Radiopharmaceutical Treatments for Cancer Therapy, Radionuclides Characteristics, Applications, and Challenges
Abstract
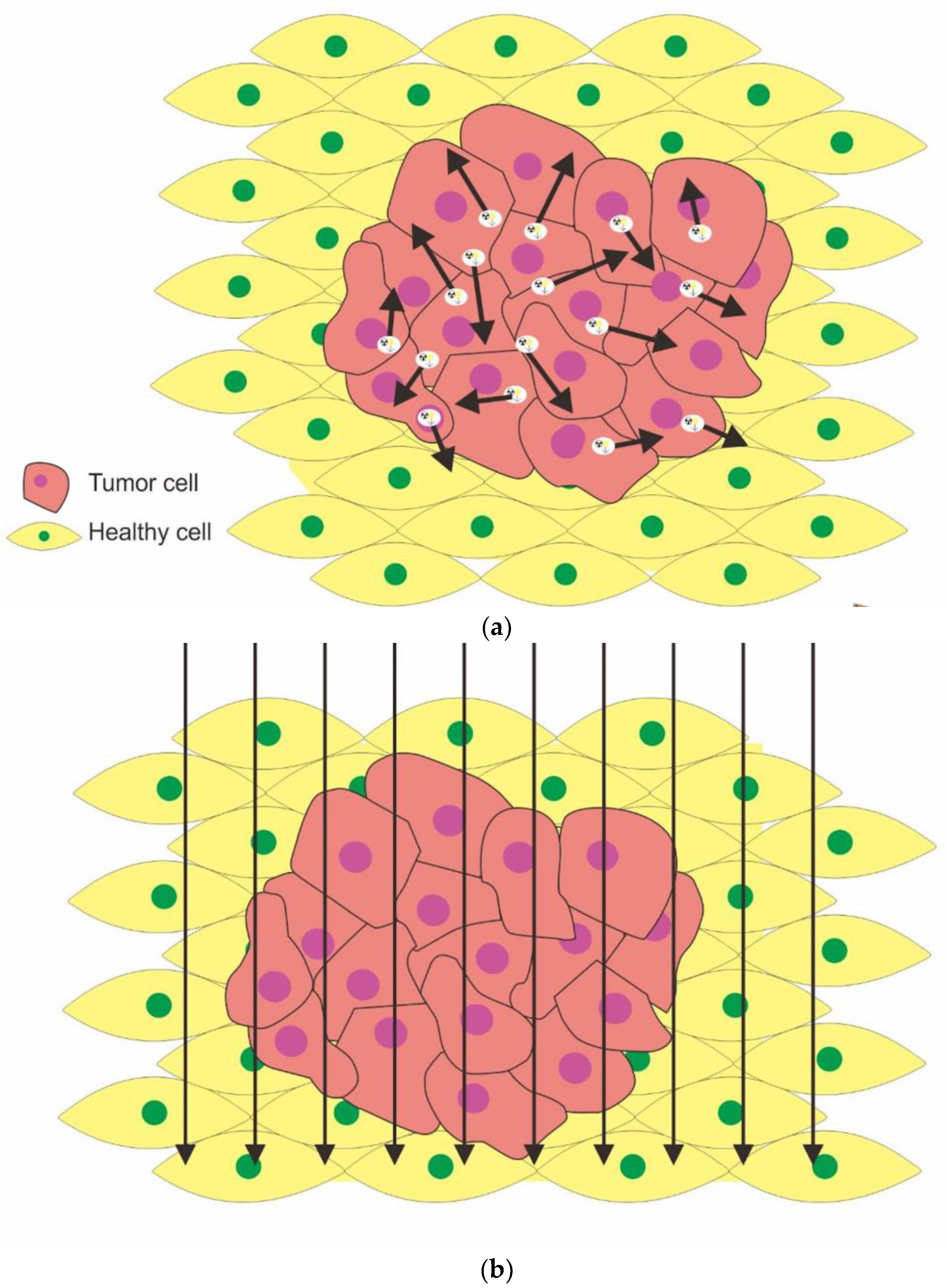
:1. Introduction
2. Radionuclide Emission Properties
2.1. Beta Particles
2.2. Alpha Particles
| Alpha Particle | Beta Particle | Auger Electron | |
|---|---|---|---|
| Type of particles | 4He nucleus | Energetic electron | Low energy electron; electron capture (ec) and/or internal conversion (ic) |
| Particle energy | 4–9 MeV | 50–2300 keV | 25–80 keV |
| Particle path length | 40–100 μm | 0.05–12 mm | Nanomicrometers |
| Linear energy transfer | ~80 keV/μm | ~0.2 keV/μm | 4–26 keV/μm |
| Hypoxic tumors | Effective | Less effective | Effective |
| Toxicity | Effective in creating double-strand breaks in DNA | High dose rates (tumor survival rates close to linear exponential). Low dose rates (single-strand breaks), repairable with shouldering the dose-response curve | Potential creation of double-strand breaks DNA, and cell membrane |
| Bystander effect/crossfire | Yes/low | Yes | Yes |
| Tumor size | Micro/small | Higher volume solid tumor | Micro |

2.3. Auger Electrons
3. Therapy Application
3.1. Antibodies
3.2. Prostate-Specific Membrane Antigen (PSMA)
3.3. Peptide Receptor Radionuclide Therapy (PRRT)
3.4. Radioiodine Concentration via Sodium Iodide Symporter
3.5. Nanotargeted Radionuclides
4. Challenges in Radiopharmaceutical Therapy
5. Conclusions and Future Direction
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goldsmith, S.J. Targeted Radionuclide Therapy: A Historical and Personal Review. Semin. Nucl. Med. 2020, 50, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Yeong, C.H.; Cheng, M.H.; Ng, K.H. Therapeutic Radionuclides in Nuclear Medicine: Current and Future Prospects. J. Zhejiang Univ. Sci. B 2014, 15, 845–863. [Google Scholar] [CrossRef] [PubMed]
- Ercan, M.T.; Caglar, M. Therapeutic Radiopharmaceuticals. In Current Pharmaceutical Design; Bentham Science Publishers: Sharjah, United Arab Emirates, 2000; Volume 6, pp. 1085–1121. [Google Scholar]
- Gabriel, M. Radionuclide therapy beyond radioiodine. Wien. Med. Wochenschr. 2012, 162, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Hillegonds, D.J.; Franklin, S.; Shelton, D.K.; Vijayakumar, S.; Vijayakumar, V. The management of painful bone metastases with an emphasis on radionuclide therapy. J. Natl. Med. Assoc. 2007, 99, 785–794. [Google Scholar] [PubMed]
- Asadian, S.; Mirzaei, H.; Kalantari, B.A.; Davarpanah, M.R.; Mohamadi, M.; Shpichka, A.; Nasehi, L.; Es, H.A.; Timashev, P.; Najimi, M.; et al. β-radiating radionuclides in cancer treatment, novel insight into promising approach. Pharmacol. Res. 2020, 160, 105070. [Google Scholar] [CrossRef] [PubMed]
- Sgouros, G.; Bodei, L.; McDevitt, M.R.; Nedrow, J.R. Radiopharmaceutical therapy in cancer: Clinical advances and challenges. Nat. Rev. Drug Discov. 2020, 19, 589–608. [Google Scholar] [CrossRef] [PubMed]
- Elliyanti, A. Radiopharmaceuticals in Modern Cancer Therapy. In Radiopharmaceutical Current Research for Better Diagnosis, Therapy, Environmental and Pharmaceutical Applications, 1st ed.; Badria, F.A., Ed.; Intechopen: London, UK, 2021. [Google Scholar] [CrossRef]
- Kramer-Marek, G.; Capala, J. The role of nuclear medicine in modern therapy of cancer. Tumor Biol. 2012, 33, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, K.; Schwaiger, M.; Lewis, J.S.; Solomon, S.B.; McNeil, B.J.; Baumann, M.; Gambhir, S.S.; Hricak, H.; Weissleder, R. Radiotheranostics: A roadmap for future development. Lancet Oncol. 2020, 21, e146–e156. [Google Scholar] [CrossRef]
- Kumar, C.; Shetake, N.; Desai, S.; Kumar, A.; Samuel, G.; Pandey, B.N. Relevance of radiobiological concepts in radionuclide therapy of cancer. Int. J. Radiat. Biol. 2016, 92, 173–186. [Google Scholar] [CrossRef]
- Wulbrand, C.; Seidl, C.; Gaertner, F.C.; Bruchertseifer, F.; Morgenstern, A.; Essler, M.; Senekowitsch-Schmidtke, R. Alpha-particle emitting 213Bi-anti- EGFR immunoconjugates eradicate tumor cells independent of oxygenation. PLoS ONE 2013, 8, e64730. [Google Scholar] [CrossRef]
- Calais, P.J.; Turner, J.H. Outpatient 131I-rituximab radoimmunotherapy for non-Hodgkin lymphoma: A study in safety. Clin. Nucl. Med. 2012, 37, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Qaim, S.M. Therapeutic radionuclides and nuclear data. Radiochim. Acta 2001, 89, 297–304. [Google Scholar] [CrossRef]
- Ferrier, M.G.; Radchenko, V. An appendix of radionuclides used in targeted alpha therapy. J. Med. Imaging Radiat. Sci. 2019, 50, S58–S65. [Google Scholar] [CrossRef] [PubMed]
- Baskar, R.; Dai, J.; Wenlong, N.; Yeo, R.; Yeoh, K.W. Biological response of cancer cells to radiation treatment. Front. Mol. Biosci. 2014, 1, 24. [Google Scholar] [CrossRef]
- Komal, S.; Nadeem, S.; Faheem, Z.; Raza, A.; Sarwer, K.; Umer, H.; Roohi, S.; Naqvi, S.A.R. Localization Mechanisms of Radiopharmaceuticals. In Medical Isotopes, 1st ed.; Naqvi, S.A.R., Imran, M.B., Eds.; Intechopen: London, UK, 2021. [Google Scholar] [CrossRef]
- Elliyanti, A. An introduction to nuclear medicine in oncological molecular imaging. In Proceedings of the AIP Conference Proceedings, Padang, Indonesia, 10 December 2019. [Google Scholar] [CrossRef]
- Arslan, N.; Emi, M.; Alagöz, E.; Üstünsöz, B.; Oysul, K.; Arpacı, F.; Uğurel, Ş.; Beyzadeoğlu, M.; Ozgüven, M.A. Selective intraarterial radionuclide therapy with Yttrium-90 (Y-90) microspheres for hepatic neuroendocrine metastases: Initial experience at a single center. Vojnosanit. Pregl. 2011, 68, 341–348. [Google Scholar] [CrossRef]
- Kucuk, O.N.; Soydal, C.; Lacin, S.; Ozkan, E.; Bilgic, S. Selective intraarterial radionuclide therapy with yttrium-90 (Y-90) microspheres for unresectable primary and metastatic liver tumors. World J. Surg. Oncol. 2011, 9, 86. [Google Scholar] [CrossRef]
- Houle, S.; Yip, T.K.; Shepherd, F.A.; Rotstein, L.E.; Sniderman, K.W.; Theis, E.; Cawthorn, R.H.; Richmond-Cox, K. Hepatocellular carcinoma: Pilot trial of treatment with Y-90 microspheres. Radiology 1989, 172, 857–860. [Google Scholar] [CrossRef]
- Thamboo, T.; Tan, K.B.; Wang, S.C.; Salto-Tellez, M. Extrahepatic embolisation of Y-90 microspheres from selective internal radiation therapy (SIRT) of the liver. Pathology 2003, 35, 351–353. [Google Scholar]
- Widel, M.; Przybyszewski, W.M.; Cieslar-Pobuda, A.; Saenko, Y.V.; Rzeszowska-Wolny, J. Bystander normal human fibroblasts reduce damage response in radiation targeted cancer cells through intercellular ROS level modulation. Mutat. Res. 2012, 731, 117–124. [Google Scholar] [CrossRef]
- Ku, A.; Facca, V.J.; Cai, Z.; Reilly, R.M. Auger electrons for cancer therapy—A review. EJNMMI Radiopharm. Chem. 2019, 4, 27. [Google Scholar] [CrossRef]
- Elliyanti, A. Molecular Radiobiology and Radionuclides Therapy Concepts. In The Evolutionof Radionanotargeting towards Clinical Precission Oncology: A Festschrift in Honor of Kalevi Kairemo; Jekunen, A., Ed.; Bentham Science: Sharjah, United Arab Emirates, 2022; pp. 395–408. [Google Scholar] [CrossRef]
- Navalkissoor, S.; Grossman, A. Targeted alpha particle therapy for neuroendocrine tumours: The next generation of peptide receptor radionuclide therapy. Neuroendocrinology 2019, 108, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Elliyanti, A. Radioiodine for Graves’ Disease Therapy. In Graves’ Diseas, 1st ed.; Gensure, R., Ed.; Intechopen: London, UK, 2021. [Google Scholar] [CrossRef]
- Slonimsky, E.; Tulchinsky, M. Radiotheragnostics Paradigm for Radioactive Iodine (Iodide) Management of Differentiated Thyroid Cancer. Curr. Pharm. Des. 2020, 26, 3812. [Google Scholar] [CrossRef] [PubMed]
- Luster, M.; Pfestroff, A.; Hänscheid, H.; Verburg, F.A. Radioiodine Therapy. Semin. Nucl. Med. 2017, 47, 126–134. [Google Scholar] [CrossRef]
- Kendi, A.T.; Moncayo, V.M.; Nye, J.A.; Galt, J.R.; Halkar, R.; Schuster, D.M. Radionuclide therapies in molecular imaging and precision medicine. PET Clin. 2017, 12, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Koziorowski, J.; Ballinger, J. Theragnostic radionuclides: A clinical perspective. Q. J. Nucl. Med. Mol. Imaging 2021, 65, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Kassis, I.A.; Adelstein, S.J. Radiobiologic principles of radionuclide therapy. J. Nucl. Med. 2005, 46, 4S–12S. [Google Scholar] [PubMed]
- Gholami, Y.H.; Maschmeyer, R.; Kuncic, Z. Radio-enhancement effects by radiolabeled nanoparticles. Sci. Rep. 2019, 9, 14346. [Google Scholar] [CrossRef]
- Pouget, J.P.; Navarro-Teulon, I.; Bardiès, M.; Chouin, N.; Cartron, G.; Pèlegrin, A.; Azria, D. Clinical Radioimmunotherapy—The role of radiobiology. Nat. Rev. Clin. Oncol. 2011, 8, 720–734. [Google Scholar] [CrossRef]
- Jia, Z.; Wang, W. Yttrium-90 radioembolization for unresectable metastatic neuroendocrine liver tumor: A systematic review. Eur. J. Radiol. 2018, 100, 23–29. [Google Scholar] [CrossRef]
- Filippi, L.; Schillaci, O.; Cianni, R.; Bagni, O. Yttrium-90 resin microspheres and their use in the treatment of intrahepatic cholangiocarcinoma. Future Oncol. 2018, 14, 809–818. [Google Scholar] [CrossRef]
- Oei, A.L.; Verheijen, R.H.; Seiden, M.V.; Benigno, B.B.; Lopes, A.D.B.; Soper, J.T.; Epenetos, A.A.; Massuger, L.F. Decreased intraperitoneal disease recurrence in epithelial ovarian cancer patients receiving intraperitoneal consolidation treatment with yttrium-90-labeled murine HMFG1 without improvement in overall survival. Int. J. Cancer 2007, 120, 2710–2714. [Google Scholar] [CrossRef] [PubMed]
- Waldmann, T.; White, J.; Carrasquillo, J.; Reynolds, J.; Paik, C.; Gansow, O.; Brechbiel, M.; Jaffe, E.; Fleisher, T.; Goldman, C. Radioimmunotherapy of interleukin-2R alpha-expressing adult T-cell leukemia with yttrium-90-labeled anti-Tac. Blood 1995, 86, 4063–4075. [Google Scholar] [CrossRef] [PubMed]
- Nisa, L.; Savelli, G.; Giubbini, R. Yttrium-90 DOTATOC therapy in GEP-NET and other SST2 expressing tumors: A selected review. Ann. Nucl. Med. 2011, 25, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Li, C.; Rosenkrans, Z.T.; Huo, N.; Chen, Z.; Ehlerding, E.B.; Huo, Y.; Ferreira, C.A.; Barnhart, T.E.; Engle, J.W.; et al. CD38-Targeted Theranostics of Lymphoma with 89Zr/177 Lu-Labeled Daratumumab. Adv. Sci. 2021, 8, 2001879. [Google Scholar] [CrossRef]
- Da Silva, T.N.; van Velthuysen, M.L.F.; van Eijck, C.H.J.; Teunissen, J.J.; Hofland, J. Successful neoadjuvant peptide receptor radionuclide therapy for an inoperable pancreatic neuroendocrine tumour. Endocrinol. Diabetes Metab. Case Rep. 2018, 11, 18-0015. [Google Scholar] [CrossRef]
- Sartor, O. Overview of samarium Sm 153 lexidronam in the treatment of painful metastatic bone disease. Rev. Urol. 2004, 6, S3–S12. [Google Scholar]
- Sgouros, G. Alpha-particles for targeted therapy. Adv. Drug Deliv. Rev. 2008, 60, 1402–1406. [Google Scholar] [CrossRef]
- Manafi-Farid, R.; Masoumi, F.; Divband, G.; Saidi, B.; Ataeinia, B.; Hertel, F.; Schweighofer-Zwink, G.; Morgenroth, A.; Beheshti, M. Targeted Palliative Radionuclide Therapy for Metastatic Bone Pain. J. Clin. Med. 2020, 9, 2622. [Google Scholar] [CrossRef]
- Guerra Liberal, F.D.C.; O’Sullivan, J.M.; McMahon, S.J.; Prise, K.M. Targeted Alpha Therapy: Current Clinical Applications. Cancer Biother. Radiopharm. 2020, 35, 404–417. [Google Scholar] [CrossRef]
- Jurcic, J.G.; Levy, M.; Park, J.; Ravandi, F.; Perl, A.; Pagel, J.; Smith, B.D.; Orozco, J.; Estey, E.; Kantarjian, H.; et al. Trial in progress: A phase I/II study of lintuzumab-Ac225 in older patients with untreated acute myeloid leukemia. Clin. Lymphoma Myeloma Leuk. 2017, 17, S277. [Google Scholar] [CrossRef]
- Kleynhans, J.; Sathekge, M.; Ebenhan, T. Obstacles and Recommendations for Clinical Translation of Nanoparticle System-Based Targeted Alpha-Particle Therapy. Materials 2021, 14, 4784. [Google Scholar] [CrossRef] [PubMed]
- Goyal, J.; Antonarakis, E.S. Bone-targeting radiopharma ceuticals for the treatment of prostate cancer with bone metastases. Cancer Lett. 2012, 323, 135. [Google Scholar] [CrossRef] [PubMed]
- Filippi, L.; Chiaravalloti, A.; Schillaci, O.; Cianni, R.; Bagni, O. Theranostic approaches in nuclear medicine: Current status and future prospects. Expert Rev. Med. Devices 2020, 17, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Bertolet, A.; Ramos-Méndez, J.; Paganetti, H.; Schuemann, J. The relation between microdosimetry and induction of direct damage to DNA by alpha particles. Phys. Med. Biol. 2021, 66, 155016. [Google Scholar] [CrossRef] [PubMed]
- Liberini, V.; Huellner, M.W.; Grimaldi, S.; Finessi, M.; Thuillier, P. The Challenge of Evaluating Response to Peptide Receptor Radionuclide Therapy in Gastroenteropancreatic Neuroendocrine Tumors: The Present and the Future. Diagnostics 2020, 10, 1083. [Google Scholar] [CrossRef]
- McDevitt, M.R.; Sgouros, G.; Sofou, S. Targeted and nontargeted α-particle therapies. Annu. Rev. Biomed. Eng. 2018, 20, 73–93. [Google Scholar] [CrossRef]
- Gustafsson-Lutz, A.; Bäck, T.; Aneheim, E.; Hultborn, R.; Palm, S.; Jacobsson, L.; Morgenstern, A.; Bruchertseifer, F.; Albertsson, P.; Lindegren, S. Therapeutic efficacy of α-radioimmunotherapy with different activity levels of the 213Bi-labeled monoclonal antibody MX35 in an ovarian cancer model. EJNMMI Res. 2017, 7, 38. [Google Scholar] [CrossRef]
- Ahenkorah, S.; Cassells, I.; Deroose, C.; Cardinaels, T.; Burgoyne, A.; Bormans, G.; Ooms, M.; Cleeren, F. Bismuth-213 for Targeted Radionuclide Therapy: From Atom to Bedside. Pharmaceutics 2021, 3, 599. [Google Scholar] [CrossRef]
- Silindir-Gunay, M.; Karpuz, M.; Ozer, A.Y. Targeted alpha therapy and Nanocarrier approach. Cancer Biother. Radiopharm. 2020, 35, 446–458. [Google Scholar] [CrossRef]
- Rosar, F.; Krause, J.; Bartholomä, M.; Maus, S.; Stemler, T.; Hierlmeier, I.; Linxweiler, J.; Ezziddin, S.; Khreish, F. Efficacy and safety of [225Ac] Ac-PSMA-617 augmented [177Lu] Lu-PSMA-617 Radioligand therapy in patients with highly advanced mCRPC with poor prognosis. Pharmaceutics 2021, 13, 722. [Google Scholar] [CrossRef]
- Reissig, F.; Wunderlich, G.; Runge, R.; Freudenberg, R.; Lühr, A.; Kotzerke, J. The effect of hypoxia on the induction of strand breaks in plasmid DNA by alpha-, beta- and Auger electron-emitters 223Ra, 188Re, 99mTc and DNA-binding 99mTc-labeled pyrene. Nucl. Med. Biol. 2020, 80–81, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Stéen, E.J.L.; Edem, P.E.; Nørregaard, K.; Jørgensen, J.T.; Shalgunov, V.; Kjaer, A.; Herth, M.M. Pretargeting in nuclear imaging and radionuclide therapy: Improving efficacy of theranostics and nanomedicines. Biomaterials 2018, 179, 209–245. [Google Scholar] [CrossRef] [PubMed]
- Kennel, S.J.; Mirzadeh, S.; Eckelman, W.C.; Waldmann, T.A.; Garmestani, K.; Yordanov, A.T.; Stabin, M.G.; Brechbiel, M.W. Vascular-targeted radioimmunotherapy with the alpha- particle emitter 211At. Radiat. Res. 2002, 157, 633–641. [Google Scholar] [CrossRef]
- Persson, L. The Auger electron effect in radiation dosimetry. Health Phys. 1994, 67, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Widel, M. Radionuclides in radiation-induced bystander effect; may it share in radionuclide therapy? Neoplasma 2017, 64, 641–654. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, D.G.; Diehn, M.; Kesarwala, A.; Maity, A.; Morgan, M.A.; Schwarz, J.K.; Bristow, R.; DeMaria, S.; Eke, I.; Griffin, R.J.; et al. The Future of Radiobiology. J. Natl. Cancer Inst. 2018, 110, 329–340. [Google Scholar] [CrossRef]
- Paillas, S.; Ladjohounlou, R.; Lozza, C.; Pichard, A.; Boudousq, V.; Jarlier, M.; Sevestre, S.; Le Blay, M.; Deshayes, E.; Sosabowski, J.; et al. Localized irradiation of cell membrane by auger electrons is cytotoxic through oxidative stress-mediated nontargeted effects. Antioxid. Redox Signal. 2016, 25, 467–484. [Google Scholar] [CrossRef]
- Pandit-Taskar, N. Targeted Radioimmunotherapy and Theranostics with Alpha Emitters. J. Med. Imaging Radiat. Sci 2019, 50, S41–S44. [Google Scholar] [CrossRef]
- White, J.M.; Escorcia, F.E.; Viola, N.T. Perspectives on metals-based radioimmunotherapy (RIT): Moving forward. Theranostics 2021, 11, 6293–6314. [Google Scholar] [CrossRef]
- Fendler, W.P.; Rahbar, K.; Herrmann, K.; Kratochwil, C.; Eiber, M. 177Lu-PSMA Radioligand Therapy for Prostate Cancer. J. Nucl. Med. 2017, 58, 1196–1200. [Google Scholar] [CrossRef]
- Nevedomskaya, E.; Baumgart, S.J.; Haendler, B. Recent advances in prostate Cancer treatment and drug discovery. Int. J. Mol. Sci. 2018, 19, 1359. [Google Scholar] [CrossRef] [PubMed]
- Lunger, L.; Tauber, R.; Feuerecker, B.; Gschwend, J.E.; Eiber, M. Narrative review: Prostate-specific membrane antigen-radioligand therapy in metastatic castration-resistant prostate cancer. Transl. Androl. Urol. 2021, 10, 3963–3971. [Google Scholar] [CrossRef] [PubMed]
- Kairemo, K.; Joensuu, T. Lu-177-PSMA treatment for metastatic prostate cancer: Case examples of major responses. Clin. Transl Imaging 2018, 6, 223–237. [Google Scholar] [CrossRef]
- Sathekge, M.; Bruchertseifer, F.; Knoesen, O.; Reyneke, F.; Lawal, I.; Lengana, T.; Davis, C.; Mahapane, J.; Corbett, C.; Vorster, M.; et al. 225Ac-PSMA-617 in chemotherapy-naive patients with advanced prostate cancer: A pilot study. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Khreish, F.; Ebert, N.; Ries, M.; Maus, S.; Rosar, F.; Bohnenberger, H.; Stemler, T.; Saar, M.; Bartholomä, M.; Ezziddin, S. 225Ac-PSMA-617/177 Lu-PSMA-617 tandem therapy of metastatic castration-resistant prostate cancer: Pilot experience. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 721–728. [Google Scholar] [CrossRef]
- Kratochwil, C.; Haberkorn, U.; Giesel, F.L. 225Ac-PSMA-617 for Therapy of Prostate Cancer. Semin. Nucl. Med. 2020, 50, 133–140. [Google Scholar] [CrossRef]
- Basu, S.; Parghane, R.V.; Chakrabarty, S. Peptide Receptor Radionuclide Therapy of Neuroendocrine Tumors. Semin. Nucl. Med. 2020, 50, 447–464. [Google Scholar] [CrossRef]
- Kunikowska, J.; Królicki, L. Targeted α-Emitter Therapy of Neuroendocrine Tumors. Semin. Nucl. Med. 2020, 50, 171–176. [Google Scholar] [CrossRef]
- Elliyanti, A.; Rustam, R.; Tofrizal, T.; Yenita, Y.; Susanto, Y.D.B. Evaluating the Natrium iodide Symporter expressions in thyroid Tumors. Open Access Maced. J. Med. Sci. 2021, 9, 18–23. [Google Scholar] [CrossRef]
- Elliyanti, A.; Rusnita, D.; Afriani, N.; Susanto, Y.D.B.; Susilo, V.Y.; Setiyowati, S.; Harahap, W.A. Analysis natrium iodide symporter expression in breast cancer subtypes for radioiodine therapy response. Nucl. Med. Mol. Imaging 2020, 54, 35–42. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Lin, Y.; Liang, J. Radioactive Iodine-Refractory Differentiated Thyroid Cancer and Redifferentiation Therapy. Endocrinol. Metab. 2019, 34, 215–225. [Google Scholar] [CrossRef]
- Schug, C.; Gupta, A.; Urnauer, S.; Steiger, K.; Cheung, P.F.Y.; Neander, C.; Savvatakis, K.; Schmohl, K.A.; Trajkovic-Arsic, M.; Schwenk, N. A Novel Approach for Image-Guided 131I Therapy of Pancreatic Ductal Adenocarcinoma Using Mesenchymal Stem Cell-Mediated NIS Gene Delivery. Mol. Cancer Res. 2019, 17, 310–320. [Google Scholar] [CrossRef]
- Bayoumi, N.A.; El-Kolaly, M.T. Utilization of nanotechnology in targeted radionuclide cancer therapy: Monotherapy, combined therapy and radiosensitization. Radiochim. Acta 2021, 109, 459–475. [Google Scholar] [CrossRef]
- Mirshojaei, S.F.; Ahmadi, A.; Morales-Avila, E.; Ortiz-Reynoso, M.; Reyes-Perez, H. Radiolabelled nanoparticles: Novel classification of radiopharmaceuticals for molecular imaging of cancer. J. Drug Target. 2016, 24, 91–101. [Google Scholar] [CrossRef]
- Ting, G.; Chang, C.H.; Wang, H.E.; Lee, T.W. Nanotargeted radionuclides for cancer nuclear imaging and internal radiotherapy. J. Biomed. Biotechnol. 2010, 2010, 953537. [Google Scholar] [CrossRef]
- Farzin, L.; Sheibani, S.; Moassesi, M.E.; Shamsipur, M. An overview of nanoscale radionuclides and radiolabeled nanomaterials commonly used for nuclear molecular imaging and therapeutic functions. J. Biomed. Mater. Res. A 2019, 107, 251–285. [Google Scholar] [CrossRef]
- Chang, C.H.; Chang, M.C.; Chang, Y.J.; Chen, L.C.; Lee, T.W.; Ting, G. Translating Research for the Radiotheranostics of Nanotargeted 188Re-Liposome. Int. J. Mol. Sci. 2021, 22, 3868. [Google Scholar] [CrossRef]
- Salvanou, E.-A.; Stellas, D.; Tsoukalas, C.; Mavroidi, B.; Paravatou-Petsotas, M.; Kalogeropoulos, N.; Xanthopoulos, S.; Denat, F.; Laurent, G.; Bazzi, R.; et al. A Proof-of-Concept Study on the Therapeutic Potential of Au Nanoparticles Radiolabeled with the Alpha-Emitter Actinium-225. Pharmaceutics 2020, 12, 188. [Google Scholar] [CrossRef]
- Wong, C.H.; Siah, K.W.; Lo, A.W. Estimation of clinical trial success rates and related parameters. Biostatistics 2018, 20, 273–286. [Google Scholar] [CrossRef]
| Radionuclides | Emitting | Physical Half-Life | Mean Eα/β- (MeV) | Primary Eα/β- (MeV) (%) | Mean Range in Soft Tissue (mm) | Indication | References | ||
|---|---|---|---|---|---|---|---|---|---|
| Max | Min | Mean | |||||||
| 131I | β | 8.02 d | 0.606 MeV | 0.069 MeV | 0.356 MeV | 0.3645 MeV (81%) | 0.4 mm | Hyperthyroid, thyroid cancer, Radioimmunotherapy (RIT) for NHL and neuroblastoma, pheochromocytoma, carcinoid, medullary thyroid cancer | [2,3,6,8,22,24] |
| 32P | β | 14.26 d | 1.71 MeV | 0.695 MeV | 1.015 MeV | - | 2.6 mm | Polycythemia vera, keloid, cystic craniopharyngioma, | [2,3,23] |
| 89Sr | β | 50.53 d | 1.491 MeV | 0.583 MeV | 0.908 MeV | 0.91 MeV (0.01%) | 2.4 mm | Bone pain palliation | [2,3,6,8,23] |
| 90Y | β | 64.10 d | 2.284 MeV | 0.935 MeV | 1.349 MeV | (0.01%) | 3.6 mm | Liver metastasis, hepatocellular carcinoma, RIT for NHL, neuroendocrine tumor | [2,3,6,8,22,23] |
| 153Sm | β | 46.50 h | 0.8082 MeV | - | - | 0.1032 MeV (29.8%) | 0.7 mm | Bone pain palliation, synovitis | [2,3,6,8] |
| 169Er | β | 9.4 d | 0.35 MeV | - | - | 0.084 MeV (0.16%) | 0.3 mm | Synovitis | [2,3] |
| 177Lu | β | 6.73 d | 0.497 MeV | 0.047 MeV | 0.208 MeV | 0.208 MeV (11%) | 0.28 mm | Synovitis and RIT for various cancer | [2,6,8,22,23,24] |
| 186Re | β | 3.72 d | 1.077 MeV | 0.308 MeV | 0. 769 MeV | 0.137 MeV (9.4%) | 1.2 mm | Bone pain palliation, arthritis | [2,6,8,23] |
| 188Re | β | 17 h | 2.12 MeV | 0.528 MeV | 1.592 MeV | 0.155 MeV (15%) | 2.1 mm | Bone pain palliation, RIT for various cancer, rheumatoid arthritis | [2,3,8,22,23] |
| 223Ra | α | 11.44 d | 5.9792 MeV | - | 6.59 MeV | 0.154 MeV (5.59%) | 0.054 mm | Bone pain palliation | [2,5,13] |
| 211At | α | 7.2 h | - | - | 6.79 MeV | (5.87%) | 0.057 mm | RIT leukemia, brain tumor, RLT prostate cancer | [2,3,23,25] |
| 213Bi | α | 46 mins | - | - | 8.32MeV | (26%) | 0.078 mm | RIT leukemia, brain tumor | [3,22,23,25] |
| 225Ac | α | 10 d | - | - | 0.218MeV | (11.4%) | 0.05–0.08 mm | Radioligand (RLT) prostate cancer | [2,8,24] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salih, S.; Alkatheeri, A.; Alomaim, W.; Elliyanti, A. Radiopharmaceutical Treatments for Cancer Therapy, Radionuclides Characteristics, Applications, and Challenges. Molecules 2022, 27, 5231. https://doi.org/10.3390/molecules27165231
Salih S, Alkatheeri A, Alomaim W, Elliyanti A. Radiopharmaceutical Treatments for Cancer Therapy, Radionuclides Characteristics, Applications, and Challenges. Molecules. 2022; 27(16):5231. https://doi.org/10.3390/molecules27165231
Chicago/Turabian StyleSalih, Suliman, Ajnas Alkatheeri, Wijdan Alomaim, and Aisyah Elliyanti. 2022. "Radiopharmaceutical Treatments for Cancer Therapy, Radionuclides Characteristics, Applications, and Challenges" Molecules 27, no. 16: 5231. https://doi.org/10.3390/molecules27165231
APA StyleSalih, S., Alkatheeri, A., Alomaim, W., & Elliyanti, A. (2022). Radiopharmaceutical Treatments for Cancer Therapy, Radionuclides Characteristics, Applications, and Challenges. Molecules, 27(16), 5231. https://doi.org/10.3390/molecules27165231






