Facile Synthesis of Micro-Mesoporous Copper Phyllosilicate Supported on a Commercial Carrier and Its Application for Catalytic Hydrogenation of Nitro-Group in Trinitrobenzene
Abstract
:1. Introduction
- Cu2+ selective adsorption from an aqueous salt solution on silica [25];
2. Results
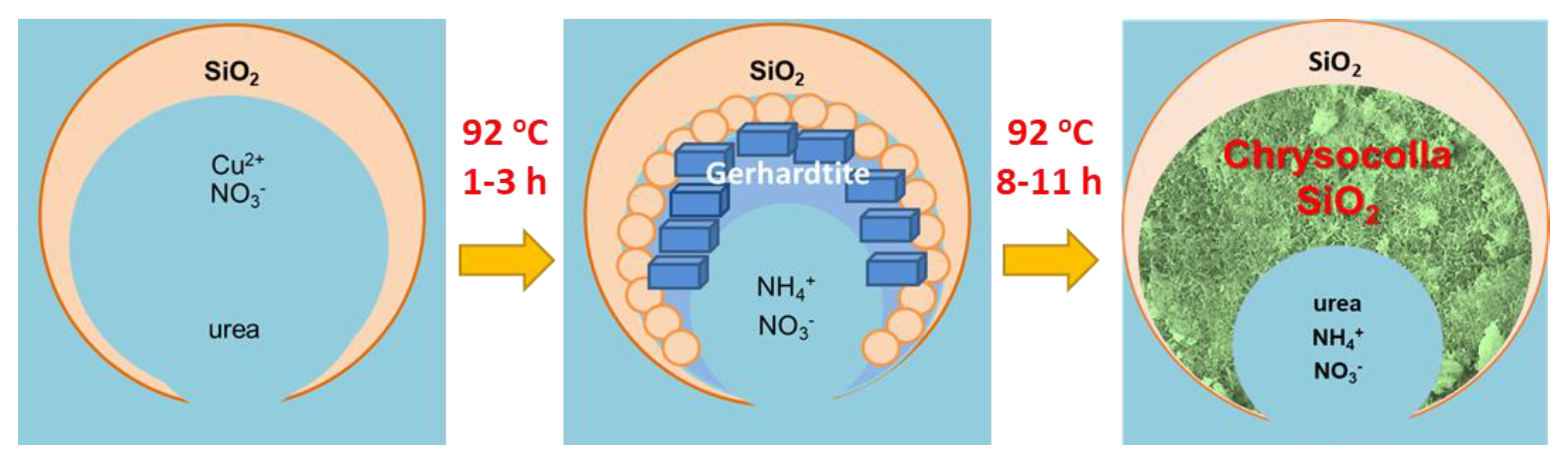
2.1. Thermal Analysis
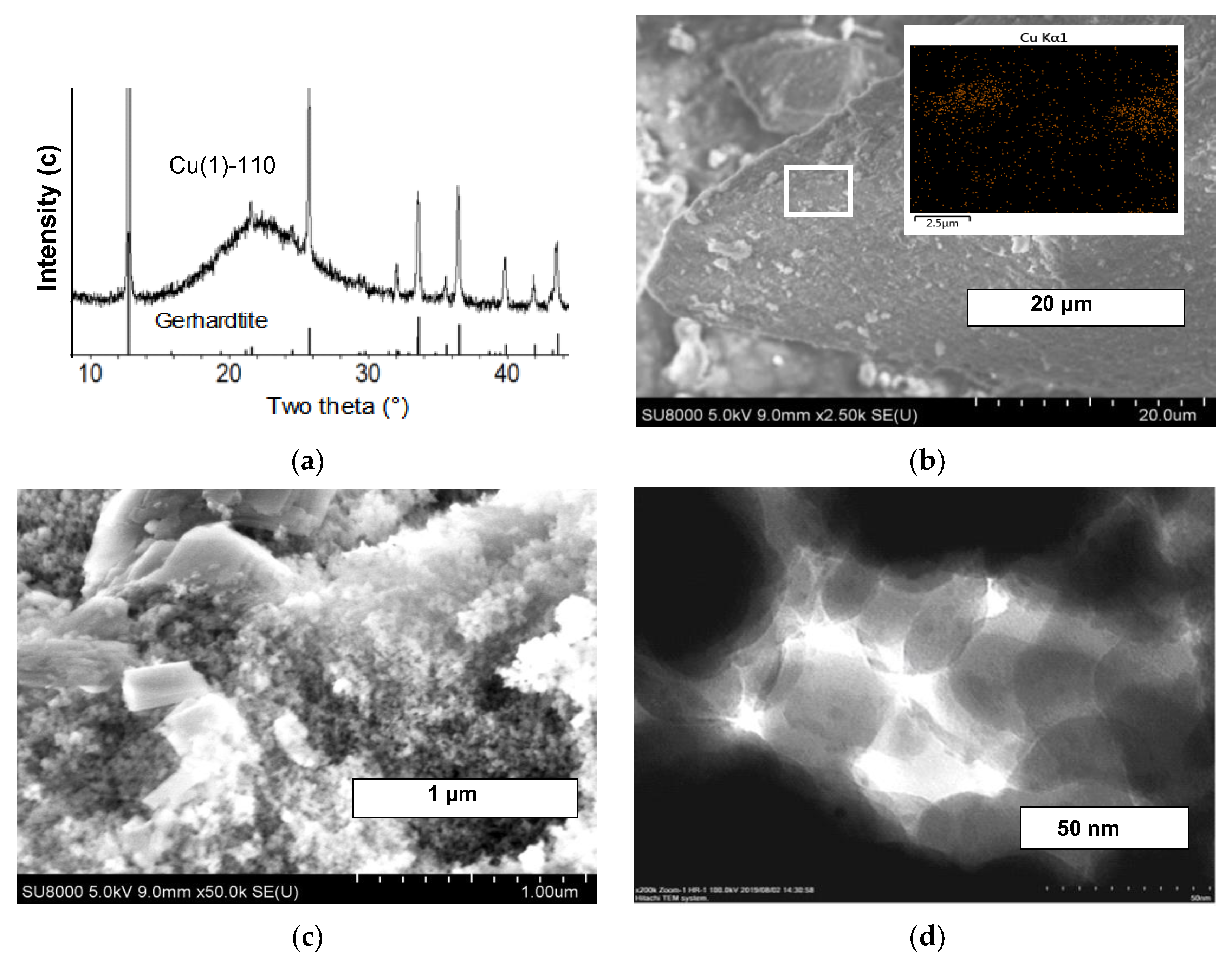
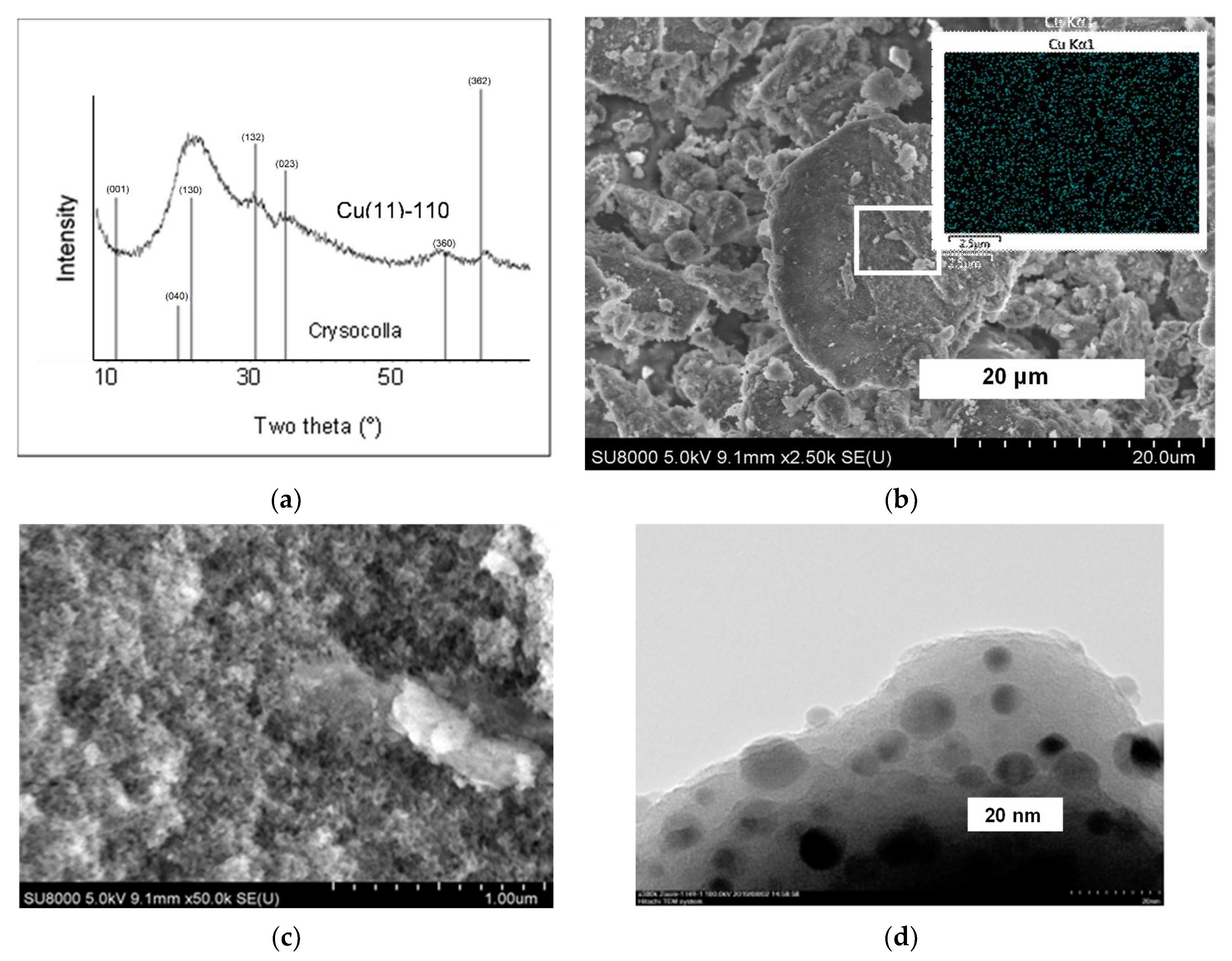
2.2. Structure and Morphology
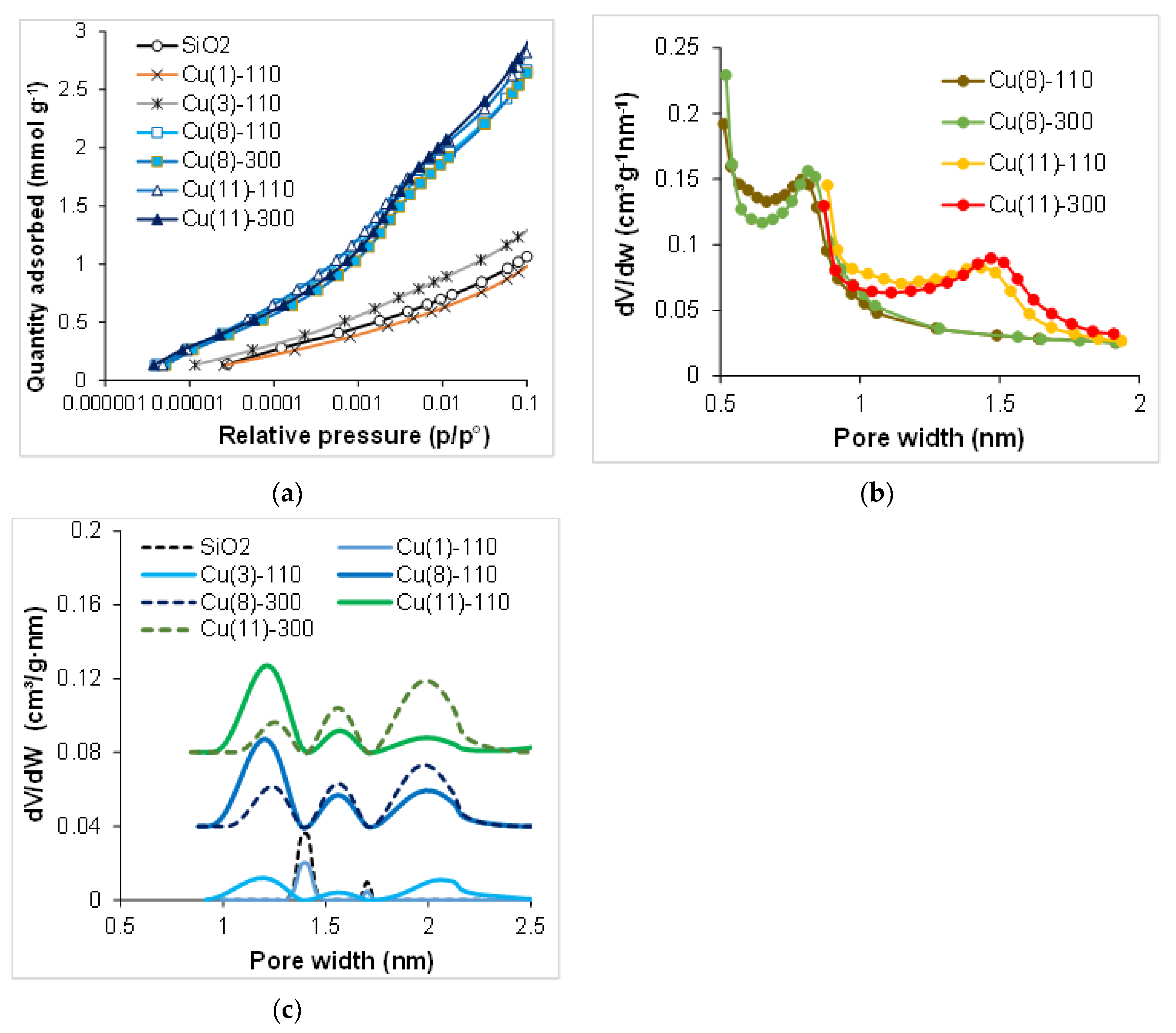
2.3. Characterization of the Texture
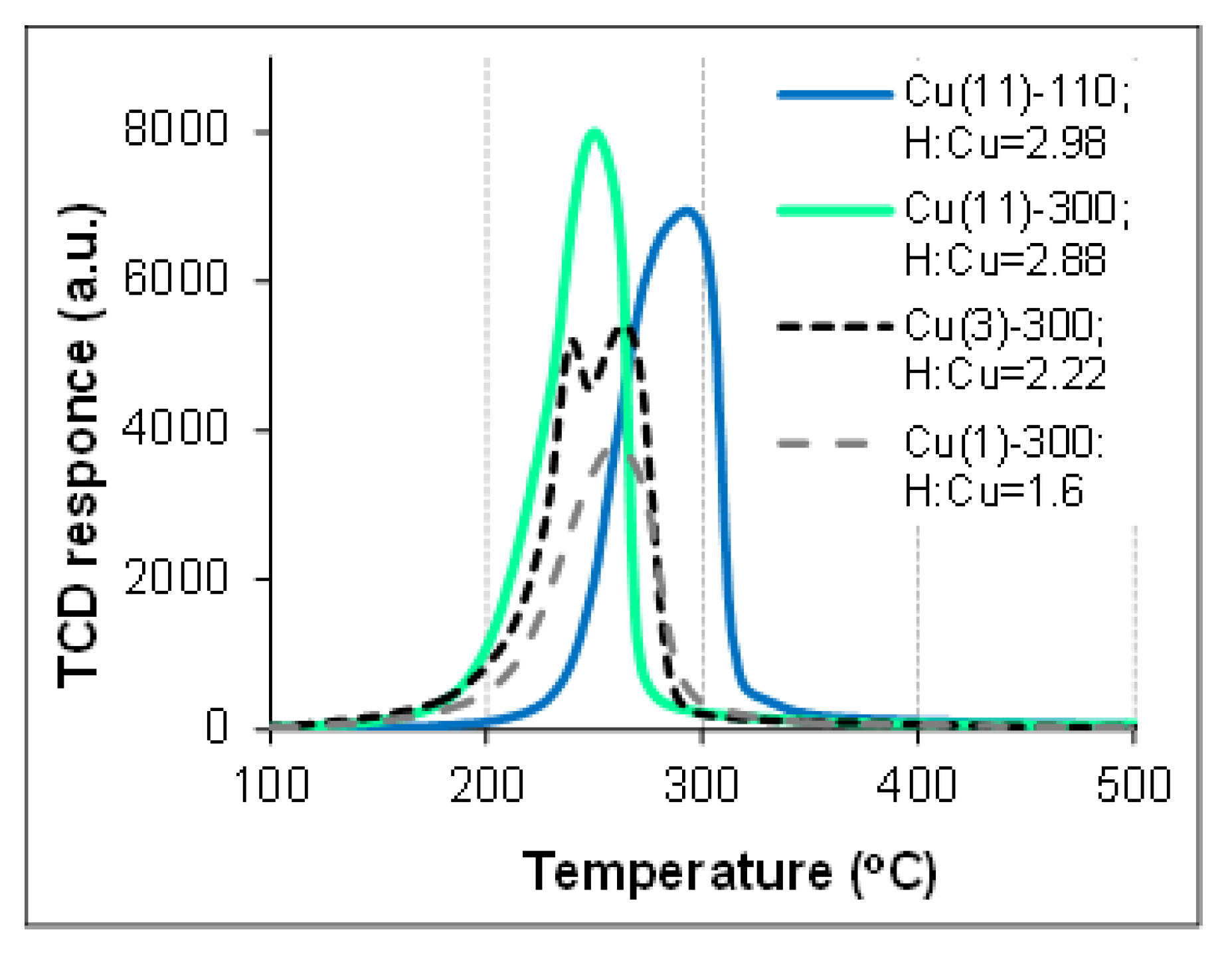
2.4. TPR-H2 Analysis
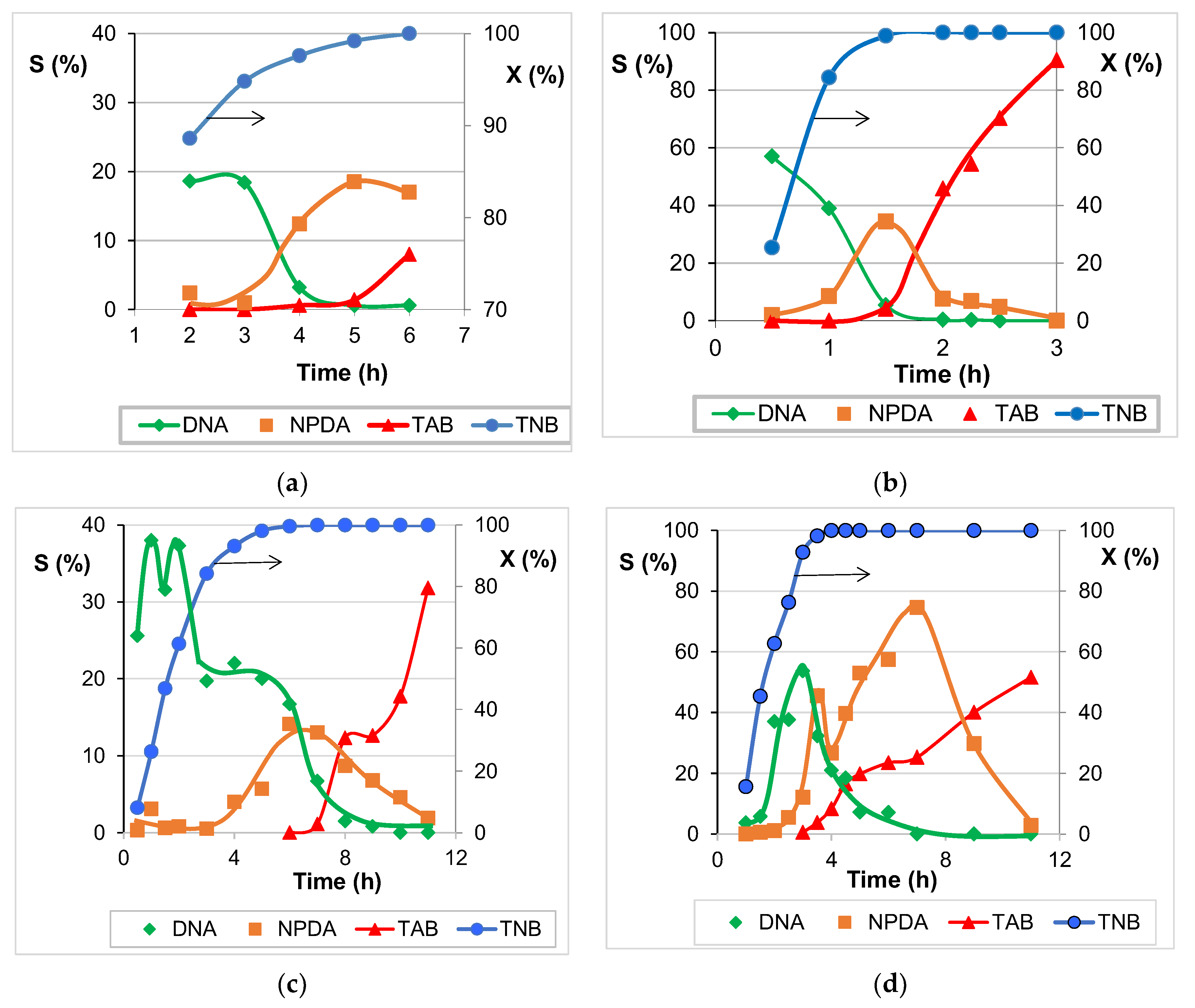
2.5. Catalytic Activity
3. Discussion
4. Materials and Methods
4.1. Materials and Synthesis
4.2. Characterization
4.3. Catalytic Activity Test
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Khalil, M.; Kadja, G.T.M.; Ilmi, M.M. Advanced nanomaterials for catalysis: Current progress in fine chemical synthesis, hydrocarbon processing, and renewable energy. J. Ind. Eng. Chem. 2021, 93, 78–100. [Google Scholar] [CrossRef]
- Rajabzadeha, M.; Khalifeh, R.; Eshghi, H.; Hafizi, A. Design and synthesis of CuO@SiO2 multi-yolk@shell and its application as a new catalyst for CO2 fixation reaction under solventless condition. J. Ind. Eng. Chem. 2020, 89, 458–469. [Google Scholar] [CrossRef]
- Bian, Z.; Kawi, S. Preparation, characterization and catalytic application of phyllosilicate: A Review. Catal. Today 2020, 339, 3–23. [Google Scholar] [CrossRef]
- Teržan, J.; Huš, M.; Likozar, B.; Djinović, P. Propylene epoxidation using molecular oxygen over copper- and silver-based catalysts: A Review. ACS Catal. 2020, 10, 13415–13436. [Google Scholar] [CrossRef]
- Xu, C.; Chen, G.; Zhao, Y.; Liu, P.; Duan, X.; Gu, L.; Fu, G.; Yuan, Y.; Zheng, N. Interfacing with silica boosts the catalysis of copper. Nat. Commun. 2018, 9, 3367–3377. [Google Scholar]
- Lu, Z.; Gao, D.; Yin, H.; Wang, A.; Liu, S. Methanol dehydrogenation to methyl formate catalyzed by SiO2-hydroxyapatite- and MgO-supported copper catalysts and reaction kinetics. J. Ind. Eng. Chem. 2015, 31, 301–308. [Google Scholar] [CrossRef]
- Pompe, C.E.; Slagter, M.; de Jongh, P.E.; de Jong, K.P. Impact of heterogeneities in silica-supported copper catalysts on their stability for methanol synthesis. J. Catal. 2018, 365, 1–9. [Google Scholar] [CrossRef]
- van den Berg, R.; Zečević, J.; Sehested, J.; Helveg, S.; de Jongh, P.E.; de Jong, K.P. Impact of the synthesis route of supported copper catalysts on the performance in the methanol synthesis reaction. Catal. Today 2016, 272, 87–93. [Google Scholar] [CrossRef]
- Bian, Z.; Zhong, W.; Yu, Y.; Jiang, B.; Kawi, S. Cu/SiO2 derived from copper phyllosilicate for low-temperature water-gas shift reaction: Role of Cu+ sites. Int. J. Hydrog. Energy 2020, 45, 27078–27088. [Google Scholar] [CrossRef]
- Amiri, T.Y.; Moghaddas, J. Cogeled copper-silica aerogel as a catalyst in hydrogen production from methanol steam reforming. Int. J. Hydrog. Energy 2015, 40, 1472–1480. [Google Scholar] [CrossRef]
- Zhang, H.; Tan, H.-R.; Jaenicke, S.; Chuah, G.-K. Highly efficient and robust Cu catalyst for non-oxidative dehydrogenation of ethanol to acetaldehyde and hydrogen. J. Catal. 2020, 389, 19–28. [Google Scholar] [CrossRef]
- Vasiliadou, E.S.; Eggenhuisen, T.M.; Munnik, P.; de Jongh, P.E.; de Jong, K.P.; Lemonidou, A.A. Synthesis and performance of highly dispersed Cu/SiO2 catalysts for the hydrogenolysis of glycerol. Appl. Catal. B Environ. 2014, 145, 108–119. [Google Scholar] [CrossRef]
- Qiu, K.; Shu, Y.; Zhang, J.; Gao, L.; Xiao, G. Effective and Stable Zeolite Imidazole Framework-Supported Copper Nanoparticles (Cu/ZIF-8) for Glycerol to Lactic Acid. Catal. Lett. 2022, 152, 172–186. [Google Scholar] [CrossRef]
- To, D.-T.; Lin, Y.-C. Copper Phyllosilicates-Derived Catalysts in the Production of Alcohols from Hydrogenation of Carboxylates, Carboxylic Acids, Carbonates, Formyls, and CO2: A Review. Catalysts 2021, 11, 255. [Google Scholar] [CrossRef]
- Wang, Z.-Q.; Xu, Z.-N.; Peng, S.-Y.; Zhang, M.-J.; Lu, G.; Chen, Q.-S.; Chen, Y.; Guo, G.-C. High-performance and long-lived Cu/SiO2 nanocatalyst for CO2 hydrogenation. ACS Catal. 2015, 5, 4255–4259. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, S.; Wang, Y.; Shan, B.; Zhang, J.; Wang, S.; Ma, X. Efficient tuning of surface copper species of Cu/SiO2 catalyst for hydrogenation of dimethyl oxalate to ethylene glycol. Chem. Eng. J. 2017, 313, 7592013768. [Google Scholar] [CrossRef]
- Popa, T.; Zhang, Y.; Jin, E.; Fan, M. An environmentally benign and low-cost approach to synthesis of thermally stable industrial catalyst Cu/SiO2 for the hydrogenation of dimethyl oxalate to ethylene glycol. Appl. Catal. A Gen. 2015, 505, 52–61. [Google Scholar] [CrossRef]
- Chen, L.-F.; Guo, P.-J.; Qiao, M.-H.; Yan, S.-R.; Li, H.-X.; Shen, W.; Xu, H.-L.; Fan, K.-N. Cu/SiO2 catalysts prepared by the ammonia-evaporation method: Texture, structure, and catalytic performance in hydrogenation of dimethyl oxalate to ethylene glycol. J. Catal. 2008, 257, 172–180. [Google Scholar] [CrossRef]
- Yu, J.; Cao, J.; Du, L.; Wei, Y.; Wang, T.; Tian, H. Enhancement of diethyl malonate hydrogenation to 1,3-propanediol using mesoporous Cu/SBA-15 as catalyst. Appl. Catal. A Gen. 2018, 555, 161–170. [Google Scholar] [CrossRef]
- Sheng, H.; Lobo, R.F. Iron-promotion of silica-supported copper catalysts for furfural hydrodeoxygenation. ChemCatChem 2016, 8, 3402–3408. [Google Scholar] [CrossRef]
- Zhu, Y.; Kong, X.; Peng, B.; Li, L.; Fang, Z.; Zhu, Y. Efficient Cu catalyst for 5-hydroxymethylfurfural hydrogenolysis by forming Cu–O–Si bonds. Catal. Sci. Technol. 2020, 10, 7323–7330. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, A.; Yin, H.; Yan, X.; Shen, L. Reduction of 3-nitro-4-methoxy-acetylaniline to 3-amino-4-methoxyacetylaniline catalyzed by metallic Cu nanoparticles at low reaction temperature. Chem. Eng. J. 2015, 262, 427. [Google Scholar] [CrossRef]
- Formenti, D.; Ferretti, F.; Scharnag, F.K.; Beller, M. Reduction of nitro compounds using 3d-non-noble metal catalysts. Chem. Rev. 2019, 119, 2611. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Huang, Z.-F.; Pan, L.; Li, K.; Zhang, X.; Wang, L.; Zou, J.-J. Review on selective hydrogenation of nitroarene by catalytic, photocatalytic and electrocatalytic reactions. Appl. Catal. B Environ. 2018, 227, 386–408. [Google Scholar] [CrossRef]
- Toupance, T.; Kermarec, M.; Lambert, J.-F.; Louis, C. Conditions of formation of copper phyllosilicates in silica-supported copper catalysts prepared by selective adsorption. J. Phys. Chem. B 2002, 106, 2277–2286. [Google Scholar] [CrossRef]
- Toupance, T.; Kermarec, M.; Louis, C. Metal particle size in silica-supported copper catalysts: Influence of the conditions of preparation and of thermal pretreatments. J. Phys. Chem. B 2000, 104, 965–972. [Google Scholar] [CrossRef]
- Dong, X.; Ma, X.; Xu, H.; Ge, Q. Comparative study of silica-supported copper catalysts prepared by different methods: Formation and transition of copper phyllosilicate. Catal. Sci. Technol. 2016, 6, 4151–4158. [Google Scholar] [CrossRef]
- Zhang, B.; Hui, S.; Zhang, S.; Ji, Y.; Li, W.; Fang, D. Effect of copper loading on texture, structure and catalytic performance of Cu/SiO2 catalyst for hydrogenation of dimethyl oxalate to ethylene glycol. J. Nat. Gas Chem. 2012, 21, 563–570. [Google Scholar] [CrossRef]
- Di, W.; Cheng, J.; Tian, S.; Li, J.; Chen, J.; Sun, Q. Synthesis and characterization of supported copper phyllosilicate catalysts for acetic ester hydrogenation to ethanol. Appl. Catal. A Gen. 2016, 510, 244–259. [Google Scholar] [CrossRef]
- van der Grift, C.J.G.; Wielers, A.F.H.; Mulder, A.; Geus, J.W. The reduction behavior of silica-supported copper catalysts prepared by deposition-precipitation. Thermochim. Acta 1990, 171, 95–113. [Google Scholar] [CrossRef]
- van der Grift, C.J.G.; Elberse, P.A.; Mulder, A.; Geus, J.W. Preparation of silica-supported copper catalysts by means of deposition precipitation. Appl. Catal. 1990, 59, 275–289. [Google Scholar] [CrossRef]
- Shesterkina, A.; Vikanova, K.; Kostyukhin, E.; Strekalova, A.; Shuvalova, E.; Kapustin, G.; Salmi, T. Microwave synthesis of copper phyllosilicates as effective catalysts for hydrogenation of C≡C bonds. Molecules 2022, 27, 988. [Google Scholar] [CrossRef] [PubMed]
- Kirichenko, O.A.; Shuvalova, E.V.; Strekalova, A.A.; Davshan, N.A.; Kapustin, G.I.; Nissenbaum, V.D. Catalytic activity of Cu and Cu–Fe hydrosilicates in hydrogenation with molecular hydrogen. Russ. J. Phys. Chem. A 2018, 92, 2417–2423. [Google Scholar] [CrossRef]
- Kirichenko, O.A.; Shuvalova, E.V.; Redina, E.A. Low-temperature copper hydrosilicates: Catalysts for reduction of aromatic nitro compounds with molecular hydrogen. Russ. Chem. Bull. 2019, 68, 2048–2052. [Google Scholar] [CrossRef]
- Shuvalova, E.V.; Kirichenko, O.A. Hydrogenation of nitroarenes on silica-supported copper catalyst. Mendeleev Commun. 2021, 31, 875–877. [Google Scholar] [CrossRef]
- Chatterjee, S.; Deb, U.; Datta, S.; Walther, C.; Gupta, D.K. Common explosives (TNT, RDX, HMX) and their fate in the environment: Emphasizing bioremediation. Chemosphere 2017, 184, 438–451. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Bagheri, A.R.; Bhatt, P.; Chen, S. Environmental occurrence, toxicity concerns, and remediation of recalcitrant nitroaromatic compounds. J. Environ. Manag. 2021, 291, 112685. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Zhang, Y.; Zhou, F.; Lv, F.; Ye, Z.; Fan, F.; Chu, P.K. Preparation and characterization of Cu2O–ZnO immobilized on diatomite for photocatalytic treatment of red water produced from manufacturing of TNT. Chem. Eng. J. 2011, 171, 61–68. [Google Scholar] [CrossRef]
- Wu, S.; Qi, Y.; He, S.; Fan, C.; Dai, B.; Zhou, W.; Gao, L.; Huang, J. Preparation and application of novel catalytic-ceramic-filler in a coupled system for TNT manufacturing wastewater treatment. Chem. Eng. J. 2015, 280, 417–425. [Google Scholar] [CrossRef]
- Belskaya, O.B.; Mironenko, R.M.; Talsi, V.P.; Rodionov, V.A.; Sysolyatin, S.V.; Likholobov, V.A. A study of Pd/C catalysts in the liquid-phase hydrogenation of1,3,5-trinitrobenzene and 2,4,6-trinitrobenzoic acid. Selection of hydrogenation conditions for selective production of 1,3,5-triaminobenzene. Procedia Eng. 2016, 152, 110–115. [Google Scholar] [CrossRef]
- Belskaya, O.B.; Mironenko, R.M.; Talsi, V.P.; Rodionov, V.A.; Gulyaeva, T.I.; Sysolyatin, S.V.; Likholobov, V.A. The effect of preparation conditions of Pd/C catalyst on its activity and selectivity in the aqueous-phase hydrogenation of 2,4,6-trinitrobenzoic acid. Catal. Today 2018, 301, 258–265. [Google Scholar] [CrossRef]
- Ilcheva, L.; Maneva, M.; Bozadziev, P. Thermal investigation of the basic copper(II) nitrate Cu(OH)1.5(NO3)0.5. J. Therm. Anal. 1979, 16, 205–207. [Google Scholar] [CrossRef]
- Ghose, J.; Kanungo, A. Studies on the thermal decomposition of Cu(NO3)2·3H2O. J. Therm. Anal. 1981, 20, 459–462. [Google Scholar] [CrossRef]
- Hariu, T.; Arima, H.; Sugiyama, K. The structure of hydrated copper-silicate gels, an analog compound for natural chrysocolla. J. Mineral. Petrol. Sci. 2013, 108, 111–115. [Google Scholar] [CrossRef]
- Stojceva Radovanovic, B.C.; Premovic, P.I. Thermal behavior of Cu(II)-urea complex. J. Therm. Anal. 1992, 38, 715–719. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Neimark, A.V.; Sing, K.S.W.; Thommes, M. Characterization of solid catalysts: Surface area and porosity. In Handbook of Heterogeneous Catalysis; Eertl, G., Knozinger, H., Schuth, F., Weitkamp, J., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008; pp. 721–729. [Google Scholar]
- Monti, D.A.M.; Baker, A. Temperature-programmed reduction. Parametric sensitivity and estimation of kinetic parameters. J. Catal. 1983, 83, 323–335. [Google Scholar] [CrossRef]
- Malet, P.; Caballero, A. The selection of experimental conditions in temperature-programmed reduction experiments. J. Chem. Condens. Phases 1988, 84, 2369–2375. [Google Scholar] [CrossRef]
- Shuvalova, E.V.; Kirichenko, O.A.; Kapustin, G.I.; Kustov, L.M. Silica-supported copper nanoparticles as efficient catalysts for the liquid-phase selective hydrogenation of p-dinitrobenzene by molecular hydrogen. Russ. Chem. Bull. 2016, 65, 2850–2854. [Google Scholar] [CrossRef]
- Belskaya, O.B.; Talsi, V.P.; Mironenko, R.M.; Rodionov, V.A.; Sysolyatin, S.V.; Likholobov, V.A. Transformation pathways of 2,4,6-trinitrobenzoic acid in the aqueoua-phase hydrogenation over Pd/C catalyst. J. Mol. Catal. A Chem. 2016, 420, 190–199. [Google Scholar] [CrossRef]
- Mironenko, R.M.; Belskaya, O.B.; Talsi, V.P.; Rodionov, V.A.; Sysolyatin, S.V.; Likholobov, V.A. An unusual reduction route of 2,4,6-trinitrobenzoic acid under conditions of aqueous-phase hydrogenation over Pd/Sibunit catalyst. Russ. Chem. Bull. 2016, 65, 1535–1540. [Google Scholar] [CrossRef]
- Neri, G.; Musolino, M.G.; Bonaccorsi, M.G.; Donato, L.; Mercadante, L. Catalytic hydrogenation of 4-(hydroxyamino)-2-nitrotoluene and 2,4-nitroamine isomers. Ind. Eng. Chem. Res. 1997, 36, 3619–3624. [Google Scholar] [CrossRef]
- Chernyshev, V.M.; Khazipov, O.V.; Eremin, D.B.; Denisova, E.A.; Ananikov, V.P. Formation and stabilization of nanosized Pd particles in catalytic systems: Ionic nitrogen compounds as catalytic promoters and stabilizers of nanoparticles. Coord. Chem. Rev. 2021, 437, 213860. [Google Scholar] [CrossRef]
- Li, W.; Cui, X.; Junge, K.; Surkus, A.E.; Kreyenschulte, C.; Bartling, S.; Beller, M. General and chemoselective copper oxide catalysts for hydrogenation reactions. ACS Catal. 2019, 9, 4302–4307. [Google Scholar] [CrossRef]
- van der Grift, C.J.G.; Elberse, P.A.; Mulder, A.; Geus, J.W. Characterization of silica-supported copper catalysts by means of temperature-programmed reduction. Appl. Catal. 1990, 60, 181–192. [Google Scholar] [CrossRef]
- Kachala, V.V.; Khemchyan, L.L.; Kashin, A.S.; Orlov, N.V.; Grachev, A.A.; Zalesskiy, S.S.; Ananikov, V.P. Target-oriented analysis of gaseous, liquid and solid chemical systems by mass spectrometry, nuclear magnetic resonance spectroscopy and electron microscopy. Russ. Chem. Rev. 2013, 82, 648–685. [Google Scholar] [CrossRef]
- Kashin, A.S.; Ananikov, V.P. A SEM study of nanosized metal films and metal nanoparticles obtained by magnetron sputtering. Russ. Chem. Bull. 2011, 60, 2602–2607. [Google Scholar] [CrossRef]




| Sample | SBET, m2 g−1 | Vtotal 1, cm3 g−1 | Vmeso cm3 g−1 | Vmicro (Harkins–Jura), cm3 g−1 | Vmicro (DFT-Slit), cm3 g−1 | Vmicro (DFT-Cylinder), cm3 g−1 |
|---|---|---|---|---|---|---|
| SiO2 | 100 | 0.664 | 0.659 | 0.038 | 0.012 | 0.003 |
| Cu(1)-110 | 96 | 0.602 | 0.595 | - | 0.009 | 0.002 |
| Cu(3)-110 | 123 | 0.560 | 0.549 | 0.046 | 0.015 | 0.005 |
| Cu(8)-110 | 252 | 0.615 | 0.599 | 0.096 | 0.034 | 0.017 |
| Cu(8)-300 | 251 | 0.639 | 0.628 | 0.095 | 0.035 | 0.014 |
| Cu(11)-110 | 272 | 0.590 | 0.577 | 0.071 | 0.038 | 0.016 |
| Cu(11)-300 | 278 | 0.630 | 0.618 | 0.072 | 0.036 | 0.016 |
| Sample ID | Cycle | Reaction Time, h | TNB Conversion,% | Selectivity to TAB,% | Selectivity to DNA,% | Selectivity to NPDA,% |
|---|---|---|---|---|---|---|
| Cu(8)-110 | 1st 2nd | 5 5 8 | 100 98 100 | 40 0 0.07 | 0 16 14 | 0 3.1 10 |
| Cu(8)-300 | 1st 2nd | 2 2 7 | 100 76 100 | 52 0 21 | 0 57 0 | 0 5.4 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirichenko, O.; Kapustin, G.; Mishin, I.; Nissenbaum, V.; Shuvalova, E.; Redina, E.; Kustov, L. Facile Synthesis of Micro-Mesoporous Copper Phyllosilicate Supported on a Commercial Carrier and Its Application for Catalytic Hydrogenation of Nitro-Group in Trinitrobenzene. Molecules 2022, 27, 5147. https://doi.org/10.3390/molecules27165147
Kirichenko O, Kapustin G, Mishin I, Nissenbaum V, Shuvalova E, Redina E, Kustov L. Facile Synthesis of Micro-Mesoporous Copper Phyllosilicate Supported on a Commercial Carrier and Its Application for Catalytic Hydrogenation of Nitro-Group in Trinitrobenzene. Molecules. 2022; 27(16):5147. https://doi.org/10.3390/molecules27165147
Chicago/Turabian StyleKirichenko, Olga, Gennady Kapustin, Igor Mishin, Vera Nissenbaum, Elena Shuvalova, Elena Redina, and Leonid Kustov. 2022. "Facile Synthesis of Micro-Mesoporous Copper Phyllosilicate Supported on a Commercial Carrier and Its Application for Catalytic Hydrogenation of Nitro-Group in Trinitrobenzene" Molecules 27, no. 16: 5147. https://doi.org/10.3390/molecules27165147
APA StyleKirichenko, O., Kapustin, G., Mishin, I., Nissenbaum, V., Shuvalova, E., Redina, E., & Kustov, L. (2022). Facile Synthesis of Micro-Mesoporous Copper Phyllosilicate Supported on a Commercial Carrier and Its Application for Catalytic Hydrogenation of Nitro-Group in Trinitrobenzene. Molecules, 27(16), 5147. https://doi.org/10.3390/molecules27165147








