Heptanuclear Silver Hydride Clusters as Catalytic Precursors for the Reduction of 4-Nitrophenol
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of the Silver Clusters
2.2. Nuclear Magnetic Resonance (NMR) Spectroscopy
2.3. Mass Spectrometry
2.4. X-ray Crystallography
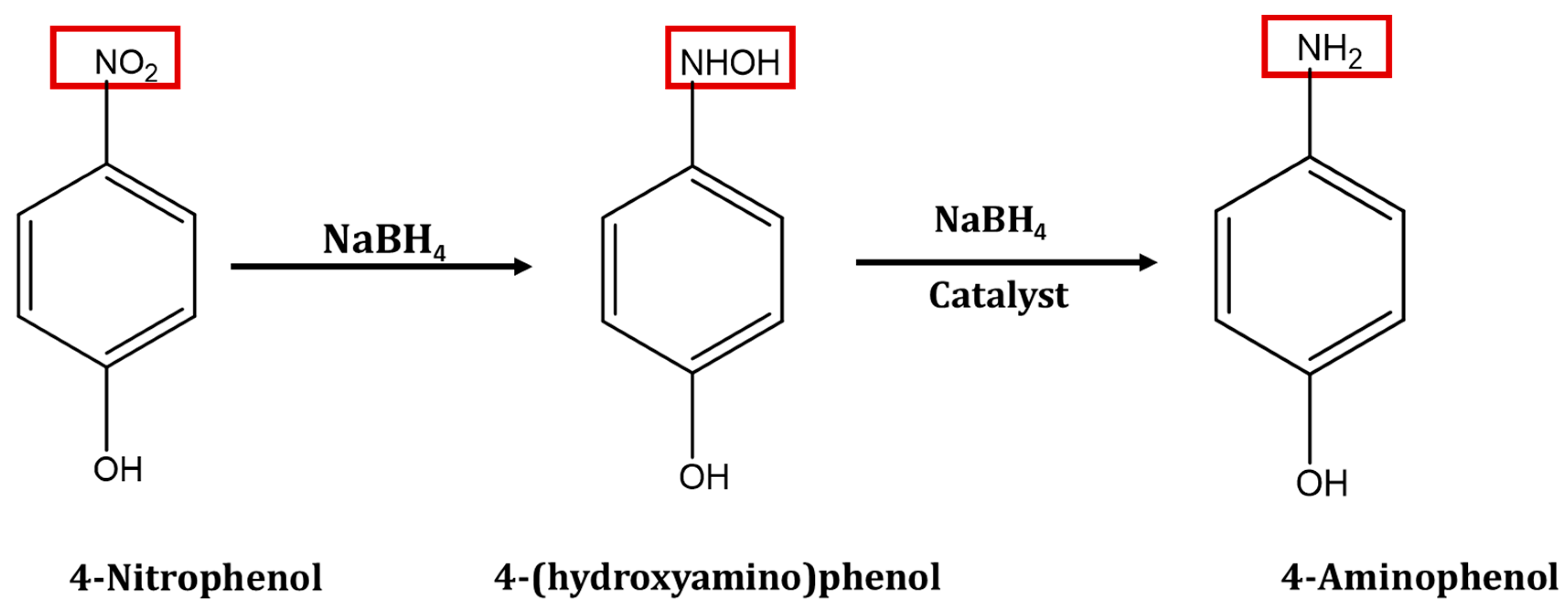
3. In Situ Production of Silver Nanoparticle and Reduction of 4-Nitrophenol
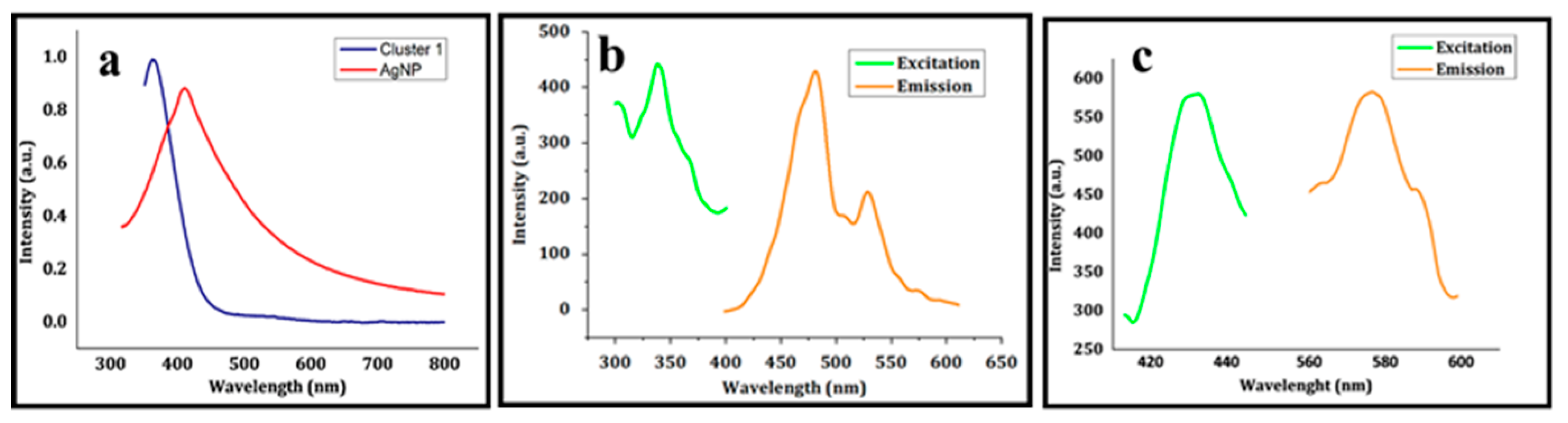
4. Properties of Cluster 1 and the Synthesized Silver Nanoparticles
5. Materials and Methods
5.1. General Procedures and Instrumentation
5.2. Synthesis of Silver Clusters
5.2.1. Synthesis of cluster 1 ([Ag7(H){(S2P(4-C6H4OMe)(OC4H9)}6])
5.2.2. Synthesis of Cluster 1D ([Ag7(D){(S2P(4-C6H4OMe)(OC4H9)}6])
5.2.3. Synthesis of Cluster 2 ([Ag7(H){S2P(4-C6H4OMe)(OC3H7)}6])
5.2.4. Synthesis of Cluster 2D ([Ag7(D) {S2P(4-C6H4OMe)(OC3H7)}6])
5.2.5. Synthesis of Cluster 3 ([Ag7(H){S2P(4-C6H4OMe)(OCH2C6H5)}6])
5.2.6. Synthesis of Cluster 4 ([Ag7(H){S2P(4-C6H4OMe)(OC5H11)}6])
5.3. Characterization of AgNP
5.4. Catalytic Reduction of 4-NP by Cluster 1 and AgNPs
5.5. X-ray Crystallographic Determination
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Wurtz, A. Sur l’hydrure de cuivre. Ann. Chim. 1844, 11, 250–252. [Google Scholar]
- Kaesz, H.D.; Saillant, R.-B. Hydride complexes of the transition metals. Chem. Rev. 1972, 72, 231–281. [Google Scholar] [CrossRef]
- Lin, Z.; Hall, M.B. Transition metal polyhydride complexes: A theoretical view. Coord. Chem. Rev. 1994, 135, 845–879. [Google Scholar] [CrossRef]
- Pillay, M.N.; Van Zyl, W.E.; Liu, C.W. A construction guide for high-nuclearity (≥50 metal atoms) coinage metal clusters at nanoscale: Bridging molecular precise constructs with the bulk materials phase. Nanoscale 2020, 12, 24331–24348. [Google Scholar] [CrossRef]
- Maseras, F.; Lledos, A.; Clot, E.; Eisenstein, O. Transition metal polyhydrides: From qualitative ideas to reliable computational studies. Chem. Rev. 2000, 100, 601–636. [Google Scholar] [CrossRef] [PubMed]
- Hoskin, A.J.; Stephan, D.W. Early transition metal hydride complexes: Synthesis and reactivity. Coord. Chem. Rev. 2002, 233, 107–129. [Google Scholar] [CrossRef]
- Morris, R.H. Dihydrogen, dihydride and in between: NMR and structural properties of iron group complexes. Coord. Chem. Rev. 2008, 252, 2381–2394. [Google Scholar] [CrossRef]
- Van Zyl, W.E.; Liu, C.W. Interstitial hydrides in nanoclusters can reduce M(I) (M = Cu, Ag, Au) to M(0) and form stable superatoms. Chem.–Eur. J. 2022, 28, e202104241. [Google Scholar] [CrossRef]
- Gloaguen, F.; Rauchfuss, T.B. Small molecule mimics of hydrogenases: Hydrides and redox. Chem. Soc. Rev. 2009, 38, 100–108. [Google Scholar] [CrossRef]
- King, R. Structure and bonding in homoleptic transition metal hydride anions. Coord. Chem. Rev. 2000, 200, 813–829. [Google Scholar] [CrossRef]
- Dhayal, R.S.; Van Zyl, W.E.; Liu, C.W. Copper hydride clusters in energy storage and conversion. Dalton Trans. 2019, 48, 3531–3538. [Google Scholar] [CrossRef] [PubMed]
- Henderson, R.A. Metal hydride intermediates in hydrogenases and nitrogenases: Enzymological and model studies. In Recent Advances in Hydride Chemistry, 1st ed.; Peruzzini, M., Poli, R., Eds.; Elsevier: Amsterdam, The Netherland, 2001; pp. 463–505. [Google Scholar]
- Angelici, R.J. Heterogeneous catalysis of the hydrodesulfurization of thiophenes in petroleum: An organometallic perspective of the mechanism. Acc. Chem. Res. 1988, 21, 387–394. [Google Scholar] [CrossRef]
- Brestensky, D.M.; Stryker, J.M. Regioselective conjugate reduction and reductive silylation of α, β-unsaturated. Tetrahedron Lett. 1989, 30, 5677–5680. [Google Scholar] [CrossRef]
- Miller, K.M.; Luanphaisarnnont, T.; Molinaro, C.; Jamison, T.F. Alkene-directed, nickel-catalyzed alkyne coupling reactions. J. Am. Chem. Soc. 2004, 126, 4130–4131. [Google Scholar] [CrossRef]
- Brayshaw, S.K.; Harrison, A.; McIndoe, J.S.; Marken, F.; Raithby, P.R.; Warren, J.E.; Weller, A.S. Sequential reduction of high hydride count octahedral rhodium clusters [Rh6(PR3)6H12][BArF4]2: Redox-switchable hydrogen storage. J. Am. Chem. Soc. 2007, 129, 1793–1804. [Google Scholar] [CrossRef]
- Shimizu, K.I.; Sato, R.; Satsuma, A. Direct C-C Cross-Coupling of Secondary and Primary Alcohols Catalyzed by a γ-Alumina-Supported Silver Subnanocluster. Angew. Chem. Int. Ed. 2009, 48, 3982–3986. [Google Scholar] [CrossRef]
- Shimizu, K.I.; Sugino, K.; Sawabe, K.; Satsuma, A. Oxidant-Free Dehydrogenation of Alcohols Heterogeneously Catalyzed by Cooperation of Silver Clusters and Acid–Base Sites on Alumina. Chem. Eur. J. 2009, 15, 2341–2351. [Google Scholar] [CrossRef]
- Shimizu, K.; Nishimura, M.; Satsuma, A. γ-Alumina-Supported Silver Cluster for N-Benzylation of Anilines with Alcohols. ChemCatChem 2009, 1, 497–503. [Google Scholar] [CrossRef]
- Shimizu, K.-I.; Satsuma, A. Selective catalytic reduction of NO over supported silver catalysts—practical and mechanistic aspects. Phys. Chem. Chem. Phys. 2006, 8, 2677–2695. [Google Scholar] [CrossRef]
- Shimizu, K.-I.; Satsuma, A. Reaction mechanism of H2-promoted selective catalytic reduction of NO with NH3 over Ag/Al2O3. J. Phys. Chem. C 2007, 111, 2259–2264. [Google Scholar] [CrossRef]
- Shimizu, K.-I.; Tsuzuki, M.; Kato, K.; Yokota, S.; Okumura, K.; Satsuma, A. Reductive activation of O2 with H2-reduced silver clusters as a key step in the H2-promoted selective catalytic reduction of NO with C3H8 over Ag/Al2O3. J. Phys. Chem. C 2007, 111, 950–959. [Google Scholar] [CrossRef]
- Liao, J.H.; Dhayal, R.S.; Wang, X.; Kahlal, S.; Saillard, J.Y.; Liu, C.W. Neutron diffraction studies of a four-coordinated hydride in near square-planar geometry. Inorg. Chem. 2014, 53, 11140–11145. [Google Scholar] [CrossRef] [PubMed]
- Dhayal, R.S.; Liao, J.H.; Lin, Y.R.; Liao, P.K.; Kahlal, S.; Saillard, J.Y.; Liu, C.W. A nanospheric polyhydrido copper cluster of elongated triangular orthobicupola array: Liberation of H2 from solar energy. J. Am. Chem. Soc. 2013, 135, 4704–4707. [Google Scholar] [CrossRef] [PubMed]
- Edwards, A.J.; Dhayal, R.S.; Liao, P.K.; Liao, J.H.; Chiang, M.H.; Piltz, R.O.; Kahlal, S.; Saillard, J.Y.; Liu, C.W. Chinese puzzle molecule: A 15 hydride, 28 copper atom nanoball. Angew. Chem. Int. Ed. 2014, 53, 7214–7218. [Google Scholar] [CrossRef]
- Dhayal, R.S.; Chen, H.-P.; Liao, J.-H.; van Zyl, W.E.; Liu, C.W. Synthesis, Structural Characterization, and H2 Evolution Study of a Spheroid-Shape Hydride-Rich Copper Nanocluster. ChemistrySelect 2018, 3, 3603–3610. [Google Scholar] [CrossRef]
- Dhayal, R.S.; Liao, J.H.; Kahlal, S.; Wang, X.; Liu, Y.C.W.; Chiang, M.H.; van Zyl, W.E.; Saillard, J.Y.; Liu, C.W. [Cu32(H)20{S2P(OiPr)2}12]: The Largest Number of Hydrides Recorded in a Molecular Nanocluster by Neutron Diffraction. Eur. J. Chem. 2015, 21, 8369–8374. [Google Scholar] [CrossRef]
- Brown, S.S.; Salter, I.D.; Šik, V.; Colquhoun, I.J.; McFarlane, W.; Bates, P.A.; Hursthouse, M.B.; Murray, M. The heteronuclear cluster chemistry of the group 1B metals. Part 9. Stereochemical non-rigidity of the metal skeletons of cluster compounds in solution. 109Ag-{1H}INEPT nuclear magnetic resonance studies on [Ag2Ru4(µ3-H)2{µ-Ph2P(CH2)nPPh2}(CO)12](n = 1, 2, or 4) and X-ray crystal structure of [Ag2Ru4(µ3-H)2(µ-Ph2PCH2PPh2)(CO)12]. J. Chem. Soc. Dalton Trans. 1988, 8, 2177–2185. [Google Scholar] [CrossRef]
- Beringhelli, T.; D’Alfonso, G.; Garavaglia, M.G.; Panigati, M.; Mercandelli, P.; Sironi, A. Synthesis, Solid-State Structure and Solution Behavior of Hydrido-Bridged Adducts between the Group 11 [M(PPh3)]+ Cations and the Triangular Cluster Anion [Re3(μ-H)4(CO)9(PPh3)]. Organometallics 2002, 21, 2705–2714. [Google Scholar] [CrossRef]
- Carreno, R.; Riera, V.; Ruiz, M.A.; Tiripicchio, A.; Tiripicchio-Camellini, M. Reactivity of [Mn2(.mu-H)2(CO)6 (.mu.-tedip)](tedip=(EtO)2POP(OEt)2) with Group 11 Alkynyl Compounds. X-ray Structures of [Ag2Mn4 (.mu.-H)6(CO)12(.mu-tedip)2] and [AuMn4(.mu.-H)5(CO)12(.mu-tedip)2]. Organometallics 1994, 13, 993–1004. [Google Scholar] [CrossRef]
- Gorol, M.; Mösch-Zanetti, N.C.; Roesky, H.W.; Noltemeyer, M.; Schmidt, H.-G. Unprecedented stabilisation of the Ag22+-ion by two hydrido-iridium (iii) complexes. Chem. Commun. 2003, 46–47. [Google Scholar] [CrossRef]
- Albinati, A.; Chaloupka, S.; Demartin, F.; Koetzle, T.F.; Ruegger, H.; Venanzi, L.M.; Wolfer, M.K. Complexes with platinum-hydrogen-silver bonds. J. Am. Chem. Soc. 1993, 115, 169–175. [Google Scholar] [CrossRef]
- Cook, A.W.; Nguyen, T.-A.D.; Buratto, W.R.; Wu, G.; Hayton, T.W. Synthesis, characterization, and reactivity of the group 11 hydrido clusters [Ag6H4(dppm)4(OAc)2] and [Cu3H(dppm)3(OAc)2]. Inorg. Chem. 2016, 55, 12435–12440. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.W.; Lin, Y.R.; Fang, C.S.; Latouche, C.; Kahlal, S.; Saillard, J.Y. [Ag7(H){E2P(OR)2}6] (E = Se, S): Precursors for the fabrication of silver nanoparticles. Inorg. Chem. 2013, 52, 2070–2077. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Ahlrichs, R.; Anson, C.E.; Rothenberger, A.; Schrodt, C.; Shafaei-Fallah, M. Reactions of P/S-containing proligands with coinage metal salts: A new route to polynuclear complexes with unusual structural types. Chem. Commun. 2005, 47, 5893–5895. [Google Scholar] [CrossRef]
- Van Zyl, W.E. Dithiophosphonates and related P/S-type ligands of group 11 metals. Comments Inorg. Chem. 2010, 31, 13–45. [Google Scholar] [CrossRef]
- Pillay, M.N.; Omondi, B.; Staples, R.J.; Van Zyl, W.E. A hexanuclear gold(I) metallatriangle derived from a chiral dithiophosphate: Synthesis, structure, luminescence and oxidative bromination reactivity. CrystEngComm 2013, 15, 4417–4421. [Google Scholar] [CrossRef]
- Lee, Y.C.; Lin, Y.R.; Liou, B.Y.; Liao, J.H.; Gusarova, N.K.; Trofimov, B.A.; Van Zyl, W.E.; Liu, C.W. Dinuclear gold(I) dithio- and diselenophosph(in)ate complexes forming mononuclear gold(III) oxidative addition complexes and reversible chemical reductive elimination products. Dalton Trans. 2014, 43, 663–670. [Google Scholar] [CrossRef]
- Ajayi, T.J.; Pillay, M.N.; Van Zyl, W.E. Solvent-free mechanochemical synthesis of dithiophosphonic acids and corresponding nickel(II) complexes. Phosphorus Sulf. Silicon Relat. Elem. 2017, 192, 1205–1211. [Google Scholar] [CrossRef]
- Aditya, T.; Pal, A.; Pal, T. Nitroarene reduction: A trusted model reaction to test nanoparticle catalysts. Chem Commun. 2015, 51, 9410–9431. [Google Scholar] [CrossRef]
- Zhao, P.; Feng, X.; Huang, D.; Yang, G.; Astruc, D. Basic concepts and recent advances in nitrophenol reduction by gold-and other transition metal nanoparticles. Coord. Chem. Rev. 2015, 287, 114–136. [Google Scholar] [CrossRef]
- Rajegaonkar, P.S.; Deshpande, B.A.; More, M.S.; Waghmare, S.S.; Sangawe, V.V.; Inamdar, A.; Shirsat, M.D.; Adhapure, N.N. Catalytic reduction of p-nitrophenol and methylene blue by microbiologically synthesized silver nanoparticles. Mater. Sci. Eng. C 2018, 93, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Ai, L.; Jiang, J. Catalytic reduction of 4-nitrophenol by silver nanoparticles stabilized on environmentally benign macroscopic biopolymer hydrogel. Bioresour. Technol. 2013, 132, 374–377. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Kuang, D.; Feng, Y.; Zhang, F.; Xu, Z.; Liu, M. A graphene oxide-based electrochemical sensor for sensitive determination of 4-nitrophenol. J. Hazard. Mater. 2012, 201, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.-Y.; Gao, Z.-W.; Yang, K.-F.; Zhang, W.-Q.; Xu, L.-W. Nanosilver as a new generation of silver catalysts in organic transformations for efficient synthesis of fine chemicals. Catal. Sci. Technol. 2015, 5, 2554–2574. [Google Scholar] [CrossRef]
- Kästner, C.; Thünemann, A.F. Catalytic reduction of 4-nitrophenol using silver nanoparticles with adjustable activity. Langmuir 2016, 32, 7383–7391. [Google Scholar] [CrossRef]
- Begum, R.; Farooqi, Z.H.; Ahmed, E.; Naseem, K.; Ashraf, S.; Sharif, A.; Rehan, R. Catalytic reduction of 4-nitrophenol using silver nanoparticles-engineered poly (N-isopropylacrylamide-co-acrylamide) hybrid microgels. Appl. Organomet. Chem. 2017, 31, e3563. [Google Scholar] [CrossRef]
- Liao, P.-K.; Fang, C.-S.; Edwards, A.J.; Kahlal, S.; Saillard, J.-Y.; Liu, C.W. Hydrido copper clusters supported by dithiocarbamates: Oxidative hydride removal and neutron diffraction analysis of [Cu7(H){S2C(aza-15-crown-5)}6]. Inorg. Chem. 2012, 51, 6577–6591. [Google Scholar] [CrossRef]
- Liu, C.W.; Chang, H.-W.; Sarkar, B.; Saillard, J.-Y.; Kahlal, S.; Wu, Y.-Y. Stable Silver(I) Hydride Complexes Supported by Diselenophosphate Ligands. Inorg. Chem. 2010, 49, 468–475. [Google Scholar] [CrossRef]
- Latouche, C.; Kahlal, S.; Lin, Y.-R.; Liao, J.-H.; Furet, E.; Liu, C.W.; Saillard, J.-Y. Anion encapsulation and geometric changes in hepta-and hexanuclear copper (I) dichalcogeno clusters: A theoretical and experimental investigation. Inorg. Chem. 2013, 52, 13253–13262. [Google Scholar] [CrossRef]
- Xie, Y.; Yan, B.; Xu, H.; Chen, J.; Liu, Q.; Deng, Y.; Zeng, H. Highly Regenerable Mussel-Inspired Fe3O4@Polydopamine-Ag Core–Shell Microspheres as Catalyst and Adsorbent for Methylene Blue Removal. ACS Appl. Mater. Int. 2014, 6, 8845–8852. [Google Scholar] [CrossRef]
- Baruah, B.; Gabriel, G.J.; Akbashev, M.J.; Booher, M.E. Facile synthesis of silver nanoparticles stabilized by cationic polynorbornenes and their catalytic activity in 4-nitrophenol reduction. Langmuir 2013, 29, 4225–4234. [Google Scholar] [CrossRef] [PubMed]
- Kubas, G.J.; Monzyk, B.; Crumblis, A.L. Tetrakis(Acetonitrile)Copper(1+) Hexafluorophosphate(1-). Inorg. Synth. 1990, 51, 68–70. [Google Scholar] [CrossRef]
- Van Zyl, W.E.; Woollins, J.D. The coordination chemistry of dithiophosphonates: An emerging and versatile ligand class. Coord. Chem. Rev. 2013, 257, 718–731. [Google Scholar] [CrossRef]
- Yusuf, T.L.; Quadri, T.W.; Tolufashe, G.F.; Olasunkanmi, L.O.; Ebenso, E.E.; Van Zyl, W.E. Synthesis and structures of divalent Co, Ni, Zn and Cd complexes of mixed dichalcogen and dipnictogen ligands with corrosion inhibition properties: Experimental and computational studies. RSC Adv. 2020, 10, 41967–41982. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G. A short history of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Sheldrick, G.A. Crystal structure refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yusuf, T.L.; Ogundare, S.A.; Pillay, M.N.; van Zyl, W.E. Heptanuclear Silver Hydride Clusters as Catalytic Precursors for the Reduction of 4-Nitrophenol. Molecules 2022, 27, 5223. https://doi.org/10.3390/molecules27165223
Yusuf TL, Ogundare SA, Pillay MN, van Zyl WE. Heptanuclear Silver Hydride Clusters as Catalytic Precursors for the Reduction of 4-Nitrophenol. Molecules. 2022; 27(16):5223. https://doi.org/10.3390/molecules27165223
Chicago/Turabian StyleYusuf, Tunde L., Segun A. Ogundare, Michael N. Pillay, and Werner E. van Zyl. 2022. "Heptanuclear Silver Hydride Clusters as Catalytic Precursors for the Reduction of 4-Nitrophenol" Molecules 27, no. 16: 5223. https://doi.org/10.3390/molecules27165223






