7-Hydroxyflavone Alleviates Myocardial Ischemia/Reperfusion Injury in Rats by Regulating Inflammation
Abstract
:1. Introduction
2. Results
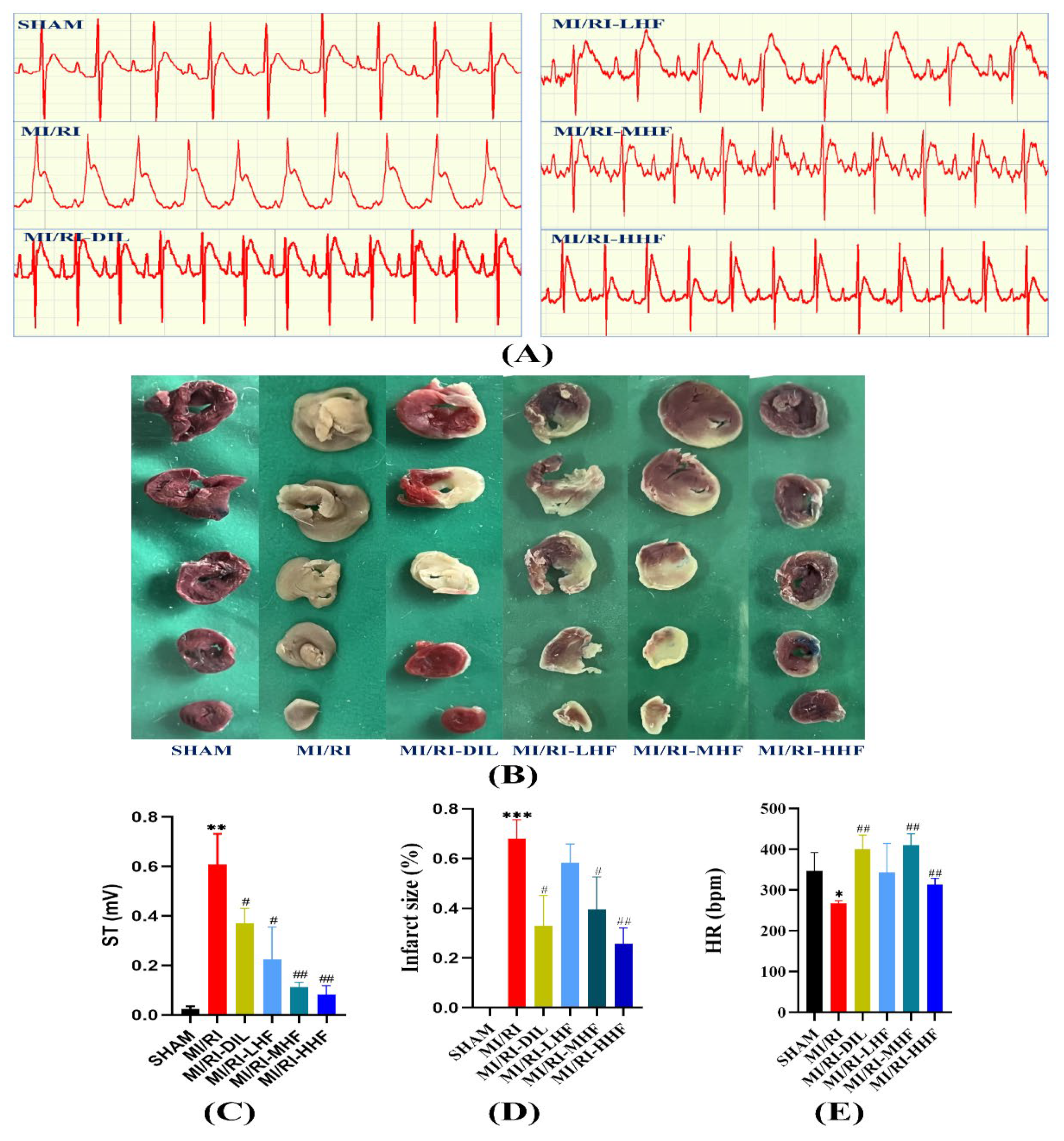
2.1. HF Improves the Anatomic Data
2.2. HF Improves the Electrocardiograph (ECG) Parameters
2.3. HF Reduces on IS in Rat MI/RI Model
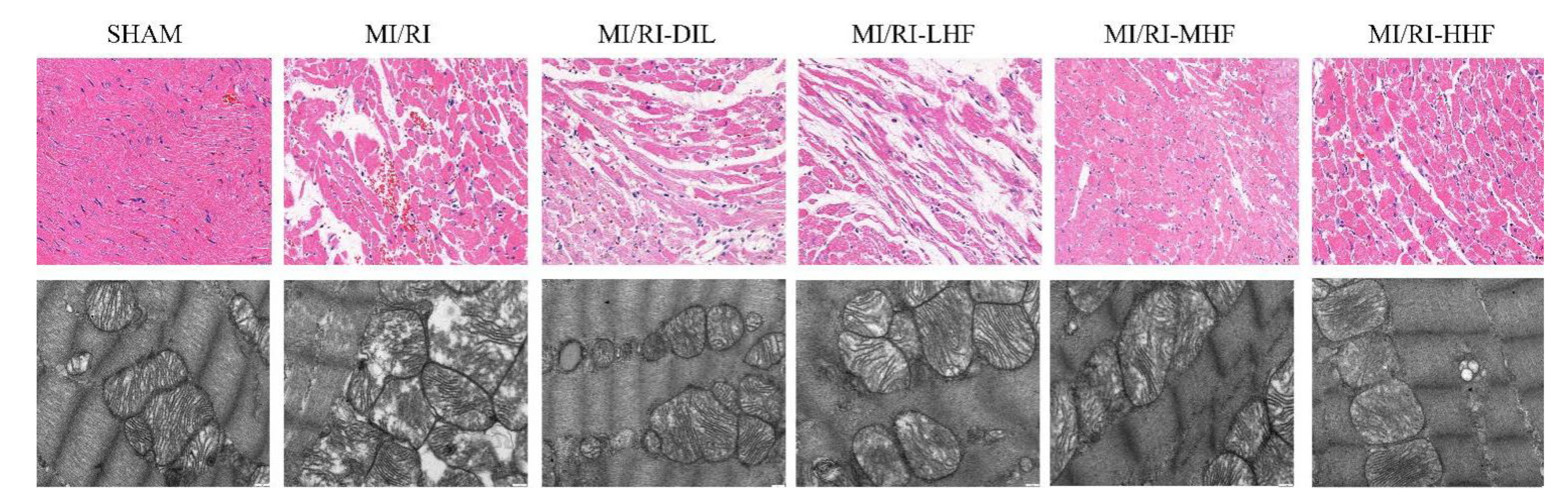
2.4. HF Recovers Myocardial Architecture after MI/RI
2.5. HF Decreases Myocardial Injury Markers after MI/RI
2.6. HF Inhibits the Inflammatory Cytokines after MI/RI
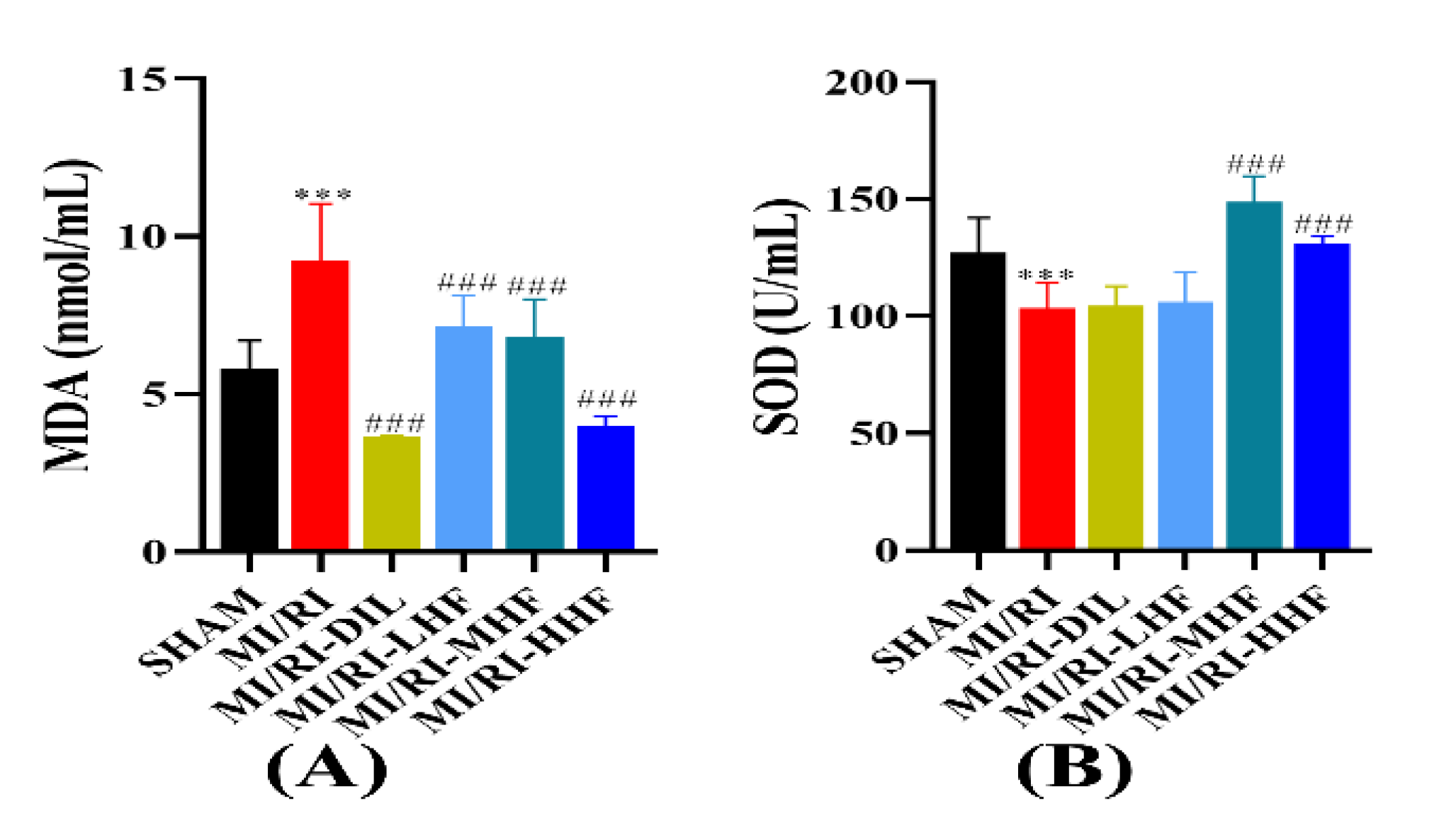
2.7. HF Modulates Superoxide Dismutase and Malondialdehyde after MI/RI
2.8. HF Attenuates Apoptosis after MI/RI
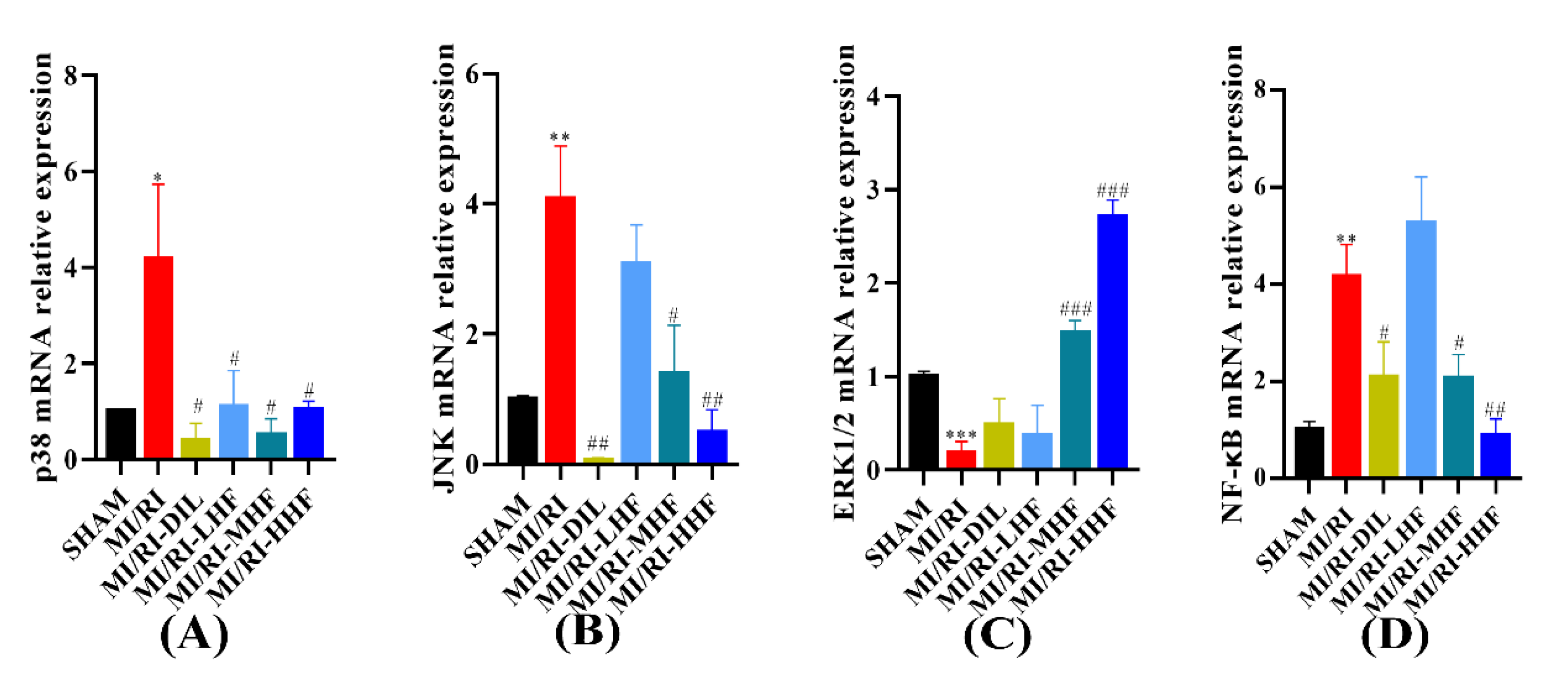
2.9. HF Regulates the Expression of MAPK/NF-κB mRNA after MI/RI
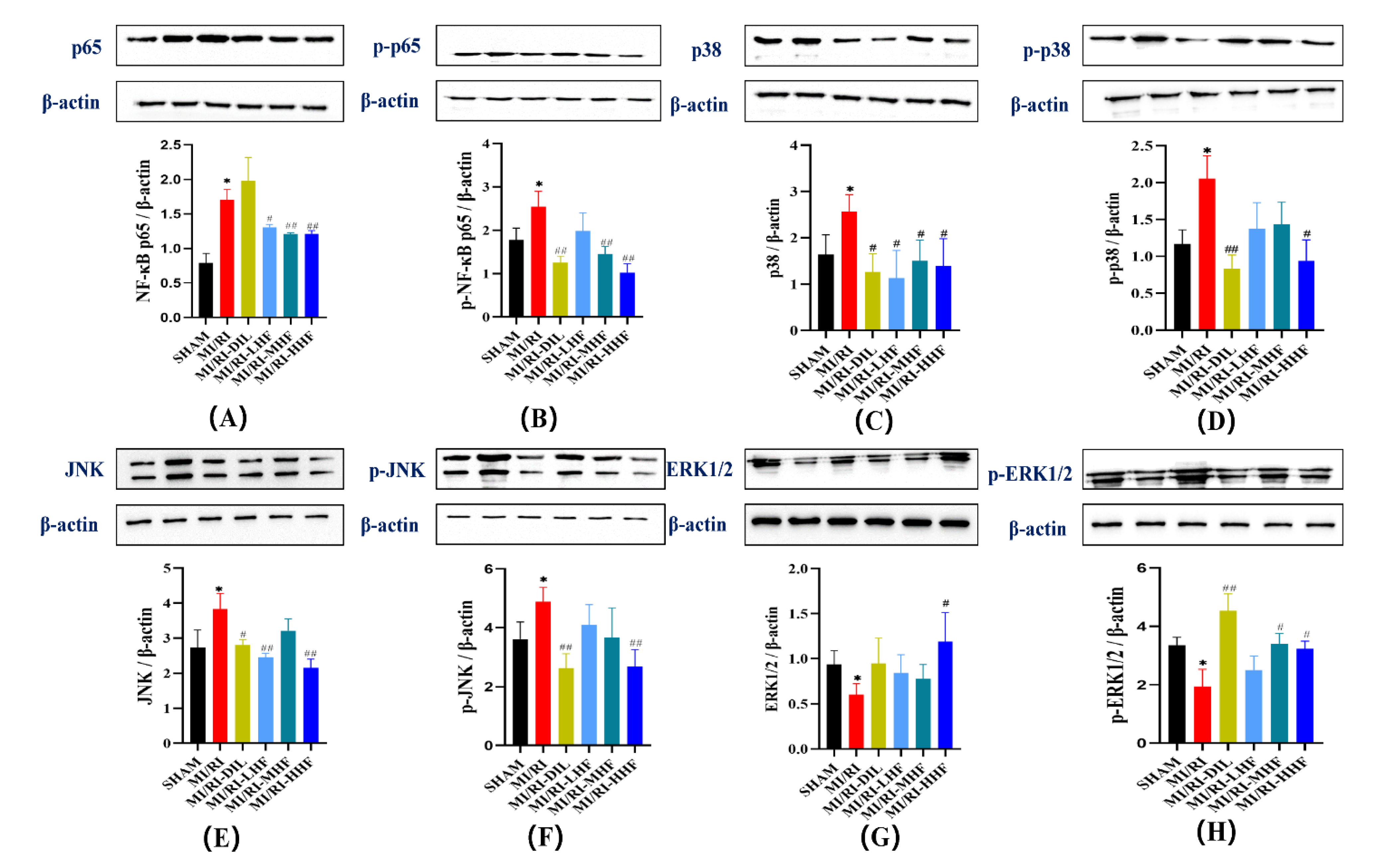
2.10. HF Modulates the Protein of the MAPK/NF-κB Signaling Pathway after MI/RI
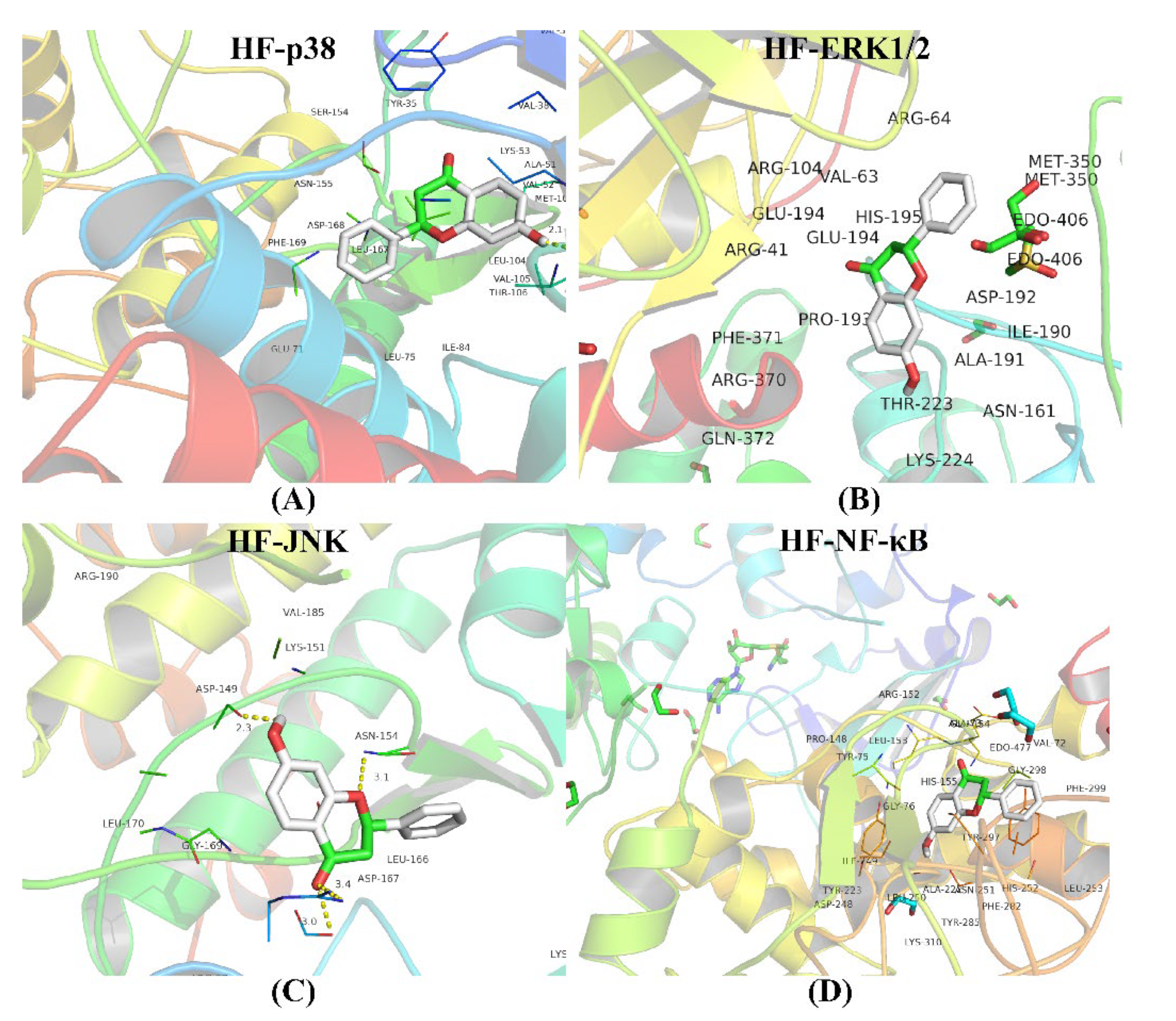
2.11. Molecular Docking
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Experimental Animals
4.3. Myocardial Infarct Area Measurement
4.4. Morphological and Histological Analysis
4.5. TEM
4.6. TUNEL Assay
4.7. Markers of Myocardial Injury Test
4.8. SOD and MDA Test
4.9. Anti-Inflammatory Activity Test
4.10. Antiapoptotic Activity Test
4.11. Quantitative Real-Time PCR
4.12. Western Blotting
4.13. Molecular Docking
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Ethics Statements
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| Aspartate transaminase | AST |
| B-cell lymphoma-2 | BCL2 |
| B-cell lymphoma-2-associated X | Bax |
| Cardiac troponin I | c-TnI |
| Caspase-3 | Casp-3 |
| c-Jun N-terminal kinase | JNK |
| Creatine kinase | CK |
| Diltiazem | DIL |
| Electrocardiograph | ECG |
| Extracellular regulated protein kinases1/2 | ERK1/2 |
| Heart rate | HR |
| Hematoxylin-Eosin | HE |
| Heme oxygenase-1 | HO-1 |
| Interleukin-1β | IL-1β |
| Interleukin-6 | IL-6 |
| Infarct size | IS |
| Ischemic heart disease | IHD |
| Interfibrillar mitochondria | IFM |
| Left anterior descending branch of the coronary artery | LAD |
| Left ventricle | LV |
| Malondialdehyde | MDA |
| Mitogen-activated protein kinase | MAPK |
| Myocardial ischemia/reperfusion injury | MI/RI |
| Myocardial ischemia/reperfusion injury-diltiazem | MI/RI-DIL |
| Myocardial ischemia/reperfusion injury-low dose of 7-hydroxyflavone | MI/RI-LHF |
| Myocardial ischemia/reperfusion injury-medium dose of 7-hydroxyflavone | MI/RI-MHF |
| Myocardial ischemia/reperfusion injury-high dose of 7-hydroxyflavone | MI/RI-MHF |
| Nuclear factor erythroid 2-related factor 2 | NRF2 |
| Nuclear factor-κB | NF-κB |
| Oxytropis falcata Bunge | O. falcata |
| Perinuclear mitochondria | PNM |
| 7-Hydroxyflavone | HF |
| Sprague Dawley | SD |
| Subsarcolemmal mitochondria | SSM |
| Superoxide dismutase | SOD |
| TdT-mediated dUTP nick end labeling | TUNEL |
| The ratio of heart weight to body weight | RHB |
| The ratio of liver weight to body weight | RLIB |
| The ratio of kidney weight to body weight | RKB |
| The ratio of spleen weight to body weight | RSB |
| The ratio of thymus weight to body weight | RTB |
| Transmission electron microscope | TEM |
| Tumor necrosis factor-α | TNF-α |
| Traditional Tibetan medicines | TTMs |
| 2,3,5-Triphenyltetrazolium chloride | TTC |
References
- Heusch, G. Myocardial ischaemia–reperfusion injury and cardioprotection in perspective. Nat. Rev. Cardiol. 2020, 17, 773–789. [Google Scholar] [CrossRef] [PubMed]
- Liebman, B.; Schwaegler, C.; Foote, A.T.; Rao, K.S.; Marquis, T.; Aronshtam, A.; Bell, S.P.; Gogo, P.; LaChapelle, R.R.; Spees, J.L. Human Growth Factor/Immunoglobulin Complexes for Treatment of Myocardial Ischemia-Reperfusion Injury. Front. Bioeng. Biotechnol. 2022, 10, 749787. [Google Scholar] [CrossRef] [PubMed]
- Lv, T.; Yan, J.; Lou, Y.; Zhang, Z.; Ye, M.; Zhou, J.; Luo, F.; Bi, C.; Lin, H.; Zhang, J.; et al. Evaluation of Melatonin Therapy in Patients with Myocardial Ischemia-Reperfusion Injury: A Systematic Review and Meta-Analysis. Oxidative Med. Cell. Longev. 2022, 2022, 4610522. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Zhang, H.; Zhang, Y.; Zhang, Z.; Yu, M.; Wang, S.; Han, F. Lingguizhugan decoction protects PC12 cells against Aβ (25–35)-induced oxidative stress and neuroinflammation by modulating NF-κB/MAPK signaling pathways. J. Ethnopharmacol. 2022, 292, 115194. [Google Scholar] [CrossRef]
- Zhong, J.; Liu, P.; Li, S.; Huang, X.; Zhang, Q.; Huang, J.; Guo, Y.; Chen, M.; Ruan, Z.; Qin, C.; et al. A comparison of three-dimensional speckle tracking echocardiography parameters in predicting left ventricular remodeling. J. Healthc. Eng. 2020, 2020, 8847144. [Google Scholar] [CrossRef]
- Ni, L.; Lu, Q.; Tang, M.; Tao, L.; Zhao, H.; Zhang, C.; Yu, Y.; Wu, X.; Liu, H.; Cui, R. Periplaneta americana extract ameliorates dextran sulfate sodium-induced ulcerative colitis via immunoregulatory and PI3K/AKT/NF-κB signaling pathways. Inflammopharmacology 2022, 30, 907–918. [Google Scholar] [CrossRef]
- Wijesekera, T.P.; Wu, Z.; Stephens, N.P.; Godula, R.; Lew, L.K.; Atkinson, N.S. A non-nuclear NF-κB modulates alcohol sensitivity but not immunity. J. Neurosci. 2022, 42, 3329–3343. [Google Scholar] [CrossRef]
- Li, T.; Lu, H.; Zhou, L.; Jia, M.; Zhang, L.; Wu, H.; Shan, L. Growth factors-based platelet lysate rejuvenates skin against ageing through NF-κB signalling pathway: In vitro and in vivo mechanistic and clinical studies. Cell Prolif. 2022, 55, e13212. [Google Scholar] [CrossRef]
- Bhuiyan, M.I.H.; Young, C.B.; Jahan, I.; Hasan, M.N.; Fischer, S.; Azlan, N.F.M.; Liu, M.; Chattopadhyay, A.; Huang, H.; Kahle, K.T.; et al. NF-κB Signaling-Mediated Activation of WNK-SPAK-NKCC1 Cascade in Worsened Stroke Outcomes of Ang II-Hypertensive Mice. Stroke 2022, 53, 1720–1734. [Google Scholar] [CrossRef]
- Myocardial ischemia-reperfusion injury and the influence of inflammation. Available online: https://www.sciencedirect.com/science/article/abs/pii/S1050173822000299 (accessed on 16 May 2022).
- Zhang, H.; Kim, H.; Park, B.W.; Noh, M.; Kim, Y.; Park, J.; Park, J.-H.; Kim, J.-J.; Sim, W.-S.; Ban, K.; et al. CU06-1004 enhances vascular integrity and improves cardiac remodeling by suppressing edema and inflammation in myocardial ischemia–reperfusion injury. Exp. Mol. Med. 2022, 54, 23–34. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Y.; Cao, X.; Wang, W.; Cui, X.; Yang, X.; Wang, Y.; Shi, J. Nrf2 Promotes Inflammation in Early Myocardial Ischemia-Reperfusion via Recruitment and Activation of Macrophages. Front. Immunol. 2021, 12, 763760. [Google Scholar] [CrossRef]
- Zhang, Q.; Guo, Y.; Zhang, B.; Liu, H.; Peng, Y.; Wang, D.; Zhang, D. Identification of hub biomarkers of myocardial infarction by single-cell sequencing, bioinformatics, and machine learning. Front. Cardiovasc. Med. 2022, 9, 939972. [Google Scholar] [CrossRef]
- Li, Q.; Xu, M.; Li, Z.; Li, T.; Wang, Y.; Chen, Q.; Wang, Y.; Feng, J.; Yin, X.; Lu, C. Maslinic Acid Attenuates Ischemia/Reperfusion Injury-Induced Myocardial Inflammation and Apoptosis by Regulating HMGB1-TLR4 Axis. Front. Cardiovasc. Med. 2021, 8, 768947. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, B.-Y.; Peng, Y.-F.; Chang, L.C.; Li, Z.-Q.; Zhang, X.-X.; Zhang, D.-J. Mechanism of Action of Flavonoids of Oxytropis falcata on the Alleviation of Myocardial Ischemia–Reperfusion Injury. Molecules 2022, 27, 1706. [Google Scholar] [CrossRef]
- Ullah, R.; Ali, G.; Rasheed, A.; Subhan, F.; Khan, A.; Halim, S.A.; Al-Harrasi, A. The 7-Hydroxyflavone attenuates chemotherapy-induced neuropathic pain by targeting inflammatory pathway. Int. Immunopharmacol. 2022, 107, 108674. [Google Scholar] [CrossRef]
- Soliman, M.S.M.; Abdella, A.; Khidr, Y.A.; Hassan, G.O.O.; Al-Saman, M.A.; Elsanhoty, R.M. Pharmacological Activities and Characterization of Phenolic and Flavonoid Compounds in Methanolic Extract of Euphorbia cuneata Vahl Aerial Parts. Molecules 2021, 26, 7345. [Google Scholar] [CrossRef]
- Jamal, S.; Hassan, J.; Kichloo, A.; Ijaz, S.H.; Mahmoud, M.; Ajmal, M.; Paul, T.K.; Bailey, B. Impact of Ischemic Heart Disease on Inpatient Outcomes of Atrial Fibrillation. Circulation 2021, 144, A9826. [Google Scholar]
- Chen, Z.; Wu, J.; Li, S.; Liu, C.; Ren, Y. Inhibition of Myocardial Cell Apoptosis Is Important Mechanism for Ginsenoside in the Limitation of Myocardial Ischemia/Reperfusion Injury. Front. Pharmacol. 2022, 13, 806216. [Google Scholar] [CrossRef]
- Zhang, Q.; Guo, Y.; Zhang, D. Network Pharmacology Integrated with Molecular Docking Elucidates the Mechanism of Wuwei Yuganzi San for the Treatment of Coronary Heart Disease. Nat. Prod. Commun. 2022, 17, 1934578X221093907. [Google Scholar] [CrossRef]
- Zou, R.; Nie, C.; Pan, S.; Wang, B.; Hong, X.; Xi, S.; Bai, J.; Yu, M.; Liu, J.; Yang, W. Co-administration of hydrogen and metformin exerts cardioprotective effects by inhibiting pyroptosis and fibrosis in diabetic cardiomyopathy. Free Radic. Biol. Med. 2022, 183, 35–50. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Z.; Wang, P.; Han, Y.; Liu, L.; Li, J.; Chen, Y.; Liu, D.; Wang, J.; Tian, X.; et al. Bawei Chenxiang Wan Ameliorates Cardiac Hypertrophy by Activating AMPK/PPAR-α Signaling Pathway Improving Energy Metabolism. Front. Pharmacol. 2021, 12, 653901. [Google Scholar] [CrossRef]
- Zhao, F.; Bai, R.; Li, J.; Feng, X.; Jiao, S.; Wuken, S.; Ge, F.; Zhang, Q.; Zhou, X.; Tu, P.; et al. Meconopsis horridula Hook. f. & Thomson extract and its alkaloid oleracein E exert cardioprotective effects against acute myocardial ischaemic injury in mice. J. Ethnopharmacol. 2020, 258, 112893. [Google Scholar]
- Zhang, D.J.; Yuan, W.T.; Zhang, B.Y.; Hong, E.K.; Shi, P.; Yang, Z.T.; Zhang, Y.H.; Wang, H.L. Analysis of chemical constituents in the extract and rat serum from the chloroform extract of Oxytropis falcata Bunge by HPLC-MS. Pak. J. Pharm. Sci. 2020, 33, 669–674. [Google Scholar]
- Sengupta, B.; Reilly, S.M.; Davis, D.E., Jr.; Harris, K.; Wadkins, R.M.; Ward, D.; Gholar, D.; Hampton, C. Excited state proton transfer of natural flavonoids and their chromophores in duplex and tetraplex DNAs. J. Phys. Chem. B 2015, 119, 2546–2556. [Google Scholar] [CrossRef] [Green Version]
- Vajragupta, O.; Boonchoong, P.; Sumanont, Y.; Watanabe, H.; Wongkrajang, Y.; Kammasud, N. Manganese-based complexes of radical scavengers as neuroprotective agents. Bioorg. Med. Chem. 2003, 11, 2329–2337. [Google Scholar] [CrossRef]
- Sengupta, B.; Sahihi, M.; Dehkhodaei, M.; Kelly, D.; Arany, I. Differential roles of 3-Hydroxyflavone and 7-Hydroxyflavone against nicotine-induced oxidative stress in rat renal proximal tubule cells. PLoS ONE 2017, 12, e0179777. [Google Scholar] [CrossRef] [Green Version]
- Jin, Z.; Yang, Y.Z.; Chen, J.X.; Tang, Y.Z. Inhibition of pro-inflammatory mediators in RAW264.7 cells by 7-hydroxyflavone and 7,8-dihydroxyflavone. J. Pharm. Pharmacol. 2017, 69, 865–874. [Google Scholar] [CrossRef]
- Yue, R.C.; Lu, S.Z.; Luo, Y.; Wang, T.; Liang, H.; Zeng, J.; Liu, J.; Hu, H.X. Calpain silencing alleviates myocardial ischemia-reperfusion injury through the NLRP3/ASC/Caspase-1 axis in mice. Life Sci. 2019, 233, 116631. [Google Scholar] [CrossRef]
- Zhu, L.; Wei, T.; Gao, J.; Chang, X.; He, H.; Luo, F.; Zhou, R.; Ma, C.; Liu, Y.; Yan, T. The cardioprotective effect of salidroside against myocardial ischemia reperfusion injury in rats by inhibiting apoptosis and inflammation. Apoptosis 2015, 20, 1433–1443. [Google Scholar] [CrossRef]
- Chen, C.; Lu, W.; Wu, G.; Lv, L.; Chen, W.; Huang, L.; Wu, X.; Xu, N.; Wu, Y. Cardioprotective effects of combined therapy with diltiazem and superoxide dismutase on myocardial ischemia-reperfusion injury in rats. Life Sci. 2017, 183, 50–59. [Google Scholar] [CrossRef]
- Luo, Y.; Pan, Y.Z.; Zeng, C.; Li, G.L.; Lei, X.M.; Liu, Z.; Zhou, S.F. Altered serum creatine kinase level and cardiac function in ischemia-reperfusion injury during percutaneous coronary intervention. Med. Sci. Monit. 2011, 17, Cr474–Cr479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabe, S.A.; Feng, J.; Sellke, F.W.; Abid, M.R. Mechanisms and Clinical Implications of Endothelium-dependent Vasomotor Dysfunction in Coronary Microvasculature. Am. J. Physiol. Heart Circ. Physiol. 2022, 322, H819–H841. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Thai, P.N.; Lu, S.; Pu, J.; Bers, D.M. Intrafibrillar and perinuclear mitochondrial heterogeneity in adult cardiac myocytes. J. Mol. Cell. Cardiol. 2019, 136, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Chen, K.; Lv, Z.; Shao, Y.; Zhang, W.; Zhao, X.; Li, C. Bcl-2 mediates coelomocytes apoptosis by suppressing cytochrome c release in Vibrio splendidus challenged Apostichopus japonicus. Dev. Comp. Immunol. 2020, 103, 103533. [Google Scholar] [CrossRef] [PubMed]
- Zhai, K.-F.; Duan, H.; Chen, Y.; Khan, G.J.; Cao, W.-G.; Gao, G.-Z.; Shan, L.-L.; Wei, Z.-J. Apoptosis effects of imperatorin on synoviocytes in rheumatoid arthritis through mitochondrial/caspase-mediated pathways. Food Funct. 2018, 9, 2070–2079. [Google Scholar] [CrossRef] [PubMed]
- Zhai, K.F.; Duan, H.; Khan, G.J.; Xu, H.; Han, F.K.; Cao, W.G.; Gao, G.Z.; Shan, L.L.; Wei, Z.J. Salicin from Alangium chinense Ameliorates Rheumatoid Arthritis by Modulating the Nrf2-HO-1-ROS Pathways. J. Agric. Food Chem. 2018, 66, 6073–6082. [Google Scholar] [CrossRef] [PubMed]
- Yi, C.; Song, M.; Sun, L.; Si, L.; Yu, D.; Li, B.; Lu, P.; Wang, W.; Wang, X. Asiatic Acid Alleviates Myocardial Ischemia-Reperfusion Injury by Inhibiting the ROS-Mediated Mitochondria-Dependent Apoptosis Pathway. Oxidative Med. Cell. Longev. 2022, 2022, 3267450. [Google Scholar] [CrossRef]
- Lyu, M.; Cui, Y.; Zhao, T.; Ning, Z.; Ren, J.; Jin, X.; Fan, G.; Zhu, Y. Tnfrsf12a-Mediated Atherosclerosis Signaling and Inflammatory Response as a Common Protection Mechanism of Shuxuening Injection Against Both Myocardial and Cerebral Ischemia-Reperfusion Injuries. Front. Pharmacol. 2018, 9, 312. [Google Scholar] [CrossRef] [Green Version]
- Zhai, K.F.; Duan, H.; Luo, L.; Cao, W.G.; Han, F.K.; Shan, L.L.; Fang, X.M. Protective effects of paeonol on inflammatory response in IL-1β-induced human fibroblast-like synoviocytes and rheumatoid arthritis progression via modulating NF-κB pathway. Inflammopharmacology 2017, 25, 523–532. [Google Scholar] [CrossRef]
- Sun, N.; Wang, H.; Wang, L. Protective effects of ghrelin against oxidative stress, inducible nitric oxide synthase and inflammation in a mouse model of myocardial ischemia/reperfusion injury via the HMGB1 and TLR4/NF-κB pathway. Mol. Med. Rep. 2016, 14, 2764–2770. [Google Scholar] [CrossRef] [Green Version]
- Yue, J.; López, J.M. Understanding MAPK Signaling Pathways in Apoptosis. Int. J. Mol. Sci. 2020, 21, 2346. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Ding, Y.; Peng, Y.; Yu, J.; Pan, C.; Cai, Y.; Dong, Q.; Zhong, Y.; Zhu, R.; Yu, K.; et al. Effects of IL-38 on Macrophages and Myocardial Ischemic Injury. Front. Immunol. 2022, 13, 894002. [Google Scholar] [CrossRef]
- Thomas, C.J.; Ng, D.C.; Patsikatheodorou, N.; Limengka, Y.; Lee, M.W.; Darby, I.A.; Woodman, O.L.; May, C.N. Cardioprotection from ischaemia–reperfusion injury by a novel flavonol that reduces activation of p38 MAPK. Eur. J. Pharmacol. 2011, 658, 160–167. [Google Scholar] [CrossRef]
- Guo, W.; Liu, X.; Li, J.; Shen, Y.; Zhou, Z.; Wang, M.; Xie, Y.; Feng, X.; Wang, L.; Wu, X. Prdx1 alleviates cardiomyocyte apoptosis through ROS-activated MAPK pathway during myocardial ischemia/reperfusion injury. Int. J. Biol. Macromol. 2018, 112, 608–615. [Google Scholar] [CrossRef]
- Zhou, L.; Zhao, D.; An, H.; Zhang, H.; Jiang, C.; Yang, B. Melatonin prevents lung injury induced by hepatic ischemia–reperfusion through anti-inflammatory and anti-apoptosis effects. Int. Immunopharmacol. 2015, 29, 462–467. [Google Scholar] [CrossRef]
- Xie, D.; Zhao, J.; Guo, R.; Jiao, L.; Zhang, Y.; Lau, W.B.; Lopez, B.; Christopher, T.; Gao, E.; Cao, J.; et al. Sevoflurane Pre-conditioning Ameliorates Diabetic Myocardial Ischemia/Reperfusion Injury Via Differential Regulation of p38 and ERK. Sci. Rep. 2020, 10, 23. [Google Scholar] [CrossRef]
- Liu, J.; Feng, X.; Li, B.; Sun, Y.; Jin, T.; Feng, M.; Ni, Y.; Liu, M. Lactobacillus rhamnosus GR-1 Alleviates Escherichia coli-Induced Inflammation via NF-κB and MAPKs Signaling in Bovine Endometrial Epithelial Cells. Front. Cell Infect. Microbiol. 2022, 12, 809674. [Google Scholar] [CrossRef]
- Yan, X.; Zhao, X.; Zhou, M.; Sun, Y.; Xu, T. IRF4b and IRF8 Negatively Regulate RLR-Mediated NF-κB Signaling by Targeting MITA for Degradation in Teleost Fish. Front. Immunol. 2022, 13, 858179. [Google Scholar] [CrossRef]
- Zeng, S.; Yi, R.; Tan, F.; Sun, P.; Cheng, Q.; Zhao, X. Lactobacillus plantarum HFY05 Attenuates Carrageenan-Induced Thrombosis in Mice by Regulating NF-κB Pathway-Associated Inflammatory Responses. Front. Nutr. 2022, 9, 813899. [Google Scholar] [CrossRef]
- Yang, Z.; Yin, X.; Chen, C.; Huang, S.; Li, X.; Yan, J.; Sun, Q. CircOAS3 Regulates Keratinocyte Proliferation and Psoriatic Inflammation by Interacting with Hsc70 via the JNK/STAT3/NF-κB Signaling Pathway. Inflammation 2022, 2022, 1–12. [Google Scholar] [CrossRef]
- Alsahly, M.B.; Zakari, M.O.; Koch, L.G.; Britton, S.; Katwa, L.C.; Lust, R.M. Influence of Intrinsic Aerobic Exercise Capacity and Sex on Cardiac Injury Following Acute Myocardial Ischemia and Reperfusion. Front. Cardiovasc. Med. 2021, 8, 751864. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Peng, Y.; Liu, J.; Yang, Y.; Hu, Z.; Zhou, Y.; Ma, J.; Zhang, D. 7-Hydroxyflavone Alleviates Myocardial Ischemia/Reperfusion Injury in Rats by Regulating Inflammation. Molecules 2022, 27, 5371. https://doi.org/10.3390/molecules27175371
Zhang Q, Peng Y, Liu J, Yang Y, Hu Z, Zhou Y, Ma J, Zhang D. 7-Hydroxyflavone Alleviates Myocardial Ischemia/Reperfusion Injury in Rats by Regulating Inflammation. Molecules. 2022; 27(17):5371. https://doi.org/10.3390/molecules27175371
Chicago/Turabian StyleZhang, Qunhui, Yanfeng Peng, Jiangyu Liu, Yongjing Yang, Zhangjie Hu, Yi Zhou, Jing Ma, and Dejun Zhang. 2022. "7-Hydroxyflavone Alleviates Myocardial Ischemia/Reperfusion Injury in Rats by Regulating Inflammation" Molecules 27, no. 17: 5371. https://doi.org/10.3390/molecules27175371
APA StyleZhang, Q., Peng, Y., Liu, J., Yang, Y., Hu, Z., Zhou, Y., Ma, J., & Zhang, D. (2022). 7-Hydroxyflavone Alleviates Myocardial Ischemia/Reperfusion Injury in Rats by Regulating Inflammation. Molecules, 27(17), 5371. https://doi.org/10.3390/molecules27175371






