Diindolylmethane Inhibits Cadmium-Induced Autophagic Cell Death via Regulation of Oxidative Stress in HEL299 Human Lung Fibroblasts
Abstract
:1. Introduction
2. Results
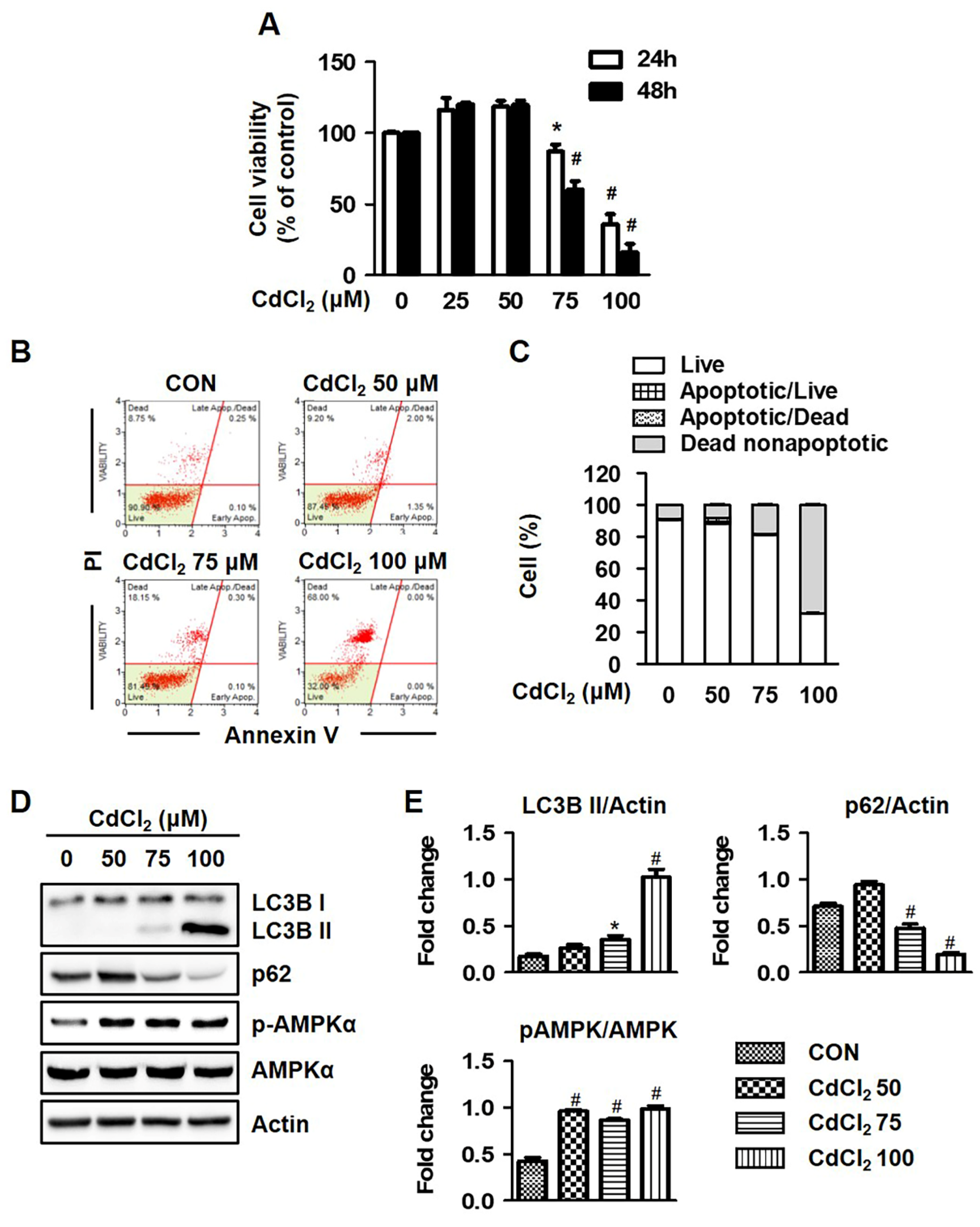
2.1. Cadmium Chloride (Cd) Induces Autophagic Cell Death in HEL299 Cell Lines
2.2. Cadmium Induces Oxidative Stress in Lung Fibroblast
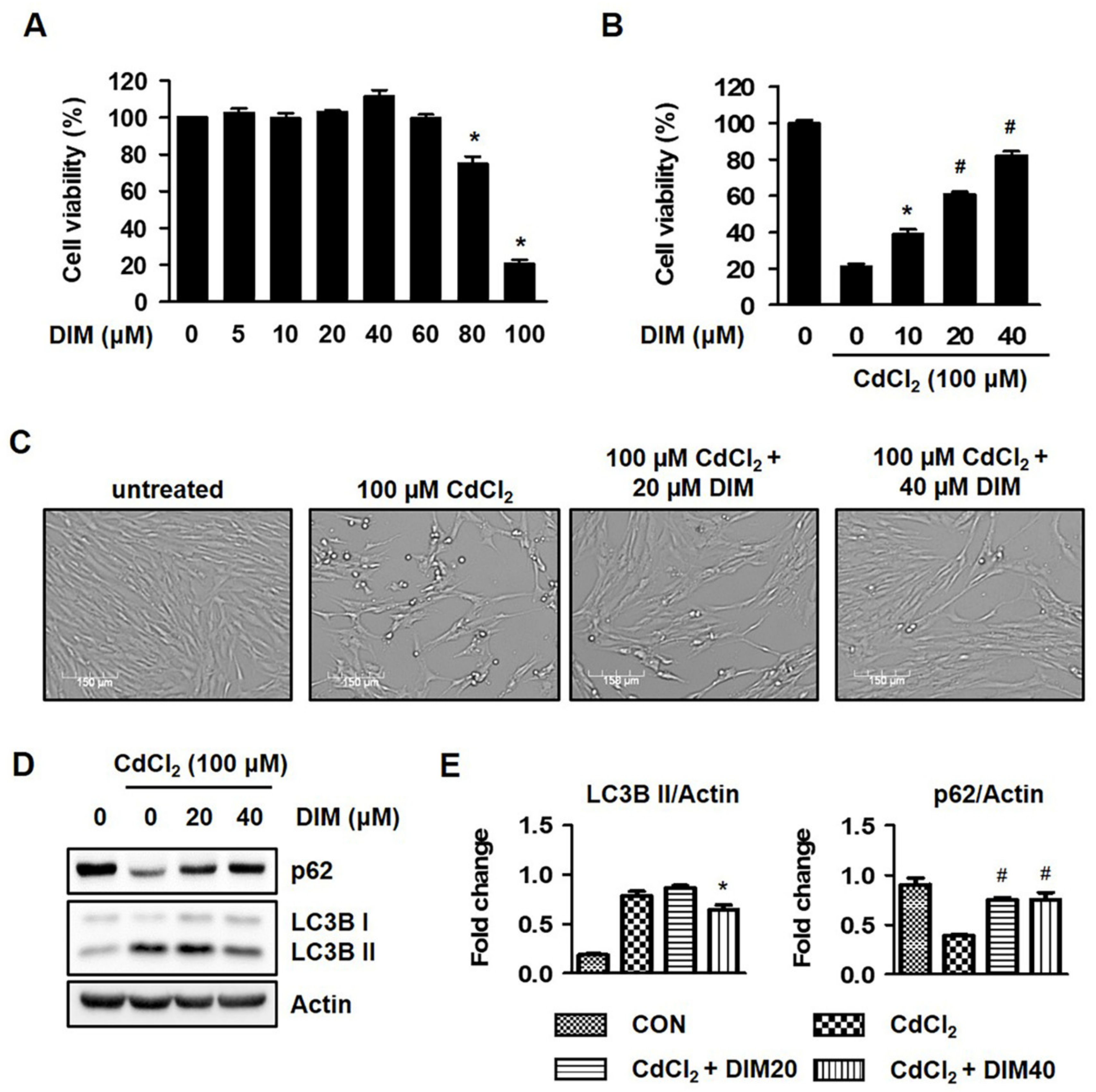
2.3. Diindolylmethane (DIM) Inhibits Cd-Mediated Autophagic Cell Death of HEL299 Cells
2.4. DIM Inhibits Cd-Mediated Autophagic Cell Death by Regulating the Intracellular ROS and ER Stress Levels
3. Discussion
4. Materials and Methods
4.1. Antibodies, Reagents, and Chemicals
4.2. Cell Culture and Treatment
4.3. Cell Viability Assay
4.4. Western Blot Analysis
4.5. Measurement of Intracellular Level of ROS
4.6. Annexin V and Dead Cell Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bajpai, V.K.; Alam, M.B.; Quan, K.T.; Kwon, K.-R.; Ju, M.-K.; Choi, H.-J.; Lee, J.S.; Yoon, J.-I.; Majumder, R.; Rather, I.A.; et al. Antioxidant Efficacy and the Upregulation of Nrf2-Mediated Ho-1 Expression by (+)-Lariciresinol, a Lignan Isolated from Rubia Philippinensis, through the Activation of P38. Sci. Rep. 2017, 7, 46035. [Google Scholar] [CrossRef] [PubMed]
- Godt, J.; Scheidig, F.; Grosse-Siestrup, C.; Esche, V.; Brandenburg, P.; Reich, A.; Groneberg, D.A. The toxicity of cadmium and resulting hazards for human health. J. Occup. Med. Toxicol. 2006, 1, 22. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-S.; Kim, S.-H.; Jeon, D.; Kim, H.-Y.; Han, J.-Y.; Kim, B.; Lee, K. Low-dose cadmium exposure exacerbates polyhexamethylene guanidine-induced lung fibrosis in mice. J. Toxicol. Environ. Health Part A 2018, 81, 384–396. [Google Scholar] [CrossRef] [PubMed]
- Chargui, A.; Zekri, S.; Jacquillet, G.; Rubera, I.; Ilie, M.; Belaid, A.; Duranton, C.; Tauc, M.; Hofman, P.; Poujeol, P.; et al. Cadmium-Induced Autophagy in Rat Kidney: An Early Biomarker of Subtoxic Exposure. Toxicol. Sci. 2011, 121, 31–42. [Google Scholar] [CrossRef]
- Gobe, G.; Crane, D. Mitochondria, reactive oxygen species and cadmium toxicity in the kidney. Toxicol. Lett. 2010, 198, 49–55. [Google Scholar] [CrossRef]
- Hutchinson, D. Cadmium lung adsorption, citrullination and an enhanced risk of COPD. Eur. Respir. Rev. 2018, 27, 180054. [Google Scholar] [CrossRef]
- Tinkov, A.A.; Filippini, T.; Ajsuvakova, O.P.; Aaseth, J.; Gluhcheva, Y.G.; Ivanova, J.M.; Bjørklund, G.; Skalnaya, M.G.; Gatiatulina, E.R.; Popova, E.V.; et al. The role of cadmium in obesity and diabetes. Sci. Total Environ. 2017, 601–602, 741–755. [Google Scholar] [CrossRef]
- Zhang, L.; Xia, Q.; Zhou, Y.; Li, J. Endoplasmic reticulum stress and autophagy contribute to cadmium-induced cytotoxicity in retinal pigment epithelial cells. Toxicol. Lett. 2019, 311, 105–113. [Google Scholar] [CrossRef]
- Cuypers, A.; Plusquin, M.; Remans, T.; Jozefczak, M.; Keunen, E.; Gielen, H.; Opdenakker, K.; Nair, A.R.; Munters, E.; Artois, T.; et al. Cadmium stress: An oxidative challenge. BioMetals 2010, 23, 927–940. [Google Scholar] [CrossRef]
- Ivanova, J.; Gluhcheva, Y.; Tsanova, D.; Piskova, A.; Djaleva, R.; Mokresheva, S.; Kamenova, D.; Mitewa, M. On the effect of chelating agents and antioxidants on cadmium-induced organ toxicity. An overview. Eur. J. Chem. 2013, 4, 74–84. [Google Scholar] [CrossRef]
- Wang, T.T.; Schoene, N.W.; Milner, J.A.; Kim, Y.S. Broccoli-Derived Phytochemicals Indole-3-Carbinol and 3,3′-Diindolylmethane Exerts Concentration-Dependent Pleiotropic Effects on Prostate Cancer Cells: Comparison with Other Cancer Preventive Phytochemicals. Mol. Carcinog. 2012, 51, 244–256. [Google Scholar] [CrossRef]
- Marques, M.; Laflamme, L.; Benassou, I.; Cissokho, C.; Guillemette, B.; Gaudreau, L. Low Levels of 3,3′-Diindolylmethane Activate Estrogen Receptor Alpha and Induce Proliferation of Breast Cancer Cells in the Absence of Estradiol. BMC Cancer 2014, 14, 524. [Google Scholar] [CrossRef]
- Kim, S.M. Cellular and Molecular Mechanisms of 3,3′-Diindolylmethane in Gastrointestinal Cancer. Int. J. Mol. Sci. 2016, 17, 1155. [Google Scholar] [CrossRef]
- Thomson, C.A.; Ho, E.; Strom, M.B. Chemopreventive Properties of 3,3′-Diindolylmethane in Breast Cancer: Evidence from Experimental and Human Studies. Nutr. Rev. 2016, 74, 432–443. [Google Scholar] [CrossRef]
- Thomson, C.A.; Chow, H.H.S.; Wertheim, B.C.; Roe, D.J.; Stopeck, A.; Maskarinec, G.; Altbach, M.; Chalasani, P.; Huang, C.; Strom, M.B.; et al. A randomized, placebo-controlled trial of diindolylmethane for breast cancer biomarker modulation in patients taking tamoxifen. Breast Cancer Res. Treat. 2017, 165, 97–107. [Google Scholar] [CrossRef]
- Morales-Prieto, D.M.; Herrmann, J.; Osterwald, H.; Kochhar, P.S.; Schleussner, E.; Markert, U.R.; Oettel, M. Comparison of Dienogest Effects Upon 3,3′-Diindolylmethane Supplementation in Models of Endometriosis and Clinical Cases. Reprod. Biol. 2018, 18, 252–258. [Google Scholar] [CrossRef]
- Castanon, A.; Tristram, A.; Mesher, D.; Powell, N.; Beer, H.; Ashman, S.; Rieck, G.; Fielder, H.; Fiander, A.; Sasieni, P. Effect of Diindolylmethane Supplementation on Low-Grade Cervical Cytological Abnormalities: Double-Blind, Randomised, Controlled Trial. Br. J. Cancer 2012, 106, 45–52. [Google Scholar] [CrossRef]
- Yang, H.; Seo, S.G.; Shin, S.H.; Min, S.; Kang, M.J.; Yoo, R.; Kwon, J.Y.; Yue, S.; Kim, K.H.; Cheng, J.-X.; et al. 3,3′-Diindolylmethane suppresses high-fat diet-induced obesity through inhibiting adipogenesis of pre-adipocytes by targeting USP2 activity. Mol. Nutr. Food Res. 2017, 61, 1700119. [Google Scholar] [CrossRef]
- Ampofo, E.; Schmitt, B.M.; Menger, M.D.; Laschke, M.W. Targeting the Microcirculation by In-dole-3-Carbinol and Its Main Derivate 3,3,′-Diindolylmethane: Effects on Angiogenesis, Thrombosis and Inflammation. Mini Rev. Med. Chem. 2018, 18, 962–968. [Google Scholar] [CrossRef]
- Rzemieniec, J.; Wnuk, A.; Lason, W.; Bilecki, W.; Kajta, M. The Neuroprotective Action of 3,3′-Diindolylmethane against Ischemia Involves an Inhibition of Apoptosis and Autophagy That Depends on Hdac and Ahr/Cyp1a1 but Not Eralpha/Cyp19a1 Signaling. Apoptosis 2019, 24, 435–452. [Google Scholar] [CrossRef]
- Hajra, S.; Basu, A.; Singha Roy, S.; Patra, A.R.; Bhattacharya, S. Attenuation of Doxorubicin-Induced Cardi-otoxicity and Genotoxicity by an Indole-Based Natural Compound 3,3′-Diindolylmethane (Dim) through Activation of Nrf2/Are Signaling Pathways and Inhibiting Apoptosis. Free Radic. Res. 2017, 51, 812–827. [Google Scholar] [CrossRef]
- Ma, Q. Role of Nrf2 in Oxidative Stress and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Lionaki, E.; Markaki, M.; Palikaras, K.; Tavernarakis, N. Mitochondria, autophagy and age-associated neurodegenerative diseases: New insights into a complex interplay. Biochim. Biophys. Acta—Bioenerg. 2015, 1847, 1412–1423. [Google Scholar] [CrossRef]
- Choi, S.; Kim, H. The Remedial Potential of Lycopene in Pancreatitis through Regulation of Autophagy. Int. J. Mol. Sci. 2020, 21, 5775. [Google Scholar] [CrossRef]
- Glick, D.; Barth, S.; MacLeod, K.F. Autophagy: Cellular and molecular mechanisms. J. Pathol. 2010, 221, 3–12. [Google Scholar] [CrossRef]
- Mizushima, N.; Levine, B. Autophagy in mammalian development and differentiation. Nat. Cell Biol. 2010, 12, 823–830. [Google Scholar] [CrossRef]
- Chang, Z.; Shi, G.; Jin, J.; Guo, H.; Guo, X.; Luo, F.; Song, Y.; Jia, X. Dual PI3K/mTOR inhibitor NVP-BEZ235-induced apoptosis of hepatocellular carcinoma cell lines is enhanced by inhibitors of autophagy. Int. J. Mol. Med. 2013, 31, 1449–1456. [Google Scholar] [CrossRef]
- Son, Y.-O.; Wang, X.; Hitron, J.A.; Zhang, Z.; Cheng, S.; Budhraja, A.; Ding, S.; Lee, J.-C.; Shi, X. Cadmium induces autophagy through ROS-dependent activation of the LKB1–AMPK signaling in skin epidermal cells. Toxicol. Appl. Pharmacol. 2011, 255, 287–296. [Google Scholar] [CrossRef]
- Liu, W.J.; Ye, L.; Huang, W.F.; Guo, L.J.; Xu, Z.G.; Wu, H.L.; Yang, C.; Liu, H.F. p62 links the autophagy pathway and the ubiqutin–proteasome system upon ubiquitinated protein degradation. Cell. Mol. Biol. Lett. 2016, 21, 29. [Google Scholar] [CrossRef]
- Lee, J. 3,3′-Diindolylmethane Inhibits Tnf-α- and Tgf-β-Induced Epithelial-Mesenchymal Transition in Breast Cancer Cells. Nutr. Cancer 2019, 71, 992–1006. [Google Scholar] [CrossRef]
- Huang, Z.; Zuo, L.; Zhang, Z.; Liu, J.; Chen, J.; Dong, L.; Zhang, J. 3,3′-Diindolylmethane decreases VCAM-1 expression and alleviates experimental colitis via a BRCA1-dependent antioxidant pathway. Free Radic. Biol. Med. 2011, 50, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, M.C.; Zhang, D.D. The emerging role of the Nrf2–Keap1 signaling pathway in cancer. Genes Dev. 2013, 27, 2179–2191. [Google Scholar] [CrossRef] [PubMed]
- Bartolini, D.; Dallaglio, K.; Torquato, P.; Piroddi, M.; Galli, F. Nrf2-p62 autophagy pathway and its response to oxidative stress in hepatocellular carcinoma. Transl. Res. 2018, 193, 54–71. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, S.; Saito, T.; Obata, M.; Koide, R.H.; Ichimura, Y.; Komatsu, M. Negative Regulation of the Keap1-Nrf2 Pathway by a P62/Sqstm1 Splicing Variant. Mol. Cell. Biol. 2018, 38, e00642-17. [Google Scholar] [CrossRef]
- Fan, S.; Meng, Q.; Saha, T.; Sarkar, F.H.; Rosen, E.M. Low Concentrations of Diindolylmethane, a Metabolite of Indole-3-Carbinol, Protect against Oxidative Stress in a BRCA1-Dependent Manner. Cancer Res. 2009, 69, 6083–6091. [Google Scholar] [CrossRef]
- He, J.; Huang, T.; Zhao, L. 3,3′-Diindolylmethane mitigates lipopolysaccharide-induced acute kidney injury in mice by inhibiting NOX-mediated oxidative stress and the apoptosis of renal tubular epithelial cells. Mol. Med. Rep. 2019, 19, 5115–5122. [Google Scholar] [CrossRef]
- Lee, H.-S.; Safe, S.; Lee, S.-O. Inactivation of the orphan nuclear receptor NR4A1 contributes to apoptosis induction by fangchinoline in pancreatic cancer cells. Toxicol. Appl. Pharmacol. 2017, 332, 32–39. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, Y.-S.; Lee, H.J.; Hyun, M.; Kim, H.-J.; Kim, J.-H.; Hwang, K.-H.; Kim, W.-S.; Choi, J.; Heo, J.D. Diindolylmethane Inhibits Cadmium-Induced Autophagic Cell Death via Regulation of Oxidative Stress in HEL299 Human Lung Fibroblasts. Molecules 2022, 27, 5215. https://doi.org/10.3390/molecules27165215
Jung Y-S, Lee HJ, Hyun M, Kim H-J, Kim J-H, Hwang K-H, Kim W-S, Choi J, Heo JD. Diindolylmethane Inhibits Cadmium-Induced Autophagic Cell Death via Regulation of Oxidative Stress in HEL299 Human Lung Fibroblasts. Molecules. 2022; 27(16):5215. https://doi.org/10.3390/molecules27165215
Chicago/Turabian StyleJung, Yeon-Seop, Ho Jeong Lee, Moonjung Hyun, Hye-Jin Kim, Je-Hein Kim, Kwang-Hyun Hwang, Woong-Soo Kim, Jungil Choi, and Jeong Doo Heo. 2022. "Diindolylmethane Inhibits Cadmium-Induced Autophagic Cell Death via Regulation of Oxidative Stress in HEL299 Human Lung Fibroblasts" Molecules 27, no. 16: 5215. https://doi.org/10.3390/molecules27165215





