyd. Res. 1986, 154, 177–187. [Google Scholar] [CrossRef]
- Fares, K.; Aissaoui, N.; Zaki, A.; R’Zina, Q.; Mauch, W. Evaluation des incidences du séjournement de la betterave sous climat méditerranéen. In Proceedings of the 20th General Assembly of Commission Internationale Technique de Sucrerie, Munich, Germany, 26–30 June 1995; pp. 170–184. [Google Scholar]
- Fares, K.; Catherine, M.G.C.; Crepeau, R.M.-J.; Thibault, J.-F. Characterization of hemiceluloses of sugar beet roots grown in Morocco. Int. J. Food Sci. Technol. 2004, 39, 303–309. [Google Scholar] [CrossRef]
- Hanau, S.; Almugadam, S.H.; Sapienza, E.; Cacciari, B.; Manfrinato, M.C.; Trentini, A.; Kennedy, J.F. Schematic overview of oligosaccharides, with survey on their major physiological effects and a focus on milk ones. Carbohydr. Polym. Technol. Appl. 2020, 1, 100013. [Google Scholar] [CrossRef]
- Meyer, M.; Melim, T.S.; Miguel, A.S.; Rodriguez Fernandez, D.E.; Dellamora Ortiz, G.M. Biotechnological production of oligosaccharides—Applications in the food industry. In Food Production and Industry, Intech Open Science/Open Minds; Eissa, A.A., Ed.; IntechOpen Limited: London, UK, 2015. [Google Scholar] [CrossRef]
- Lambertz, J.; Weiskirchen, S.; Landert, S.; Weiskirchen, R. Fructose: A dietary sugar in crosstalk with microbiota contributing to the development and progression of non-alcoholic liver disease. Front. Immunol. 2017, 8, 1159. [Google Scholar] [CrossRef]
- Campbell, J.M.; Bauer, L.L.; Fahey, G.C.; Hogarth, A.J.C.L.; Wolf, B.W.; Hunter, D.E. Selected Fructooligosaccharide (1-Kestose, Nystose, and 1F-β-Fructofuranosylnystose) Composition of Foods and Feeds. J. Agric. Food Chem. 1997, 45, 3076–3082. [Google Scholar] [CrossRef]
- Singh, R.S.; Singh, T.; Kennedy, J.F. Enzymatic synthesis of fructooligosaccharides from inulin in a batch system. Carbohydr. Polym. Technol. Appl. 2020, 1, 100009. [Google Scholar] [CrossRef]
- Picazo, B.; Flores-Gallegos, A.C.; Muñiz-Márquez, D.B.; Flores-Maltos, A.; Michel-Michel, M.R.; de la Rosa, O.; Rodríguez-Jasso, R.M.; Rodríguez-Herrera, R.; Aguilar-González, C.N. Enzymes for fructooligosaccharides production: Achievements and opportunities. In Enzymes in Food Biotechnology; Academic Press: London, UK, 2019; Volume 18, pp. 303–320. [Google Scholar]
- Babaei, B.; Abdollahian-Noghabi, M.; Noshad, H.; Vahedi, S. Optimization of cellulose extraction from sugar beet pulp. J. Sugar Beet 2012, 27, 197–210. [Google Scholar] [CrossRef]
- Shodex NH2P-50 series columns. In Analysis of Saccharides in Food Industry; Technical Notebook No 2; Showa Denko Europe GmbH: Wiesbaden, Germany, 2015.
- Ravn, H.C.; Sorensen, O.B.; Meyer, A.S. Time of harvest affects the yield of soluble polysaccharides extracted enzymatically from potato pulp. Food Bioprod. Process. 2015, 93, 77–83. [Google Scholar] [CrossRef]
- Khodaei, N.; Karboune, S. Extraction and structural characterization of rhamnogalacturonan I-type pectic polysaccharides from potato cell wall. Food Chem. 2013, 139, 617–623. [Google Scholar] [CrossRef] [PubMed]
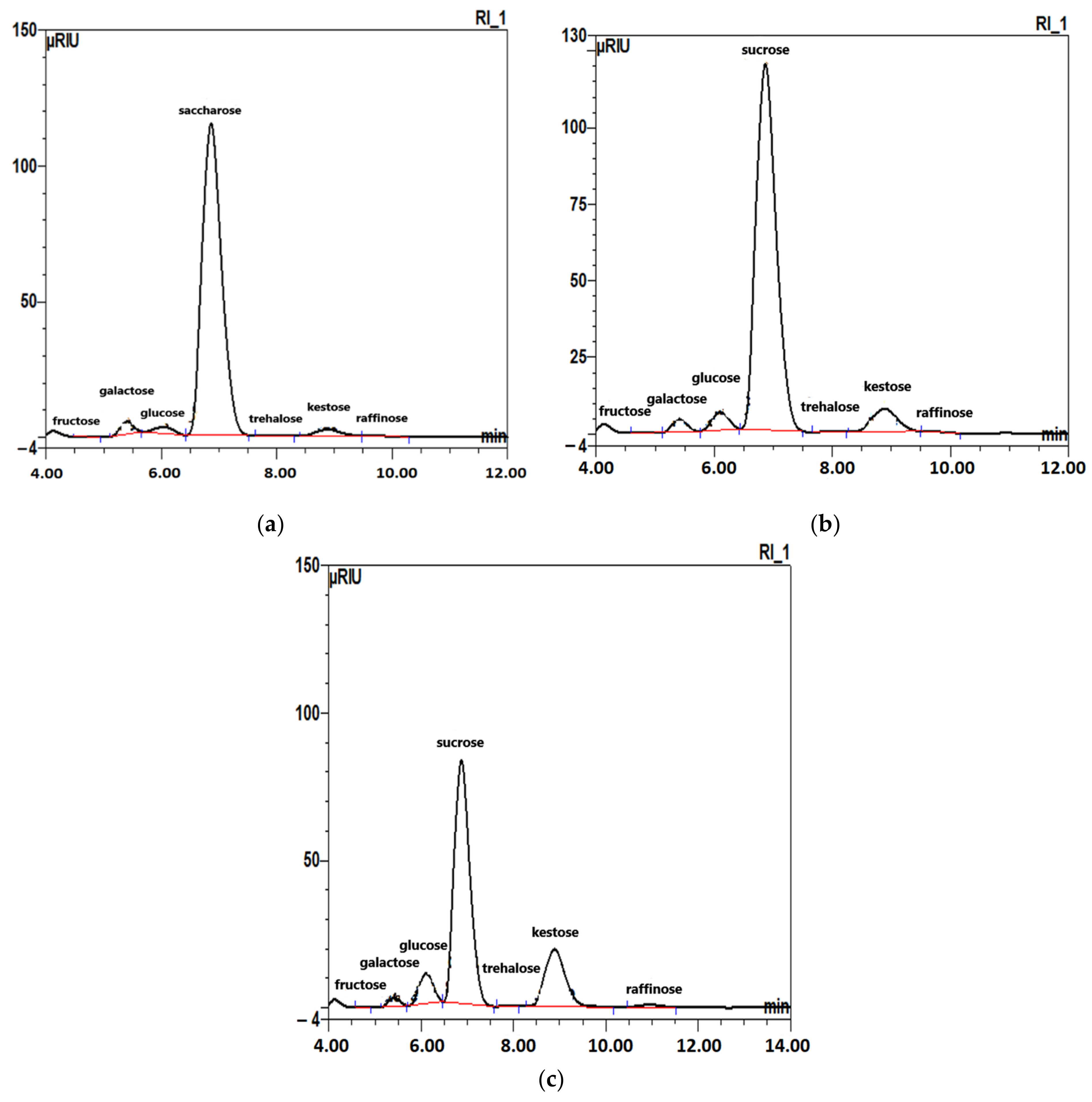
| Carbohydrates | Fresh Roots | Roots Stored for Three Months in | |
|---|---|---|---|
| Uncovered Mounds | Covered Mounds | ||
| Fructose | 0.01 ± 0.00 | 0.01 ± 0.00 | ND |
| Glucose | 0.26 ± 0.01 | 0.70 ± 0.01 | 1.25 ± 0.02 |
| Galactose | 0.54 ± 0.01 | 0.65 ± 0.01 | 0.45 ± 0.01 |
| Saccharose | 18.77 ± 0.18 | 25.18 ± 0.24 | 16.18 ± 0.20 |
| Trehalose | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 |
| Kestose | 0.30 ± 0.01 | 1.24 ± 0.01 | 3.39 ± 0.03 |
| Raffinose | 0.01 ± 0.00 | 0.04 ± 0.00 | 0.19 ± 0.01 |
| Carbohydrates | Fresh Roots | Roots Stored for Three Months in | |
|---|---|---|---|
| Uncovered Mounds | Covered Mounds | ||
| Glucose | 4.95 ± 0.05 | 4.55 ± 0.04 | 8.37 ± 0.11 |
| Xylose | 0.90 ± 0.01 | 0.33 ± 0.02 | 1.15 ± 0.02 |
| Galactose | 0.06 ± 0.00 | 0.05 ± 0.01 | 0.20 ± 0.01 |
| Arabinose | 2.81 ± 0.03 | 2.96 ± 0.03 | 3.11 ± 0.03 |
| Sample | Mass (kDa) | Percentage (%) |
|---|---|---|
| Fresh sugar beet roots | 89.21 ± 0.80 9.28 ± 0.10 1.76 ± 0.02 1.40 ± 0.02 1.03 ± 0.01 0.34 ± 0.01 | 0.10 ± 0.01 0.61 ± 0.01 0.92 ± 0.02 0.76 ± 0.01 0.88 ± 0.01 96.73 ± 0.71 |
| Roots stored for three months (uncovered mounds) | 109.38 ± 0.89 5.31 ± 0.03 1.43 ± 0.02 1.00 ± 0.01 0.34 ± 0.01 | 0.12 ± 0.01 1.87 ± 0.02 0.65 ± 0.01 0.83 ± 0.01 96.53 ± 0.70 |
| Roots stored for three months (covered mounds) | 44.14 ± 0.35 5.59 ± 0.02 1.83 ± 0.01 1.54 ± 0.01 0.34 ± 0.01 | 0.08 ± 0.01 1.69 ± 0.01 0.44 ± 0.01 0.36 ± 0.01 97.43 ± 0.60 |
| Month | Temperature (°C) | Total Rainfall (mm) | Insolation (h) | ||
|---|---|---|---|---|---|
| Average | Minimum | Maximum | |||
| September | 15 | 3 | 26 | 60–70 | 160–180 |
| October | 10 | −4 | 21 | 40–55 | 120–140 |
| November | 6 | −3 | 14 | <20 | 40–50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gruska, R.M.; Baryga, A.; Kunicka-Styczyńska, A.; Brzeziński, S.; Rosicka-Kaczmarek, J.; Miśkiewicz, K.; Sumińska, T. Fresh and Stored Sugar Beet Roots as a Source of Various Types of Mono- and Oligosaccharides. Molecules 2022, 27, 5125. https://doi.org/10.3390/molecules27165125
Gruska RM, Baryga A, Kunicka-Styczyńska A, Brzeziński S, Rosicka-Kaczmarek J, Miśkiewicz K, Sumińska T. Fresh and Stored Sugar Beet Roots as a Source of Various Types of Mono- and Oligosaccharides. Molecules. 2022; 27(16):5125. https://doi.org/10.3390/molecules27165125
Chicago/Turabian StyleGruska, Radosław Michał, Andrzej Baryga, Alina Kunicka-Styczyńska, Stanisław Brzeziński, Justyna Rosicka-Kaczmarek, Karolina Miśkiewicz, and Teresa Sumińska. 2022. "Fresh and Stored Sugar Beet Roots as a Source of Various Types of Mono- and Oligosaccharides" Molecules 27, no. 16: 5125. https://doi.org/10.3390/molecules27165125
APA StyleGruska, R. M., Baryga, A., Kunicka-Styczyńska, A., Brzeziński, S., Rosicka-Kaczmarek, J., Miśkiewicz, K., & Sumińska, T. (2022). Fresh and Stored Sugar Beet Roots as a Source of Various Types of Mono- and Oligosaccharides. Molecules, 27(16), 5125. https://doi.org/10.3390/molecules27165125






