Thermally Driven Structural Order of Oligo(Ethylene Glycol)-Terminated Alkanethiol Monolayers on Au(111) Prepared by Vapor Deposition
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals and Preparation of Au(111) Substrates
2.2. Preparation of CH3(EG)2S SAMs
2.3. STM and CV Measurements
3. Results and Discussion
3.1. Deposition Temperature-Dependent Structural Transitions of EG2 SAMs on Au(111) Formed by Vapor Deposition
3.2. Deposition Temperature-Dependent Changes to the Number and Size of VIs in EG2 SAMs on Au(111) Formed by Vapor Deposition
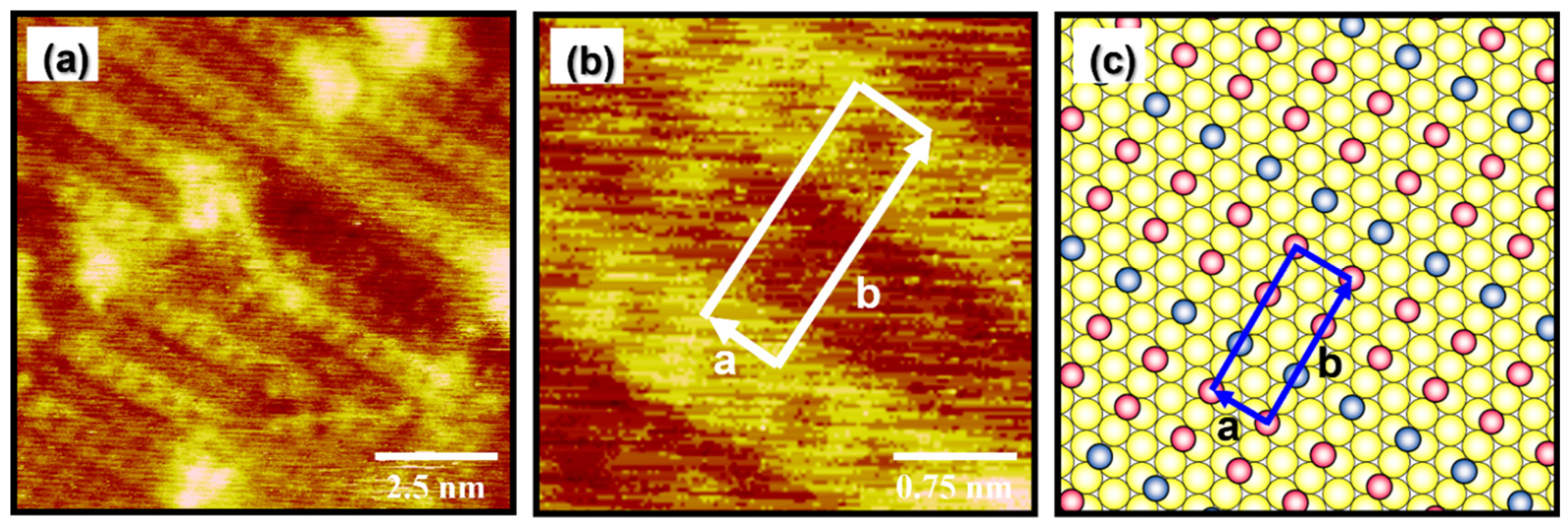
3.3. Packing Structure of Well-Ordered EG2 SAMs on Au(111) Formed by Vapor Deposition at 348 K
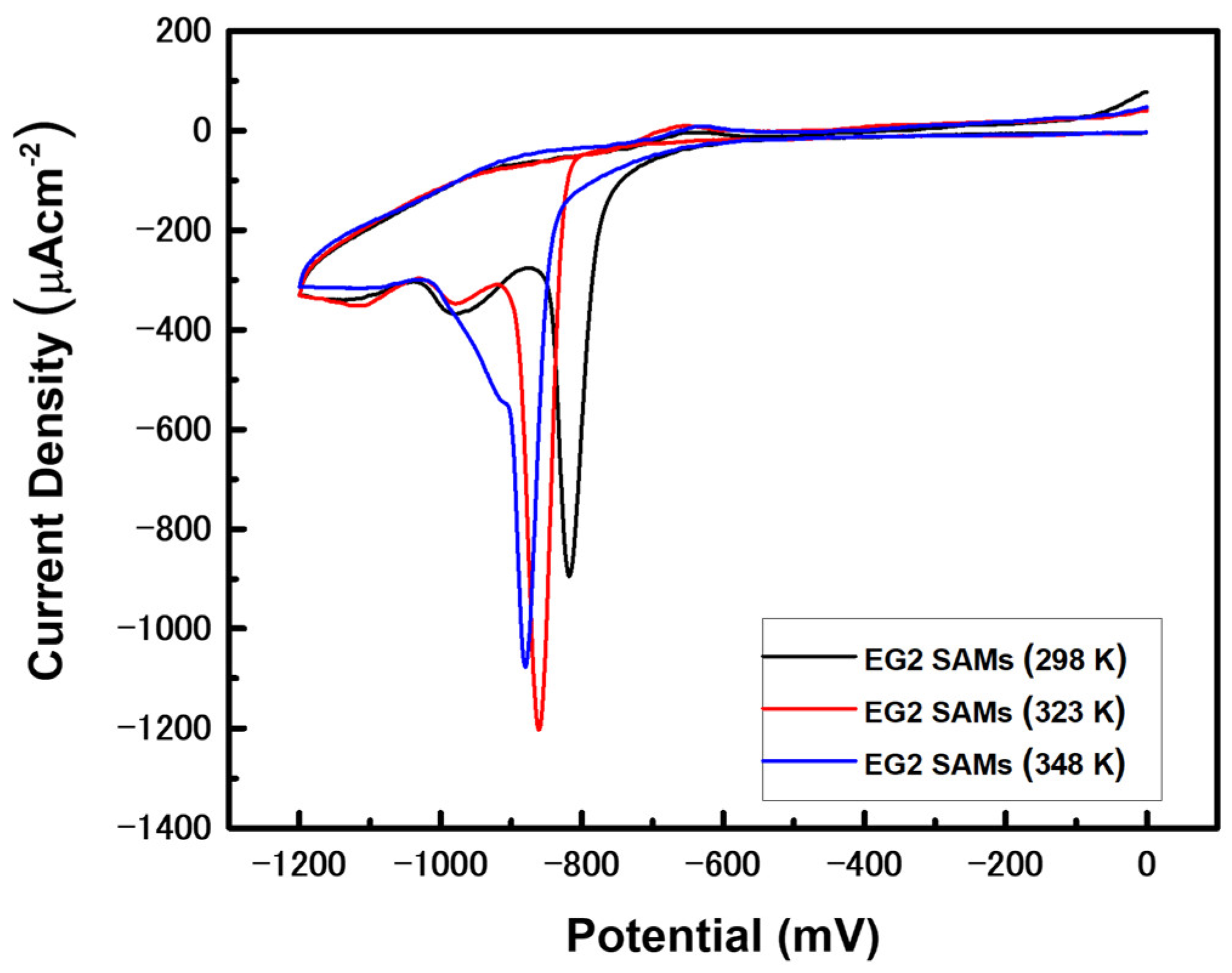
3.4. Reductive Desorption (RD) Behaviors of EG2 SAMs on Au(111) Prepared by Vapor Deposition
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Love, J.C.; Estroff, L.A.; Kriebel, J.K.; Nuzzo, R.G.; Whitesides, G.M. Self-assembled monolayers of thiolates on metals as a form of nanotechnology. Chem. Rev. 2005, 105, 1103–1170. [Google Scholar] [CrossRef] [PubMed]
- Romashov, L.V.; Ananikov, V.P. Self-assembled selenium monolayers: From nanotechnology to materials science and adaptive catalysis. Chem. Eur. J. 2013, 19, 17640–17660. [Google Scholar] [CrossRef] [PubMed]
- Azzam, W.; Subaihi, A. Influence of an alkyl spacer on the formation and structure of 4-fluorobenzenethiol and 4-fluorobenzenemethanethiol self-assembled monolayers on Au(111). Surf. Interfaces 2020, 20, 100544. [Google Scholar] [CrossRef]
- Kang, H.; Jeong, H.; Seong, S.; Han, S.; Son, Y.J.; Tahara, H.; Hayashi, T.; Yoon, H.J.; Noh, J. Formation and superlattice of long-range and highly ordered alicyclic selenolate monolayers on Au(111) studied by scanning tunneling microscopy. Appl. Surf. Sci. 2022, 572, 151454. [Google Scholar] [CrossRef]
- Lee, N.S.; Kim, D.; Kang, H.; Park, D.K.; Han, S.W.; Noh, J. Structural transitions of octanethiol self-assembled monolayers on gold nanoplates after mild thermal annealing. J. Phys. Chem. C 2011, 115, 5868–5874. [Google Scholar] [CrossRef]
- Jiang, T.M.; Malone, W.; Tong, Y.F.; Dragoe, D.; Bendounan, A.; Kara, A.; Esaulov, V.A. Thiophene derivatives on gold and molecular dissociation processes. J. Phys. Chem. C 2017, 121, 27923–27935. [Google Scholar] [CrossRef]
- Han, S.; Seong, S.; Son, Y.J.; Yokota, Y.; Hayashi, T.; Hara, M.; Noh, J. Formation and surface structures of highly ordered self-assembled monolayers of alkyl selenocyanates on Au(111) via ambient-pressure vapor deposition. J. Phys. Chem. C 2020, 124, 26730–26740. [Google Scholar] [CrossRef]
- Han, M.S.; Seong, S.; Han, S.; Lee, N.S.; Noh, J. Molecular self-assembly of phenylselenyl chloride on a Au(111) surface. Bull. Korean Chem. Soc. 2020, 41, 1048–1051. [Google Scholar] [CrossRef]
- Asyuda, A.; Das, S.; Zharnikov, M. Thermal stability of alkanethiolate and aromatic thiolate self-assembled monolayers on Au(111): An X-ray photoelectron spectroscopy study. J. Phys. Chem. C 2021, 125, 21754–21763. [Google Scholar] [CrossRef]
- Ito, E.; Noh, J.; Hara, M. Different adsorption states between thiophene and alpha-bithiophene thin films prepared by self-assembly method. Jpn. J. Appl. Phys. 2003, 42, L852–L855. [Google Scholar] [CrossRef]
- Kato, H.S.; Yoshimoto, S.; Ueda, A.; Yamamoto, S.; Kanematsu, Y.; Tachikawa, M.; Mori, H.; Yoshinobu, J.; Matsuda, I. Strong hydrogen bonds at the interface between proton-donating and-accepting self-assembled monolayers on Au(111). Langmuir 2018, 34, 2189–2197. [Google Scholar] [CrossRef] [PubMed]
- Seong, S.; Kwon, S.; Han, S.; Son, Y.J.; Lee, G.; Yang, T.; Lee, N.S.; Noh, J. Steric effects on the formation of self-assembled monolayers of alicyclic thiol derivatives on Au(111). Bull. Korean Chem. Soc. 2021, 42, 1259–1264. [Google Scholar] [CrossRef]
- Park, T.; Kang, H.; Ito, E.; Noh, J. Self-assembled monolayers of alkanethioacetates on Au(111) in ammonium hydroxide solution. Bull. Korean Chem. Soc. 2021, 42, 252–257. [Google Scholar] [CrossRef]
- Azzam, W.; Al-Rawashdeh, N.A.F.; Al-Refaie, N.; Shekhah, O.; Bashir, A. On the influence of the aliphatic linker on fabrication of highly ordered and orientated self-assembled monolayers of aromatic selenols on Au(111). J. Phys. Chem. C 2014, 118, 4846–4859. [Google Scholar] [CrossRef]
- Azzam, W.; Zharnikov, M.; Rohwerde, M.; Bashir, A. Functional group selective STM imaging in self-assembled monolayers: Benzeneselenol on Au(111). Appl. Surf. Sci. 2018, 427, 581–586. [Google Scholar] [CrossRef]
- Lee, S.Y.; Ito, E.; Kang, H.; Hara, M.; Lee, H.; Noh, J. Surface structure, adsorption, and thermal desorption behaviors of methaneselenolate monolayers on Au(111) from dimethyl diselenides. J. Phys. Chem. C 2014, 118, 8322–8330. [Google Scholar] [CrossRef]
- Ito, E.; Ito, H.; Kang, H.; Hayashi, T.; Hara, M.; Noh, J. Influence of surface morphology and substrate on thermal stability and desorption behavior of octanethiol self-assembled monolayers: Cu, Ag, and Au. J. Phys. Chem. C 2012, 116, 17586–17593. [Google Scholar] [CrossRef]
- Seong, S.; Kang, H.; Han, S.; Son, Y.J.; Jang, J.; Yoon, H.J.; Maeda, S.; Song, S.; Palai, D.; Hayashi, T.; et al. Surface structure and work function change of pentafluorobenzeneselenolate self-assembled monolayers on Au(111). Surf. Interfaces 2022, 33, 102228. [Google Scholar] [CrossRef]
- Krzykawska, A.; Ossowski, J.; Żaba, T.; Cyganik, P. Binding groups for highly ordered SAM formation: Carboxylic versus thiol. Chem. Commun. 2017, 53, 5748–5751. [Google Scholar] [CrossRef]
- Zaba, T.; Noworolska, A.; Bowers, C.M.; Breiten, B.; Whitesides, G.M.; Cyganik, P. Formation of highly ordered self-assembled monolayers of alkynes on Au(111) substrate. J. Am. Chem. Soc. 2014, 136, 11918–11921. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.; Takeda, S.; Nakajima, K.; Noh, J.; Choi, J.; Hara, M.; Nagamune, T. Rectified photocurrent in a protein based molecular photo-diode consisting of a cytochrome b562-green fluorescent protein chimera self-assembled monolayer. Biosens. Bioelectron. 2004, 19, 1169–1174. [Google Scholar] [CrossRef] [PubMed]
- Phares, N.; White, R.J.; Plaxco, K.W. Improving the stability and sensing of electrochemical biosensors by employing trithiol-anchoring groups in a six-carbon self-assembled monolayer. Anal. Chem. 2009, 81, 1095–1100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patrikar, K.; Bothra, U.; Rao, V.R.; Kabra, D. Charge carrier doping as mechanism of self-assembled monolayers functionalized electrodes in organic field effect transistors. Adv. Mater. Interfaces 2022, 9, 2101377. [Google Scholar] [CrossRef]
- Kang, H.; Seong, S.; Ito, E.; Isoshima, T.; Hara, M.; Yoon, H.J.; Noh, J. Comparative study of structural order, thermal desorption behavior, and work function change of self-assembled monolayers of pentafluorobenzenethiols and tetrafluorobenzenethiols on Au(111). Appl. Surf. Sci. 2021, 555, 149671. [Google Scholar] [CrossRef]
- Yi, R.W.; Mao, Y.Y.; Shen, Y.B.; Chen, L.W. Self-assembled monolayers for batteries. J. Am. Chem. Soc. 2021, 143, 12897–12912. [Google Scholar] [CrossRef]
- Jesper, M.; Alt, M.; Schinke, J.; Hillebrandt, S.; Angelova, I.; Rohnacher, V.; Pucci, A.; Lemmer, U.; Jaegermann, W.; Kowalsky, W.; et al. Dipolar SAMs reduce charge carrier injection barriers in n-channel organic field effect transistors. Langmuir 2015, 31, 10303–10309. [Google Scholar] [CrossRef]
- Kim, G.-H.; de Arquer, F.P.G.; Yoon, Y.J.; Lan, X.; Liu, M.; Voznyy, O.; Yang, Z.; Fan, F.; Ip, A.H.; Kanjanaboos, P.; et al. High-efficiency colloidal quantum dot photovoltaics via robust self-assembled monolayers. Nano Lett. 2015, 15, 7691–7696. [Google Scholar] [CrossRef]
- Casalini, S.; Bortolotti, C.A.; Leonardi, F.; Biscarini, F. Self-assembled monolayers in organic electronics. Chem. Soc. Rev. 2017, 46, 40–71. [Google Scholar] [CrossRef]
- Ostuni, E.; Chapman, R.G.; Holmlin, R.E.; Takayama, S.; Whitesides, G.M. A survey of structure-property relationships of surfaces that resist the adsorption of protein. Langmuir 2001, 17, 5605–5620. [Google Scholar] [CrossRef]
- Herrwerth, S.; Eck, W.; Reinhardt, S.; Grunze, M. Factors that determine the protein resistance of oligoether self-assembled monolayers-Internal hydrophilicity, terminal hydrophilicity, and lateral packing density. J. Am. Chem. Soc. 2003, 125, 9359–9366. [Google Scholar] [CrossRef]
- Valiokas, R.; Svedhem, S.; Ostblom, M.; Svensson, S.C.T.; Liedberg, B. Influence of specific intermolecular interactions on the self-assembly and phase behavior of oligo(ethylene glycol)-terminated alkanethiolates on gold. J. Phys. Chem. B 2001, 105, 5459–5469. [Google Scholar] [CrossRef]
- Harder, P.; Grunze, M.; Dahint, R.; Whitesides, G.M.; Laibinis, P.E. Molecular conformation in oligo(ethylene glycol)-terminated self-assembled monolayers on gold and silver surfaces determines their ability to resist protein adsorption. J. Phys. Chem. B 1998, 102, 426–436. [Google Scholar] [CrossRef]
- Kankate, L.; Werner, U.; Turchanin, A.; Gölzhäuser, A.; Großmann, H.; Tampé, R. Protein resistant oligo(ethylene glycol) terminated self-assembled monolayers of thiols on gold by vapor deposition in vacuum. Biointerphases 2010, 5, 30–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baghbanzadeh, M.; Rappoport, D.; Bowers, C.M.; Rappoport, D.; Żaba, T.; Yuan, L.; Kang, K.; Liao, K.-C.; Gonidec, M.; Rothemund, P.; et al. Anomalously rapid tunneling: Charge transport across self-assembled monolayers of oligo(ethylene glycol). J. Am. Chem. Soc. 2017, 139, 7624–7631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, P.S.; Goel, M.; Abbott, N.L.; Himpsel, F.J. Helical versus all-trans conformations of oligo(ethylene glycol)-terminated alkanethiol self-assembled monolayers. Langmuir 2014, 30, 10263–10269. [Google Scholar] [CrossRef]
- Zwahlen, M.; Herrwerth, S.; Eck, W.; Grunze, M.; Hähner, G. Conformational order in oligo(ethylene glycol)-terminated self-assembled monolayers on gold determined by soft X-ray absorption. Langmuir 2003, 19, 9305–9310. [Google Scholar] [CrossRef]
- Zolk, M.; Eisert, F.; Pipper, J.; Herrwerth, S.; Eck, W.; Buck, M.; Grunze, M. Solvation of oligo(ethylene glycol)-terminated self-assembled monolayers studied by vibrational sum frequency spectroscopy. Langmuir 2000, 16, 5849–5852. [Google Scholar] [CrossRef]
- Inada, N.; Asakawa, H.; Matsumoto, Y.; Fukuma, T. Molecular-scale surface structures of oligo(ethylene glycol)-terminated self-assembled monolayers investigated by frequency modulation atomic force microscopy in aqueous solution. Nanotechnology 2014, 25, 305602. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.; Han, S.; Seong, S.; Son, Y.J.; Ito, E.; Hara, M.; Noh, J. Unique mixed phases and structures of self-assembled monolayers on Au(111) derived from methoxy-terminated mono(ethylene glycol)ethanethiols. J. Phys. Chem. C 2017, 121, 18021–18029. [Google Scholar] [CrossRef]
- Poirier, G.E. Coverage-dependent phases and phase stability of decanethiol on Au(111). Langmuir 1999, 15, 1167–1175. [Google Scholar] [CrossRef]
- Poirier, G.E.; Pylant, E.D. The self-assembly mechanism of alkanethiols on Au(111). Science 1996, 272, 1145–1148. [Google Scholar] [CrossRef] [PubMed]
- Yamada, R.; Wano, H.; Uosaki, K. Effect of temperature on structure of the self-assembled monolayer of decanethiol on Au(111) surface. Langmuir 2000, 16, 5523–5525. [Google Scholar] [CrossRef]
- Lee, N.-S.; Kang, H.; Seong, S.; Noh, J. Effect of immersion time on the structure of octanethiol self-assembled monolayers on Au(111) at an elevated solution temperature. Bull. Korean Chem. Soc. 2019, 40, 1152–1153. [Google Scholar] [CrossRef]
- Mamun, A.H.A.; Hahn, J.R. Effects of immersion temperature on self-assembled monolayers of octanethiol on Au(111). Surf. Sci. 2012, 606, 664–669. [Google Scholar] [CrossRef]
- Azzam, W.; Al-Momani, L. A new striped-phase of decanethiol self-assembled monolayers on Au(111) formed at a high solution temperature. Appl. Surf. Sci. 2013, 266, 239–244. [Google Scholar] [CrossRef]
- Choi, Y.; Seong, S.; Son, Y.J.; Han, S.; Ito, E.; Mondarte, E.A.Q.; Chang, R.; Hayashi, T.; Hara, M.; Noh, J. Formation of long-range-ordered self-assembled monolayers of dodecyl thiocyanates on Au(111) via ambient-pressure vapor deposition. Colloids Surf. A 2019, 583, 123969. [Google Scholar] [CrossRef]
- Lee, S.Y.; Choi, Y.; Ito, E.; Hara, M.; Lee, H.; Noh, J. Growth, solvent effects, and thermal desorption behavior of octylthiocyanate self-assembled monolayers on Au(111). Phys. Chem. Chem. Phys. 2013, 15, 3609–3617. [Google Scholar] [CrossRef]
- Kwon, S.; Choi, J.; Lee, H.; Noh, J. Molecular-scale investigation of octanethiol self-assembled monolayers on Au(111) prepared by solution and vapor deposition at high temperature. Colloids Surf. A 2008, 313–314, 324–327. [Google Scholar] [CrossRef]
- Mamun, A.H.A.; Hahn, J.R. Effects of solvent on the formation of octanethiol self-assembled monolayers on Au(111) at high temperatures in a closed vessel: A scanning tunneling microscopy and X-ray photoelectron spectroscopy study. J. Phys. Chem. C 2012, 116, 22441–22448. [Google Scholar] [CrossRef]
- Yang, G.; Liu, G.-Y. New insights for self-assembled monolayers of organothiols on Au(111) revealed by scanning tunneling microscopy. J. Phys. Chem. B 2003, 107, 8746–8759. [Google Scholar] [CrossRef]
- Deering, A.L.; Van Lue, S.M.; Kandel, S.A. Ambient-pressure vapor deposition of octanethiol self-assembled monolayers. Langmuir 2005, 21, 10260–10263. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.J.; Seong, S.; Noh, J. Formation of a highly-ordered thiophene monolayer on Au(111) via vapor phase deposition. Bull. Korean Chem. Soc. 2019, 40, 619–620. [Google Scholar] [CrossRef]
- Lee, S.Y.; Noh, J.; Ito, E.; Lee, H.; Hara, M. Solvent effect on formation of cysteamine self-assembled monolayers on Au(111). Jpn. J. Appl. Phys. 2003, 42, 236–241. [Google Scholar] [CrossRef]
- Dai, J.Y.; Li, Z.G.; Jin, J.; Cheng, J.J.; Kong, J.; Bi, S.P. Study of the solvent effect on the quality of dodecanethiol self-assembled monolayers on polycrystalline gold. J. Electroanal. Chem. 2008, 624, 315–322. [Google Scholar] [CrossRef]
- Lee, N.S.; Kang, H.; Ito, E.; Hara, M.; Noh, J. Effects of solvent on the structure of octanethiol self-assembled monolayers on Au(111) at a high solution temperature. Bull. Korean Chem. Soc. 2010, 31, 2137–2138. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.; Seong, S.; Han, S.; Son, Y.J.; Park, J.; Noh, J. Negative thermal effects on the structural order of methoxy–terminated mono(ethylene glycol) ethanethiol Self-assembled Monolayers on Au(111). Bull. Korean Chem. Soc. 2019, 40, 299–300. [Google Scholar] [CrossRef]
- Poirier, G.E.; Tarlov, M.J. Molecular ordering and gold migration observed in butanethiol self-assembled monolayers using scanning tunneling microscopy. J. Phys. Chem. 1995, 99, 10966–10970. [Google Scholar] [CrossRef]
- Cavalleri, O.; Hirstein, A.; Kern, K. Ostwald ripening of vacancy islands at thiol covered Au(111). Surf. Sci. 1995, 340, L960–L964. [Google Scholar] [CrossRef]
- Poirier, G.E. Mechanism of formation of Au vacancy islands in alkanethiol monolayers on Au(111). Langmuir 1997, 13, 2019–2026. [Google Scholar] [CrossRef]
- Arisnabarreta, N.; Ruano, G.D.; Lingenfelder, M.; Patrito, E.M.; Cometto, F.P. Comparative study of the adsorption of thiols and selenols on Au(111) and Au(100). Langmuir 2017, 33, 13733–13739. [Google Scholar] [CrossRef]
- Kang, H.; Noh, J. Influence of thiol molecular backbone structure on the formation and reductive desorption of self-assembled aromatic and alicyclic thiol monolayers on Au(111) Surface. Bull. Korean Chem. Soc. 2013, 34, 1383–1387. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.-F.; Lee, Y.-L. Adsorption characteristics of OH-terminated alkanethiol and arenethiol on Au(111) surfaces. Nanoscale 2012, 4, 2093–2100. [Google Scholar] [CrossRef] [PubMed]
- Salvarezza, R.C.; Carro, P. The electrochemical stability of thiols on gold surfaces. J. Electroanal. Chem. 2018, 819, 234–239. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Son, Y.J.; Kang, H.; Seong, S.; Han, S.; Lee, N.-S.; Noh, J. Thermally Driven Structural Order of Oligo(Ethylene Glycol)-Terminated Alkanethiol Monolayers on Au(111) Prepared by Vapor Deposition. Molecules 2022, 27, 5377. https://doi.org/10.3390/molecules27175377
Son YJ, Kang H, Seong S, Han S, Lee N-S, Noh J. Thermally Driven Structural Order of Oligo(Ethylene Glycol)-Terminated Alkanethiol Monolayers on Au(111) Prepared by Vapor Deposition. Molecules. 2022; 27(17):5377. https://doi.org/10.3390/molecules27175377
Chicago/Turabian StyleSon, Young Ji, Hungu Kang, Sicheon Seong, Seulki Han, Nam-Suk Lee, and Jaegeun Noh. 2022. "Thermally Driven Structural Order of Oligo(Ethylene Glycol)-Terminated Alkanethiol Monolayers on Au(111) Prepared by Vapor Deposition" Molecules 27, no. 17: 5377. https://doi.org/10.3390/molecules27175377
APA StyleSon, Y. J., Kang, H., Seong, S., Han, S., Lee, N.-S., & Noh, J. (2022). Thermally Driven Structural Order of Oligo(Ethylene Glycol)-Terminated Alkanethiol Monolayers on Au(111) Prepared by Vapor Deposition. Molecules, 27(17), 5377. https://doi.org/10.3390/molecules27175377






