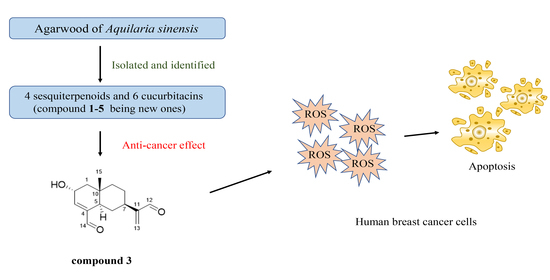
Anti-Cancer Effect of Sesquiterpene and Triterpenoids from Agarwood of Aquilaria sinensis
Abstract
:1. Introduction
2. Results and Discussion
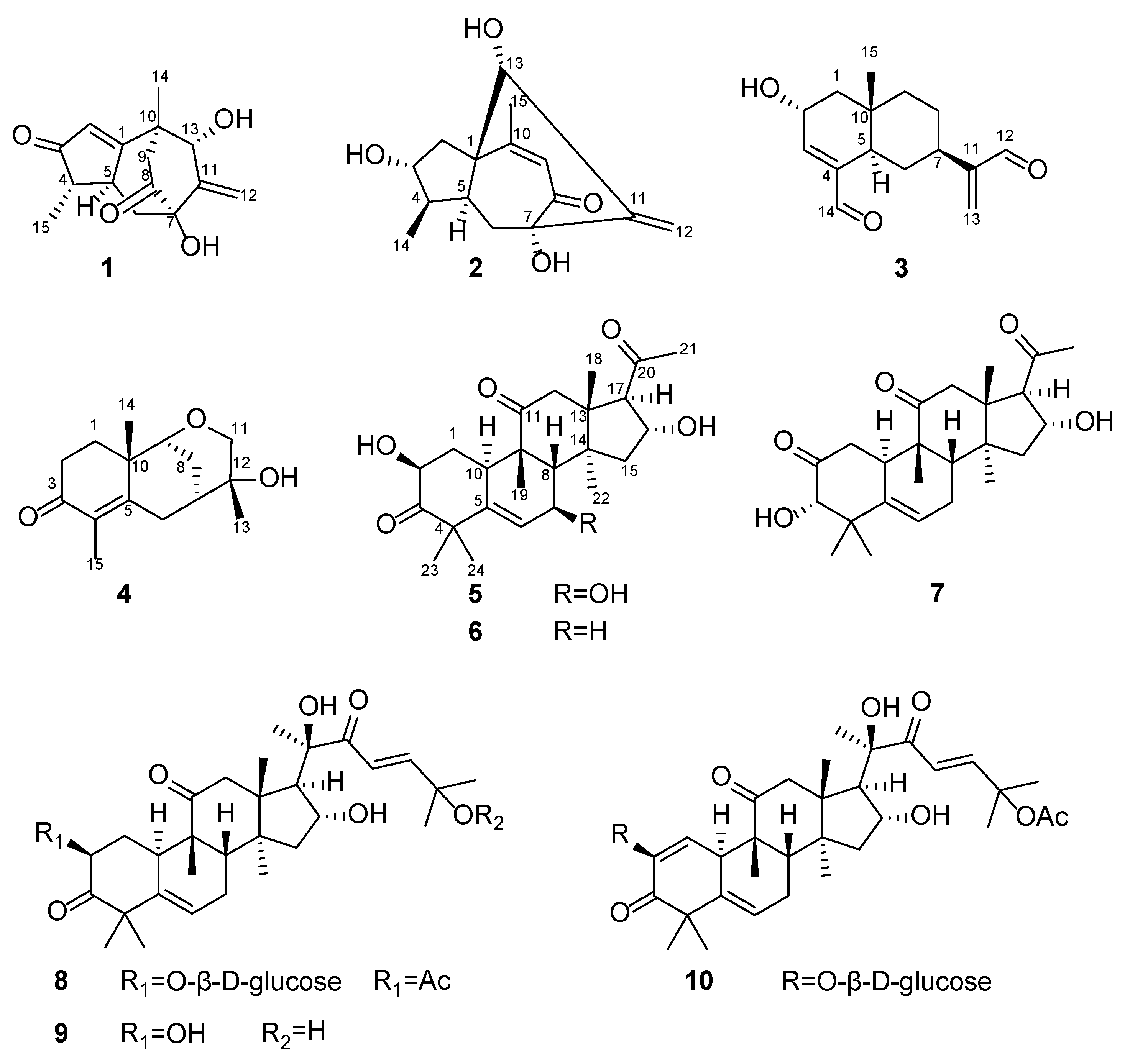
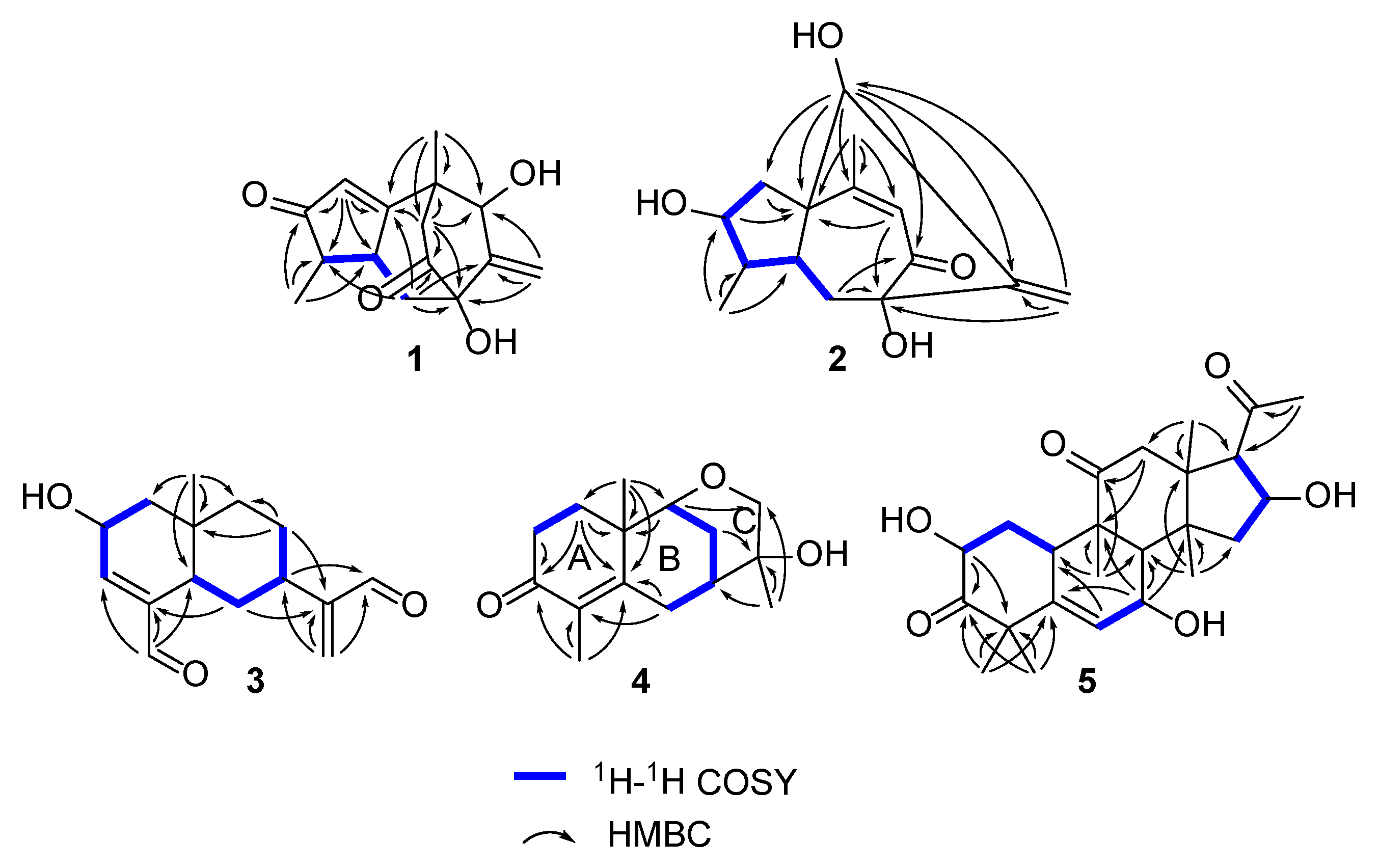
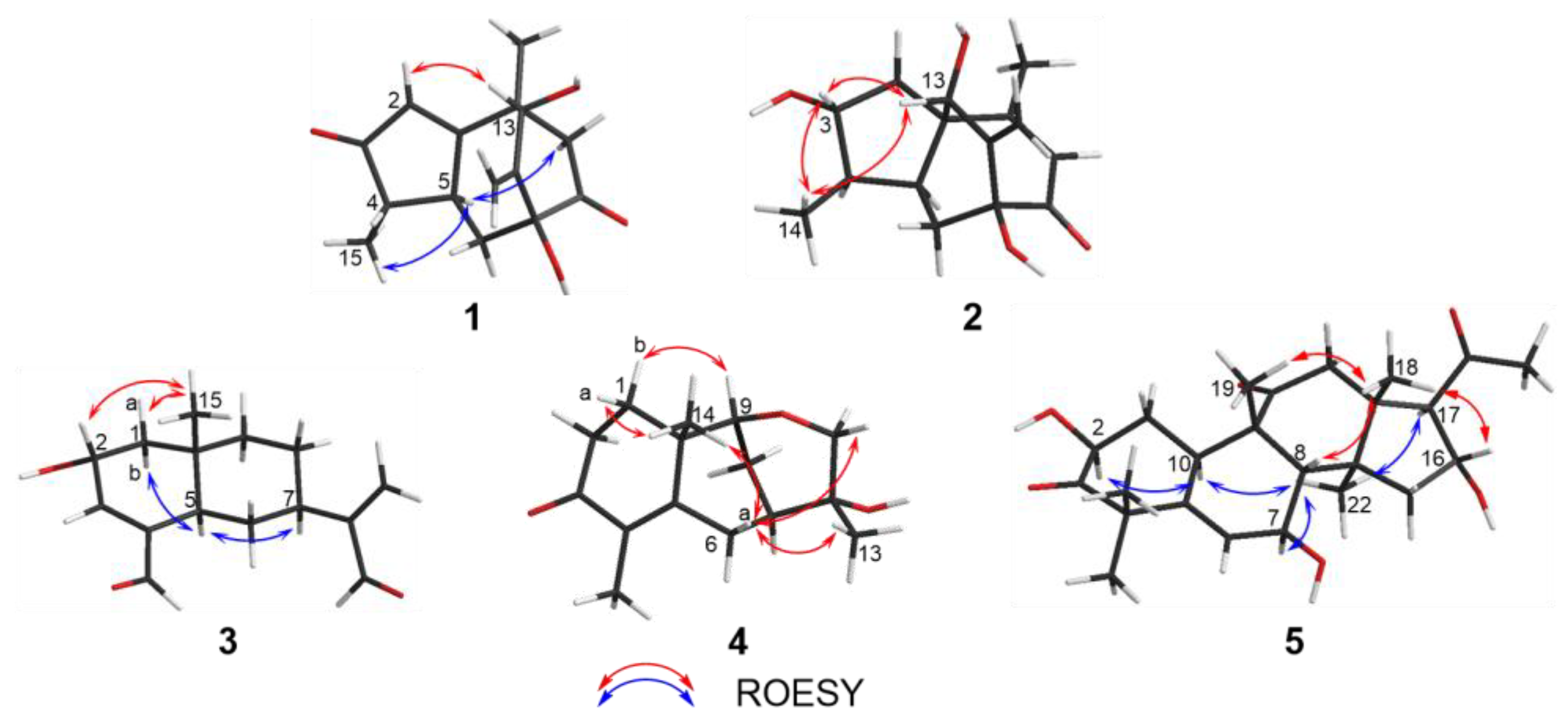
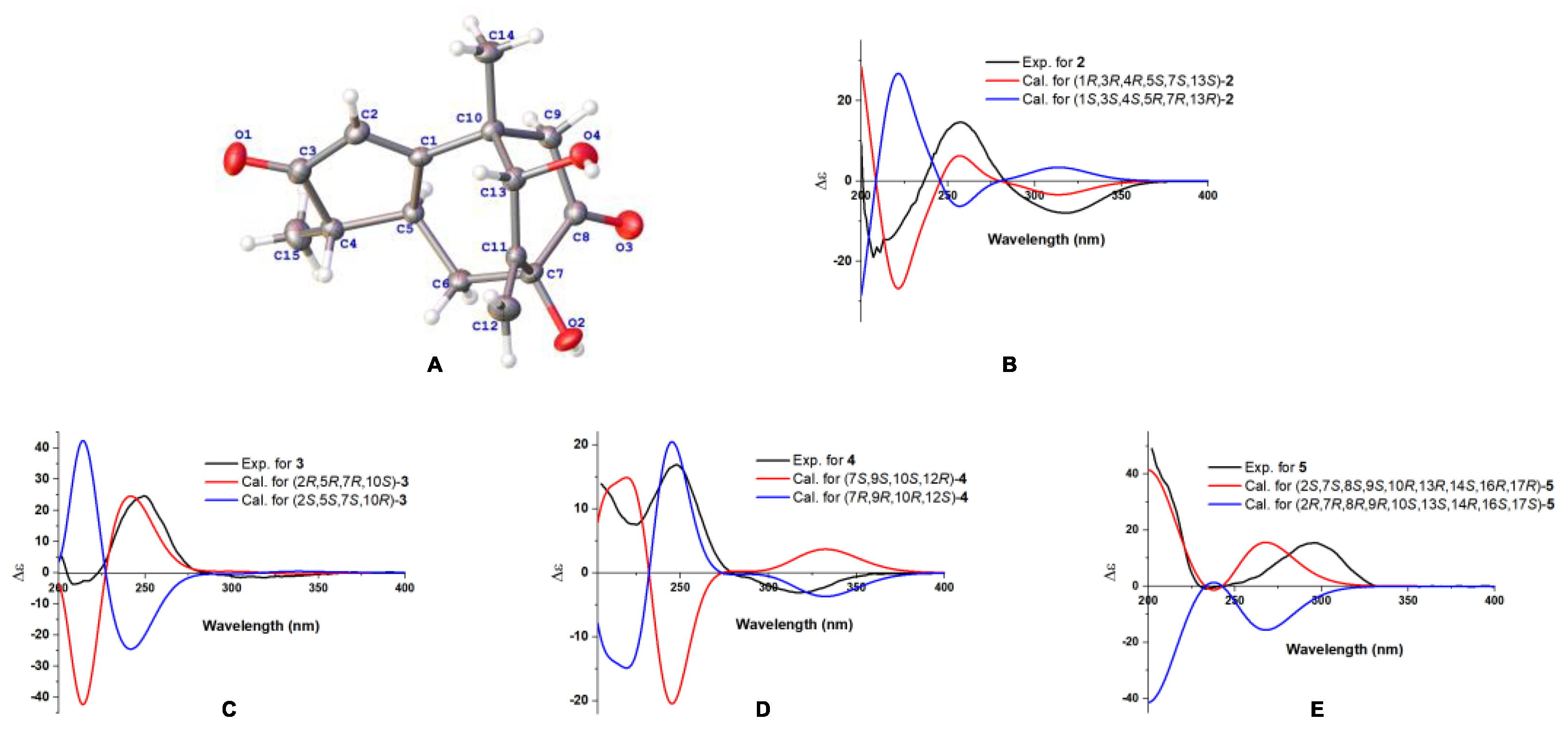
2.1. Structure Elucidation of the Compounds
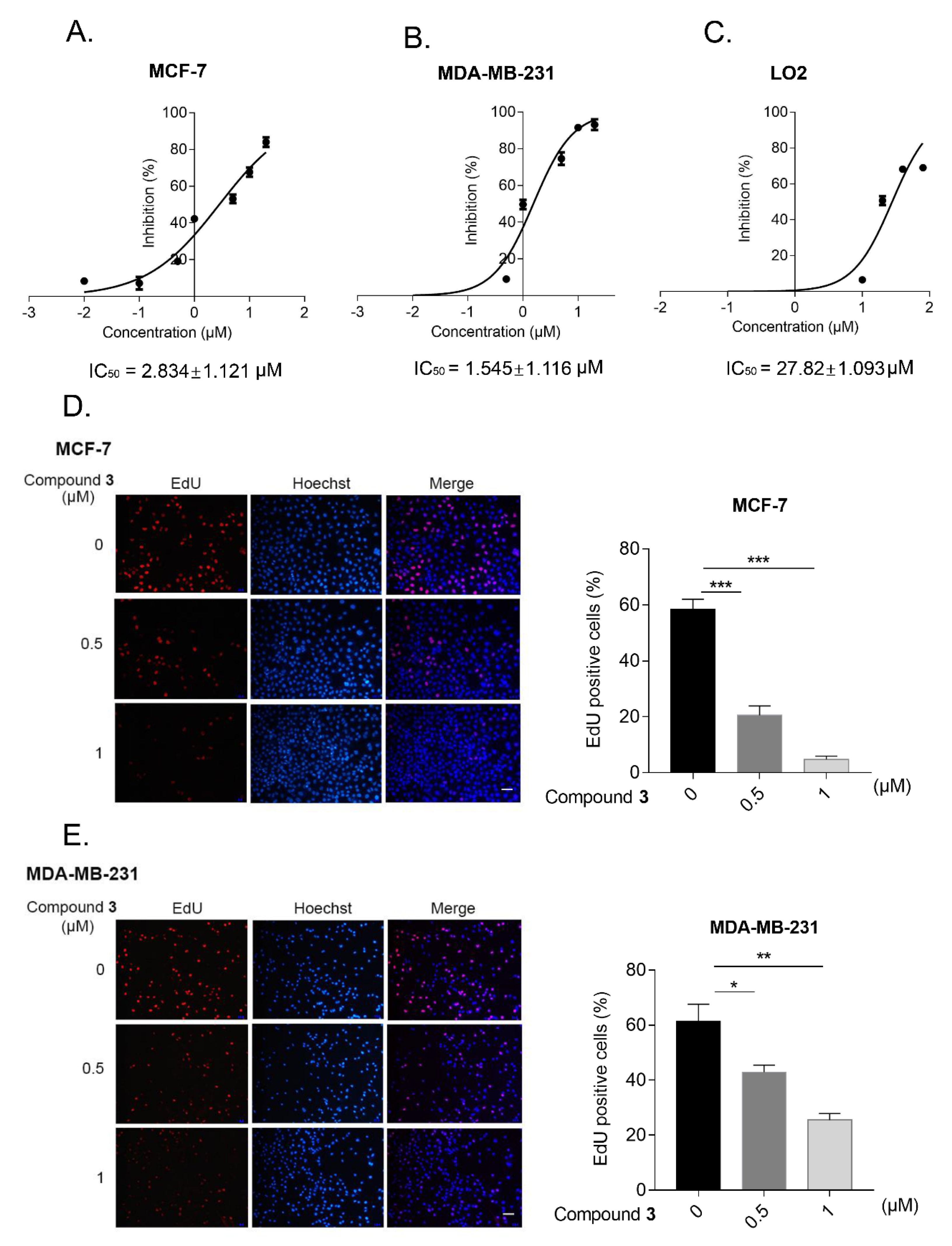
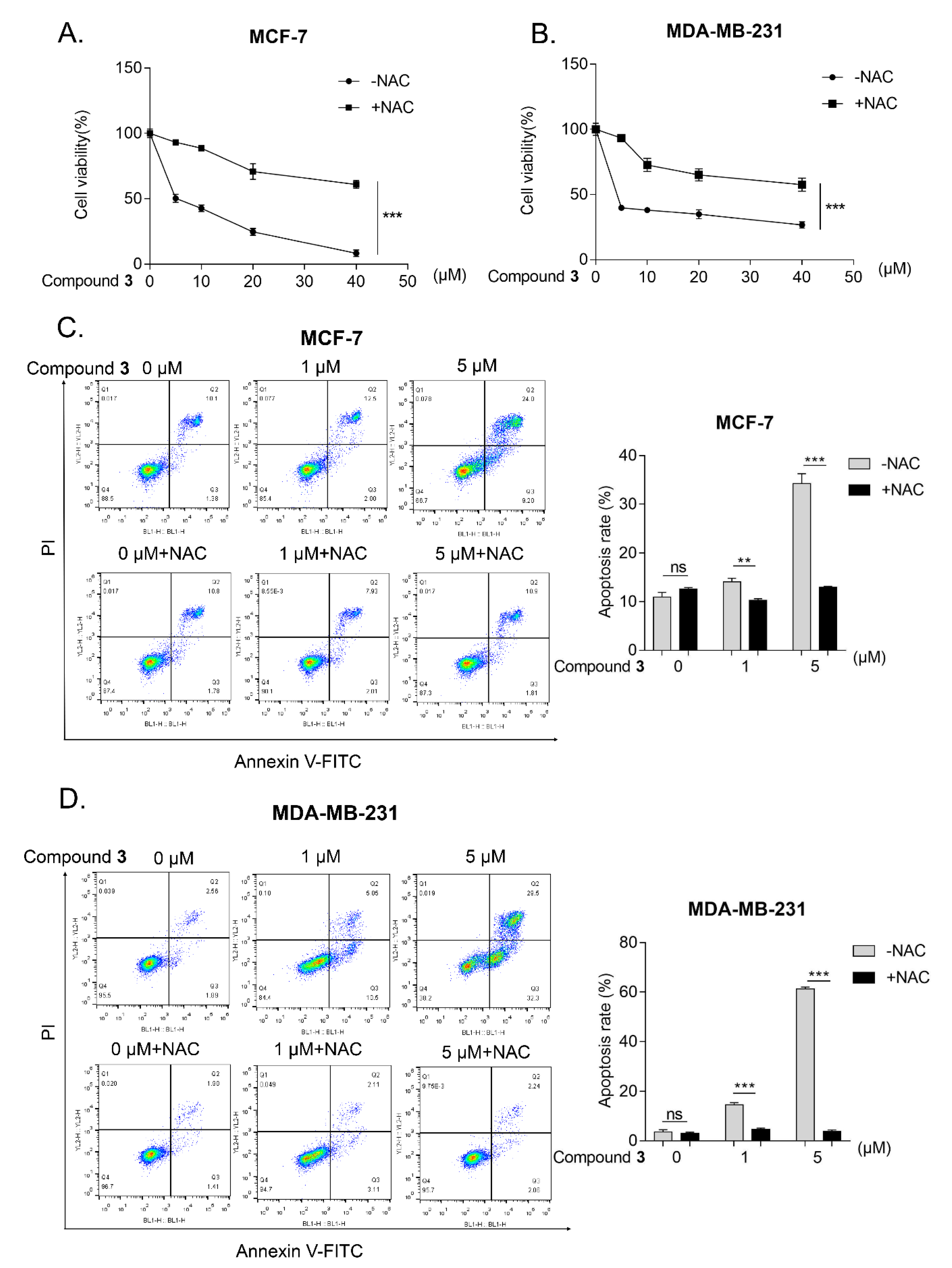
2.2. Biological Evaluation
3. Experimental Section
3.1. General Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Compound Characterization Data
3.5. ECD Calculation for Compounds 1–5
3.6. Bioactivity Assay
3.6.1. Cell Culture
3.6.2. Cell Viability Assay
3.6.3. EdU Assay
3.6.4. Flow Cytometry
3.6.5. Measurement of Reactive Oxygen Species (ROS)
3.6.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Kubczak, M.; Szustka, A.; Rogalińska, M. Molecular Targets of Natural Compounds with Anti-Cancer Properties. Int. J. Mol. Sci. 2021, 22, 13659. [Google Scholar] [CrossRef]
- Tan, C.S.; Isa, N.M.; Ismail, I.; Zainal, Z. Agarwood Induction: Current Developments and Future Perspectives. Front. Plant. Sci. 2019, 10, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.C.; Hussain, M.; Jiang, Z.B.; Wang, Z.H.; Gao, J.; Ye, F.X.; Mao, R.Q.; Li, H. Aquilaria Species (Thymelaeaceae) Distribution, Volatile and Non-Volatile Phytochemicals, Pharmacological Uses, Agarwood Grading System, and Induction Methods. Molecules 2021, 26, 7708. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.L.; Yan, Y.M.; Wang, D.W.; Cheng, Y.X. Spiroaquilarenes A–E: Unprecedented anti-inflammatory sesquiterpene polymers from agarwood of Aquilaria sinensis. Org. Chem. Front. 2022, 9, 2070–2078. [Google Scholar] [CrossRef]
- Naziz, P.S.; Das, R.; Sen, S. The Scent of Stress: Evidence From the Unique Fragrance of Agarwood. Front. Plant. Sci. 2019, 10, 840. [Google Scholar] [CrossRef]
- Shivanand, P.; Arbie, N.F.; Krishnamoorthy, S.; Ahmad, N. Agarwood—The Fragrant Molecules of a Wounded Tree. Molecules 2022, 27, 3386. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, H.Q.; Wang, H.; Mei, W.L.; Dai, H.F. Natural products in agarwood and Aquilaria plants: Chemistry, biological activities and biosynthesis. Nat. Prod. Rep. 2021, 38, 528–565. [Google Scholar] [CrossRef]
- Huang, X.L.; Zhou, Y.T.; Yan, Y.M.; Cheng, Y.X. Sesquiterpenoid-Chromone Heterohybrids from Agarwood of Aquilaria sinensis as Potent Specific Smad3 Phosphorylation Inhibitors. J. Org. Chem. 2022, 87, 7643–7648. [Google Scholar] [CrossRef]
- Gao, M.; Han, X.; Sun, Y.; Chen, H.; Yang, Y.; Liu, Y.; Meng, H.; Gao, Z.; Xu, Y.; Zhang, Z.; et al. Overview of sesquiterpenes and chromones of agarwood originating from four main species of the genus Aquilaria. RSC Adv. 2019, 9, 4113–4130. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Wang, J.; Li, W.; Dong, W.; Mei, W.; Dai, H. New guaiane and acorane sesquiterpenes in high quality agarwood “Qi-Nan” from Aquilaria sinensis. Phytochem. Lett. 2016, 17, 94–99. [Google Scholar] [CrossRef]
- Li, W.; Cai, C.H.; Guo, Z.K.; Wang, H.; Zuo, W.J.; Dong, W.H.; Mei, W.L.; Dai, H.F. Five new eudesmane-type sesquiterpenoids from Chinese agarwood induced by artificial holing. Fitoterapia 2015, 100, 44–49. [Google Scholar] [CrossRef]
- Zhang, X.; Li, H.; Wang, W.; Chen, T.; Xuan, L. Lipid-Lowering Activities of Cucurbitacins Isolated from Trichosanthes cucumeroides and Their Synthetic Derivatives. J. Nat. Prod. 2020, 83, 3536–3544. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, N.; Kurata, A.; Hakamatsuka, T.; Sekita, S.; Satake, M. Two New Cucurbitacin Glucosides, Opercurins A and B, from the Brazilian Folk Medicine “Buchinha” (Luffa operculata). Chem. Pharm. Bull. 2004, 52, 1018–1020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.J.; Sun, Y.; Yuan, Z.K.; Li, Y.J.; Wang, L.B.; Wu, L.J. Isolation and identification of the chemical constituents of Pedicellus Melo. J. Shenyang Pharm. Univ. 2014, 31, 351–354. [Google Scholar]
- Maatooq, G.; Sharkawy, S.E.; Afifi, M.S.; Rosazza, J.P.N. Microbial Transformation of Cucurbitacin E 2-O-β-D-Glucopyranoside. J. Nat. Prod. 1995, 58, 165–171. [Google Scholar] [CrossRef]
- Ghosh, S. Cisplatin: The first metal based anticancer drug. Bioorg. Chem. 2019, 88, 102925. [Google Scholar] [CrossRef] [PubMed]
- Park, W.H. The effect of MAPK inhibitors and ROS modulators on cell growth and death of H2O2-treated HeLa cells. Mol. Med. Rep. 2013, 8, 557–564. [Google Scholar] [CrossRef]
- Qian, C.; Wang, Y.; Zhong, Y.; Tang, J.; Zhang, J.; Li, Z.; Wang, Q.; Hu, R. Wogonin-enhanced reactive oxygen species-induced apoptosis and potentiated cytotoxic effects of chemotherapeutic agents by suppression Nrf2-mediated signaling in HepG2 cells. Free Radic. Res. 2014, 48, 607–621. [Google Scholar] [CrossRef]
- Curtin, J.F.; Donovan, M.; Cotter, T.G. Regulation and measurement of oxidative stress in apoptosis. J. Immunol. Methods 2002, 265, 49–72. [Google Scholar] [CrossRef] [Green Version]
- Cui, L.; Bu, W.Q.; Song, J.; Feng, L.; Xu, T.T.; Liu, D.; Ding, W.B.; Wang, J.H.; Li, C.Y.; Ma, B.G.; et al. Apoptosis induction by alantolactone in breast cancer MDA-MB-231 cells through reactive oxygen species-mediated mitochondrion-dependent pathway. Arch. Pharm. Res. 2018, 41, 299–313. [Google Scholar] [CrossRef]
- Kim, K.Y.; Yu, S.N.; Lee, S.Y.; Chun, S.S.; Choi, Y.L.; Park, Y.M.; Song, C.S.; Chatterjee, B.; Ahn, S.C. Salinomycin-induced apoptosis of human prostate cancer cells due to accumulated reactive oxygen species and mitochondrial membrane depolarization. Biochem. Biophys. Res. Commun. 2011, 413, 80–86. [Google Scholar] [CrossRef]
- Halasi, M.; Wang, M.; Chavan, T.S.; Gaponenko, V.; Hay, N.; Gartel, A.L. ROS inhibitor N-acetyl-L-cysteine antagonizes the activity of proteasome inhibitors. Biochem. J. 2013, 454, 201–208. [Google Scholar] [CrossRef] [Green Version]
- Wu, B.; He, S.; Wu, X.D.; Wu, D.K.; Pan, Y.J. Cadinane and eudesmane sesquiterpenoids from Chloranthas henryi. Helv. Chim. Acta 2007, 90, 1586–1592. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A. Gaussian 09; Version 09; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Liu, Y.Y.; Wei, J.H.; Gao, Z.H.; Zhang, Z.; Lyu, J.C. A review of quality assessment and grading for agarwood. Chin. Herb. Med. 2017, 9, 22–30. [Google Scholar] [CrossRef]
| 1 | 2 | ||||
|---|---|---|---|---|---|
| no. | δH | δC | no. | δH | δC |
| 1 | 187.7, C | 1 | 55.6, C | ||
| 2 | 6.12 (d, 1.72) | 129.5, CH | 2 | Ha: 2.45 (dd, 14.02, 6.46) | 39.6, CH2 |
| Hb: 2.05 (dd, 14.02, 9.23) | |||||
| 3 | 213.0, C | 3 | 4.20 (dt, 9.23, 6.46) | 74.9, CH | |
| 4 | 2.00 (qd, 7.44, 2.05) | 51.2, CH | 4 | 2.23 (m) | 41.3, CH |
| 5 | 2.83 (ddt, 11.04, 9.03, 2.05) | 47.4, CH | 5 | 2.16 (dt, 12.27, 8.89) | 49.0, CH |
| 6 | Ha: 2.42 (dd, 12.27, 9.03) | 49.9, CH2 | 6 | Ha: 1.97 (t, 12.27) | 32.0, CH2 |
| Hb: 1.63 (dd, 12.27, 11.04) | Hb: 1.57 (dd, 12.27, 8.89) | ||||
| 7 | 81.1, C | 7 | 83.3, C | ||
| 8 | 211.0, C | 8 | 201.0, C | ||
| 9 | Ha: 2.72 (d, 19.40) | 44.0, CH2 | 9 | 6.06 (d, 1.41) | 128.3, CH |
| Hb: 2.66 (dd, 19.40, 0.72) | |||||
| 10 | 43.4, C | 10 | 173.4, C | ||
| 11 | 154.2, C | 11 | 151.5, C | ||
| 12 | Ha: 5.59 (br s) | 116.8, CH2 | 12 | Ha: 5.45 (dd, 2.64, 1.41) | 111.2, CH2 |
| Hb: 5.30 (br s) | Hb: 5.32 (t, 1.41) | ||||
| 13 | 4.09 (br s) | 76.7, CH | 13 | 4.65 (t, 2.64) | 77.0, CH |
| 14 | 1.40 (s) | 23.7, CH3 | 14 | 0.96 (d, 7.34) | 9.7, CH3 |
| 15 | 1.14 (d, 7.44) | 15.5, CH3 | 15 | 2.08 (d, 1.41) | 26.4, CH3 |
| 3 | 4 | ||||
|---|---|---|---|---|---|
| no. | δH | δC | no. | δH | δC |
| 1 | Ha: 1.88 (ddd, 12.36, 6.60, 1.42) | 47.4, CH2 | 1 | Ha: 2.11 (dt, 13.52, 4.65) | 34.6, CH2 |
| Hb: 1.33 (m) | Hb: 1.78 (overlap) | ||||
| 2 | 4.55 (m) | 66.6, CH | 2 | Ha: 2.47 (ddd, 16.73, 13.90, 5.10) | 34.3, CH2 |
| Hb: 2.36 (dt, 16.73, 4.65) | |||||
| 3 | 6.64 (td, 3.12, 1.42) | 154.1, CH | 3 | 201.5, C | |
| 4 | 143.2, C | 4 | 131.0, C | ||
| 5 | 2.34 (dq, 12.76, 3.12) | 45.1, CH | 5 | 164.2, C | |
| 6 | Ha: 2.67 (dt, 12.76, 3.12) | 27.5, CH2 | 6 | Ha: 2.78 (dd, 12.92, 2.53) | 29.3, CH2 |
| Hb: 1.19 (q-like, 12.76) | Hb: 2.01 (t-like, 12.92) | ||||
| 7 | 2.58 (m) | 38.5, CH | 7 | 1.66 (m) | 42.1, CH |
| 8 | 1.61 (m) | 27.2, CH2 | 8 | Ha: 1.92 (m) | 31.1, CH2 |
| Hb: 1.67 (m) | |||||
| 9 | Ha: 1.55 (m) | 40.3, CH2 | 9 | 3.36 (dd, 10.91, 4.27) | 79.1, CH |
| Hb: 1.45 (td, 12.92, 4.87) | |||||
| 10 | 36.0, C | 10 | 43.0, C | ||
| 11 | 156.3, C | 11 | 74.6, C | ||
| 12 | 9.51 (s) | 196.2, CH | 12 | Ha: 3.51 (d, 10.80) | 68.8, CH2 |
| Hb: 3.47 (d, 10.80) | |||||
| 13 | Ha: 6.36 (br s) | 134.6, CH2 | 13 | 1.18 (s) | 21.7, CH3 |
| Hb: 6.08 (br s) | |||||
| 14 | 9.46 (s) | 196.7, CH | 14 | 1.16 (s) | 16.3, CH3 |
| 15 | 0.93 (s) | 16.7, CH3 | 15 | 1.78 (s) | 11.3, CH3 |
| 5 | |||||
|---|---|---|---|---|---|
| no. | δH | δC | no. | δH | δC |
| 1 | Ha: 2.12 (ddd, 12.52, 5.76, 3.74) | 36.5, CH2 | 13 | 50.4, C | |
| Hb: 1.26 (q, 12.52) | 14 | 48.8, C | |||
| 2 | 4.60 (dd, 12.81, 5.76) | 72.7, CH | 15 | Ha: 2.17 (m) | 46.0, CH2 |
| 3 | 213.3, C | Hb: 1.58 (br d, 13.42) | |||
| 4 | 51.8, C | 16 | 4.86 (overlap) | 72.5, CH | |
| 5 | 146.1, C | ||||
| 6 | 122.7, CH | 17 | 3.20 (d, 6.76) | 67.8, CH | |
| 7 | 4.12 (dd, 5.34, 1.07) | 66.9, CH | 18 | 0.70 (s) | 20.2, CH3 |
| 8 | 2.08 (br s) | 53.5, CH | 19 | 1.16 (s) | 21.4, CH3 |
| 9 | 49.4, C | 20 | 210.6, C | ||
| 10 | 3.04 (ddt, 12.96, 3.74, 1.98) | 35.6, CH | 21 | 2.20 (s) | 31.8, CH3 |
| 11 | 213.9, C | 22 | 1.32 (s) | 19.8, CH3 | |
| 12 | Ha: 2.45 (d, 14.72) | 48.2, CH2 | 23 | 1.36 (s) | 21.9, CH3 |
| Hb: 3.46 (d, 14.72) | 24 | 1.33 (s) | 30.3, CH3 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Liu, Y.; Li, Y.; Yin, W.; Cheng, Y. Anti-Cancer Effect of Sesquiterpene and Triterpenoids from Agarwood of Aquilaria sinensis. Molecules 2022, 27, 5350. https://doi.org/10.3390/molecules27165350
Chen L, Liu Y, Li Y, Yin W, Cheng Y. Anti-Cancer Effect of Sesquiterpene and Triterpenoids from Agarwood of Aquilaria sinensis. Molecules. 2022; 27(16):5350. https://doi.org/10.3390/molecules27165350
Chicago/Turabian StyleChen, Lili, Yunyun Liu, Yifei Li, Wu Yin, and Yongxian Cheng. 2022. "Anti-Cancer Effect of Sesquiterpene and Triterpenoids from Agarwood of Aquilaria sinensis" Molecules 27, no. 16: 5350. https://doi.org/10.3390/molecules27165350
APA StyleChen, L., Liu, Y., Li, Y., Yin, W., & Cheng, Y. (2022). Anti-Cancer Effect of Sesquiterpene and Triterpenoids from Agarwood of Aquilaria sinensis. Molecules, 27(16), 5350. https://doi.org/10.3390/molecules27165350