Secondary Electrons in Gold Nanoparticle Clusters and Their Role in Therapeutic Ratio: The Outcome of a Monte Carlo Simulation Study
Abstract
:1. Introduction
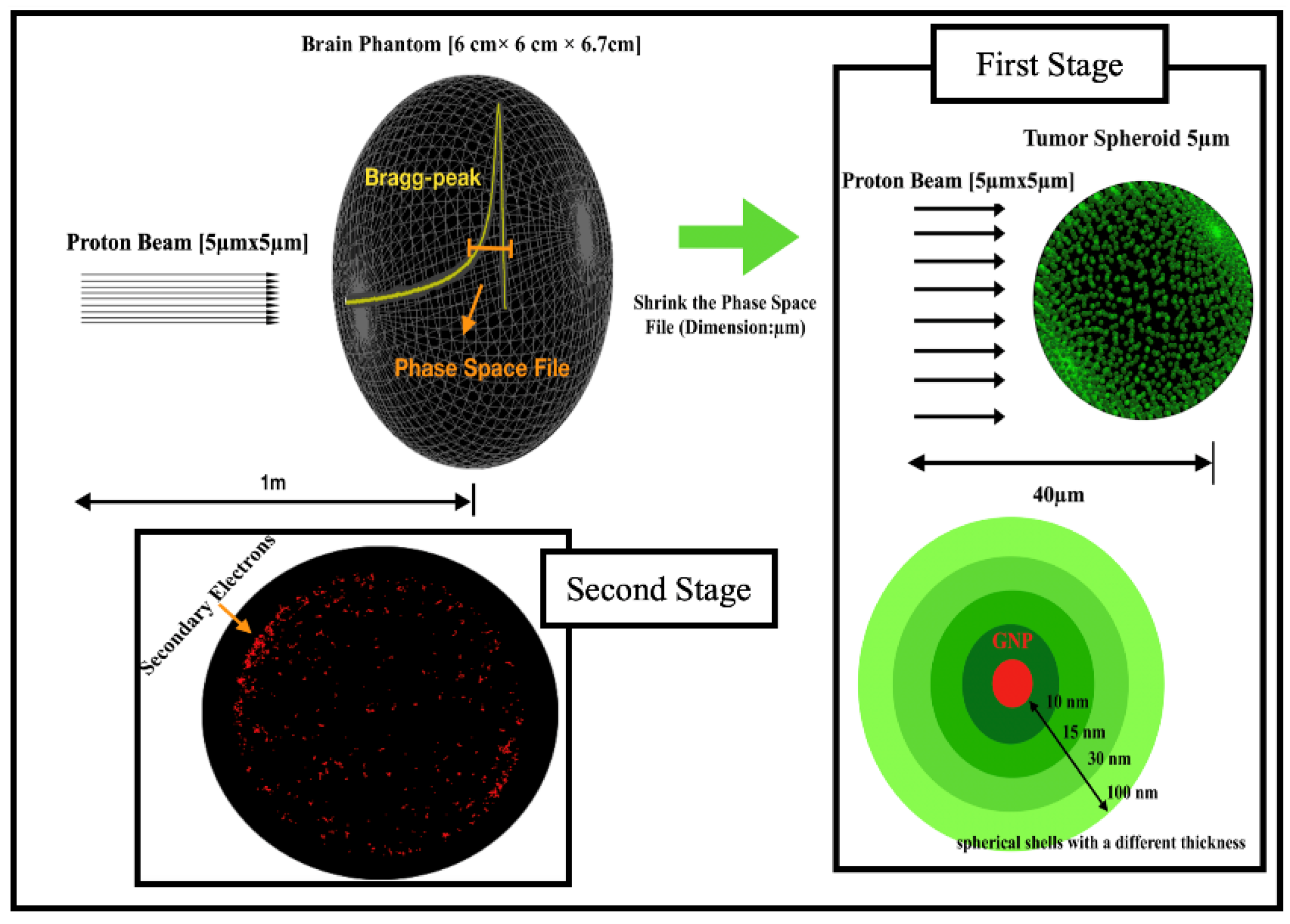
2. Materials and Methods
Simulation Setup
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morozov, V.N.; Belousov, A.V.; Krusanov, G.A.; Kolyvanova, M.A.; Chernyaev, A.P.; Shtil, A.A. Secondary Electron Spectral Changes of Irradiated Gold Nanoparticle Caused by PEGylation. KnE Energy 2018, 3, 278. [Google Scholar] [CrossRef]
- Meola, A.; Rao, J.; Chaudhary, N.; Sharma, M.; Chang, S.D. Gold Nanoparticles for Brain Tumor Imaging: A Systematic Review. Front. Neurol. 2018, 9, 328. [Google Scholar] [CrossRef]
- Butterworth, K.T.; Coulter, J.A.; Jain, S.; Forker, J.; McMahon, S.J.; Schettino, G.; Prise, K.M.; Currell, F.J.; Hirst, D.G. Evaluation of cytotoxicity and radiation enhancement using 1.9 nm gold particles: Potential application for cancer therapy. Nanotechnology 2010, 21, 295101. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Hirst, D.G.; O’Sullivan, J.M. Gold nanoparticles as novel agents for cancer therapy. Br. J. Radiol. 2012, 85, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.J.; Wang, C.H.; Chen, S.T.; Chen, H.H.; Leng, W.H.; Chien, C.C.; Wang, C.L.; Kempson, I.M.; Hwu, Y.; Lai, T.C.; et al. Enhancement of cell radiation sensitivity by pegylated gold nanoparticles. Phys. Med. Biol. 2010, 55, 931–945. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.; Karamitros, M.; Ivanchenko, V.; Guatelli, S.; McKinnon, S.; Murakami, K.; Sasaki, T.; Okada, S.; Bordage, M.; Francis, Z.; et al. Geant4 Monte Carlo simulation of absorbed dose and radiolysis yields enhancement from a gold nanoparticle under MeV proton irradiation. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2016, 373, 126–139. [Google Scholar] [CrossRef]
- Herold, M.; Das, I.J.; Stobbe, C.C. Gold microspheres: A selective technique for producing biologically effective dose enhancement. Int. J. Radiat. Biol. 2000, 76, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Hainfeld, J.F.; Slatkin, D.N.; Smilowitz, H.M. The use of gold nanoparticles to enhance radiotherapy in mice. Phys. Med. Biol. 2004, 49, N309–N315. [Google Scholar] [CrossRef]
- Kim, J.K.; Seo, S.J.; Kim, H.T.; Kim, K.H.; Chung, M.H.; Kim, K.R.; Ye, S.J. Enhanced proton treatment in mouse tumors through proton irradiated nanoradiator effects on metallic nanoparticles. Phys. Med. Biol. 2012, 57, 8309–8323. [Google Scholar] [CrossRef]
- Lin, Y.; McMahon, S.J.; Paganetti, H.; Schuemann, J. Biological modeling of gold nanoparticle enhanced radiotherapy for proton therapy. Phys. Med. Biol. 2015, 60, 4149–4168. [Google Scholar] [CrossRef]
- Leung, M.K.K.; Chow, J.C.L.; Chithrani, B.D.; Lee, M.J.G.; Oms, B.; Jaffray, D.A. Irradiation of gold nanoparticles by x-rays: Monte Carlo simulation of dose enhancements and the spatial properties of the secondary electrons production. Med. Phys. 2011, 38, 624–631. [Google Scholar] [CrossRef]
- Wälzlein, C.; Scifoni, E.; Krämer, M.; Durante, M. Simulations of dose enhancement for heavy atom nanoparticles irradiated by protons. Phys. Med. Biol. 2014, 59, 1441–1458. [Google Scholar] [CrossRef]
- Lin, Y.; McMahon, S.J.; Scarpelli, M.; Paganetti, H.; Schuemann, J. Comparing gold nano-particle enhanced radiotherapy with protons, megavoltage photons and kilovoltage photons: A Monte Carlo simulation. Phys. Med. Biol. 2014, 59, 7675–7689. [Google Scholar] [CrossRef]
- Jones, B.L.; Krishnan, S.; Cho, S.H. Estimation of microscopic dose enhancement factor around gold nanoparticles by Monte Carlo calculations. Med. Phys. 2010, 37, 3809–3816. [Google Scholar] [CrossRef]
- Carter, J.D.; Cheng, N.N.; Qu, Y.; Suarez, G.D.; Guo, T. Nanoscale Energy Deposition by X-ray Absorbing Nanostructures. J. Phys. Chem. B 2007, 111, 11622–11625. [Google Scholar] [CrossRef] [PubMed]
- Peukert, D.; Kempson, I.; Douglass, M.; Bezak, E. Gold Nanoparticle Enhanced Proton Therapy: Monte Carlo Modeling of Reactive Species’ Distributions Around a Gold Nanoparticle and the Effects of Nanoparticle Proximity and Clustering. Int. J. Mol. Sci. 2019, 20, 4280. [Google Scholar] [CrossRef]
- Kwon, J.; Sutherland, K.; Hashimoto, T.; Shirato, H.; Date, H. Spatial distributions of dose enhancement around a gold nanoparticle at several depths of proton Bragg peak. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2016, 384, 113–120. [Google Scholar] [CrossRef]
- Sotiropoulos, M.; Taylor, M.J.; Henthorn, N.T.; Warmenhoven, J.W.; Mackay, R.I.; Kirkby, K.J.; Merchant, M.J. Geant4 interaction model comparison for dose deposition from gold nanoparticles under proton irradiation. Biomed. Phys. Eng. Express 2017, 3, 025025. [Google Scholar] [CrossRef]
- Francis, Z.; Montarou, G.; Incerti, S.; Bernal, M.; Zein, S. A simulation study of gold nanoparticles localisation effects on radiation enhancement at the mitochondrion scale. Phys. Med. 2019, 67, 148–154. [Google Scholar] [CrossRef]
- Hadrontherapy. 2014. Available online: https://twiki.cern.ch/twiki/bin/view/Geant4/AdvancedExamplesHadrontherapy (accessed on 18 August 2022).
- Bhatnagar, S.; Sirisha, S. Geant4—Study of Dose Curve Parameters of Tumor in Human Tissues Using Passive Proton Beam. In Proceedings of the 2014 International Conference on Computational Intelligence and Communication Networks, Toronto, ON, Canada, 10–12 January 2014; pp. 178–185. [Google Scholar] [CrossRef]
- Hashemi, Z.; Tatari, M.; Naik, H. Simulation of dose distribution and secondary particle production in proton therapy of brain tumor. Rep. Pract. Oncol. Radiother. 2020, 25, 927–933. [Google Scholar] [CrossRef]
- Feng, A.H.; Li, X.; Wang, X.F.; Wang, X.W. Microdosimetric Evaluation on the Metallic Nanoparticle-Mediated Dose Enhancement in Radiotherapeutic Proton Irradiation. Chin. Phys. Lett. 2018, 35, 068701. [Google Scholar] [CrossRef]
- Douglass, M.; Bezak, E.; Penfold, S. Monte Carlo investigation of the increased radiation deposition due to gold nanoparticles using kilovoltage and megavoltage photons in a 3D randomized cell model. Med. Phys. 2013, 40, 071710. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.H.; Chien, C.C.; Petibois, C.; Wang, C.L.; Chu, Y.S.; Lai, S.F.; Hua, T.E.; Chen, Y.Y.; Cai, X.; Kempson, I.M.; et al. Quantitative analysis of nanoparticle internalization in mammalian cells by high resolution X-ray microscopy. J. Nanobiotechnol. 2011, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Rothen-Rutishauser, B.; Kuhn, D.A.; Ali, Z.; Gasser, M.; Amin, F.; Parak, W.J.; Vanhecke, D.; Fink, A.; Gehr, P.; Brandenberger, C. Quantification of gold nanoparticle cell uptake under controlled biological conditions and adequate resolution. Nanomedicine 2014, 9, 607–621. [Google Scholar] [CrossRef] [PubMed]





| Geometry Configuration (Number of Gold Nanoparticles) | Distance from GNP Surface (nm) | Number of per Proton | Dose Deposition (Gy) |
|---|---|---|---|
| 1918 | 10 | 17 | 1313 |
| 15 | 15 | 791 | |
| 30 | 6 | 391 | |
| 100 | 31 | 165 | |
| 3832 | 10 | 41 | 2642 |
| 15 | 44 | 1477 | |
| 30 | 46 | 786 | |
| 100 | 75 | 307 | |
| 5752 | 10 | 32 | 3905 |
| 15 | 41 | 2429 | |
| 30 | 43 | 1110 | |
| 100 | 71 | 441 | |
| 9588 | 10 | 97 | 7194 |
| 15 | 86 | 3682 | |
| 30 | 126 | 1816 | |
| 100 | 211 | 626 | |
| 19,172 | 10 | 136 | 12,412 |
| 15 | 247 | 7562 | |
| 30 | 215 | 3321 | |
| 100 | 113 | 812 | |
| 57,518 | 10 | 494 | 32,581 |
| 15 | 661 | 18,804 | |
| 30 | 565 | 7477 | |
| 100 | 204 | 952 | |
| 76,692 | 10 | 814 | 42,174 |
| 15 | 1036 | 21,317 | |
| 30 | 635 | 6706 | |
| 100 | 427 | 898 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akhdar, H.; Alanazi, R.; Alanazi, N.; Alodhayb, A. Secondary Electrons in Gold Nanoparticle Clusters and Their Role in Therapeutic Ratio: The Outcome of a Monte Carlo Simulation Study. Molecules 2022, 27, 5290. https://doi.org/10.3390/molecules27165290
Akhdar H, Alanazi R, Alanazi N, Alodhayb A. Secondary Electrons in Gold Nanoparticle Clusters and Their Role in Therapeutic Ratio: The Outcome of a Monte Carlo Simulation Study. Molecules. 2022; 27(16):5290. https://doi.org/10.3390/molecules27165290
Chicago/Turabian StyleAkhdar, Hanan, Reem Alanazi, Nadyah Alanazi, and Abdullah Alodhayb. 2022. "Secondary Electrons in Gold Nanoparticle Clusters and Their Role in Therapeutic Ratio: The Outcome of a Monte Carlo Simulation Study" Molecules 27, no. 16: 5290. https://doi.org/10.3390/molecules27165290






