Adsorption Characteristics of Ionic Surfactants on Anthracite Surface: A Combined Experimental and Modeling Study
Abstract
:1. Introduction
2. Experiments and Simulations
2.1. Materials
2.2. Experiment
2.2.1. Surfactant Adsorption
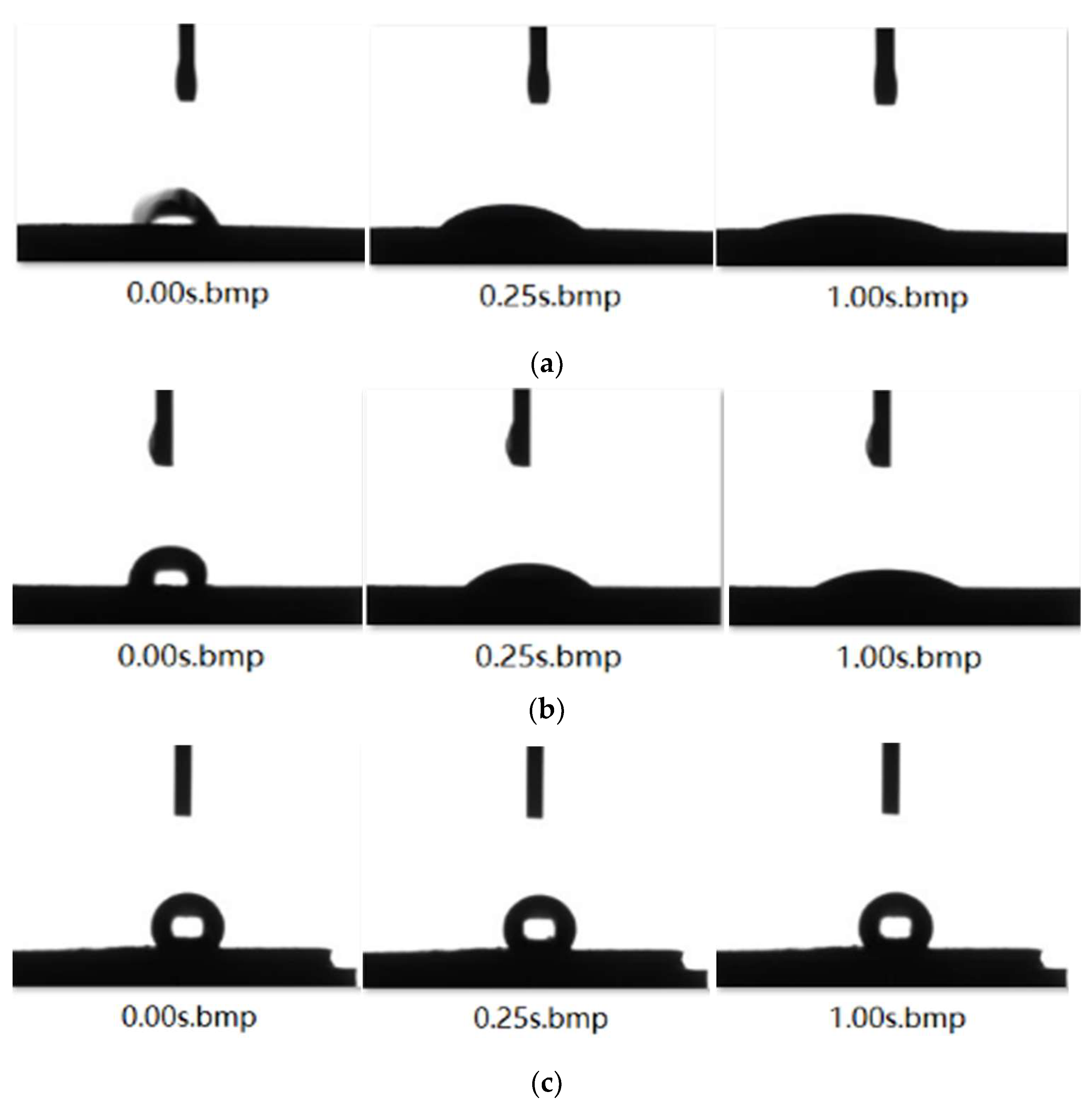
2.2.2. Contact Angle Measurement
2.2.3. XPS Measurements
2.2.4. FTIR Measurements
2.2.5. Molecular Dynamics Simulation
3. Results and Discussion
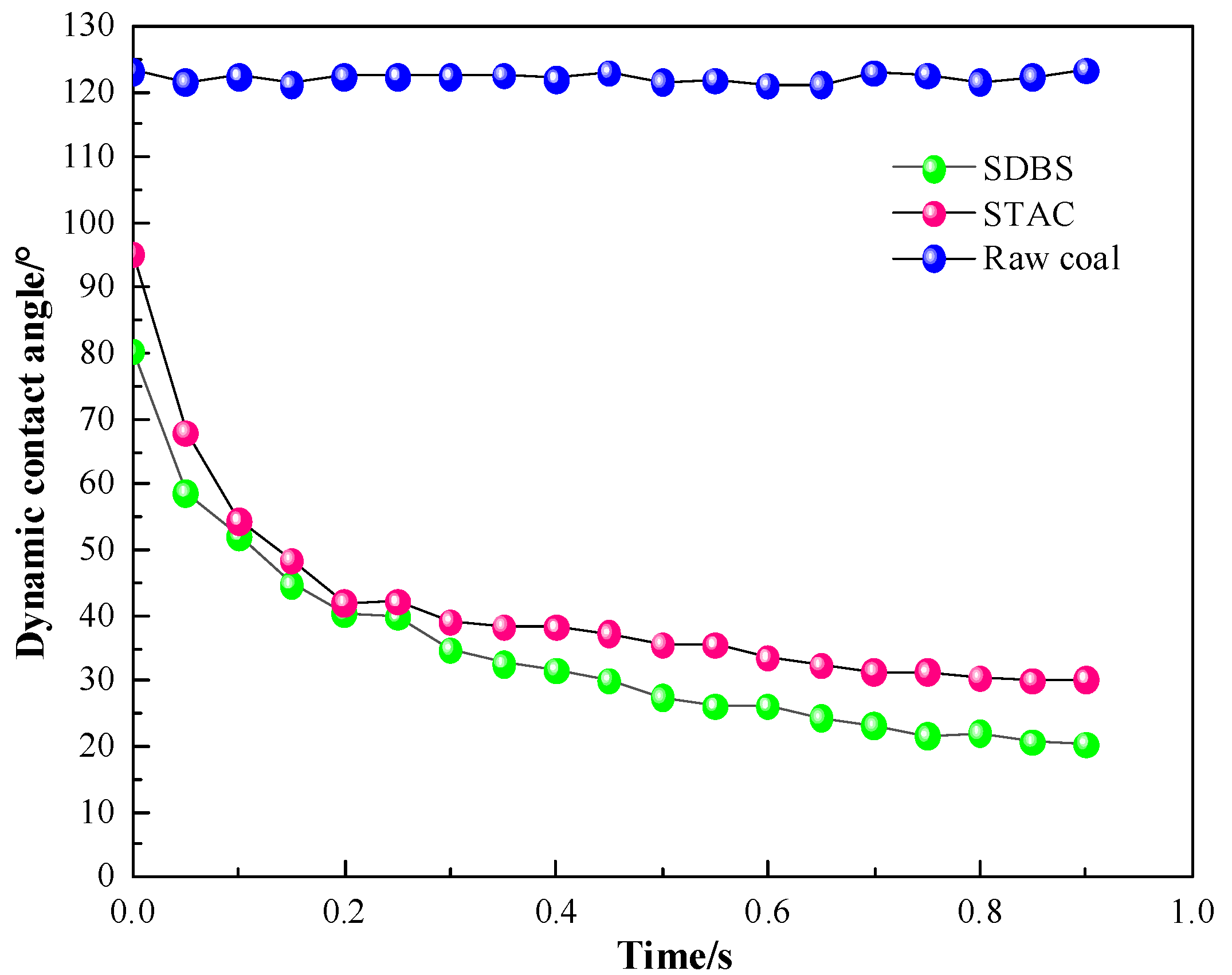
3.1. Contact Angle Analysis
3.2. XPS Analysis
3.3. FTIR Analysis
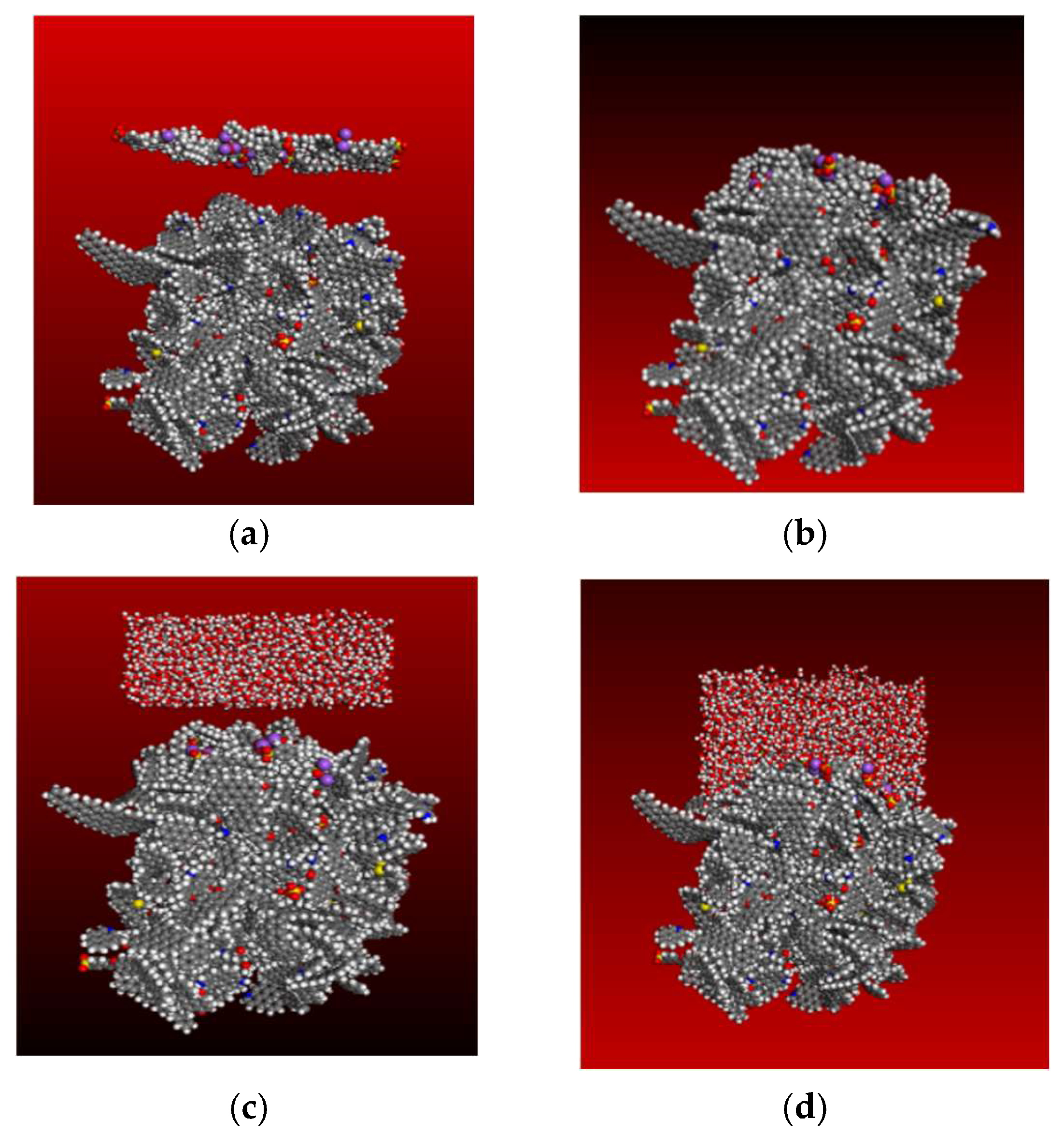
3.4. Molecular Dynamics Simulation
3.4.1. Contact Surface Area
3.4.2. Mass Density
3.4.3. Adsorption Energy
3.4.4. Mean Square Displacement
4. Conclusions
- (1)
- By measuring the contact angles of the two surfactants on the surface of anthracite and comparing with deionized water, it is concluded that STAC has stronger adsorption capacity on the surface of anthracite.
- (2)
- Through XPS experiment, the element changes in the SDBS system and STAC system were compared with those of raw coal system, and it was concluded that the adsorption capacity of STAC on the surface of anthracite was much higher than that of SDBS. Moreover, STAC forms a denser surfactant layer on the surface of anthracite through stronger electrostatic interaction, hydrophobic bonding and self-polymerization, which better covers the surface of the coal, so that the adsorption strength of STAC on the surface of anthracite is higher than that of SDBS.
- (3)
- The functional groups and chemical bond forms on the surface of the samples after adsorption were analyzed by FTIR experiments, and it was found that the adsorption efficiency of STAC on the surface of anthracite is better than that of SDBS, and the strength after adsorption is higher than that of SDBS.
- (4)
- Through a molecular dynamics’ simulation, CSA, MSD, adsorption energy and mass density were calculated. The study showed that STAC is more closely adsorbed on the surface of anthracite, and the distribution is more uniform at the coal–water interface. The surface of anthracite modified by STAC has a stronger binding ability to water molecules.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Cheng, J.W.; Zheng, X.R.; Lei, Y.D.; Luo, W.; Wang, Y.; Borowski, M.; Li, X.C.; Song, W.T.; Wang, Z.; Wang, K. A compound binder of coal dust wetting and suppression for coal pile. Process Saf. Environ. Prot. 2021, 147, 92–102. [Google Scholar] [CrossRef]
- Wang, H.T.; Wang, D.M.; Tang, Y.; Wang, Q.G. Foaming agent self-suction properties of a jet-type foam preparation device used in mine dust suppression. Process Saf. Environ. Prot. 2015, 98, 231–238. [Google Scholar] [CrossRef]
- Li, Q.Z.; Lin, B.Q.; Zhao, S.; Dai, H.M. Surface physical properties and its effects on the wetting behaviors of respirable coal mine dust. Powder Technol. 2013, 233, 137–145. [Google Scholar] [CrossRef]
- Yan, J.Y.; Nie, W.; Xiu, Z.H.; Yuan, M.Y.; Zhou, W.W.; Bao, Q.; Peng, H.T.; Niu, W.J.; Yu, F.N. Development and characterization of a dust suppression spray agent based on an adhesive NaAlg− gln− poly/polysaccharide polymer. Sci. Total Environ. 2021, 785, 147192. [Google Scholar] [CrossRef]
- Zhou, Q.; Xu, G.; Chen, Y.P.; Qin, B.T.; Zhao, Z.D.; Guo, C.W. The development of an optimized evaluation system for improving coal dust suppression efficiency using aqueous solution sprays. Colloids Surf. A-Physicochem. Eng. Asp. 2020, 602, 125104. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, G.; Qian, X.; Yuan, M.; Sun, Y.; Wang, D. Diffuse pollution characteristics of respirable dust in fully-mechanized mining face under various velocities based on CFD investigation. J. Clean. Prod. 2018, 184, 239–250. [Google Scholar] [CrossRef]
- Dou, G.; Xu, C. Comparison of effects of sodium carboxymethylcellulose and superabsorbent polymer on coal dust wettability by surfactants. J. Dispers. Sci. Technol. 2017, 38, 1542–1546. [Google Scholar] [CrossRef]
- Wang, Y.; Hou, B.; Cao, X.; Zhang, J.; Song, X.; Ding, M.; Chen, W. Interaction between polymer and anionic/nonionic surfactants and its mechanism of enhanced oil recovery. J. Dispers. Sci. Technol. 2018, 39, 1178–1184. [Google Scholar] [CrossRef]
- Khan, M.Y.; Samanta, A.; Ojha, K.; Mandal, A. Interaction between aqueous solutions of polymer and surfactant and its effect on physicochemical properties. Asia-Pac. J. Chem. Eng. 2008, 3, 579–585. [Google Scholar] [CrossRef]
- Omane, D.; Liu, W.V.; Pourrahimian, Y. Comparison of chemical suppressants under different atmospheric temperatures for the control of fugitive dust emission on mine hauls roads. Atmos. Pollut. Res. 2018, 9, 561–568. [Google Scholar] [CrossRef]
- Wang, P.F.; Tian, C.; Liu, R.H.; Wang, J. Mathematical model for multivariate nonlinear prediction of SMD of X-type swirl pressure nozzles. Process Saf. Environ. Prot. 2019, 125, 228–237. [Google Scholar] [CrossRef]
- Zhang, H.H.; Nie, W.; Liu, Y.H.; Wang, H.K.; Jin, H.; Bao, Q. Synthesis and performance measurement of environment-friendly solidified dust suppressant for open pit coalmine. J. Appl. Polym. Sci. 2018, 135, 46505. [Google Scholar] [CrossRef]
- Zhang, H.H.; Nie, W.; Yan, J.Y.; Bao, Q.; Wang, H.K.; Jin, H.; Peng, H.T.; Chen, D.W.; Liu, Z.Q.; Liu, Q. Preparation and performance study of a novel polymeric spraying dust suppression agent with enhanced wetting and coagulation properties for coal mine. Powder Technol. 2020, 364, 901–914. [Google Scholar] [CrossRef]
- Polok-Rubiniec, M.; Wlodarczyk-Fligier, A.; Chmielnicki, B. The properties of a polypropylene matrix composite with anthracite filler. Arch. Metall. Mater. 2021, 66, 305–311. [Google Scholar]
- Wang, H.; Zhang, L.; Wang, D.; He, X. Experimental investigation on the wettability of respirable coal dust based on infrared spectroscopy and contact angle analysis. Adv. Powder Technol. 2017, 28, 3130–3139. [Google Scholar] [CrossRef]
- Wang, X.; Yuan, S.; Jiang, B. Experimental investigation of the wetting ability of surfactants to coals dust based on physical chemistry characteristics of the different coal samples. Adv. Powder Technol. 2019, 30, 1696–1708. [Google Scholar] [CrossRef]
- Xia, W.; Li, Y. Role of Roughness Change on Wettability of Taixi Anthracite Coal Surface before and after the Heating Process. Energy Fuels 2016, 30, 281–284. [Google Scholar] [CrossRef]
- Zhang, X.; Cheng, J.; Kang, T.; Zhou, X.; Zhang, L. Electrochemical Modification on CH4 and H2O Wettability of Qinshui Anthracite Coal: A Combined Experimental and Molecular Dynamics Simulation Study. ACS Omega 2021, 6, 24147–24155. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Nie, W.; Cai, P.; Liu, Y.H.; Peng, H.T.; Liu, Q. Pattern characterization concerning spatial and temporal evolution of dust pollution associated with two typical ventilation methods at fully mechanized excavation faces in rock tunnels. Powder Technol. 2018, 334, 117–131. [Google Scholar] [CrossRef]
- Liu, Q.; Nie, W.; Hua, Y.; Peng, H.T.; Liu, C.Q.; Wei, C.H. Research on tunnel ventilation systems: Dust Diffusion and Pollution Behaviour by air curtains based on CFD technology and field measurement. Build. Environ. 2019, 147, 444–460. [Google Scholar] [CrossRef]
- Zhang, R.; Xing, Y.W.; Xia, Y.C.; Luo, J.Q.; Tan, J.L.; Rong, G.Q.; Gui, X.H. New insight into surface wetting of coal with varying coalification degree: An experimental and molecular dynamics simulation study. Appl. Surf. Sci. 2020, 511, 145610. [Google Scholar] [CrossRef]
- You, X.F.; He, M.; Zhu, X.C.; Wei, H.B.; Cao, X.Q.; Wang, P.; Li, L. Influence of surfactant for improving dewatering of brown coal: A comparative experimental and MD simulation study. Sep. Purif. Technol. 2019, 210, 473–478. [Google Scholar] [CrossRef]
- Xia, Y.C.; Zhang, R.; Xing, Y.W.; Gui, X.H. Improving the adsorption of oily collector on the surface of low-rank coal during flotation using a cationic surfactant: An experimental and molecular dynamics simulation study. Fuel 2019, 235, 687–695. [Google Scholar] [CrossRef]
- Gui, X.H.; Xing, Y.W.; Wang, T.X.; Cao, Y.J.; Miao, Z.Y.; Xu, M.D. Intensification mechanism of oxidized coal flotation by using oxygen-containing collector alpha-furanacrylic acid. Powder Technol. 2017, 305, 109–116. [Google Scholar] [CrossRef]
- Xia, Y.C.; Rong, G.Q.; Xing, Y.W.; Gui, X.H. Synergistic adsorption of polar and nonpolar reagents on oxygen-containing graphite surfaces: Implications for low-rank coal flotation. J. Colloid Interface Sci. 2019, 557, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.C.; Yang, Z.L.; Zhang, R.; Xing, Y.W.; Gui, X.H. Enhancement of the surface hydrophobicity of low-rank coal by adsorbing DTAB: An experimental and molecular dynamics simulation study. Fuel 2019, 239, 145–152. [Google Scholar] [CrossRef]
- He, M.; Zhang, W.; Cao, X.; You, X.; Li, L. Adsorption Behavior of Surfactant on Lignite Surface: A Comparative Experimental and Molecular Dynamics Simulation Study. Int. J. Mol. Sci. 2018, 19, 437. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.L.; Yan, G.C.; Xu, G.; Yang, X.L.; Li, J.J.; Bai, X.Y. Influence of the Branched Structure of Polyoxyethylene Units in Nonionic Surfactants on the Wettability of Anthracite: A Combined Modeling and Experimental Study. Adsorpt. Sci. Technol. 2022, 2022, 4249949. [Google Scholar] [CrossRef]
- Liu, S.Y.; Liu, X.Y.; Guo, Z.Y.; Liu, Y.T.; Guo, J.Y.; Zhang, S.H. Wettability modification and restraint of moisture re-adsorption of lignite using cationic gemini surfactant. Colloids Surf. A-Physicochem. Eng. Asp. 2016, 508, 286–293. [Google Scholar] [CrossRef]
- Liu, X.Y.; Liu, S.Y.; Cheng, Y.C.; Xu, G.J. Decrease in hydrophilicity and moisture readsorption of lignite: Effects of surfactant structure. Fuel 2020, 273, 117812. [Google Scholar] [CrossRef]
- Shen, M.C.; Song, B.; Zeng, G.M.; Zhang, Y.X.; Teng, F.Y.; Zhou, C.Y. Surfactant changes lead adsorption behaviors and mechanisms on microplastics. Chem. Eng. J. 2021, 405, 126989. [Google Scholar] [CrossRef]
- Yang, Y.; Ma, Z.K.; Xia, F.J.; Li, X.B. Adsorption behavior of oil -displacing surfactant at oil/water interface: Molecular simulation and experimental. J. Water Process Eng. 2020, 36, 101292. [Google Scholar] [CrossRef]
- Zhang, P.Y.; Sun, L.Q.; Wang, H.R.; Cui, J.W.; Hao, J.C. Surfactant-assistant atmospheric acid leaching of laterite ore for the improvement of leaching efficiency of nickel and cobalt. J. Clean. Prod. 2019, 228, 1–7. [Google Scholar] [CrossRef]
- Chen, R.X.; Dong, X.S.; Fan, Y.P.; Ma, X.M.; Dong, Y.D.; Chang, M. Interaction between STAC and coal/kaolinite in tailing dewatering: An experimental and molecular-simulation study. Fuel 2020, 279, 118224. [Google Scholar] [CrossRef]
- Eren Boncu, T.; Ozdemir, N. The Effects of Polymeric Molar Mass, Concentration, and Adding of Different Surfactants on The Electrospun Poly (Vinyl Alcohol) Nanofibers. Lat. Am. J. Pharm. 2019, 38, 1552–1561. [Google Scholar]
- Jiang, L.; Cao, Y.; Ni, X.; Zhang, M.; Cao, G. Influences of the concentration and the molar ratio of mixed surfactants on the performance of vesicle pseudostationary phase. Electrophoresis 2018, 39, 1794–1801. [Google Scholar] [CrossRef]
- Liang, X.D.; Liu, Y.F.; Zhou, D.; Yu, W.; Yin, J.Z. Critical Microemulsion Concentration and Molar Ratio of Water-to-Surfactant of Supercritical CO2 Microemulsions with Commercial Nonionic Surfactants: Experiment and Molecular Dynamics Simulation. J. Chem. Eng. Data 2016, 61, 3135–3143. [Google Scholar] [CrossRef]
- Aschaffenburg, D.J.; Kawasaki, S.; Das Pemmaraju, C.; Cuk, T. Accuracy in Resolving the First Hydration Layer on a Transition-Metal Oxide Surface: Experiment (AP-XPS) and Theory. J. Phys. Chem. C 2020, 124, 21407–21417. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Zhou, G.; Li, S.L.; Wang, C.M.; Liu, R.L.; Jiang, W.J. Molecular dynamics simulation and experimental characterization of anionic surfactant: Influence on wettability of low-rank coal. Fuel 2020, 279, 118323. [Google Scholar] [CrossRef]
- Akintola, G.O.; Amponsah-Dacosta, F.; Rupprecht, S.; Palaniyandy, N.; Mhlongo, S.E.; Gitari, W.M.; Edokpayi, J.N. Methanogenesis Potentials: Insights from Mineralogical Diagenesis, SEM and FTIR Features of the Permian Mikambeni Shale of the Tuli Basin, Limpopo Province of South Africa. Minerals 2021, 11, 651. [Google Scholar] [CrossRef]
- Walker, R.L.T.; Cauda, E.; Chubb, L.; Krebs, P.; Stach, R.; Mizaikoff, B.; Johnston, C. Complexity of Respirable Dust Found in Mining Operations as Characterized by X-ray Diffraction and FTIR Analysis. Minerals 2021, 11, 383. [Google Scholar] [CrossRef]
- D’Amico, F.; Musso, M.E.; Berger, R.J.F.; Cefarin, N.; Birarda, G.; Tondi, G.; Menezes, D.B.; Reyer, A.; Scarabattoli, L.; Sepperer, T.; et al. Chemical constitution of polyfurfuryl alcohol investigated by FTIR and Resonant Raman spectroscopy. Spectrochim. Acta Part A-Mol. Biomol. Spectrosc. 2021, 262, 120090. [Google Scholar] [CrossRef] [PubMed]
- Sandt, C.; Waeytens, J.; Deniset-Besseau, A.; Nielsen-Leroux, C.; Rejasse, A. Use and misuse of FTIR spectroscopy for studying the bio-oxidation of plastics. Spectrochim. Acta Part A-Mol. Biomol. Spectrosc. 2021, 258, 119841. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Ren, G.; Bai, L.; Feng, J.; Zhang, Z. Molecular Model Construction and Evaluation of Jincheng Anthracite. ACS Omega 2020, 5, 10663–10670. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, C.; Yan, K. Adsorption of collectors on model surface of Wiser bituminous coal: A molecular dynamics simulation study. Miner. Eng. 2015, 79, 31–39. [Google Scholar] [CrossRef]
- Marsalek, R.; Pospisil, J.; Taraba, B. The influence of temperature on the adsorption of CTAB on coals. Colloids Surf. A-Physicochem. Eng. Asp. 2011, 383, 80–85. [Google Scholar] [CrossRef]
- Tyrode, E.; Rutland, M.W.; Bain, C.D. Adsorption of CTAB on Hydrophilic Silica Studied by Linear and Nonlinear Optical Spectroscopy. J. Am. Chem. Soc. 2008, 130, 17434–17445. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.J.; Liu, J.Z.; Wang, R.K.; Zhou, J.H.; Cen, K.F. Effect of hydrothermal dewatering on the slurryability of brown coals. Energy Convers. Manag. 2012, 57, 8–12. [Google Scholar] [CrossRef]
- Zhao, F.; Du, Y.K.; Yang, P.; Li, X.C.; Tang, J.A. Adsorption behavior of hexadecyltrimethylammonium bromide (CTAB) to mica substrates as observed by atomic force microscopy. Sci. China Ser. B-Chem. 2005, 48, 101–106. [Google Scholar] [CrossRef]
- Ni, G.H.; Li, Z.; Sun, Q.; Li, S.; Dong, K. Effects of Bmim Cl ionic liquid with different concentrations on the functional groups and wettability of coal. Adv. Powder Technol. 2019, 30, 610–624. [Google Scholar]
- Khan, M.I.; Madni, A.; Ahmad, S.; Khan, A.; Rehmanand, M.; Mahmood, M.A. ATR-FTIR Based Pre and Post Formulation Compatibility Studies for the Design of Niosomal Drug Delivery System Containing Nonionic Amphiphiles and Chondroprotective Drug. J. Chem. Soc. Pak. 2015, 37, 527–534. [Google Scholar]
- Xu, C.H.; Wang, D.M.; Wang, H.T.; Xin, H.H.; Ma, L.Y.; Zhu, X.L.; Zhang, Y.; Wang, Q.G. Effects of chemical properties of coal dust on its wettability. Powder Technol. 2017, 318, 33–39. [Google Scholar] [CrossRef]
- Chen, X.L.; Yan, G.C.; Yang, X.L.; Xu, G.; Wei, S. Microscopic Diffusion Characteristics of Linear Alkylbenzene Sulfonates on the Surface of Anthracite: The Influence of Different Attachment Sites of Benzene Ring in the Backbone. Minerals 2021, 11, 1045. [Google Scholar] [CrossRef]
- Cai, P.; Nie, W.; Hua, Y.; Wei, W.L.; Jin, H. Diffusion and pollution of multi-source dusts in a fully mechanized coal face. Process Saf. Environ. Prot. 2018, 118, 93–105. [Google Scholar] [CrossRef]

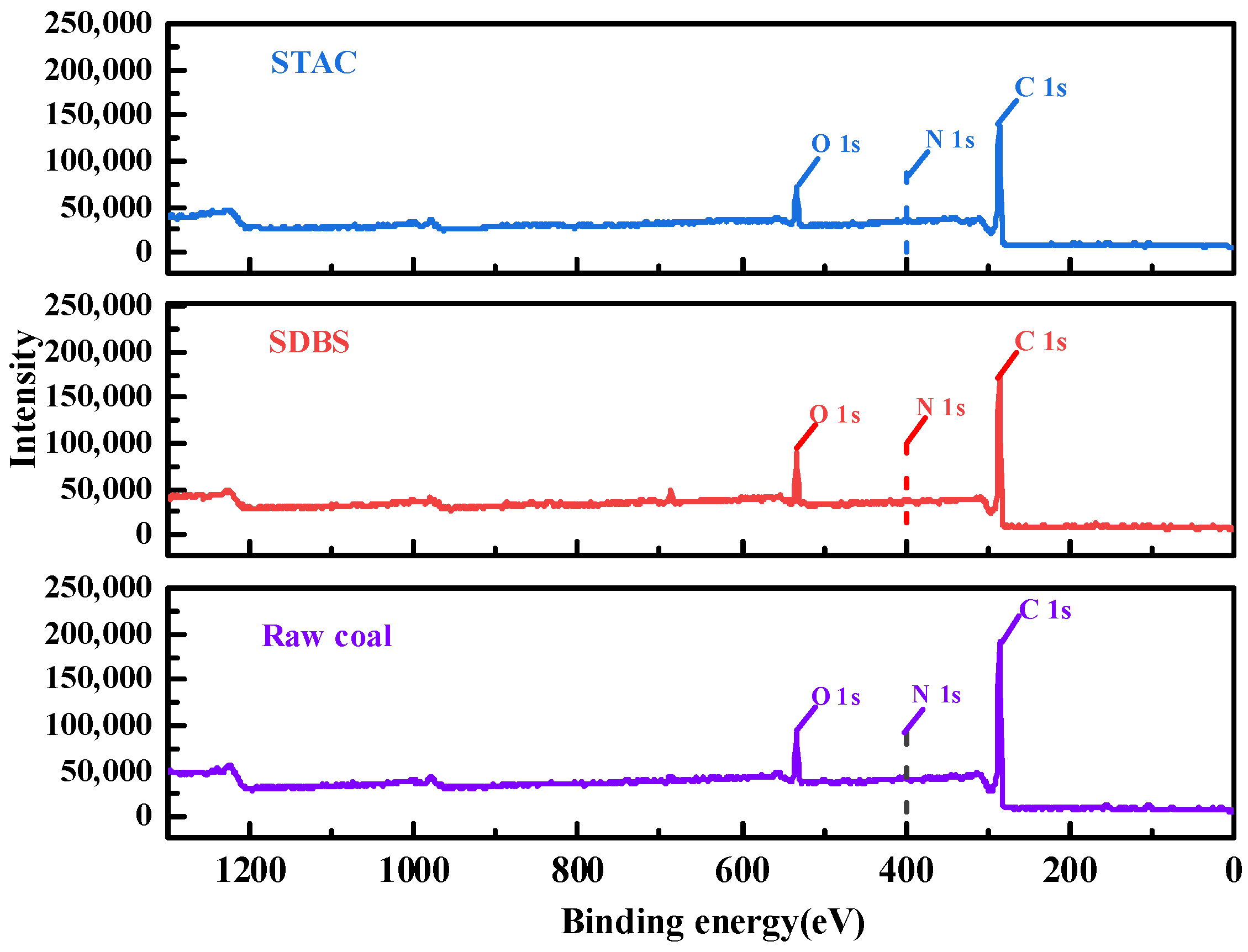
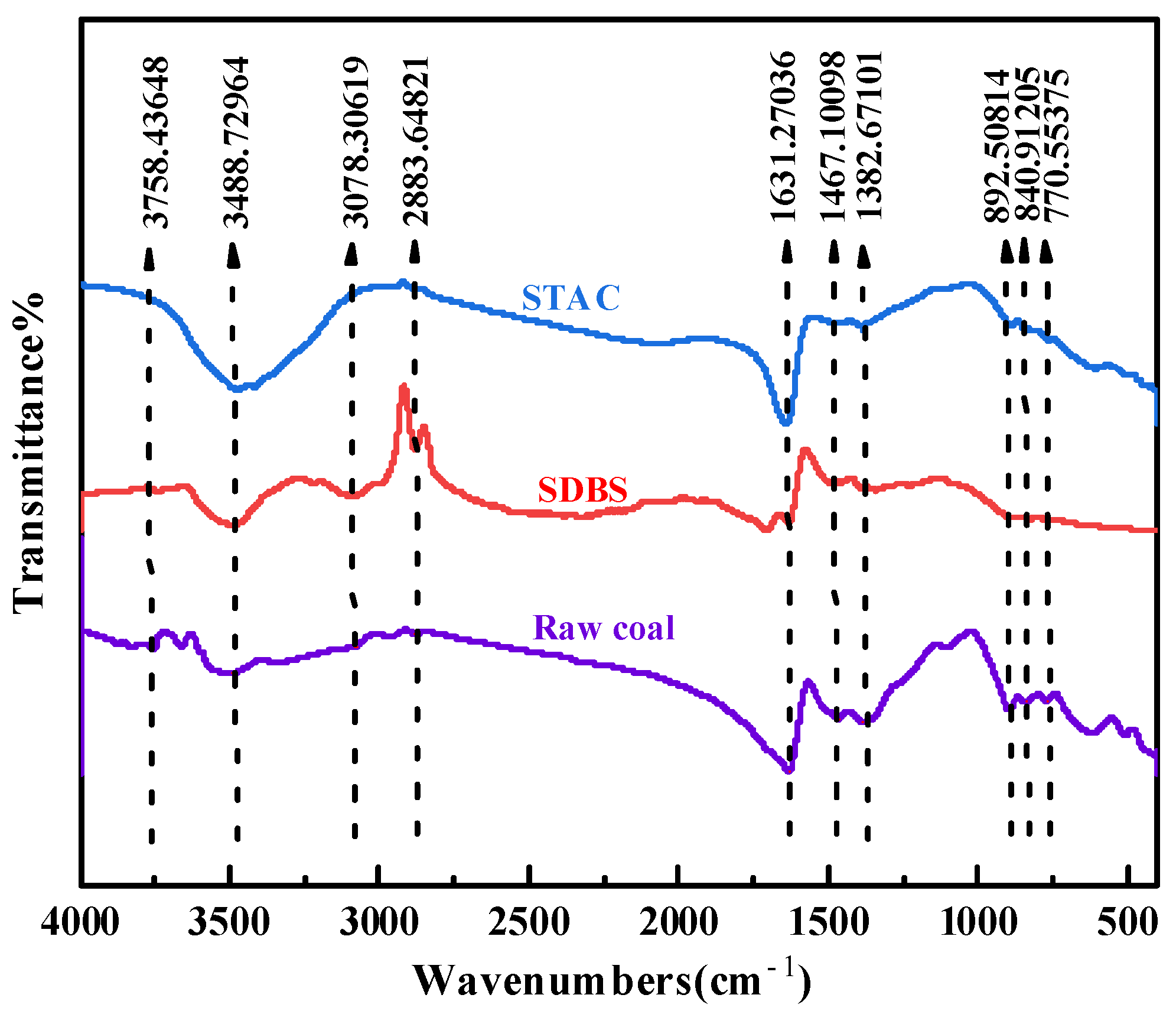
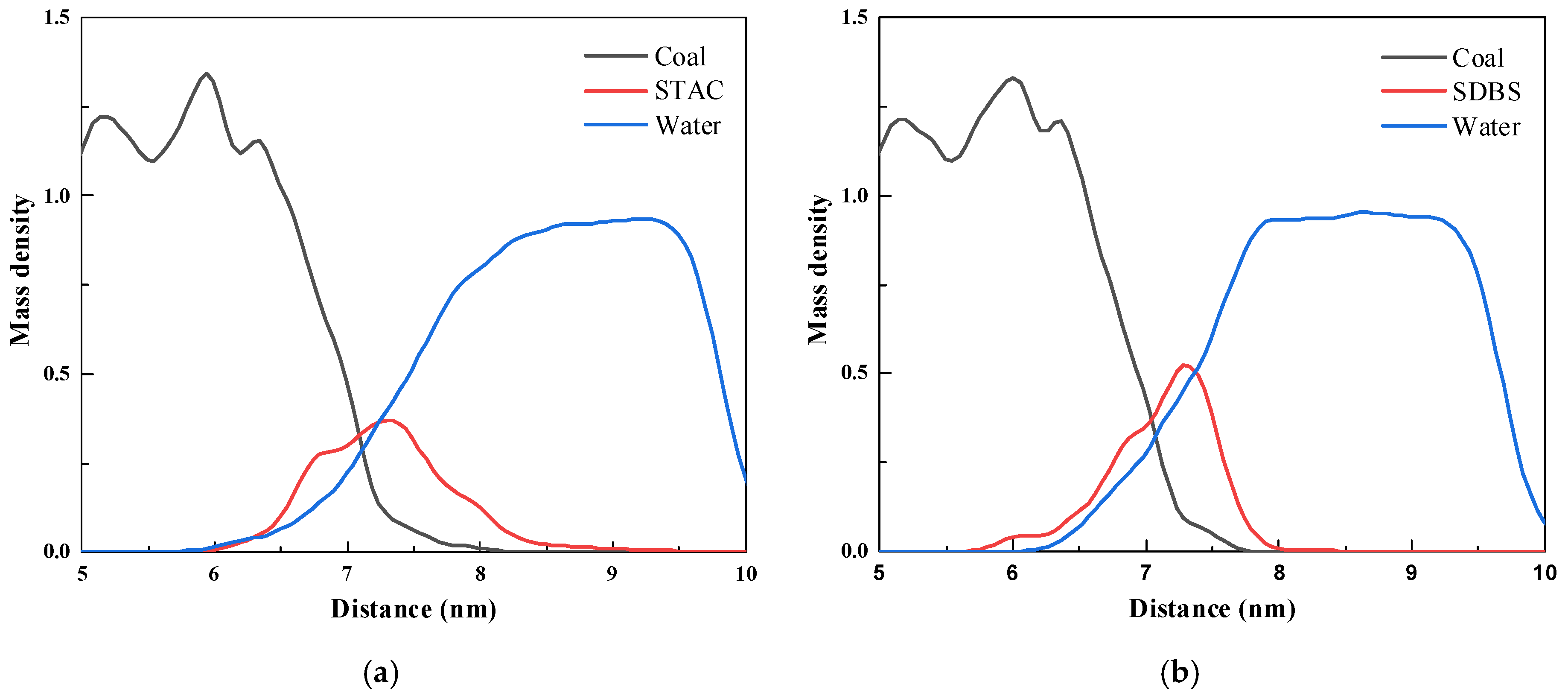
| Samples | Relative Atomic Number of Major Elements (%) | ||
|---|---|---|---|
| C | O | N | |
| Raw Coal | 85.65 | 13.30 | 1.05 |
| SDBS | 84.18 | 14.78 | 1.04 |
| STAC | 87.85 | 10.45 | 1.70 |
| Model | EV/(kcal·mol−1) | EE/(kcal·mol−1) | E/(kcal·mol−1) |
|---|---|---|---|
| Water–STAC–anthracite | −0.14 | −5329.68 | −5329.82 |
| Water–SDBS–anthracite | −88.53 | −1571.70 | −1660.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, X.; Yan, G.; Chen, X.; Li, J. Adsorption Characteristics of Ionic Surfactants on Anthracite Surface: A Combined Experimental and Modeling Study. Molecules 2022, 27, 5314. https://doi.org/10.3390/molecules27165314
Bai X, Yan G, Chen X, Li J. Adsorption Characteristics of Ionic Surfactants on Anthracite Surface: A Combined Experimental and Modeling Study. Molecules. 2022; 27(16):5314. https://doi.org/10.3390/molecules27165314
Chicago/Turabian StyleBai, Xuyang, Guochao Yan, Xuanlai Chen, and Jiajun Li. 2022. "Adsorption Characteristics of Ionic Surfactants on Anthracite Surface: A Combined Experimental and Modeling Study" Molecules 27, no. 16: 5314. https://doi.org/10.3390/molecules27165314
APA StyleBai, X., Yan, G., Chen, X., & Li, J. (2022). Adsorption Characteristics of Ionic Surfactants on Anthracite Surface: A Combined Experimental and Modeling Study. Molecules, 27(16), 5314. https://doi.org/10.3390/molecules27165314





