Bacopa Protects against Neurotoxicity Induced by MPP+ and Methamphetamine
Abstract
:1. Introduction
2. Results
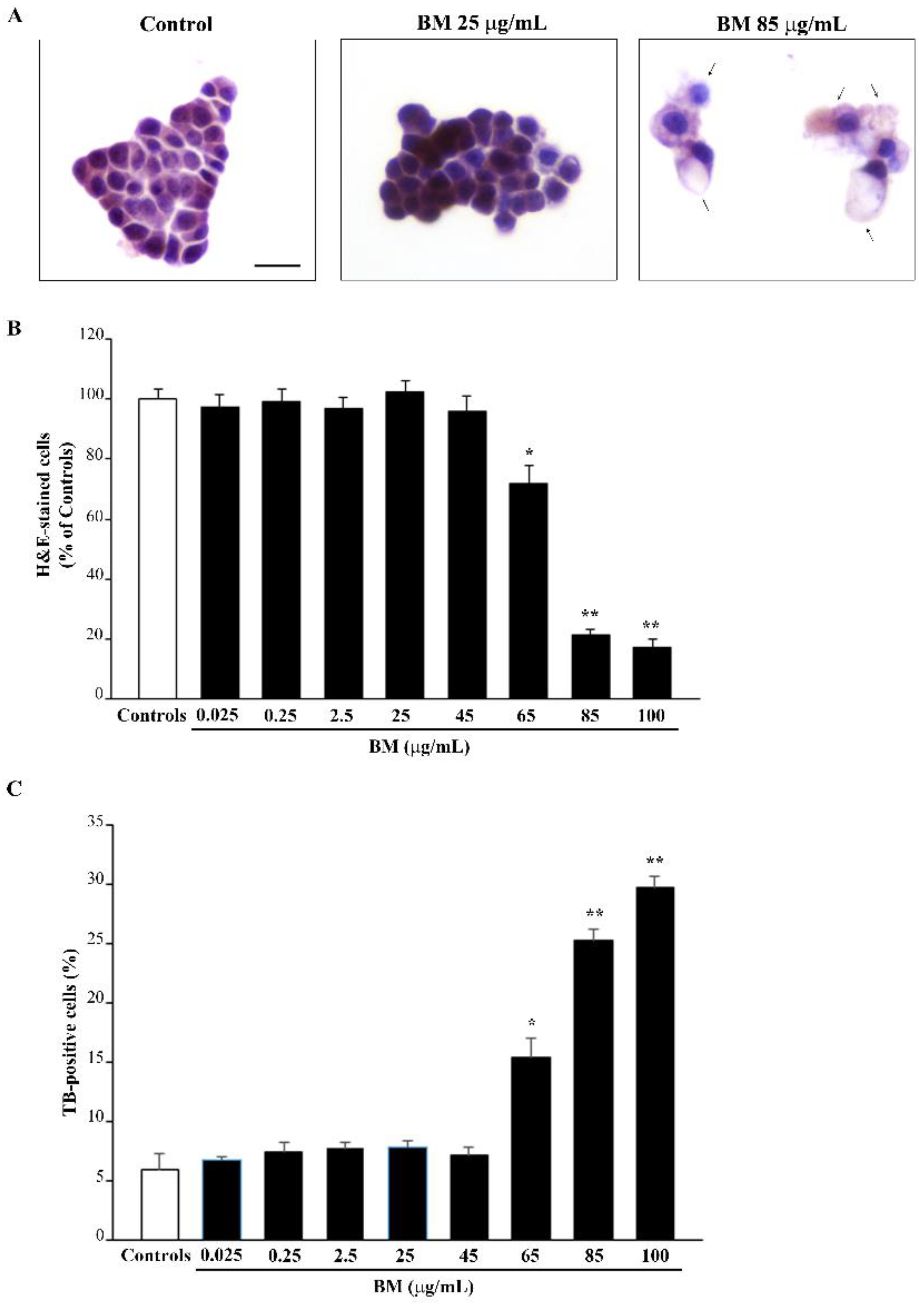
2.1. Dose-Dependent Effects of Bacopa Monnieri (BM) on Cell Viability
2.2. BM Dose-Dependently Protects PC12 Cells against METH- and MPP+-Induced Toxicity
2.3. BM Dose-Dependently Prevents METH- and MPP+-Induced ROS
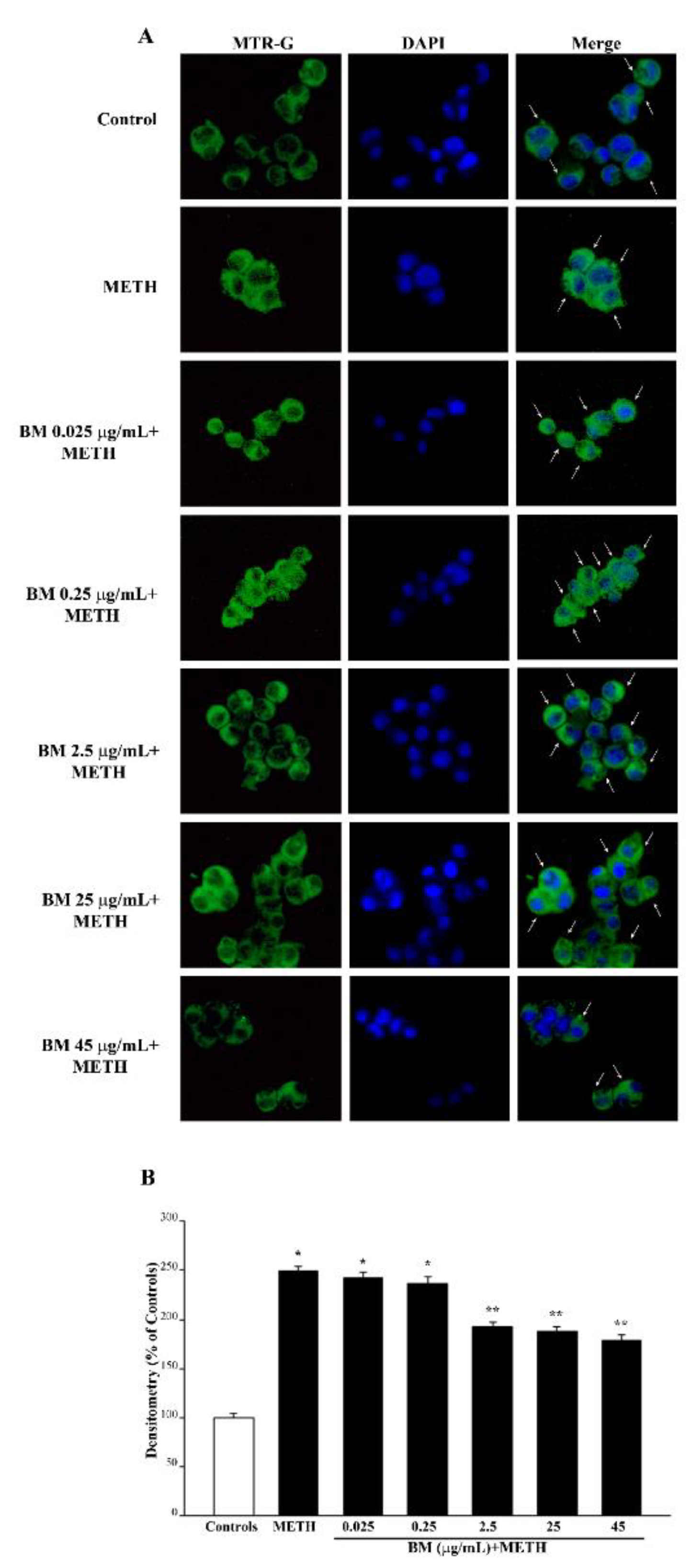
2.4. BM Dose-Dependently Increases Healthy (MTR-R) and Total (MTR-G) Mitochondria When Given Alone or in Combination with METH or MPP+
2.5. In Situ Counts of Mitochondrial Morphometry at TEM Confirms Data Obtained with MTR Histofluorescence
3. Discussion
4. Materials and Methods
4.1. Cell Cultures
4.2. Cell Treatments and Experimental Design
4.3. Trypan Blue (TB) Staining
4.4. Hematoxylin and Eosin (H&E) Histochemistry
4.5. Fluoro Jade B (FJB) Histofluorescence
4.6. Measurement of Reactive Oxygen Species (ROS)
4.7. Mitochondrial Labelling
4.8. Transmission Electron Microscopy (TEM)
4.9. Ultrastructural Morphometry of Mitochondria
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Seiden, L.S.; MacPhail, R.C.; Oglesby, M.W. Catecholamines and drug-behavior interactions. Fed. Proc. 1975, 34, 1823–1831. [Google Scholar] [PubMed]
- Seiden, L.S.; Fischman, M.W.; Schuster, C.R. Long-term methamphetamine induced changes in brain catecholamines in tolerant rhesus monkeys. Drug Alcohol Depend. 1976, 1, 215–219. [Google Scholar] [CrossRef]
- Finnegan, K.T.; Ricaurte, G.; Seiden, L.S.; Schuster, C.R. Altered sensitivity to d-methylamphetamine, apomorphine, and haloperidol in rhesus monkeys depleted of caudate dopamine by repeated administration of d-methylamphetamine. Psychopharmacology 1982, 77, 43–52. [Google Scholar] [CrossRef]
- Ricaurte, G.A.; Guillery, R.W.; Seiden, L.S.; Schuster, C.R.; Moore, R.Y. Dopamine nerve terminal degeneration produced by high doses of methylamphetamine in the rat brain. Brain Res. 1982, 235, 93–103. [Google Scholar] [CrossRef]
- Langston, J.W.; Ballard, P.; Tetrud, J.W.; Irwin, I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science 1983, 219, 979–980. [Google Scholar] [CrossRef]
- Langston, J.W.; Irwin, I.; Langston, E.B.; Forno, L.S. 1-Methyl-4-phenylpyridinium ion (MPP+): Identification of a metabolite of MPTP, a toxin selective to the substantia nigra. Neurosci. Lett. 1984, 48, 87–92. [Google Scholar] [CrossRef]
- Javitch, J.A.; D’Amato, R.J.; Strittmatter, S.M.; Snyder, S.H. Parkinsonism-inducing neurotoxin N-methyl-4-phenyl-l,2,3,6-tetrahydropyridine. Uptake of the metabolite N-methyl-4-phenyldropyridine by dopamine neurons explains selective toxicity. Proc. Natl. Acad. Sci. USA 1985, 827, 2173–2177. [Google Scholar] [CrossRef]
- Pileblad, E.; Carlsson, A. Catecholamine-uptake inhibitors prevent the neurotoxicity of 1-methyl-4-phenyl-l,2,3,6-tetrahydropyridine (MPTP) in mouse brain. Neuropharmacology 1985, 24, 689–692. [Google Scholar] [CrossRef]
- Seniuk, N.A.; Tatton, W.G.; Greenwood, C.E. Dose-dependent destruction of the coeruleus-cortical and nigral-striatal projections by MPTP. Brain Res. 1990, 527, 7–20. [Google Scholar] [CrossRef]
- Javitch, J.A.; Uhl, G.R.; Snyder, S.H. Parkinsonism-inducing neurotoxin, N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine: Characterization and localization of receptor binding sites in rat and human brain. Proc. Natl. Acad. Sci. USA 1984, 81, 4591–4595. [Google Scholar] [CrossRef]
- Schmidt, C.J.; Gibb, J.W. Role of the dopamine uptake carrier in the neurochemical response to methamphetamine: Effects of amfonelic acid. Eur. J. Pharmacol. 1985, 109, 73–80. [Google Scholar] [CrossRef]
- Pifl, C.; Hornykiewicz, O.; Giros, B.; Caron, M.G. Catecholamine transporters and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine neurotoxicity: Studies comparing the cloned human noradrenaline and human dopamine transporter. J. Pharmacol. Exp. Ther. 1996, 277, 1437–1443. [Google Scholar] [PubMed]
- Fleckenstein, A.E.; Metzger, R.R.; Gibb, J.W.; Hanson, G.R. A rapid and reversible change in dopamine transporters induced by methamphetamine. Eur. J. Pharmacol. 1997, 323, R9–R10. [Google Scholar] [CrossRef]
- Kokoshka, J.M.; Vaughan, R.A.; Hanson, G.R.; Fleckenstein, A.E. Nature of methamphetamine-induced rapid and reversible changes in dopamine transporters. Eur. J. Pharmacol. 1998, 361, 269–275. [Google Scholar] [CrossRef]
- Rothman, R.B.; Partilla, J.S.; Baumann, M.H.; Dersch, C.M.; Carroll, F.I.; Rice, K.C. Neurochemical neutralization of methamphetamine with high-affinity nonselective inhibitors of biogenic amine transporters: A pharmacological strategy for treating stimulant abuse. Synapse 2000, 35, 222–227. [Google Scholar] [CrossRef]
- Rothman, R.B.; Baumann, M.H. Monoamine transporters and psychostimulant drugs. Eur. J. Pharmacol. 2003, 479, 23–40. [Google Scholar] [CrossRef] [PubMed]
- Fleckenstein, A.E.; Volz, T.J.; Riddle, E.L.; Gibb, J.W.; Hanson, G.R. New insights into the mechanism of action of amphetamines. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 681–698. [Google Scholar] [CrossRef] [PubMed]
- Ricaurte, G.A.; Langston, J.W.; DeLanney, L.E.; Irwin, I.; Brooks, J.D. Dopamine uptake blockers protect against the dopamine depleting effect of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in the mouse striatum. Neurosci. Lett. 1985, 59, 259–264. [Google Scholar] [CrossRef]
- Sundstrom, E.; Jonsson, G. Pharmacological interference with the neurotoxic action of 1-methyl-4-phenyl-l,2,3,6-tetrahydropyridine (MPTP) on central catecholamine neurons in the mouse. Eur. J. Pharmacol. 1985, 110, 293–299. [Google Scholar] [CrossRef]
- Marek, G.J.; Vosmer, G.; Seiden, L.S. Dopamine uptake inhibitors block long-term neurotoxic effects of methamphetamine upon dopaminergic neurons. Brain Res. 1990, 513, 274–279. [Google Scholar] [CrossRef]
- Fumagalli, F.; Gainetdinov, R.R.; Valenzano, K.J.; Caron, M.G. Role of dopamine transporter in methamphetamine-induced neurotoxicity: Evidence from mice lacking the transporter. J. Neurosci. 1998, 18, 4861–4869. [Google Scholar] [CrossRef]
- Nicklas, W.J.; Vyas, I.; Heikkila, R.E. Inhibition of NADH-linked oxidation in brain mitochondria by 1-methyl-4-phenylpyridine, a metabolite of the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Life Sci. 1985, 36, 2503–2508. [Google Scholar] [CrossRef]
- Azzard, A.J.; Rutledge, C.O. Selectivity of release of norepinephrine, dopamine and 5-hydroxytryptamine by amphetamine in various regions of rat brain. Biochem. Pharmacol. 1973, 22, 2801–2813. [Google Scholar] [CrossRef]
- O’Dell, S.J.; Weihmuller, F.B.; Marshall, J.F. Multiple methamphetamine injections induce marked increases in extracellular striatal dopamine which correlate with subsequent neurotoxicity. Brain Res. 1991, 564, 256–260. [Google Scholar] [CrossRef]
- Heikkila, R.E.; Nicklas, W.J.; Vyas, I.; Duvoisin, R.C. Dopaminergic toxicity of rotenone and the 1-methyl-4-phenylpyridinium ion after their stereotaxic administration to rats: Implication for the mechanism of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine toxicity. Neurosci. Lett. 1985, 62, 389–394. [Google Scholar] [CrossRef]
- Ramsay, R.R.; Singer, T.P. Energy-dependent uptake of 1-methyl-4-phenylpyridinium, the neurotoxic metabolite of 1-methyl-4-phenyl-l,2,3,6-tetrahydropyridine, by mitochondria. J. Biol. Chem. 1986, 261, 7885–7887. [Google Scholar] [CrossRef]
- Krueger, M.J.; Singer, T.P.; Casida, J.E.; Ramsay, R.R. Evidence that the blockade of mitochondrial respiration by the neurotoxin 1-methyl-4-phenylpyridinium (MPP+) involves binding at the same site as the respiratory inhibitor, rotenone. Biochem. Biophys. Res. Commun. 1990, 169, 123–128. [Google Scholar] [CrossRef]
- Sulzer, D.; Rayport, S. Amphetamine and other psychostimulants reduce pH gradients in midbrain dopaminergic neurons and chromaffin granules: A mechanism of action. Neuron 1990, 5, 797–808. [Google Scholar] [CrossRef]
- Cubells, J.F.; Rayport, S.; Rajendran, G.; Sulzer, D. Methamphetamine neurotoxicity involves vacuolation of endocytic organelles and dopamine-dependent intracellular oxidative stress. J. Neurosci. 1994, 14, 2260–2271. [Google Scholar] [CrossRef]
- Kogan, F.J.; Nichols, W.K.; Gibb, J.W. Influence of methamphetamine on nigral and striatal tyrosine hydroxylase activity and on striatal dopamine levels. Eur. J. Pharmacol. 1976, 36, 363–371. [Google Scholar] [CrossRef]
- Lazzeri, G.; Lenzi, P.; Busceti, C.L.; Ferrucci, M.; Falleni, A.; Bruno, V.; Paparelli, A.; Fornai, F. Mechanisms involved in the formation of dopamine-induced intracellular bodies within striatal neurons. J. Neurochem. 2007, 101, 1414–1427. [Google Scholar] [CrossRef]
- Denton, T.; Howard, B.D. A dopaminergic cell line variant resistant to the neurotoxin 1-methyl-4-phenyl-l,2,3,6-tetrahydropyridine. J. Neurochem. 1987, 49, 622–629. [Google Scholar] [CrossRef]
- Nicklas, W.J.; Youngster, S.K.; Kindt, M.V.; Heikkila, R.E. MPTP, MPP+ and mitochondrial function. Life Sci. 1987, 40, 721–729. [Google Scholar] [CrossRef]
- Tsai, M.J.; Lee, E.H. Nitric oxide donors protect cultured rat astrocytes from 1-methyl-4-phenylpyridinium-induced toxicity. Free Radic Biol. Med. 1998, 24, 705–713. [Google Scholar] [CrossRef]
- Lee, C.S.; Han, E.S.; Jang, Y.Y.; Han, J.H.; Ha, H.W.; Kim, D.E. Protective effect of harmalol and harmaline on MPTP neurotoxicity in the mouse and dopamine-induced damage of brain mitochondria and PC12 cells. J. Neurochem. 2000, 75, 521–531. [Google Scholar] [CrossRef]
- Parrado, J.; Absi, E.; Ayala, A.; Castaño, A.; Cano, J.; Machado, A. The endogenous amine 1-methyl-1,2,3,4-tetrahydroisoquinoline prevents the inhibition of complex I of the respiratory chain produced by MPP(+). J. Neurochem. 2000, 75, 65–71. [Google Scholar] [CrossRef]
- Honma, T. [Mechanism of methamphetamine toxicity in grouped mice and the effects of centrally acting drugs on its toxicity (author’s transl)]. Nihon Yakurigaku Zasshi 1978, 74, 27–36. (In Japanese) [Google Scholar] [CrossRef]
- Wagner, G.C.; Lucot, J.B.; Schuster, C.R.; Seiden, L.S. Alpha-methyltyrosine attenuates and reserpine increases methamphetamine- induced neuronal changes. Brain Res. 1983, 270, 285–288. [Google Scholar] [CrossRef]
- Schmidt, C.J.; Ritter, J.K.; Sonsalla, P.K.; Hanson, G.R.; Gibb, J.W. Role of dopamine in the neurotoxic effects of methamphetamine. J. Pharmacol. Exp. Ther. 1985, 233, 539–544. [Google Scholar]
- Gibb, J.W.; Johnson, M.; Hanson, G.R. Neurochemical basis of neurotoxicity. Neurotoxicology 1990, 11, 317–321. [Google Scholar]
- Reinhard, J.F., Jr.; Carmichael, S.W.; Daniels, A.J. Mechanisms of toxicity and cellular resistance to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and 1-methyl-4-phenylpyridinium in adrenomedullary chromaffin cell cultures. J. Neurochem. 1990, 55, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Fornai, F.; Schlüter, O.M.; Lenzi, P.; Gesi, M.; Ruffoli, R.; Ferrucci, M.; Lazzeri, G.; Busceti, C.L.; Pontarelli, F.; Battaglia, G.; et al. Parkinson-like syndrome induced by continuous MPTP infusion: Convergent roles of the ubiquitin-proteasome system and alpha-synuclein. Proc. Natl. Acad. Sci. USA 2005, 102, 3413–3418. [Google Scholar] [CrossRef] [PubMed]
- Schlüter, O.M.; Fornai, F.; Alessandrí, M.G.; Takamori, S.; Geppert, M.; Jahn, R.; Südhof, T.C. Role of alpha-synuclein in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced parkinsonism in mice. Neuroscience 2003, 118, 985–1002. [Google Scholar] [CrossRef]
- Fornai, F.; Lenzi, P.; Gesi, M.; Soldani, P.; Ferrucci, M.; Lazzeri, G.; Capobianco, L.; Battaglia, G.; De Blasi, A.; Nicoletti, F.; et al. Methamphetamine produces neuronal inclusions in the nigrostriatal system and in PC12 cells. J. Neurochem. 2004, 88, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Kita, T.; Miyazaki, I.; Asanuma, M.; Takeshima, M.; Wagner, G.C. Dopamine-induced behavioral changes and oxidative stress in methamphetamine-induced neurotoxicity. Int. Rev. Neurobiol. 2009, 88, 43–64. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.G.; Tiffany, S.M.; Vogel, F.S. The Toxicity of Melanin Precursors. J. Investig. Dermatol. 1978, 70, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Cohen, G. Oxy-radical toxicity in catecholamine neurons. Neurotoxicology 1984, 5, 77–82. [Google Scholar]
- Hastings, T.G.; Lewis, D.A.; Zigmond, M.J. Role of oxidation in the neurotoxic effects of intrastriatal dopamine injections. Proc. Natl. Acad. Sci. USA 1996, 93, 1956–1961. [Google Scholar] [CrossRef]
- LaVoie, M.J.; Hastings, T.G. Dopamine quinone formation and protein modification associated with the striatal neurotoxicity of methamphetamine: Evidence against a role for extracellular dopamine. J. Neurosci. 1999, 19, 1484–1491. [Google Scholar] [CrossRef]
- Cleeter, M.W.; Cooper, J.M.; Schapira, A.H. Irreversible inhibition of mitochondrial complex I by 1-methyl-4-phenylpyridinium: Evidence for free radical involvement. J. Neurochem. 1992, 58, 786–789. [Google Scholar] [CrossRef]
- Liu, W.B.; Zhou, J.; Qu, Y.; Li, X.; Lu, C.T.; Xie, K.L.; Sun, X.L.; Fei, Z. Neuroprotective effect of osthole on MPP+-induced cytotoxicity in PC12 cells via inhibition of mitochondrial dysfunction and ROS production. Neurochem. Int. 2010, 57, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Herraiz, T.; Guillén, H. Inhibition of the bioactivation of the neurotoxin MPTP by antioxidants, redox agents and monoamine oxidase inhibitors. Food Chem. Toxicol. 2011, 49, 1773–1781. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Murthy, V.; Ramassamy, C. Standardized extracts of Bacopa monniera protect against MPP+- and paraquat-induced toxicity by modulating mitochondrial activities, proteasomal functions, and redox pathways. Toxicol. Sci. 2012, 125, 219–232. [Google Scholar] [CrossRef]
- Singh, B.; Pandey, S.; Verma, R.; Ansari, J.A.; Mahdi, A.A. Comparative evaluation of extract of Bacopa monnieri and Mucuna pruriens as neuroprotectant in MPTP model of Parkinson’s disease. Indian J. Exp. Biol. 2016, 54, 758–766. [Google Scholar] [PubMed]
- Singh, B.; Pandey, S.; Rumman, M.; Mahdi, A.A. Neuroprotective effects of Bacopa monnieri in Parkinson’s disease model. Metab. Brain Dis. 2020, 35, 517–525. [Google Scholar] [CrossRef]
- Singh, B.; Pandey, S.; Rumman, M.; Kumar, S.; Kushwaha, P.P.; Verma, R.; Mahdi, A.A. Neuroprotective and Neurorescue Mode of Action of Bacopa monnieri (L.) Wettst in 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine-Induced Parkinson’s Disease: An In Silico and In Vivo Study. Front. Pharmacol. 2021, 12, 616413. [Google Scholar] [CrossRef]
- Anjaneyulu, J.; Vidyashankar, R.; Godbole, A. Differential effect of Ayurvedic nootropics on C. elegans models of Parkinson’s disease. J. Ayurveda Integr. Med. 2020, 11, 440–447. [Google Scholar] [CrossRef]
- Lazzeri, G.; Busceti, C.L.; Biagioni, F.; Fabrizi, C.; Morucci, G.; Giorgi, F.S.; Ferrucci, M.; Lenzi, P.; Puglisi-Allegra, S.; Fornai, F. Norepinephrine Protects against Methamphetamine Toxicity through β2-Adrenergic Receptors Promoting LC3 Compartmentalization. Int. J. Mol. Sci. 2021, 22, 7232. [Google Scholar] [CrossRef]
- Lazzeri, G.; Biagioni, F.; Fulceri, F.; Busceti, C.L.; Scavuzzo, M.C.; Ippolito, C.; Salvetti, A.; Lenzi, P.; Fornai, F. mTOR Modulates Methamphetamine-Induced Toxicity through Cell Clearing Systems. Oxid. Med. Cell Longev. 2018, 2018, 6124745. [Google Scholar] [CrossRef]
- Tripathi, M.K.; Rasheed, M.S.U.; Mishra, A.K.; Patel, D.K.; Singh, M.P. Silymarin Protects against Impaired Autophagy Associated with 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine-Induced Parkinsonism. J. Mol. Neurosci. 2020, 70, 276–283. [Google Scholar] [CrossRef]
- Zhang, Z.N.; Hui, Z.; Chen, C.; Liang, Y.; Tang, L.L.; Wang, S.L.; Xu, C.C.; Yang, H.; Zhao, Y.; Zhang, J.S. Mechanism of Autophagy Regulation in MPTP-Induced PD Mice via the mTOR Signaling Pathway by Echinacoside. Neuropsychiatr Dis. Treat. 2021, 17, 1397–1411. [Google Scholar] [CrossRef] [PubMed]
- Ryskalin, L.; Puglisi-Allegra, S.; Lazzeri, G.; Biagioni, F.; Busceti, C.L.; Balestrini, L.; Fornasiero, A.; Leone, S.; Pompili, E.; Ferrucci, M.; et al. Neuroprotective Effects of Curcumin in Methamphetamine-Induced Toxicity. Molecules 2021, 26, 2493. [Google Scholar] [CrossRef] [PubMed]
- Ryskalin, L.; Biagioni, F.; Busceti, C.L.; Polzella, M.; Lenzi, P.; Frati, A.; Ferrucci, M.; Fornai, F. Lactoferrin Protects against Methamphetamine Toxicity by Modulating Autophagy and Mitochondrial Status. Nutrients 2021, 13, 3356. [Google Scholar] [CrossRef] [PubMed]
- Kanthasamy, K.; Gordon, R.; Jin, H.; Anantharam, V.; Ali, S.; Kanthasamy, A.G.; Kanthasamy, A. Neuroprotective effect of resveratrol against methamphetamine-induced dopaminergic apoptotic cell death in a cell culture model of neurotoxicity. Curr. Neuropharmacol. 2011, 9, 49–53. [Google Scholar] [CrossRef]
- Sun, D.; Yue, Q.; Guo, W.; Li, T.; Zhang, J.; Li, G.; Liu, Z.; Sun, J. Neuroprotection of resveratrol against neurotoxicity induced by methamphetamine in mouse mesencephalic dopaminergic neurons. Biofactors 2015, 41, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Rajapakse, N.; Shimizu, K.; Payne, M.; Busija, D. Isolation and characterization of intact mitochondria from neonatal rat brain. Brain Res. Brain Res. Protoc. 2001, 8, 176–183. [Google Scholar] [CrossRef]
- Ghadially, F.N. Ultrastructural Pathology of the Cell and Matrix, 3rd ed.; Wellington Butterworths: London, UK, 1988; pp. 191–328. [Google Scholar]
- Bazylianska, V.; Sharma, A.; Chauhan, H.; Schneider, B.; Moszczynska, A. Dopamine and Methamphetamine Differentially Affect Electron Transport Chain Complexes and Parkin in Rat Striatum: New Insight into Methamphetamine Neurotoxicity. Int. J. Mol. Sci. 2021, 23, 363. [Google Scholar] [CrossRef]
- Sulzer, D.; Pothos, E.; Sung, H.M.; Maidment, N.T.; Hoebel, B.G.; Rayport, S. Weak base model of amphetamine action. Ann. N. Y. Acad. Sci. 1992, 654, 525–528. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.M.; Hanson, G.R.; Fleckenstein, A.E. Methamphetamine rapidly decreases vesicular dopamine uptake. J. Neurochem. 2000, 74, 2221–2223. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, V.; Riddle, E.L.; Hanson, G.R.; Fleckenstein, A.E. Methylphenidate alters vesicular monoamine transport and prevents methamphetamine-induced dopaminergic deficits. J. Pharmacol. Exp. Ther 2003, 304, 1181–1187. [Google Scholar] [CrossRef]
- Fazeli, G.; Oli, R.G.; Schupp, N.; Stopper, H. The role of the dopamine transporter in dopamine-induced DNA damage. Brain Pathol. 2011, 21, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Sango, J.; Kakihana, T.; Takahashi, M.; Katsuragi, Y.; Anisimov, S.; Komatsu, M.; Fujii, M. USP10 inhibits the dopamine-induced reactive oxygen species-dependent apoptosis of neuronal cells by stimulating the antioxidant Nrf2 activity. J. Biol. Chem. 2022, 298, 101448. [Google Scholar] [CrossRef] [PubMed]
- Keller, J.N.; Huang, F.F.; Dimayuga, E.R.; Maragos, W.F. Dopamine induces proteasome inhibition in neural PC12 cell line. Free Radic Biol. Med. 2000, 29, 1037–1042. [Google Scholar] [CrossRef]
- Chiba, K.; Trevor, A.; Castagnoli, N., Jr. Metabolism of the neurotoxic tertiary amine, MPTP, by brain monoamine oxidase. Biochem. Bioph Res. Commun. 1984, 120, 574–578. [Google Scholar] [CrossRef]
- Kopin, I.J. MPTP: An industrial chemical and contaminant of illicit narcotics stimulates a new era in research on Parkinson’s disease. Environ. Health Perspect. 1987, 75, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Kim-Han, J.S.; Antenor-Dorsey, J.A.; O’Malley, K.L. The parkinsonian mimetic, MPP+, specifically impairs mitochondrial transport in dopamine axons. J. Neurosci. 2011, 31, 7212–7221. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.H.; Gusdon, A.M.; Cimen, H.; Van Houten, B.; Koc, E.; Chu, C.T. Impaired mitochondrial biogenesis contributes to depletion of functional mitochondria in chronic MPP+ toxicity: Dual roles for ERK1/2. Cell Death Dis. 2012, 3, e312. [Google Scholar] [CrossRef]
- Choi, J.W.; Song, M.Y.; Park, K.S. Quantitative proteomic analysis reveals mitochondrial protein changes in MPP(+)-induced neuronal cells. Mol. Biosyst. 2014, 10, 1940–1947. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, M.F.; Wagner, G.C. Neurochemical and functional consequences following 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine (MPTP) and methamphetamine. Life Sci. 1985, 36, 249–254. [Google Scholar] [CrossRef]
- Sonsalla, P.K.; Nicklas, W.J.; Heikkila, R.E. Role for excitatory amino acids in methamphetamine-induced nigrostriatal dopaminergic toxicity. Science 1989, 243, 398–400. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.; Hanson, G.R.; Gibb, J.W. Effect of MK-801 on the decrease in tryptophan hydroxylase induced by methamphetamine and its methylenedioxy analog. Eur. J. Pharmacol. 1989, 165, 315–318. [Google Scholar] [CrossRef]
- Marshall, J.F.; O’Dell, S.J.; Wethmuller, F.B. Dopamine-glutamate interactions in methamphetamine-induced neurotoxicity. J. Neural. Transm. (Gen. Sec.) 1993, 91, 241–254. [Google Scholar] [CrossRef]
- Kupsch, A.; Löschmann, P.A.; Sauer, H.; Arnold, G.; Renner, P.; Pufal, D.; Burg, M.; Wachtel, H.; ten Bruggencate, G.; Oertel, W.H. Do NMDA receptor antagonists protect against MPTP-toxicity? Biochemical and immunocytochemical analyses in black mice. Brain Res. 1992, 592, 74–83. [Google Scholar] [CrossRef]
- Sonsalla, P.K.; Zeevalk, G.D.; Manzino, L.; Giovanni, A.; Nicklas, W.J. MK-801 fails to protect against the dopaminergic neuropathology produced by systemic 1-methyl-4-phenyl-l,2,3,6-tetrahydropyridine in mice or intranigral 1-methyl-4-phenylpyridinium in rats. J. Neurochem. 1992, 58, 1979–1982. [Google Scholar] [CrossRef]
- Tipton, K.F.; Singer, T.P. Advances in our understanding of the mechanisms of the neuroloxicity of MPTP and related compounds. J. Neurochem. 1993, 61, 1191–1206. [Google Scholar] [CrossRef]
- Singh, M.; Murthy, V.; Ramassamy, C. Neuroprotective mechanisms of the standardized extract of Bacopa monniera in a paraquat/diquat-mediated acute toxicity. Neurochem. Int. 2013, 62, 530–539. [Google Scholar] [CrossRef]
- Pandareesh, M.D.; Anand, T. Neuroprotective and anti-apoptotic propensity of Bacopa monniera extract against sodium nitroprusside induced activation of iNOS, heat shock proteins and apoptotic markers in PC12 cells. Neurochem. Res. 2014, 39, 800–814. [Google Scholar] [CrossRef]
- Pandareesh, M.D.; Anand, T. Attenuation of smoke induced neuronal and physiological changes by bacoside rich extract in Wistar rats via down regulation of HO-1 and iNOS. Neurotoxicology 2014, 40, 33–42. [Google Scholar] [CrossRef]
- Pandareesh, M.D.; Anand, T.; Bhat, P.V. Cytoprotective propensity of Bacopa monniera against hydrogen peroxide induced oxidative damage in neuronal and lung epithelial cells. Cytotechnology 2016, 68, 157–172. [Google Scholar] [CrossRef]
- Singh, B.; Pandey, S.; Yadav, S.K.; Verma, R.; Singh, S.P.; Mahdi, A.A. Role of ethanolic extract of Bacopa monnieri against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) induced mice model via inhibition of apoptotic pathways of dopaminergic neurons. Brain Res. Bull. 2017, 135, 120–128. [Google Scholar] [CrossRef]
- Fornai, F.; Lenzi, P.; Lazzeri, G.; Ferrucci, M.; Fulceri, F.; Giorgi, F.S.; Falleni, A.; Ruggieri, S.; Paparelli, A. Fine ultrastructure and biochemistry of PC12 cells: A comparative approach to understand neurotoxicity. Brain Res. 2007, 1129, 174–190. [Google Scholar] [CrossRef] [PubMed]
- Nellore, J.; Pauline, C.; Amarnath, K. Bacopa monnieri Phytochemicals Mediated Synthesis of Platinum Nanoparticles and Its Neurorescue Effect on 1-Methyl 4-Phenyl 1,2,3,6 Tetrahydropyridine-Induced Experimental Parkinsonism in Zebrafish. J. Neurodegener. Dis. 2013, 2013, 972391. [Google Scholar] [CrossRef]
- Mishra, A.; Mishra, A.K.; Jha, S. Effect of traditional medicine brahmi vati and bacoside A-rich fraction of Bacopa monnieri on acute pentylenetetrzole-induced seizures, amphetamine-induced model of schizophrenia, and scopolamine-induced memory loss in laboratory animals. Epilepsy Behav. 2018, 80, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Achliya, G.S.; Wadodkar, S.G.; Dorle, A.K. Evaluation of sedative and anticonvulsant activities of Unmadnashak Ghrita. J. Ethnopharmacol. 2004, 94, 77–83. [Google Scholar] [CrossRef]
- Jash, R.; Chowdary, K.A. Ethanolic extracts of Alstonia Scholaris and Bacopa Monniera possess neuroleptic activity due to anti-dopaminergic effect. Pharmacogn. Res. 2014, 6, 46–51. [Google Scholar] [CrossRef]
- Bhatia, G.; Singh, J.; Nehru, B. Neuroprotective effects of hydro-alcoholic extract of Eclipta alba against 1-methyl-4-phenylpyridinium-induced in vitro and in vivo models of Parkinson’s disease. Environ. Sci. Pollut. Res. Int. 2021, 28, 9390–9406. [Google Scholar] [CrossRef] [PubMed]
- Perez Visñuk, D.; Teran, M.D.M.; Savoy de Giori, G.; LeBlanc, J.G.; de Moreno de LeBlanc, A. Neuroprotective Effect of Riboflavin Producing Lactic Acid Bacteria in Parkinsonian Models. Neurochem. Res. 2022, 47, 1269–1279. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.L.; Wang, C.D.; Wang, T.; Ding, H.; Zhou, M.; Yang, N.; Liu, Y.Y.; Chan, P. Ganoderma lucidum extract ameliorates MPTP-induced parkinsonism and protects dopaminergic neurons from oxidative stress via regulating mitochondrial function, autophagy, and apoptosis. Acta Pharmacol. Sin. 2019, 40, 441–450. [Google Scholar] [CrossRef]
- Zhu, Z.; Liu, L.F.; Su, C.F.; Liu, J.; Tong, B.C.; Iyaswamy, A.; Krishnamoorthi, S.; Sreenivasmurthy, S.G.; Guan, X.J.; Kan, Y.X.; et al. Corynoxine B derivative CB6 prevents Parkinsonian toxicity in mice by inducing PIK3C3 complex-dependent autophagy. Acta Pharmacol. Sin. 2022, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Fleming, A.; Bourdenx, M.; Fujimaki, M.; Karabiyik, C.; Krause, G.J.; Lopez, A.; Martín-Segura, A.; Puri, C.; Scrivo, A.; Skidmore, J.; et al. The different autophagy degradation pathways and neurodegeneration. Neuron 2022, 110, 935–966. [Google Scholar] [CrossRef]
- Song, X.; Violin, J.D.; Seidler, F.J.; Slotkin, T.A. Modeling the Developmental Neurotoxicity of Chlorpyrifosin Vitro: Macromolecule Synthesis in PC12 Cells. Toxicol. Appl. Pharmacol. 1998, 151, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Qiao, D.; Seidler, F.J.; Slotkin, T.A. Developmental neurotoxicity of chlorpyrifos modeled in vitro: Comparative effects of metabolites and other cholinesterase inhibitors on DNA synthesis in PC12 and C6 cells. Environ. Health Perspect. 2001, 109, 909–913. [Google Scholar] [CrossRef] [PubMed]
- Phrompittayarat, W.; Wittaya-areekul, S.; Jetiyanon, K.; Putalun, W.; Tanaka, H.; Ingkaninan, K. Stability studies of saponins in Bacopa monnieri dried ethanolic extracts. Planta Med. 2008, 74, 1756–1763. [Google Scholar] [CrossRef] [PubMed]
- Mendhulkar, V.D.; Patade, P.S.; Singh, S.N. Dmso induced product recovery of bacoside a in cell suspension culture of bacopa monnieri linn. Int. J. Pharm. Sci. Res. 2011, 2, 3006–3009. [Google Scholar]
- Brimson, J.M.; Prasanth, M.I.; Plaingam, W.; Tencomnao, T. Bacopa monnieri (L.) wettst. Extract protects against glutamate toxicity and increases the longevity of Caenorhabditis elegans. J. Tradit. Complement. Med. 2019, 10, 460–470. [Google Scholar] [CrossRef]
- Huangteerakul, C.; Aung, H.M.; Thosapornvichai, T.; Duangkaew, M.; Jensen, A.N.; Sukrong, S.; Ingkaninan, K.; Jensen, L.T. Chemical-Genetic Interactions of Bacopa monnieri Constituents in Cells Deficient for the DNA Repair Endonuclease RAD1 Appear Linked to Vacuolar Disruption. Molecules 2021, 26, 1207. [Google Scholar] [CrossRef]
- Leung, H.W.; Foo, G.; Banumurthy, G.; Chai, X.; Ghosh, S.; Mitra-Ganguli, T.; VanDongen, A.M.J. The effect of Bacopa monnieri on gene expression levels in SH-SY5Y human neuroblastoma cells. PLoS ONE 2017, 12, e0182984. [Google Scholar] [CrossRef]
- Schmued, L.C.; Hopkins, K.J. Fluoro-Jade B: A high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 2000, 874, 123–130. [Google Scholar] [CrossRef]
- Giovanni, A.; Liang, L.P.; Hastings, T.G.; Zigmond, M.J. Estimating hydroxyl radical content in rat brain using systemic andintraventricular salicylate: Impact of methamphetamine. J. Neurochem. 1995, 64, 1819–1825. [Google Scholar] [CrossRef]
- Battaglia, G.; Fornai, F.; Busceti, C.L.; Aloisi, G.; Cerrito, F.; De Blasi, A.; Melchiorri, D.; Nicoletti, F. Selective blockade of mGlu5 metabotropic glutamate receptors is protective against methamphetamine neurotoxicity. J. Neurosci. 2002, 22, 2135–2141. [Google Scholar] [CrossRef] [PubMed]
- Pendergrass, W.; Wolf, N.; Poot, M. Efficacy of MitoTracker Green and CMXrosamine to measure changes in mitochondrial membrane potentials in living cells and tissues. Cytometry 2004, 61, 162–169. [Google Scholar] [CrossRef]
- Gautam, N.; Sankaran, S.; Yason, J.A.; Tan, K.S.W.; Gascoigne, N.R.J. A high content imaging flow cytometry approach to study mitochondria in T cells: MitoTracker Green FM dye concentration optimization. Methods 2018, 134–135, 11–19. [Google Scholar] [CrossRef]
- Ferese, R.; Lenzi, P.; Fulceri, F.; Biagioni, F.; Fabrizi, C.; Gambardella, S.; Familiari, P.; Frati, A.; Limanaqi, F.; Fornai, F. Quantitative Ultrastructural Morphometry and Gene Expression of mTOR-Related Mitochondriogenesis within Glioblastoma Cells. Int. J. Mol. Sci. 2020, 21, 4570. [Google Scholar] [CrossRef] [PubMed]













Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrucci, M.; Busceti, C.L.; Lazzeri, G.; Biagioni, F.; Puglisi-Allegra, S.; Frati, A.; Lenzi, P.; Fornai, F. Bacopa Protects against Neurotoxicity Induced by MPP+ and Methamphetamine. Molecules 2022, 27, 5204. https://doi.org/10.3390/molecules27165204
Ferrucci M, Busceti CL, Lazzeri G, Biagioni F, Puglisi-Allegra S, Frati A, Lenzi P, Fornai F. Bacopa Protects against Neurotoxicity Induced by MPP+ and Methamphetamine. Molecules. 2022; 27(16):5204. https://doi.org/10.3390/molecules27165204
Chicago/Turabian StyleFerrucci, Michela, Carla Letizia Busceti, Gloria Lazzeri, Francesca Biagioni, Stefano Puglisi-Allegra, Alessandro Frati, Paola Lenzi, and Francesco Fornai. 2022. "Bacopa Protects against Neurotoxicity Induced by MPP+ and Methamphetamine" Molecules 27, no. 16: 5204. https://doi.org/10.3390/molecules27165204
APA StyleFerrucci, M., Busceti, C. L., Lazzeri, G., Biagioni, F., Puglisi-Allegra, S., Frati, A., Lenzi, P., & Fornai, F. (2022). Bacopa Protects against Neurotoxicity Induced by MPP+ and Methamphetamine. Molecules, 27(16), 5204. https://doi.org/10.3390/molecules27165204








