Gas Sensors Based on Single-Wall Carbon Nanotubes
Abstract
:1. Introduction
2. Working Mechanism of SWCNT-Based Gas Sensors
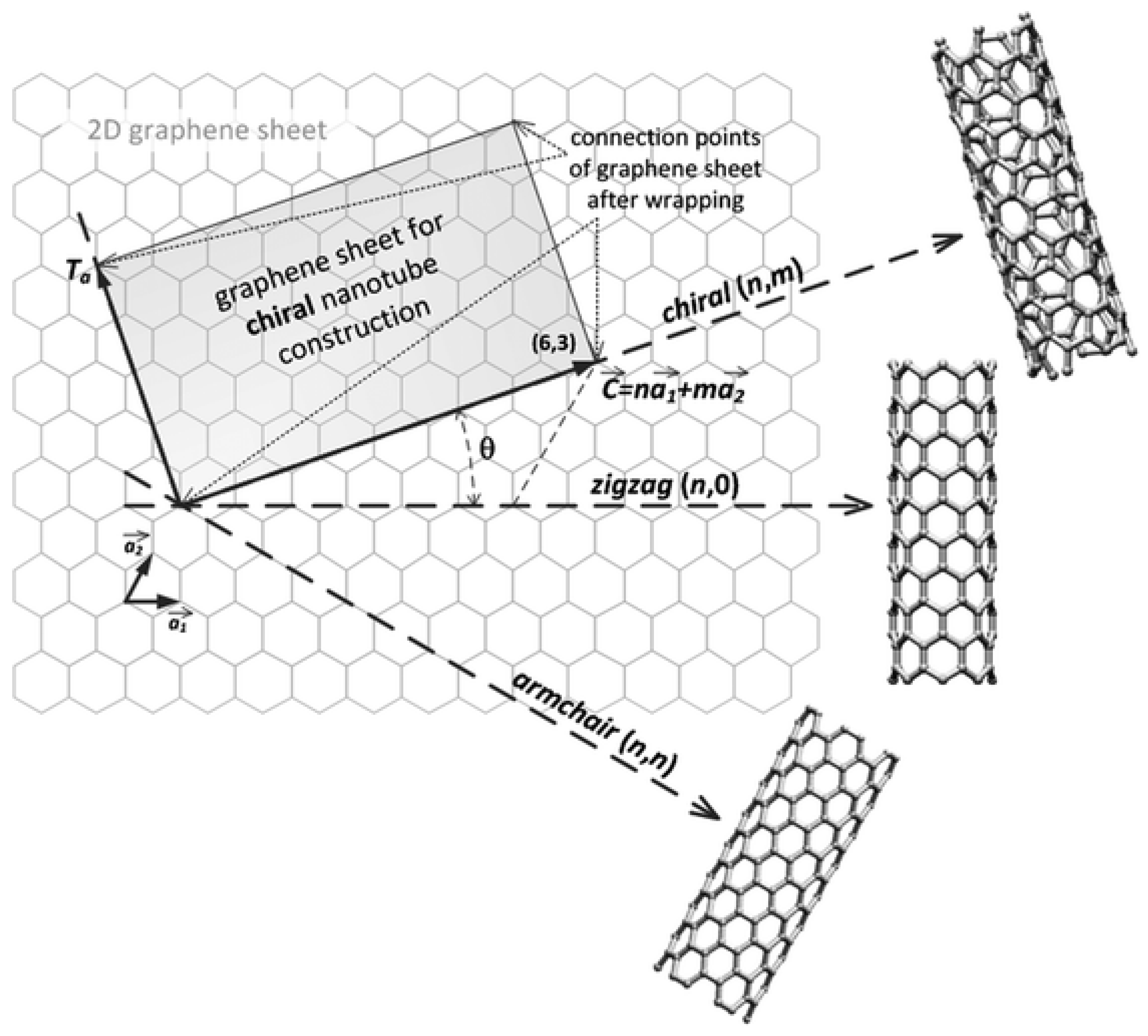
2.1. SWCNT Structure
2.2. Sensing Principle
2.3. Important Figure of Merit of Gas Sensors
2.3.1. Sensitivity
2.3.2. Response Time and Recovery Time
2.3.3. Limit of Detection
2.3.4. Drift
2.3.5. Selectivity
2.3.6. Device Structures
- (i)
- Field effect transistor
- (ii)
- Two-Electrode Sensors
2.4. The Origin of the Sensing Response
3. Approaches to and Progress in Improving the Sensing Performance
3.1. Pure SWCNT-Based Sensors Produced by Tuning the Structure
3.1.1. Individual Tubes or a Network Sensor
3.1.2. Quality and Defects
3.1.3. Electrical Conductivity type of SWCNTs
3.2. Functionalization of SWCNTs
3.2.1. Covalent Functionalization
3.2.2. Noncovalent Functionalization
3.2.3. Decoration with Nanoparticles
4. Challenges and Outlook
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Korotcenkov, G. Handbook of Gas Sensor Materials; Springer: New York, NY, USA, 2013; Volume 1, pp. 1–454. [Google Scholar]
- Duk-Dong, L.; Dae-Sik, L. Environmental gas sensors. IEEE Sens. J. 2001, 1, 214–224. [Google Scholar] [CrossRef]
- Wang, Y.; Yeow, J.T.W. A Review of Carbon Nanotubes-Based Gas Sensors. J. Sens. 2009, 2009, 493904. [Google Scholar] [CrossRef]
- Kohl, D. Function and applications of gas sensors. J. Phys. D Appl. Phys. 2001, 34, R125–R149. [Google Scholar] [CrossRef]
- Yang, S.; Jiang, C.; Wei, S.-h. Gas sensing in 2D materials. Appl. Phys. Rev. 2017, 4, 021304. [Google Scholar] [CrossRef]
- Meyyappan, M. Carbon Nanotube-Based Chemical Sensors. Small 2016, 12, 2118–2129. [Google Scholar] [CrossRef]
- Ryabtsev, S.V.; Shaposhnick, A.V.; Lukin, A.N.; Domashevskaya, E.P. Application of semiconductor gas sensors for medical diagnostics. Sens. Actuators B 1999, 59, 26–29. [Google Scholar] [CrossRef]
- James, D.; Scott, S.M.; Ali, Z.; O’Hare, W.T. Chemical Sensors for Electronic Nose Systems. Microchim. Acta 2005, 149, 1–17. [Google Scholar] [CrossRef]
- Prasek, J.; Drbohlavova, J.; Chomoucka, J.; Hubalek, J.; Jasek, O.; Adam, V.; Kizek, R. Methods for carbon nanotubes synthesis—Review. J. Mater. Chem. 2011, 21, 15872–15884. [Google Scholar] [CrossRef]
- Kauffman, D.R.; Star, A. Carbon Nanotube Gas and Vapor Sensors. Angew. Chem. Int. Ed. 2008, 47, 6550–6570. [Google Scholar] [CrossRef]
- Bai, Y.; Zhang, R.; Ye, X.; Zhu, Z.; Xie, H.; Shen, B.; Cai, D.; Liu, B.; Zhang, C.; Jia, Z.; et al. Carbon nanotube bundles with tensile strength over 80 GPa. Nat. Nanotechnol. 2018, 13, 589–595. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, X.; Shang, Y.; Chang, S.; Dai, L.; Cao, A. Application-Driven Carbon Nanotube Functional Materials. ACS Nano 2021, 15, 7946–7974. [Google Scholar] [CrossRef] [PubMed]
- Dürkop, T.; Getty, S.A.; Cobas, E.; Fuhrer, M.S. Extraordinary Mobility in Semiconducting Carbon Nanotubes. Nano Lett. 2004, 4, 35–39. [Google Scholar] [CrossRef]
- Javey, A.; Guo, J.; Wang, Q.; Lundstrom, M.; Dai, H. Ballistic carbon nanotube field-effect transistors. Nature 2003, 424, 654–657. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Zhang, T. Recent Progress of Nanostructured Sensing Materials from 0D to 3D: Overview of Structure–Property-Application Relationship for Gas Sensors. Small Methods 2021, 5, 2100515. [Google Scholar] [CrossRef]
- Mittal, M.; Kumar, A. Carbon nanotube (CNT) gas sensors for emissions from fossil fuel burning. Sens. Actuators B 2014, 203, 349–362. [Google Scholar] [CrossRef]
- Agarwal, P.B.; Alam, B.; Sharma, D.S.; Sharma, S.; Mandal, S.; Agarwal, A. Flexible NO2 gas sensor based on single-walled carbon nanotubes on polytetrafluoroethylene substrates. Flexible Printed Electron. 2018, 3, 035001. [Google Scholar] [CrossRef]
- Yoon, B.; Choi, S.-J.; Swager, T.M.; Walsh, G.F. Flexible Chemiresistive Cyclohexanone Sensors Based on Single-Walled Carbon Nanotube–Polymer Composites. ACS Sens. 2021, 6, 3056–3062. [Google Scholar] [CrossRef]
- Bezdek, M.J.; Luo, S.X.L.; Liu, R.Y.; He, Q.; Swager, T.M. Trace Hydrogen Sulfide Sensing Inspired by Polyoxometalate-Mediated Aerobic Oxidation. ACS Cent. Sci. 2021, 7, 1572–1580. [Google Scholar] [CrossRef]
- Guo, S.Y.; Hou, P.X.; Wang, H.X.; Shi, C.; Fang, H.T.; Liu, C. Transparent and flexible hydrogen sensor based on semiconducting single-wall carbon nanotube networks. Carbon 2019, 151, 156–159. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Wang, J. Non-invasive wearable electrochemical sensors: A review. Trends Biotechnol. 2014, 32, 363–371. [Google Scholar] [CrossRef]
- Kruss, S.; Hilmer, A.J.; Zhang, J.; Reuel, N.F.; Mu, B.; Strano, M.S. Carbon nanotubes as optical biomedical sensors. Adv. Drug Deliv. Rev. 2013, 65, 1933–1950. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Heller, D.A.; Jin, H.; Barone, P.W.; Song, C.; Zhang, J.; Trudel, L.J.; Wogan, G.N.; Tannenbaum, S.R.; Strano, M.S. The rational design of nitric oxide selectivity in single-walled carbon nanotube near-infrared fluorescence sensors for biological detection. Nat. Chem. 2009, 1, 473–481. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Ma, X.; Zhang, Y.; Che, Y.; Zhao, J. Detection of Amines with Fluorescent Nanotubes: Applications in the Assessment of Meat Spoilage. ACS Sens. 2016, 1, 22–25. [Google Scholar] [CrossRef]
- Snow, E.S.; Perkins, F.K.; Houser, E.J.; Badescu, S.C.; Reinecke, T.L. Chemical detection with a single-walled carbon nanotube capacitor. Science 2005, 307, 1942–1945. [Google Scholar] [CrossRef] [Green Version]
- Sivaramakrishnan, S.; Rajamani, R.; Smith, C.S.; McGee, K.A.; Mann, K.R.; Yamashita, N. Carbon nanotube-coated surface acoustic wave sensor for carbon dioxide sensing. Sens. Actuators B Chem. 2008, 132, 296–304. [Google Scholar] [CrossRef]
- Dai, J.; Ogbeide, O.; Macadam, N.; Sun, Q.; Yu, W.; Li, Y.; Su, B.-L.; Hasan, T.; Huang, X.; Huang, W. Printed gas sensors. Chem. Soc. Rev. 2020, 49, 1756–1789. [Google Scholar] [CrossRef] [PubMed]
- Paolesse, R.; Nardis, S.; Monti, D.; Stefanelli, M.; Di Natale, C. Porphyrinoids for Chemical Sensor Applications. Chem. Rev. 2017, 117, 2517–2583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandhi, T.; Chandnani, A.; Subbaraman, H.; Estrada, D. A Review of Inkjet Printed Graphene and Carbon Nanotubes Based Gas Sensors. Sensors 2020, 20, 5642. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Stolz, R.M.; Mendecki, L.; Mirica, K.A. Electrically-Transduced Chemical Sensors Based on Two-Dimensional Nanomaterials. Chem. Rev. 2019, 119, 478–598. [Google Scholar] [CrossRef]
- Javey, A.; Konj, J. Carbon Nanotube Electronics; Springer: New York, NY, USA, 2009; p. 267. [Google Scholar] [CrossRef]
- Schroeder, V.; Savagatrup, S.; He, M.; Lin, S.; Swager, T.M. Carbon Nanotube Chemical Sensors. Chem. Rev. 2019, 119, 599–663. [Google Scholar] [CrossRef]
- Bariya, M.; Nyein, H.Y.Y.; Javey, A. Wearable sweat sensors. Nat. Electron. 2018, 1, 160–171. [Google Scholar] [CrossRef]
- Guo, L.; Yang, Z.; Li, Y.; Zu, B.; Dou, X. Sensitive, real-time and anti-interfering detection of nitro-explosive vapors realized by ZnO/rGO core/shell micro-Schottky junction. Sens. Actuators B 2017, 239, 286–294. [Google Scholar] [CrossRef]
- Inaba, M.; Oda, T.; Kono, M.; Phansiri, N.; Morita, T.; Nakahara, S.; Nakano, M.; Suehiro, J. Effect of mixing ratio on NO2 gas sensor response with SnO2-decorated carbon nanotube channels fabricated by one-step dielectrophoretic assembly. Sens. Actuators B 2021, 344, 130257. [Google Scholar] [CrossRef]
- Liu, C.; Hu, J.; Wu, G.; Cao, J.; Zhang, Z.; Zhang, Y. Carbon Nanotube-Based Field-Effect Transistor-Type Sensor with a Sensing Gate for Ppb-Level Formaldehyde Detection. ACS Appl. Mater. Interfaces 2021, 13, 56309–56319. [Google Scholar] [CrossRef]
- Fennell, J.F.; Liu, S.F.; Azzarelli, J.M.; Weis, J.G.; Rochat, S.; Mirica, K.A.; Ravnsbæk, J.B.; Swager, T.M. Nanowire Chemical/Biological Sensors: Status and a Roadmap for the Future. Angew. Chem. Int. Ed. 2016, 55, 1266–1281. [Google Scholar] [CrossRef] [Green Version]
- Qi, P.; Vermesh, O.; Grecu, M.; Javey, A.; Wang, Q.; Dai, H.; Peng, S.; Cho, K.J. Toward Large Arrays of Multiplex Functionalized Carbon Nanotube Sensors for Highly Sensitive and Selective Molecular Detection. Nano Lett. 2003, 3, 347–351. [Google Scholar] [CrossRef]
- Zanolli, Z.; Charlier, J.C. Single-molecule sensing using carbon nanotubes decorated with magnetic clusters. ACS Nano 2012, 6, 10786–10791. [Google Scholar] [CrossRef]
- Song, W.; Pang, P.; He, J.; Lindsay, S. Optical and electrical detection of single-molecule translocation through carbon nanotubes. ACS Nano 2013, 7, 689–694. [Google Scholar] [CrossRef] [Green Version]
- Xia, C.; Zhang, D.; Li, H.; Li, S.; Liu, H.; Ding, L.; Liu, X.; Lyu, M.; Li, R.; Yang, J.; et al. Single-walled carbon nanotube based SERS substrate with single molecule sensitivity. Nano Res. 2022, 15, 694–700. [Google Scholar] [CrossRef]
- Savagatrup, S.; Schroeder, V.; He, X.; Lin, S.; He, M.; Yassine, O.; Salama, K.N.; Zhang, X.-X.; Swager, T.M. Bio-Inspired Carbon Monoxide Sensors with Voltage-Activated Sensitivity. Angew. Chem. Int. Ed. 2017, 56, 14066–14070. [Google Scholar] [CrossRef] [Green Version]
- Bezdek, M.J.; Luo, S.-X.L.; Ku, K.H.; Swager, T.M. A chemiresistive methane sensor. Proc. Natl. Acad. Sci. USA 2021, 118, e2022515118. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Liang, S.; Han, J.; Zhong, D.; Liu, J.; Zhang, Z.; Peng, L. Batch Fabrication of Ultrasensitive Carbon Nanotube Hydrogen Sensors with Sub-ppm Detection Limit. ACS Sens. 2018, 3, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Luo, Y.; Li, Z. A highly selective chemical gas sensor based on functionalization of multi-walled carbon nanotubes with poly(ethylene glycol). Sens. Actuators B 2007, 126, 361–367. [Google Scholar] [CrossRef]
- Boyd, A.; Dube, I.; Fedorov, G.; Paranjape, M.; Barbara, P. Gas sensing mechanism of carbon nanotubes: From single tubes to high-density networks. Carbon 2014, 69, 417–423. [Google Scholar] [CrossRef]
- Heller, I.; Janssens, A.M.; Männik, J.; Minot, E.D.; Lemay, S.G.; Dekker, C. Identifying the Mechanism of Biosensing with Carbon Nanotube Transistors. Nano Lett. 2008, 8, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Bradley, K.; Gabriel, J.-C.P.; Star, A.; Grüner, G. Short-channel effects in contact-passivated nanotube chemical sensors. Appl. Phys. Lett. 2003, 83, 3821–3823. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Luo, Z.; Han, S.; Tang, T.; Zhang, D.; Zhou, C. Band engineering of carbon nanotube field-effect transistors via selected area chemical gating. Appl. Phys. Lett. 2005, 86, 243501. [Google Scholar] [CrossRef]
- Zhang, J.; Boyd, A.; Tselev, A.; Paranjape, M.; Barbara, P. Mechanism of NO2 detection in carbon nanotube field effect transistor chemical sensors. Appl. Phys. Lett. 2006, 88, 123112. [Google Scholar] [CrossRef]
- Peng, N.; Zhang, Q.; Chow, C.L.; Tan, O.K.; Marzari, N. Sensing Mechanisms for Carbon Nanotube Based NH3 Gas Detection. Nano Lett. 2009, 9, 1626–1630. [Google Scholar] [CrossRef]
- Bondavalli, P.; Legagneux, P.; Pribat, D. Carbon nanotubes based transistors as gas sensors: State of the art and critical review. Sens. Actuators B 2009, 140, 304–318. [Google Scholar] [CrossRef]
- Chikkadi, K.; Muoth, M.; Roman, C.; Haluska, M.; Hierold, C. Advances in NO2 sensing with individual single-walled carbon nanotube transistors. Beilstein J. Nanotechnol. 2014, 5, 2179–2191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Shen, Q.; Cao, Y.; Gan, L.; Wang, Z.; Steigerwald, M.L.; Guo, X. Chemical functionalization of single-walled carbon nanotube field-effect transistors as switches and sensors. Coord. Chem. Rev. 2010, 254, 1101–1116. [Google Scholar] [CrossRef]
- Shkodra, B.; Petrelli, M.; Costa Angeli, M.A.; Garoli, D.; Nakatsuka, N.; Lugli, P.; Petti, L. Electrolyte-gated carbon nanotube field-effect transistor-based biosensors: Principles and applications. Appl. Phys. Rev. 2021, 8, 041325. [Google Scholar] [CrossRef]
- Ghaddab, B.; Sanchez, J.B.; Mavon, C.; Paillet, M.; Parret, R.; Zahab, A.A.; Bantignies, J.L.; Flaud, V.; Beche, E.; Berger, F. Detection of O3 and NH3 using hybrid tin dioxide/carbon nanotubes sensors: Influence of materials and processing on sensor’s sensitivity. Sens. Actuators B 2012, 170, 67–74. [Google Scholar] [CrossRef]
- Park, S.; Vosguerichian, M.; Bao, Z. A review of fabrication and applications of carbon nanotube film-based flexible electronics. Nanoscale 2013, 5, 1727–1752. [Google Scholar] [CrossRef] [PubMed]
- Landry, M.P.; Ando, H.; Chen, A.Y.; Cao, J.; Kottadiel, V.I.; Chio, L.; Yang, D.; Dong, J.; Lu, T.K.; Strano, M.S. Single-molecule detection of protein efflux from microorganisms using fluorescent single-walled carbon nanotube sensor arrays. Nat. Nanotechnol. 2017, 12, 368–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, T.; Wang, T.; Xu, Y.Q. Direct Measurement of π Coupling at the Single-Molecule Level using a Carbon Nanotube Force Sensor. Nano Lett. 2018, 18, 7883–7888. [Google Scholar] [CrossRef]
- Guo, X. Single-molecule electrical biosensors based on single-walled carbon nanotubes. Adv. Mater. 2013, 25, 3397–3408. [Google Scholar] [CrossRef]
- Ahmed Jamal, G.R.; Islam, M.R.; Rahman, M.A.; Meem, J.F.; Sathi, R.A. Chirality dependence of gas adsorption property of single wall carbon nanotubes. Mater. Sci. Forum 2017, 889, 248–252. [Google Scholar] [CrossRef]
- Ganzhorn, M.; Vijayaraghavan, A.; Dehm, S.; Hennrich, F.; Green, A.A.; Fichtner, M.; Voigt, A.; Rapp, M.; Von Löhneysen, H.; Hersam, M.C.; et al. Hydrogen sensing with diameter- and chirality-sorted carbon nanotubes. ACS Nano 2011, 5, 1670–1676. [Google Scholar] [CrossRef]
- Seo, K.; Park, K.A.; Kim, C.; Han, S.; Kim, B.; Lee, Y.H. Chirality- and diameter-dependent reactivity of NO2 on carbon nanotube walls. J. Am. Chem. Soc. 2005, 127, 15724–15729. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.W.; Jiang, S.; Zhu, Q.B.; Sun, Y.; Luan, J.; Hou, P.X.; Qiu, S.; Li, Q.W.; Liu, C.; Sun, D.M.; et al. Continuous Fabrication of Meter-Scale Single-Wall Carbon Nanotube Films and their Use in Flexible and Transparent Integrated Circuits. Adv. Mater. 2018, 30, 1802057. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Hou, P.-X.; Liu, C.; Cheng, H.-M. High-performance single-wall carbon nanotube transparent conductive films. J. Mater. Sci. Technol. 2019, 35, 2447–2462. [Google Scholar] [CrossRef]
- Ishikawa, F.N.; Curreli, M.; Olson, C.A.; Liao, H.I.; Sun, R.; Roberts, R.W.; Cote, R.J.; Thompson, M.E.; Zhou, C. Importance of controlling nanotube density for highly sensitive and reliable biosensors functional in physiological conditions. ACS Nano 2010, 4, 6914–6922. [Google Scholar] [CrossRef] [PubMed]
- Quang, N.H.; Van Trinh, M.; Lee, B.-H.; Huh, J.-S. Effect of NH3 gas on the electrical properties of single-walled carbon nanotube bundles. Sens. Actuators B 2006, 113, 341–346. [Google Scholar] [CrossRef]
- Zhao, J.; Buldum, A.; Han, J.; Lu, J.P. Gas molecule adsorption in carbon nanotubes and nanotube bundles. Nanotechnology 2002, 13, 195–200. [Google Scholar] [CrossRef]
- Kobashi, K.; Iizumi, Y.; Muroga, S.; Morimoto, T.; Okazaki, T. N2 Gas Adsorption Sites of Single-Walled Carbon Nanotube Bundles: Identifying Interstitial Channels at Very Low Relative Pressure. Langmuir 2021, 37, 9144–9150. [Google Scholar] [CrossRef]
- Kahng, Y.H.; Hallock, R.B.; Dujardin, E. Gas adsorption on single-wall carbon nanotube bundles and charcoal samples. Phys. Rev. B Condens. Matter Mater. Phys. 2011, 83, 115434. [Google Scholar] [CrossRef] [Green Version]
- Amorim, R.G.; Fazzio, A.; Da Silva, A.J.R.; Rocha, A.R. Confinement effects and why carbon nanotube bundles can work as gas sensors. Nanoscale 2013, 5, 2798–2803. [Google Scholar] [CrossRef]
- Thirumalai, D.; Subramani, D.; Yoon, J.-H.; Lee, J.; Paik, H.-j.; Chang, S.-C. De-bundled single-walled carbon nanotube-modified sensors for simultaneous differential pulse voltammetric determination of ascorbic acid, dopamine, and uric acid. New J. Chem. 2018, 42, 2432–2438. [Google Scholar] [CrossRef]
- Ndiaye, A.L.; Varenne, C.; Bonnet, P.; Petit, É.; Spinelle, L.; Brunet, J.; Pauly, A.; Lauron, B. Elaboration of single wall carbon nanotubes-based gas sensors: Evaluating the bundling effect on the sensor performance. Thin Solid Films 2012, 520, 4465–4469. [Google Scholar] [CrossRef]
- Hu, X.G.; Hou, P.X.; Liu, C.; Zhang, F.; Liu, G.; Cheng, H.M. Small-bundle single-wall carbon nanotubes for high-efficiency silicon heterojunction solar cells. Nano Energy 2018, 50, 521–527. [Google Scholar] [CrossRef]
- Jiang, S.; Hou, P.-X.; Chen, M.-L.; Wang, B.-W.; Sun, D.-M.; Tang, D.-M.; Jin, Q.; Guo, Q.-X.; Zhang, D.-D.; Du, J.-H.; et al. Ultrahigh-performance transparent conductive films of carbon-welded isolated single-wall carbon nanotubes. Sci. Adv. 2018, 4, eaap9264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, M.E.; LeMieux, M.C.; Bao, Z. Sorted and Aligned Single-Walled Carbon Nanotube Networks for Transistor-Based Aqueous Chemical Sensors. ACS Nano 2009, 3, 3287–3293. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.P.A.; Zheng, G.; Lieber, C.M. Subthreshold Regime has the Optimal Sensitivity for Nanowire FET Biosensors. Nano Lett. 2010, 10, 547–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charlier, J.C. Defects in Carbon Nanotubes. Acc. Chem. Res. 2002, 35, 1063–1069. [Google Scholar] [CrossRef]
- Robinson, J.A.; Snow, E.S.; Badescu, Ş.C.; Reinecke, T.L.; Perkins, F.K. Role of Defects in Single-Walled Carbon Nanotube Chemical Sensors. Nano Lett. 2006, 6, 1747–1751. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Navarro, C.; Pablo, P.J.D.; Gómez-Herrero, J.; Biel, B.; Garcia-Vidal, F.J.; Rubio, A.; Flores, F. Tuning the conductance of single-walled carbon nanotubes by ion irradiation in the Anderson localization regime. Nat. Mater. 2005, 4, 534–539. [Google Scholar] [CrossRef]
- Salehi-Khojin, A.; Khalili-Araghi, F.; Kuroda, M.A.; Lin, K.Y.; Leburton, J.P.; Masel, R.I. On the sensing mechanism in carbon nanotube chemiresistors. ACS Nano 2011, 5, 153–158. [Google Scholar] [CrossRef]
- Kim, J.; Choi, S.W.; Lee, J.H.; Chung, Y.; Byun, Y.T. Gas sensing properties of defect-induced single-walled carbon nanotubes. Sens. Actuators B Chem. 2016, 228, 688–692. [Google Scholar] [CrossRef]
- Lim, N.; Kim, K.H.; Byun, Y.T. Preparation of defected SWCNTs decorated with en-APTAS for application in high-performance nitric oxide gas detection. Nanoscale 2021, 13, 6538–6544. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Luo, H.; Cai, J.; Dong, C. Molecular and atomic adsorptions of hydrogen, oxygen, and nitrogen on defective carbon nanotubes: A first-principles study. Int. J. Hydrog. Energy 2020, 45, 26655–26665. [Google Scholar] [CrossRef]
- Lee, D.; Seol, M.L.; Moon, D.I.; Bennett, P.; Yoder, N.; Humes, J.; Bokor, J.; Choi, Y.K.; Choi, S.J. High-performance thin-film transistors produced from highly separated solution-processed carbon nanotubes. Appl. Phys. Lett. 2014, 104, 143508. [Google Scholar] [CrossRef] [Green Version]
- Yao, J.; Li, Y.; Li, Y.; Sui, Q.; Wen, H.; Cao, L.; Cao, P.; Kang, L.; Tang, J.; Jin, H.; et al. Rapid annealing and cooling induced surface cleaning of semiconducting carbon nanotubes for high-performance thin-film transistors. Carbon 2021, 184, 764–771. [Google Scholar] [CrossRef]
- Agarwal, P.B.; Sharma, R.; Mishra, D.; Thakur, N.K.; Agarwal, A.; Ajayaghosh, A. Silicon Shadow Mask Technology for Aligning and in Situ Sorting of Semiconducting SWNTs for Sensitivity Enhancement: A Case Study of NO2 Gas Sensor. ACS Appl. Mater. Interfaces 2020, 12, 40901–40909. [Google Scholar] [CrossRef]
- Zhou, S.; Xiao, M.; Liu, F.; He, J.; Lin, Y.; Zhang, Z. Sub-10 parts per billion detection of hydrogen with floating gate transistors built on semiconducting carbon nanotube film. Carbon 2021, 180, 41–47. [Google Scholar] [CrossRef]
- Tsuruta, A.; Akamatsu, T.; Naito, K.; Hirai, T.; Murase, S.; Masuda, Y. Gas Sensing Properties of High-Purity Semiconducting Single-Walled Carbon Nanotubes for NH3, H2, and NO. ECS J. Solid State Sci. Technol. 2021, 10, 121004. [Google Scholar] [CrossRef]
- Ishihara, S.; O’Kelly, C.J.; Tanaka, T.; Kataura, H.; Labuta, J.; Shingaya, Y.; Nakayama, T.; Ohsawa, T.; Nakanishi, T.; Swager, T.M. Metallic versus Semiconducting SWCNT Chemiresistors: A Case for Separated SWCNTs Wrapped by a Metallosupramolecular Polymer. ACS Appl. Mater. Interfaces 2017, 9, 38062–38067. [Google Scholar] [CrossRef]
- Hwang, S.I.; Franconi, N.G.; Rothfuss, M.A.; Bocan, K.N.; Bian, L.; White, D.L.; Burkert, S.C.; Euler, R.W.; Sopher, B.J.; Vinay, M.L.; et al. Tetrahydrocannabinol Detection Using Semiconductor-Enriched Single-Walled Carbon Nanotube Chemiresistors. ACS Sens. 2019, 4, 2084–2093. [Google Scholar] [CrossRef]
- Abbas, S.; Yi, W.; Yoo, S.; Khalid, A.; Bhalli, Z.; Si, J.; Hou, X. Highly Efficient Response of Ammonia Gas Sensor Based on Surfactant-Free Sorted-Semiconducting Single-Walled Carbon Nanotubes at Room Temperature. Phys. Status Solidi A Appl. Mater. Sci. 2022, 219, 2100529. [Google Scholar] [CrossRef]
- Umadevi, D.; Sastry, G.N. Feasibility of carbon nanomaterials as gas sensors: A computational study. Curr. Sci. 2014, 106, 1224–1234. [Google Scholar]
- Norizan, M.N.; Moklis, M.H.; Demon, S.Z.N.; Halim, N.A.; Samsuri, A.; Mohamad, I.S.; Knight, V.F.; Abdullah, N. Carbon nanotubes: Functionalisation and their application in chemical sensors. RSC Adv. 2020, 10, 43704–43732. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Norman, T.J.; Jones, R.S.; Moon, D.I.; Han, J.W.; Meyyappan, M. Carboxylated Single-Walled Carbon Nanotube Sensors with Varying pH for the Detection of Ammonia and Carbon Dioxide Using an Artificial Neural Network. ACS Appl. Nano Mater. 2019, 2, 6445–6451. [Google Scholar] [CrossRef]
- Jones, R.S.; Kim, B.; Han, J.-W.; Meyyappan, M. pH Modeling to Predict SWCNT–COOH Gas Sensor Response to Multiple Target Gases. J. Phys. Chem. C 2021, 125, 9356–9363. [Google Scholar] [CrossRef]
- Zhao, B.; Hu, H.; Haddon, R.C. Synthesis and properties of a water-soluble single-walled carbon nanotube-poly(m-aminobenzene sulfonic acid) graft copolymer. Adv. Funct. Mater. 2004, 14, 71–76. [Google Scholar] [CrossRef]
- Zhang, T.; Mubeen, S.; Bekyarova, E.; Yoo, B.Y.; Haddon, R.C.; Myung, N.V.; Deshusses, M.A. Poly(m-aminobenzene sulfonic acid) functionalized single-walled carbon nanotubes based gas sensor. Nanotechnology 2007, 18, 165504. [Google Scholar] [CrossRef]
- Tasis, D.; Tagmatarchis, N.; Bianco, A.; Prato, M. Chemistry of carbon nanotubes. Chem. Rev. 2006, 106, 1105–1136. [Google Scholar] [CrossRef]
- Şenocak, A.; Göl, C.; Basova, T.V.; Demirbaş, E.; Durmuş, M.; Al-Sagur, H.; Kadem, B.; Hassan, A. Preparation of single walled carbon nanotube-pyrene 3D hybrid nanomaterial and its sensor response to ammonia. Sens. Actuators B Chem. 2018, 256, 853–860. [Google Scholar] [CrossRef] [Green Version]
- Kong, L.; Wang, J.; Fu, X.; Zhong, Y.; Meng, F.; Luo, T.; Liu, J. p-Hexafluoroisopropanol phenyl covalently functionalized single-walled carbon nanotubes for detection of nerve agents. Carbon 2010, 48, 1262–1270. [Google Scholar] [CrossRef]
- Huang, J.; Ng, A.L.; Piao, Y.; Chen, C.F.; Green, A.A.; Sun, C.F.; Hersam, M.C.; Lee, C.S.; Wang, Y. Covalently functionalized double-walled carbon nanotubes combine high sensitivity and selectivity in the electrical detection of small molecules. J. Am. Chem. Soc. 2013, 135, 2306–2312. [Google Scholar] [CrossRef]
- Doyle, C.D.; Rocha, J.D.R.; Weisman, R.B.; Tour, J.M. Structure-dependent reactivity of semiconducting single-walled carbon nanotubes with benzenediazonium salts. J. Am. Chem. Soc. 2008, 130, 6795–6800. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.T.; Mulchandani, A. Carbon nanotubes and graphene nano field-effect transistor-based biosensors. TrAC Trends Anal. Chem. 2016, 79, 222–232. [Google Scholar] [CrossRef] [Green Version]
- Georgakilas, V.; Otyepka, M.; Bourlinos, A.B.; Chandra, V.; Kim, N.; Kemp, K.C.; Hobza, P.; Zboril, R.; Kim, K.S. Functionalization of Graphene: Covalent and Non-Covalent Approaches, Derivatives and Applications. Chem. Rev. 2012, 112, 6156–6214. [Google Scholar] [CrossRef]
- He, M.; Swager, T.M. Covalent Functionalization of Carbon Nanomaterials with Iodonium Salts. Chem. Mater. 2016, 28, 8542–8549. [Google Scholar] [CrossRef]
- Li, W.-S.; Hou, P.-X.; Liu, C.; Sun, D.-M.; Yuan, J.; Zhao, S.-Y.; Yin, L.-C.; Cong, H.; Cheng, H.-M. High-Quality, Highly Concentrated Semiconducting Single-Wall Carbon Nanotubes for Use in Field Effect Transistors and Biosensors. ACS Nano 2013, 7, 6831–6839. [Google Scholar] [CrossRef]
- Chen, R.J.; Bangsaruntip, S.; Drouvalakis, K.A.; Kam, N.W.S.; Shim, M.; Li, Y.; Kim, W.; Utz, P.J.; Dai, H. Noncovalent functionalization of carbon nanotubes for highly specific electronic biosensors. Proc. Natl. Acad. Sci. USA 2003, 100, 4984–4989. [Google Scholar] [CrossRef] [Green Version]
- Zhan, K.; Liu, H.; Zhang, H.; Chen, Y.; Ni, H.; Wu, M.; Sun, D.; Chen, Y. A facile method for the immobilization of myoglobin on multi-walled carbon nanotubes: Poly(methacrylic acid-co-acrylamide) nanocomposite and its application for direct bio-detection of H2O2. J. Electroanal. Chem. 2014, 724, 80–86. [Google Scholar] [CrossRef]
- Karajanagi, S.S.; Vertegel, A.A.; Kane, R.S.; Dordick, J.S. Structure and Function of Enzymes Adsorbed onto Single-Walled Carbon Nanotubes. Langmuir 2004, 20, 11594–11599. [Google Scholar] [CrossRef]
- Feng, Y.; Li, H.; Inoue, T.; Chiashi, S.; Rotkin, S.V.; Xiang, R.; Maruyama, S. One-Dimensional van der Waals Heterojunction Diode. ACS Nano 2021, 15, 5600–5609. [Google Scholar] [CrossRef]
- Xiang, R.; Inoue, T.; Zheng, Y.; Kumamoto, A.; Qian, Y.; Sato, Y.; Liu, M.; Tang, D.; Gokhale, D.; Guo, J.; et al. One-dimensional van der Waals heterostructures. Science 2020, 367, 537–542. [Google Scholar] [CrossRef] [Green Version]
- Xiang, R.; Maruyama, S. Heteronanotubes: Challenges and Opportunities. Small Sci. 2021, 1, 2000039. [Google Scholar] [CrossRef]
- Datta, K.; Ghosh, P.; More, M.A.; Shirsat, M.D.; Mulchandani, A. Controlled functionalization of single-walled carbon nanotubes for enhanced ammonia sensing: A comparative study. J. Phys. D Appl. Phys. 2012, 45, 355305. [Google Scholar] [CrossRef]
- Liu, S.F.; Lin, S.; Swager, T.M. An Organocobalt–Carbon Nanotube Chemiresistive Carbon Monoxide Detector. ACS Sens. 2016, 1, 354–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sayago, I.; Terrado, E.; Aleixandre, M.; Horrillo, M.C.; Fernández, M.J.; Lozano, J.; Lafuente, E.; Maser, W.K.; Benito, A.M.; Martinez, M.T.; et al. Novel selective sensors based on carbon nanotube films for hydrogen detection. Sens. Actuators B Chem. 2007, 122, 75–80. [Google Scholar] [CrossRef]
- Eder, D. Carbon Nanotube−Inorganic Hybrids. Chem. Rev. 2010, 110, 1348–1385. [Google Scholar] [CrossRef]
- Demir, S.; Fellah, M.F. A DFT study on Pt doped (4,0) SWCNT: CO adsorption and sensing. Appl. Surf. Sci. 2020, 504, 144141. [Google Scholar] [CrossRef]
- Fellah, M.F. Pt doped (8,0) single wall carbon nanotube as hydrogen sensor: A density functional theory study. Int. J. Hydrog. Energy 2019, 44, 27010–27021. [Google Scholar] [CrossRef]
- Yoosefian, M.; Raissi, H.; Mola, A. The hybrid of Pd and SWCNT (Pd loaded on SWCNT) as an efficient sensor for the formaldehyde molecule detection: A DFT study. Sens. Actuators B 2015, 212, 55–62. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, H.H. High-performance, flexible hydrogen sensors that use carbon nanotubes decorated with palladium nanoparticles. Adv. Mater. 2007, 19, 2818–2823. [Google Scholar] [CrossRef]
- Iordache, S.M.; Ionete, E.I.; Iordache, A.M.; Tanasa, E.; Stamatin, I.; Ana Grigorescu, C.E. Pd-decorated CNT as sensitive material for applications in hydrogen isotopes sensing—Application as gas sensor. Int. J. Hydrog. Energy 2021, 46, 11015–11024. [Google Scholar] [CrossRef]
- Mubeen, S.; Zhang, T.; Yoo, B.; Deshusses, M.A.; Myung, N.V. Palladium nanoparticles decorated single-walled carbon nanotube hydrogen sensor. J. Phys. Chem. C 2007, 111, 6321–6327. [Google Scholar] [CrossRef]
- Zanolli, Z.; Leghrib, R.; Felten, A.; Pireaux, J.J.; Llobet, E.; Charlier, J.C. Gas sensing with au-decorated carbon nanotubes. ACS Nano 2011, 5, 4592–4599. [Google Scholar] [CrossRef] [PubMed]
- Su, P.-G.; Li, M.-C. Recognition of binary mixture of NO2 and NO gases using a chemiresistive sensors array combined with principal component analysis. Sens. Actuators A 2021, 331, 112980. [Google Scholar] [CrossRef]
- Lee, K.; Scardaci, V.; Kim, H.-Y.; Hallam, T.; Nolan, H.; Bolf, B.E.; Maltbie, G.S.; Abbott, J.E.; Duesberg, G.S. Highly sensitive, transparent, and flexible gas sensors based on gold nanoparticle decorated carbon nanotubes. Sens. Actuators B 2013, 188, 571–575. [Google Scholar] [CrossRef]
- Mubeen, S.; Zhang, T.; Chartuprayoon, N.; Rheem, Y.; Mulchandani, A.; Myung, N.V.; Deshusses, M.A. Sensitive Detection of H2S Using Gold Nanoparticle Decorated Single-Walled Carbon Nanotubes. Anal. Chem. 2010, 82, 250–257. [Google Scholar] [CrossRef] [Green Version]
- Young, S.-J.; Liu, Y.-H.; Lin, Z.-D.; Ahmed, K.; Shiblee, M.D.N.I.; Romanuik, S.; Sekhar, P.K.; Thundat, T.; Nagahara, L.; Arya, S.; et al. Multi-Walled Carbon Nanotubes Decorated with Silver Nanoparticles for Acetone Gas Sensing at Room Temperature. J. Electrochem. Soc. 2020, 167, 167519. [Google Scholar] [CrossRef]
- Cui, S.; Pu, H.; Lu, G.; Wen, Z.; Mattson, E.C.; Hirschmugl, C.; Gajdardziska-Josifovska, M.; Weinert, M.; Chen, J. Fast and Selective Room-Temperature Ammonia Sensors Using Silver Nanocrystal-Functionalized Carbon Nanotubes. ACS Appl. Mater. Interfaces 2012, 4, 4898–4904. [Google Scholar] [CrossRef]
- Fam, D.W.H.; Tok, A.I.Y.; Palaniappan, A.; Nopphawan, P.; Lohani, A.; Mhaisalkar, S.G. Selective sensing of hydrogen sulphide using silver nanoparticle decorated carbon nanotubes. Sens. Actuators B 2009, 138, 189–192. [Google Scholar] [CrossRef]
- Asad, M.; Sheikhi, M.H. Highly sensitive wireless H2S gas sensors at room temperature based on CuO-SWCNT hybrid nanomaterials. Sens. Actuators B 2016, 231, 474–483. [Google Scholar] [CrossRef]
- Asad, M.; Sheikhi, M.H.; Pourfath, M.; Moradi, M. High sensitive and selective flexible H2S gas sensors based on Cu nanoparticle decorated SWCNTs. Sens. Actuators B 2015, 210, 1–8. [Google Scholar] [CrossRef]
- Kuganathan, N.; Chroneos, A. Ru-Doped Single Walled Carbon Nanotubes as Sensors for SO2 and H2S Detection. Chemosensors 2021, 9, 120. [Google Scholar] [CrossRef]
- Jung, D.; Han, M.; Lee, G.S. Room-temperature gas sensor using carbon nanotube with cobalt oxides. Sens. Actuators B 2014, 204, 596–601. [Google Scholar] [CrossRef]
- Wang, H.; Ma, J.; Zhang, J.; Feng, Y.; Vijjapu, M.T.; Yuvaraja, S.; Surya, S.G.; Salama, K.N.; Dong, C.; Wang, Y.; et al. Gas sensing materials roadmap. J. Phys. Condens. Matter 2021, 33, 303001. [Google Scholar] [CrossRef]
- Hoa, N.D.; Van Quy, N.; Kim, D. Nanowire structured SnOx–SWNT composites: High performance sensor for NOx detection. Sens. Actuators B 2009, 142, 253–259. [Google Scholar] [CrossRef]
- Rigoni, F.; Drera, G.; Pagliara, S.; Goldoni, A.; Sangaletti, L. High sensitivity, moisture selective, ammonia gas sensors based on single-walled carbon nanotubes functionalized with indium tin oxide nanoparticles. Carbon 2014, 80, 356–363. [Google Scholar] [CrossRef]
- Ding, M.; Sorescu, D.C.; Star, A. Photoinduced Charge Transfer and Acetone Sensitivity of Single-Walled Carbon Nanotube–Titanium Dioxide Hybrids. J. Am. Chem. Soc. 2013, 135, 9015–9022. [Google Scholar] [CrossRef]
- Seo, M.-H.; Kang, K.; Yoo, J.-Y.; Park, J.; Lee, J.-S.; Cho, I.; Kim, B.-J.; Jeong, Y.; Lee, J.-Y.; Kim, B.; et al. Chemo-Mechanically Operating Palladium-Polymer Nanograting Film for a Self-Powered H2 Gas Sensor. ACS Nano 2020, 14, 16813–16822. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Hemminger, J.C.; Penner, R.M. Catalytically Activated Palladium@Platinum Nanowires for Accelerated Hydrogen Gas Detection. ACS Nano 2015, 9, 3215–3225. [Google Scholar] [CrossRef]
- Li, X.; Le Thai, M.; Dutta, R.K.; Qiao, S.; Chandran, G.T.; Penner, R.M. Sub-6 nm Palladium Nanoparticles for Faster, More Sensitive H2 Detection Using Carbon Nanotube Ropes. ACS Sens. 2017, 2, 282–289. [Google Scholar] [CrossRef]
- Khalap, V.R.; Sheps, T.; Kane, A.A.; Collins, P.G. Hydrogen sensing and sensitivity of palladium-decorated single-walled carbon nanotubes with defects. Nano Lett. 2010, 10, 896–901. [Google Scholar] [CrossRef] [Green Version]
- Mannoor, M.S.; Tao, H.; Clayton, J.D.; Sengupta, A.; Kaplan, D.L.; Naik, R.R.; Verma, N.; Omenetto, F.G.; McAlpine, M.C. Graphene-based wireless bacteria detection on tooth enamel. Nat. Commun. 2012, 3, 763. [Google Scholar] [CrossRef] [PubMed]
- Escobedo, P.; Fernández-Ramos, M.D.; López-Ruiz, N.; Moyano-Rodríguez, O.; Martínez-Olmos, A.; Pérez de Vargas-Sansalvador, I.M.; Carvajal, M.A.; Capitán-Vallvey, L.F.; Palma, A.J. Smart facemask for wireless CO2 monitoring. Nat. Commun. 2022, 13, 72. [Google Scholar] [CrossRef] [PubMed]
- Andre, R.S.; Ngo, Q.P.; Fugikawa-Santos, L.; Correa, D.S.; Swager, T.M. Wireless Tags with Hybrid Nanomaterials for Volatile Amine Detection. ACS Sens. 2021, 6, 2457–2464. [Google Scholar] [CrossRef]
- Zhu, R.; Azzarelli, J.M.; Swager, T.M. Wireless Hazard Badges to Detect Nerve-Agent Simulants. Angew. Chem. Int. Ed. 2016, 55, 9662–9666. [Google Scholar] [CrossRef]
- Cheng, C.; Li, X.; Xu, G.; Lu, Y.; Low, S.S.; Liu, G.; Zhu, L.; Li, C.; Liu, Q. Battery-free, wireless, and flexible electrochemical patch for in situ analysis of sweat cortisol via near field communication. Biosens. Bioelectron. 2021, 172, 112782. [Google Scholar] [CrossRef]
- Lin, R.; Kim, H.J.; Achavananthadith, S.; Kurt, S.A.; Tan, S.C.C.; Yao, H.; Tee, B.C.K.; Lee, J.K.W.; Ho, J.S. Wireless battery-free body sensor networks using near-field-enabled clothing. Nat. Commun. 2020, 11, 444. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Min, J.; Yu, Y.; Wang, H.; Yang, Y.; Zhang, H.; Gao, W. Wireless battery-free wearable sweat sensor powered by human motion. Sci. Adv. 2020, 6, eaay9842. [Google Scholar] [CrossRef]











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, S.-Y.; Hou, P.-X.; Zhang, F.; Liu, C.; Cheng, H.-M. Gas Sensors Based on Single-Wall Carbon Nanotubes. Molecules 2022, 27, 5381. https://doi.org/10.3390/molecules27175381
Guo S-Y, Hou P-X, Zhang F, Liu C, Cheng H-M. Gas Sensors Based on Single-Wall Carbon Nanotubes. Molecules. 2022; 27(17):5381. https://doi.org/10.3390/molecules27175381
Chicago/Turabian StyleGuo, Shu-Yu, Peng-Xiang Hou, Feng Zhang, Chang Liu, and Hui-Ming Cheng. 2022. "Gas Sensors Based on Single-Wall Carbon Nanotubes" Molecules 27, no. 17: 5381. https://doi.org/10.3390/molecules27175381





