Assessment of the Corrosion of Steel Embedded in an Alkali-Activated Hybrid Concrete Exposed to Chlorides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Concretes
2.3. Experimental Tests
2.3.1. Compressive Strength and Resistance to Water Penetration
2.3.2. Resistance to Chloride ion Penetration
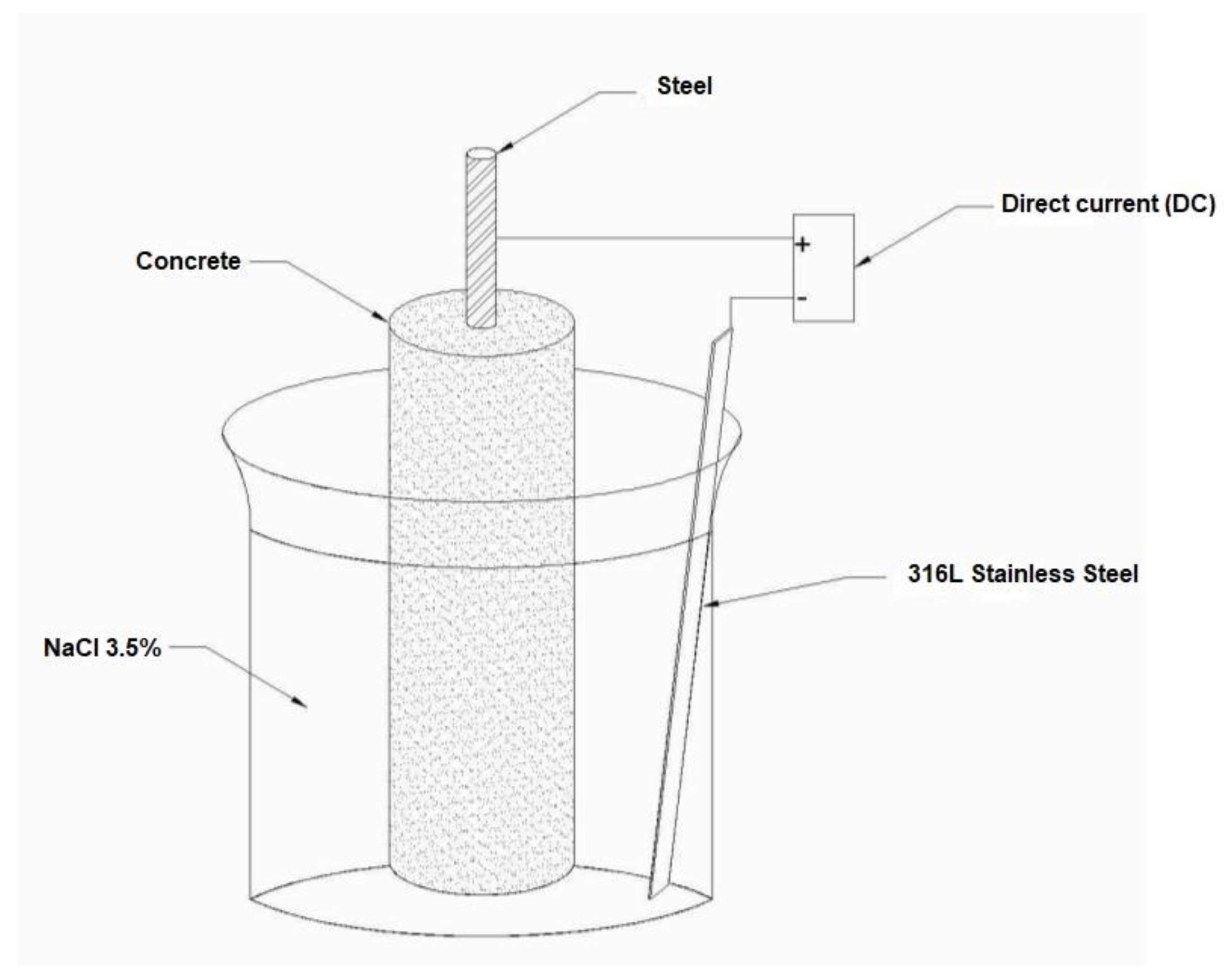
2.3.3. Corrosion Evaluation of Steel Embedded in Concrete
- -
- Polarization curves (Tafel). At exposure times of 0, 180 and 360 days of exposure, the polarization curves were carried out to evaluate the electrochemical process and to determine by means of the Tafel slopes, the constant of proportionality B, according to Equation (1).
- -
- Linear Polarization Resistance (LPR). Linear polarization resistance was determined at the end of each month of exposure, and the test was carried out for a total period of 12 months. The LPR test was performed following the ASTM G59 standard [51]. The three-electrode electrochemical cell was used, as mentioned in the Tafel test, applying overpotentials from −30 to +30 mV. The Icorr corrosion rate was calculated using the Stern-Geary equation Equation (2):
3. Results and Discussion
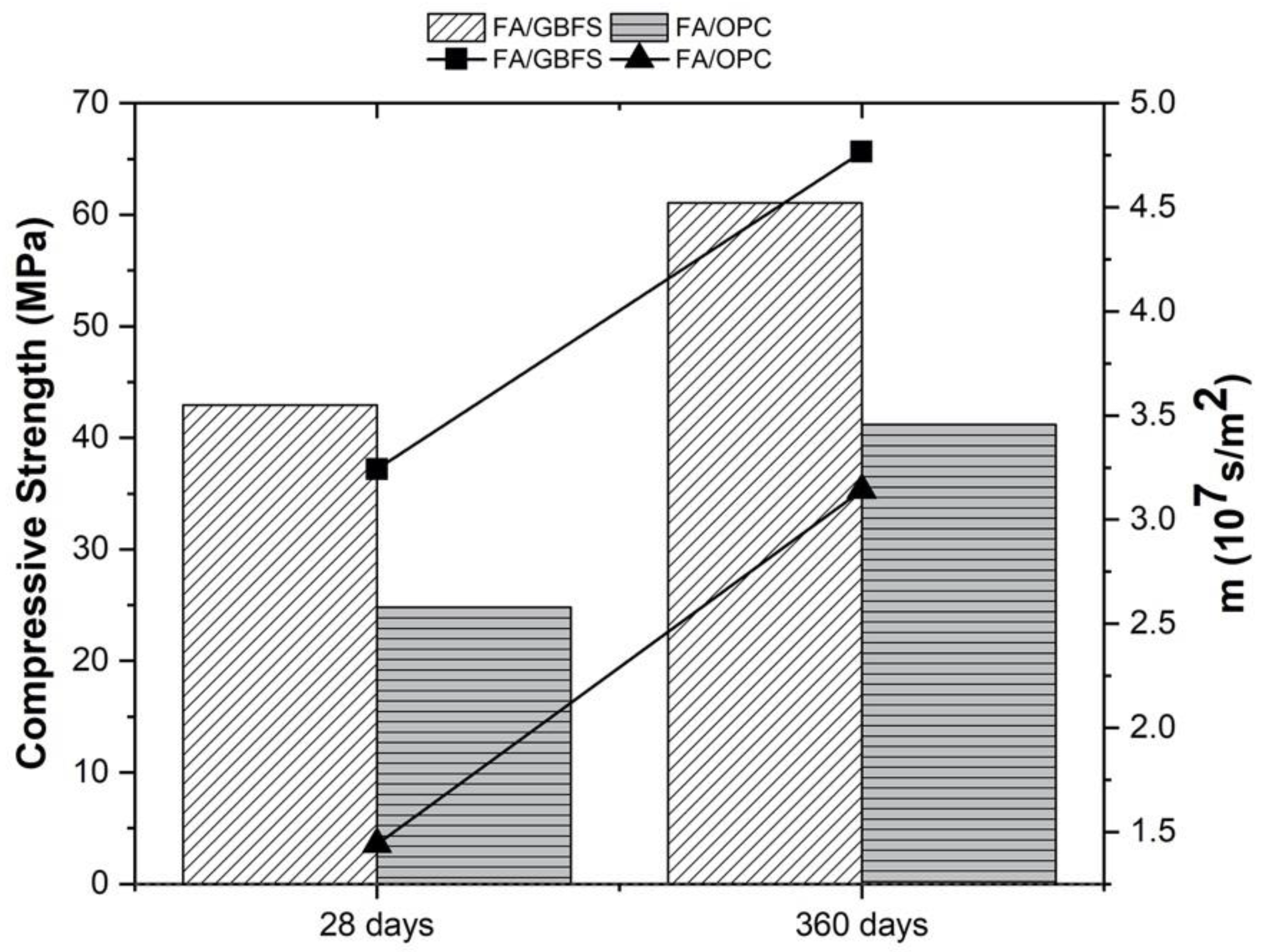
3.1. Compressive Strength and Resistance to Water Penetration
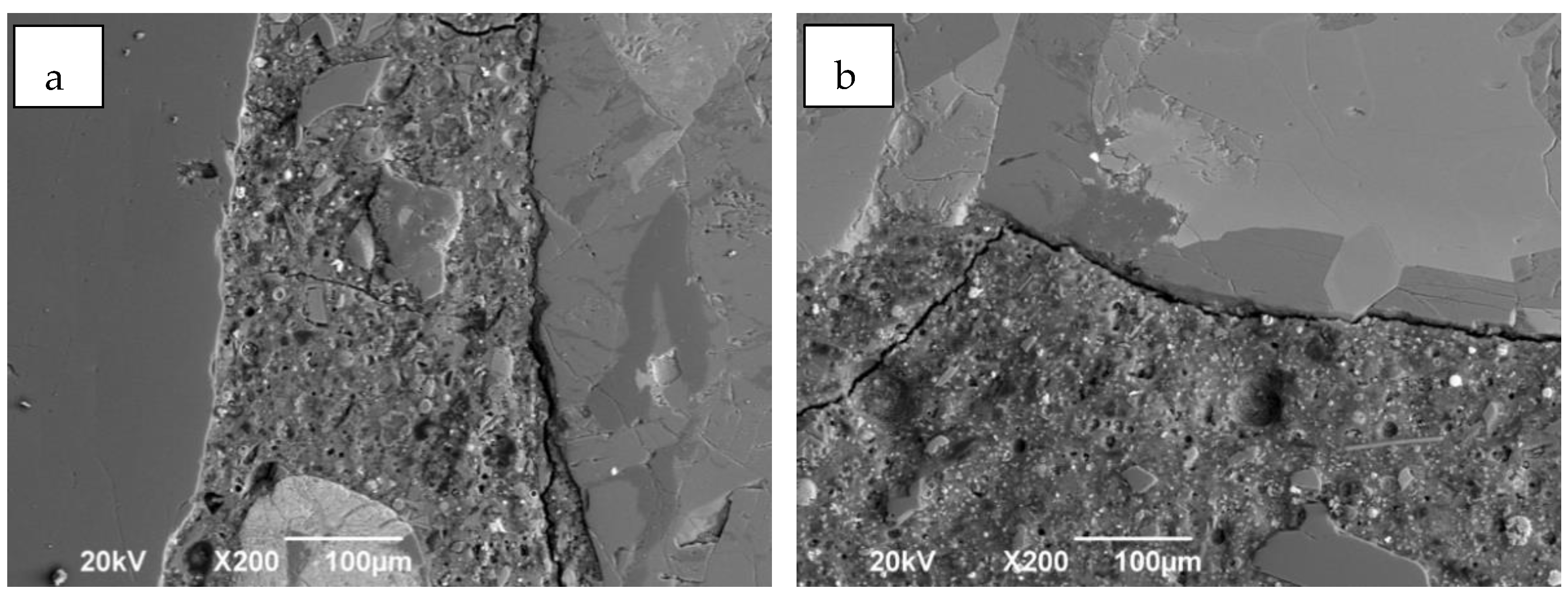
3.2. Chloride Permeability
3.3. Impressed Voltage
3.4. Immersion in Drinking Water and NaCl 3.5%
3.4.1. Polarization Curves
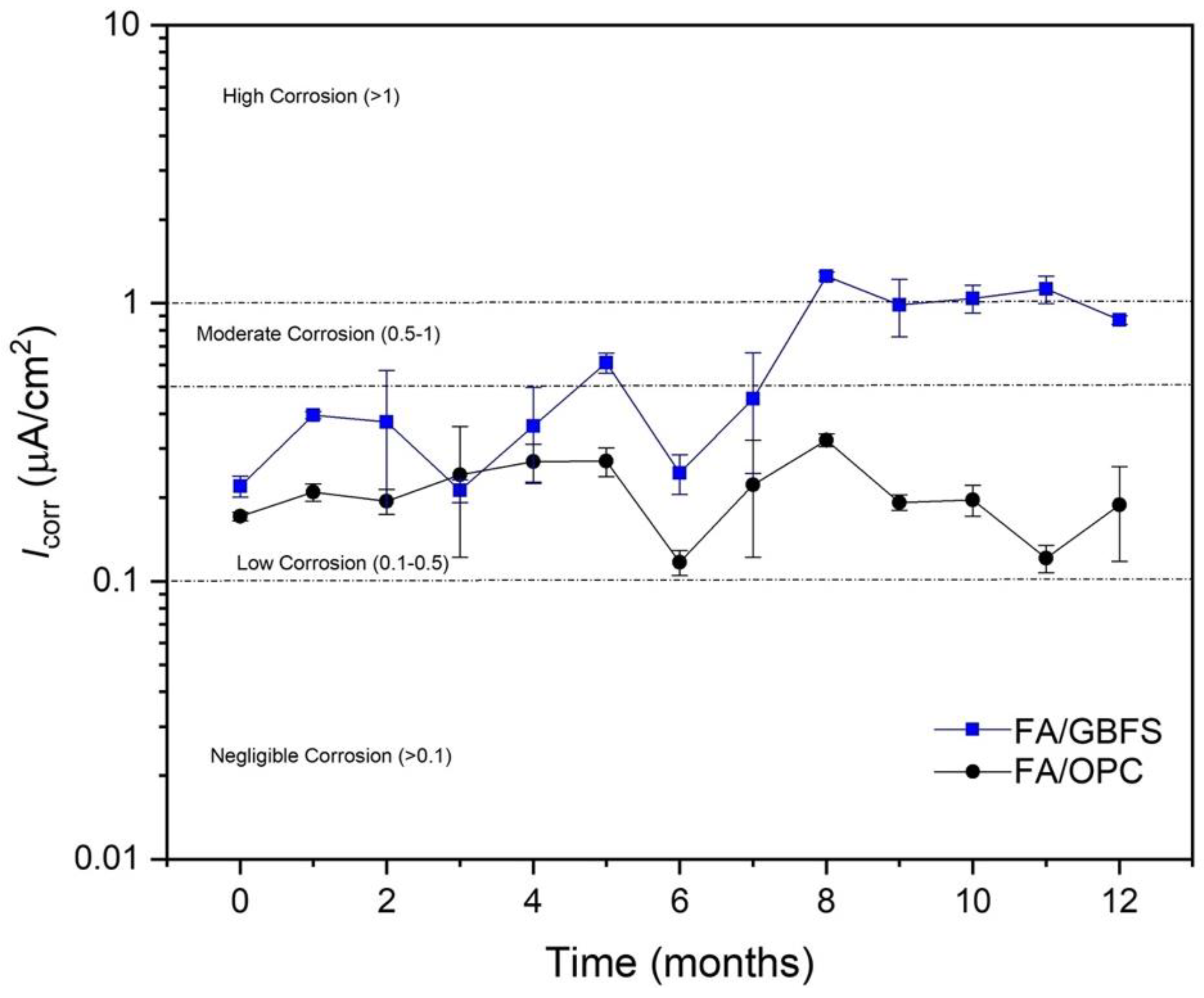
3.4.2. Linear Polarization Resistance (LPR)
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Aguirre, A.M.; Mejía de Gutiérrez, R. Durability of reinforced concrete exposed to aggressive conditions. Mater. Constr. 2013, 63, 7–38. [Google Scholar] [CrossRef]
- de Brito, J.; Kurda, R. The past and future of sustainable concrete: A critical review and new strategies on cement-based materials. J. Clean. Prod. 2021, 281, 123558. [Google Scholar] [CrossRef]
- Carreño-Gallardo, C.; Tejeda-Ochoa, A.; Perez-Ordonez, O.I.; Ledezma-Sillas, J.E.; Lardizabal-Gutierrez, D.; Prieto-Gomez, C.; Valenzuela-Grado, J.A.; Robles Hernandez, F.C.; Herrera-Ramirez, J.M. In the CO2 emission remediation by means of alternative geopolymers as substitutes for cements. J. Environ. Chem. Eng. 2018, 6, 4878–4884. [Google Scholar] [CrossRef]
- McLellan, B.C.; Williams, R.P.; Lay, J.; van Riessen, A.; Corder, G.D. Costs and carbon emissions for geopolymer pastes in comparison to ordinary portland cement. J. Clean. Prod. 2011, 19, 1080–1090. [Google Scholar] [CrossRef]
- Turner, L.K.; Collins, F.G. Carbon dioxide equivalent (CO2-e) emissions: A comparison between geopolymer and OPC cement concrete. Constr. Build. Mater. 2013, 43, 125–130. [Google Scholar] [CrossRef]
- Li, C.; Gong, X.Z.; Cui, S.P.; Wang, Z.H.; Zheng, Y.; Chi, B.C. CO2 Emissions due to Cement Manufacture. Mater. Sci. Forum 2011, 685, 181–187. [Google Scholar] [CrossRef]
- Peng, J.X.; Huang, L.; Zhao, Y.B.; Chen, P.; Zeng, L.; Zheng, W. Modeling of Carbon Dioxide Measurement on Cement Plants. Adv. Mater. Res. 2013, 610, 2120–2128. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer cement. Rev. Geopolym. Inst. Tech. Pap. 2013, 21, 1–11. [Google Scholar]
- Provis, J.L. Geopolymers and other alkali activated materials: Why, how, and what? Mater. Struct. 2014, 47, 11–25. [Google Scholar] [CrossRef]
- Shi, C.; Jiménez, A.F.; Palomo, A. New cements for the 21st century: The pursuit of an alternative to Portland cement. Cem. Concr. Res. 2011, 41, 750–763. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A. Composition and microstructure of alkali activated fly ash binder: Effect of the activator. Cem. Concr. Res. 2005, 35, 1984–1992. [Google Scholar] [CrossRef]
- Sagoe C, K.; Weng, L. Dissolution processes, hydrolysis and condensation reactions during geopolymer synthesis: Part II. High Si/Al ratio systems. J. Mater. Sci. 2007, 42, 3007–3014. [Google Scholar] [CrossRef]
- Wang, W.; Chen, H.; Li, X.; Zhu, Z. Corrosion behavior of steel bars immersed in simulated pore solutions of alkali-activated slag mortar. Constr. Build. Mater. 2017, 143, 289–297. [Google Scholar] [CrossRef]
- Robayo-Salazar, R.A.; Aguirre-Guerrero, A.M.; Mejía de Gutiérrez, R. Carbonation-induced corrosion of alkali-activated binary concrete based on natural volcanic pozzolan. Constr. Build. Mater. 2020, 232, 117189. [Google Scholar] [CrossRef]
- Palomo, A.; Fernández-Jiménez, A.; Kovalchuk, G.; Ordoñez, L.M.; Naranjo, M.C. OPC-fly ash cementitious systems: Study of gel binders produced during alkaline hydration. J. Mater. Sci. 2007, 42, 2958–2966. [Google Scholar] [CrossRef]
- Palomo, A.; Monteiro, P.; Martauz, P.; Bilek, V.; Fernandez-Jimenez, A. Hybrid binders: A journey from the past to a sustainable future (opus caementicium futurum). Cem. Concr. Res. 2019, 124, 105829. [Google Scholar] [CrossRef]
- Xue, L.; Zhang, Z.; Wang, H. Hydration mechanisms and durability of hybrid alkaline cements (HACs): A review. Constr. Build. Mater. 2021, 266, 121039. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A. Characterisation of fly ashes. Potential reactivity as alkaline cements. Fuel 2003, 82, 2259–2265. [Google Scholar] [CrossRef]
- Valencia-Saavedra, W.G.; Aguirre-Guerrero, A.M.; Mejia de Gutiérrez, R. Alkali-activated concretes based on high unburned carbon content fly ash: Carbonation and corrosion performance. Eur. J. Environ. Civ. Eng. 2022, 26, 1–21. [Google Scholar] [CrossRef]
- ASTM C-618; Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use in Concrete. ASTM Standard International: West Conshohocken, PA, USA, 2019.
- Güneyisi, E.; Özturan, T.; Gesoğlu, M. A study on reinforcement corrosion and related properties of plain and blended cement concretes under different curing conditions. Cem. Concr. Compos. 2005, 27, 449–461. [Google Scholar] [CrossRef]
- Sahmaran, M.; Li, V.C.; Andrade, C. Corrosion Resistance Performance of Steel-Reinforced Engineered Cementitious Composite Beams. ACI Mater. J. 2008, 105, 243–250. [Google Scholar]
- Angst, U.; Elsener, B.; Larsen, C.K.; Vennesland, Ø. Critical chloride content in reinforced concrete—A review. Cem. Concr. Res. 2009, 39, 1122–1138. [Google Scholar] [CrossRef]
- Aguirre-Guerrero, A.M.; Mejía de Gutiérrez, R. 13-Assessment of corrosion protection methods for reinforced concrete. In Eco-Efficient Repair and Rehabilitation of Concrete Infrastructures, Woodhead Publishing Series in Civil and Structural Engineering; Pacheco-Torgal, F., Melchers, R.E., Shi, X., Belie, N.D., Tittelboom, K.V., Sáez, A., Eds.; Woodhead Publishing: Sawston, UK, 2018; pp. 315–353. [Google Scholar] [CrossRef]
- Aperador, W.A.; Ruiz, J.H.B.; Gómez, R. Corrosion of reinforcing bars embedded in alkali-activated slag concrete subjected to chloride attack. Mater. Res. 2012, 15, 57–62. [Google Scholar] [CrossRef]
- Monticelli, C.; Natali, M.E.; Balbo, A.; Chiavari, C.; Zanotto, F.; Manzi, S.; Bignozzi, M.C. A study on the corrosion of reinforcing bars in alkali-activated fly ash mortars under wet and dry exposures to chloride solutions. Cem. Concr. Res. 2016, 87, 53–63. [Google Scholar] [CrossRef]
- Monticelli, C.; Natali, M.E.; Balbo, A.; Chiavari, C.; Zanotto, F.; Manzi, S.; Bignozzi, M.C. Corrosion behavior of steel in alkali-activated fly ash mortars in the light of their microstructural, mechanical and chemical characterization. Cem. Concr. Res. 2016, 80, 60–68. [Google Scholar] [CrossRef]
- Babaee, M.; Castel, A. Chloride-induced corrosion of reinforcement in low-calcium fly ash-based geopolymer concrete. Cem. Concr. Res. 2016, 88, 96–107. [Google Scholar] [CrossRef]
- Gunasekara, C.; Bhuiyan, S.; Law, D.; Setunge, S.; Ward, L. Corrosion resistance in different fly ash based geopolymer concretes. In Proceedings of the Conference: HPC/CIC Tromsø 2017, Norwegian Concrete Association, Tromsø, Norway, 6–8 March 2017; pp. 1–11. [Google Scholar]
- Kupwade-Patil, K.; Allouche, E.N. Examination of Chloride-Induced Corrosion in Reinforced Geopolymer Concretes. J. Mater. Civ. Eng. 2013, 25, 1465–1476. [Google Scholar] [CrossRef]
- Miranda, J.M.; Fernández-Jiménez, A.; González, J.A.; Palomo, A. Corrosion resistance in activated fly ash mortars. Cem. Concr. Res. 2005, 35, 1210–1217. [Google Scholar] [CrossRef]
- Babaee, M.; Castel, A. Chloride diffusivity, chloride threshold, and corrosion initiation in reinforced alkali-activated mortars: Role of calcium, alkali, and silicate content. Cem. Concr. Res. 2018, 111, 56–71. [Google Scholar] [CrossRef]
- Tennakoon, C.; Shayan, A.; Sanjayan, J.G.; Xu, A. Chloride ingress and steel corrosion in geopolymer concrete based on long term tests. Mater. Des. 2017, 116, 287–299. [Google Scholar] [CrossRef]
- Prusty, J.K.; Pradhan, B. Effect of GGBS and chloride on compressive strength and corrosion performance of steel in fly ash-GGBS based geopolymer concrete. Mater. Today Proc. 2020, 32, 850–855. [Google Scholar] [CrossRef]
- Donatello, S.; Maltseva, O.; Fernandez-Jimenez, A.; Palomo, A. The Early Age Hydration Reactions of a Hybrid Cement Containing a Very High Content of Coal Bottom Ash. J. Am. Ceram. Soc. 2014, 97, 929–937. [Google Scholar] [CrossRef]
- García-Lodeiro, I.; Maltseva, O.; Palomo, Á.; Fernández-Jiménez, A. Cimenturi hibride alcaline. Partea I: Fundamente. Rev. Romana Mater. 2012, 42, 330–335. [Google Scholar]
- García-Lodeiro, I.; Fernández-Jiménez, A.; Palomo, A. Variation in hybrid cements over time. Alkaline activation of fly ash–portland cement blends. Cem. Concr. Res. 2013, 52, 112–122. [Google Scholar] [CrossRef]
- Kaja, A.M.; Lazaro, A.; Yu, Q.L. Effects of Portland cement on activation mechanism of class F fly ash geopolymer cured under ambient conditions. Constr. Build. Mater. 2018, 189, 1113–1123. [Google Scholar] [CrossRef]
- Aineto, M.; Acosta, A.; Rincón, J.M.; Romero, M. Thermal expansion of slag and fly ash from coal gasification in IGCC power plant. Fuel 2006, 85, 2352–2358. [Google Scholar] [CrossRef]
- González-Acuña, R.E. Caracterización De Geopolímeros Base Ceniza Volante y Escoria Granulada De Alto Horno. Doctoral Thesis, Universidad Autónoma de Nuevo León, San Nicolás de los Garza, Monterrey, Mexico, 2012. [Google Scholar]
- Lizarazo, M.J.; Garcia, F.; Higuera, C. Activación de las propiedades cementicias de la ceniza volante mediante electromutagénesis química. Rev. Latinoam. De Metal. Mater. 2015, 35, 305–314. [Google Scholar]
- Mustafa Al Bakri, A.M.; Abdulkareem, O.A.; Kamarudin, H.; Khairul Nizar, I.; Rafiza, A.R.; Zarina, Y.; Alida, A. Microstructure Studies on the Effect of the Alkaline Activators of Fly Ash-Based Geopolymer at Elevated Heat Treatment Temperature. Appl. Mech. Mater. 2013, 421, 342–348. [Google Scholar] [CrossRef]
- Martinez Alvarado, M.J. Estudio de la Hidratación de la Escoria Granulada de alto Horno (EGAH) a Diferentes Temperaturas. Tesis de Maestría, Instituto Politécnico Nacional, Mexico, 2010. Available online: http://tesis.ipn.mx/handle/123456789/5967 (accessed on 7 July 2022).
- Salman, M.; Cizer, Ö.; Pontikes, Y.; Snellings, R.; Dijkman, J.; Sels, B.; Vandewalle, L.; Blanpain, B.; Van Balen, K. Alkali Activation of AOD Stainless Steel Slag Under Steam Curing Conditions. J. Am. Ceram. Soc. 2015, 98, 3062–3074. [Google Scholar] [CrossRef]
- Salman, M.; Cizer, Ö.; Pontikes, Y.; Snellings, R.; Vandewalle, L.; Blanpain, B.; Balen, K.V. Cementitious binders from activated stainless steel refining slag and the effect of alkali solutions. J. Hazard. Mater. 2015, 286, 211–219. [Google Scholar] [CrossRef]
- ASTM C-39; Standard Test Method for Compressive Strength of Cylindrical Concrete Specimens. ASTM Standard International: West Conshohocken, PA, USA, 2018.
- EMPA, SIA 162/1; Test No. 5-Water Conductivity. Swiss Federal Laboratories for Materials Science and Technologies: Zurich, Switzerland, 1989.
- ASTM C-1585; Standard Test Method for Measurement of Rate Absorption of Water by Hydraulic-Cement Concretes. ASTM Standard International: West Conshohocken, PA, USA, 2013.
- ASTM C-1202; Standard Test Method for Electrical Indication of Concrete’s Ability to Resist Chloride Ion Penetration. ASTM Standard International: West Conshohocken, PA, USA, 2019.
- N.T. Build 356; Nordtest Method for Concrete, Repairing Materials and Protective Coating: Embedded Steel Method, Chloride Permeability. CRC Press: Boca Raton, FL, USA, 1989.
- ASTM G-59; Standard Test Method for Conducting Potentiodynamic Polarization Resistance Measurements. ASTM Standard International: West Conshohocken, PA, USA, 2014.
- Andrade, C.; Alonso, C. Test methods for on-site corrosion rate measurement of steel reinforcement in concrete by means of the polarization resistance method. Mater. Struct. 2004, 37, 623–643. [Google Scholar] [CrossRef]
- Bernal, S.A.; Mejía de Gutiérrez, R.; Pedraza, A.L.; Provis, J.L.; Rodriguez, E.D.; Delvasto, S. Effect of binder content on the performance of alkali-activated slag concretes. Cem. Concr. Res. 2011, 41, 1–8. [Google Scholar] [CrossRef]
- Chi, M. Effects of dosage of alkali-activated solution and curing conditions on the properties and durability of alkali-activated slag concrete. Constr. Build. Mater. 2012, 35, 240–245. [Google Scholar] [CrossRef]
- Shi, C. Another Look at the Rapid Chloride Permeability Test (ASTM C1202 or ASSHTO T277); FHWA Resource Center, Federal Highway Administration: Baltimore, MD, USA, 2003; Available online: https://www.semanticscholar.org/paper/ANOTHER-LOOK-AT-THE-RAPID-CHLORIDE-PERMEABILITY-(-C-Shi/ddd80bfeffac199f2b416bb8d6d56b43236402e7 (accessed on 1 April 2022).
- Gruskovnjak, A.; Lothenbach, B.; Holzer, L.; Figi, R.; Winnefeld, F. Hydration of alkali-activated slag: Comparison with ordinary Portland cement. Adv. Cem. Res. 2006, 18, 119–128. [Google Scholar] [CrossRef]
- Lloyd, R.R.; Provis, J.L.; van Deventer, J.S.J. Pore solution composition and alkali diffusion in inorganic polymer cement. Cem. Concr. Res. 2010, 40, 1386–1392. [Google Scholar] [CrossRef]
- Song, S.; Jennings, H.M. Pore solution chemistry of alkali-activated ground granulated blast-furnace slag. Cem. Concr. Res. 1999, 29, 159–170. [Google Scholar] [CrossRef]
- Nedeljković, M.; Ghiassi, B.; van der Laan, S.; Li, Z.; Ye, G. Effect of curing conditions on the pore solution and carbonation resistance of alkali-activated fly ash and slag pastes. Cem. Concr. Res. 2019, 116, 146–158. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, C.; Zhang, Z. Chloride binding of alkali-activated slag/fly ash cements. Constr. Build. Mater. 2019, 226, 21–31. [Google Scholar] [CrossRef]
- Reddy, D.V.; Edouard, J.-B.; Sobhan, K. Durability of Fly Ash–Based Geopolymer Structural Concrete in the Marine Environment. J. Mater. Civ. Eng. 2013, 25, 781–787. [Google Scholar] [CrossRef]
- ASTM G102-89; Standard Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Measurements. ASTM Standard International: West Conshohocken, PA, USA, 2015.
- Criado, M.; Provis, J.L. Alkali activated slag mortars provide high resistance to chloride-induced corrosion of steel. Front. Mater. 2018, 5, 1–15. [Google Scholar] [CrossRef]
- Bastidas, D.M.; Fernández-Jiménez, A.; Palomo, A.; González, J.A. A study on the passive state stability of steel embedded in activated fly ash mortars. Corros. Sci. 2008, 50, 1058–1065. [Google Scholar] [CrossRef]
- Aguirre-Guerrero, A.M.; Robayo-Salazar, R.A.; Mejía de Gutiérrez, R. Corrosion resistance of alkali-activated binary reinforced concrete based on natural volcanic pozzolan exposed to chlorides. J. Build. Eng. 2021, 33, 101593. [Google Scholar] [CrossRef]








| Material | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | Na2O | SO3 | TiO2 | LOI * |
|---|---|---|---|---|---|---|---|---|---|
| FA | 28.53 | 19.18 | 8.80 | 6.68 | 2.24 | 7.94 | 2.71 | 1.62 | 20.67 |
| GBFS | 31.99 | 14.54 | 1.12 | 46.86 | 1.05 | 0.23 | 0.82 | 0.54 | 1.82 |
| OPC | 19.13 | 4.42 | 4.32 | 57.70 | 1.60 | - | 2.32 | - | 9.78 |
| Mixtures | Cement (kg) | FA (kg) | GBFS (kg) | NaOH (kg) | SS (kg) | Sand (kg) | Gravel (kg) | L/S Ratio |
|---|---|---|---|---|---|---|---|---|
| FA/GBFS | 0 | 320 | 80 | 28.5 | 158.4 | 972 | 704 | 0.48 |
| FA/OPC | 80 | 320 | 0 | 48.4 | 219.7 | 972 | 704 | 0.48 |
| Mixture | Cracking Time (h) | Maximum Anodic Current at the Time of the First Crack (mA) | Crack Size (mm) |
|---|---|---|---|
| FA/GBFS | 281 | 12 | 0.18 |
| FA/OPC | 398 | 20 | 0.25 |
| Sample | Loss of Mass (gr) | Loss of Mass (%) |
|---|---|---|
| FA/GBFS | −0.92 | 2.75 |
| FA/OPC | −1.92 | 5.74 |
| Concrete | Exposition Environment | Exposition Time (Days) | Ecorr (mV Vs Ag/AgCl) | Icorr (μA/cm2) | B |
|---|---|---|---|---|---|
| FA/OPC | Water | 0 | −179 | 0.052 | 19.2 |
| 180 | −514 | 0.092 | 13.2 | ||
| 360 | −674 | 0.794 | 17.1 | ||
| NaCl (3.5%) | 0 | −218 | 0.074 | 20.0 | |
| 180 | −643 | 0.987 | 12.7 | ||
| 360 | −788 | 1.383 | 13.9 | ||
| FA/GBFS | Water | 0 | −600 | 0.612 | 22.1 |
| 180 | −400 | 0.133 | 15.6 | ||
| 360 | −397 | 0.062 | 16.6 | ||
| NaCl (3.5%) | 0 | −657 | 0.627 | 24.1 | |
| 180 | −609 | 0.182 | 13.6 | ||
| 360 | −750 | 0.476 | 14.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valencia-Saavedra, W.; Aguirre-Guerrero, A.M.; Mejía de Gutiérrez, R. Assessment of the Corrosion of Steel Embedded in an Alkali-Activated Hybrid Concrete Exposed to Chlorides. Molecules 2022, 27, 5296. https://doi.org/10.3390/molecules27165296
Valencia-Saavedra W, Aguirre-Guerrero AM, Mejía de Gutiérrez R. Assessment of the Corrosion of Steel Embedded in an Alkali-Activated Hybrid Concrete Exposed to Chlorides. Molecules. 2022; 27(16):5296. https://doi.org/10.3390/molecules27165296
Chicago/Turabian StyleValencia-Saavedra, William, Ana María Aguirre-Guerrero, and Ruby Mejía de Gutiérrez. 2022. "Assessment of the Corrosion of Steel Embedded in an Alkali-Activated Hybrid Concrete Exposed to Chlorides" Molecules 27, no. 16: 5296. https://doi.org/10.3390/molecules27165296
APA StyleValencia-Saavedra, W., Aguirre-Guerrero, A. M., & Mejía de Gutiérrez, R. (2022). Assessment of the Corrosion of Steel Embedded in an Alkali-Activated Hybrid Concrete Exposed to Chlorides. Molecules, 27(16), 5296. https://doi.org/10.3390/molecules27165296








