Abstract
Layered vanadium-based materials are considered to be great potential electrode materials for aqueous Zn-ion batteries (AZIBs). The improvement of the electrochemical properties of vanadium-based materials is a hot research topic but still a challenge. Herein, a composite of Zn-ion pre-intercalated V2O5·nH2O combined with reduced graphene oxide (ZnVOH/rGO) is synthesized by a facile hydrothermal method and it shows improved Zn-ion storage. ZnVOH/rGO delivers a capacity of 325 mAh·g−1 at 0.1 A·g−1, and this value can still reach 210 mAh·g−1 after 100 cycles. Additionally, it exhibits 196 mAh·g−1 and keeps 161 mAh·g−1 after 1200 cycles at 4 A·g−1. The achieved performances are much higher than that of ZnVOH and VOH. All results reveal that Zn2+ as “pillars” expands the interlayer distance of VOH and facilitates the fast kinetics, and rGO improves the electron flow. They both stabilize the structure and enhance efficient Zn2+ migration. All findings demonstrate ZnVOH/rGO’s potential as a perspective cathode material for AZIBs.
1. Introduction
Nowadays, environmental problems and the energy crisis have become dominating hot topics. Researchers are engaged in exploring various high-efficiency, green energy storage and conversion devices [1,2,3]. Among various energy storage systems, aqueous Zn-ion batteries (AZIBs) have attracted increasing interest in recent years as an alternative for grid-scale energy storage systems because of their low-cost, eco-friendliness and safety [4,5,6,7]. For AZIBs, metal zinc is typically used as the anode due to its high theoretical capacity of 820 mAh·g−1 and abundance. However, a lot of work is still ongoing aimed at finding suitable cathode materials that meet the requirements of high energy density and long-cycle life [8,9,10].
So far, various cathode materials for AZIBs, such as manganese oxides, vanadium oxides and their related compounds, Prussian blue analogues, cobalt oxides, organic molecules and so on, have been widely studied [11,12,13,14,15,16]. Among them, vanadium (V)-based compounds are considered as attractive candidates because of their variable valence states, abundant reserves and open-frame structures [17,18,19,20,21]. This material can accommodate lots of Zn-ions. Layered hydration vanadium pentoxide V2O5·nH2O (abbreviation as VOH) possesses tunable structures and multivalences for large Zn2+ reserves and ingress/egress [22,23,24,25]. Although many progresses of vanadium oxides are achieved as cathode materials for AZIBs, they still suffer from intrinsic poor electroconductivity and hindered dynamics owing to the strong electrostatic attraction between Zn2+ and VO layer [26]. Thus, the structural engineering of vanadium oxides with open architecture is key to eliminating this electrostatic impact [27].
As the cathode material for AZIBs, Zn0.25V2O5∙nH2O was first reported by Nazar’s group [28]; it exhibited high capacity (ca. 300 mAh g−1 @ 50 mA g−1) and showed excellent rate performance. After that, some objects (e.g., various metal ions, different polymers) are introduced to tune the lamellar structure of VOH. After adjusting the interlayer spacing, electrochemical properties of intercalated VOH were significantly boosted [29,30,31,32,33,34]. For instance, Liu et al. reported that polyaniline-intercalated VOH shows an expanded interlayer space of ~14 Å delivering a capacity of 354 mAh·g−1 at 0.1 A·g−1 and good cycle stability [35]. Other reports prove the utility of object-intercalated VOH with enhanced electrochemical properties for AZIBs. Although Zn0.25V2O5∙nH2O for AZIBs has been reported, Zn2+-intercalated VOH combined with rGO has been rarely reported to the best of our knowledge [36]. As is well known, recombination rGO with metal oxides enhances the conductivity, facilitates electron flow and stabilizes the structure of the composites [37,38].
Herein, a composite with Zn2+-intercalated VOH integrated with rGO (denoted as ZnVOH/rGO) is developed and it exhibits enhanced Zn-ion storage with a capacity of 325 mAh·g−1 at 0.1 A·g−1 and 210 mAh·g−1 after 100 cycles. The capacity is not higher than that reported by Nazar (0.05 A·g−1) because of our value obtained at a very high rate 0.1 A·g−1. The achieved performances of ZnVOH/rGO are much higher than that of ZnVOH and VOH, and even surpass some state-of-the-art cathodes for the application of AZIBs.
2. Results and Discussion
ZnVOH/rGO was prepared by a hydrothermal route combination with freeze-drying treatment. V-based complexes were first formed by reacting between V2O5 and H2O2 to form a clear solution [35]. In hydrothermal route, VOH nanobelts were formed, meanwhile, Zn-ions were intercalated into a VOH interlayer, expanding the distance of interlayer spacing, like other metal ions [30]. During this process, GO was transformed to rGO and ZnVOH was connected/fixed with rGO by hydroxyl bonding or electrostatic attraction [38]. ZnVOH/rGO was formed by this hydrothermal route and finally obtained by freeze-drying treatment.
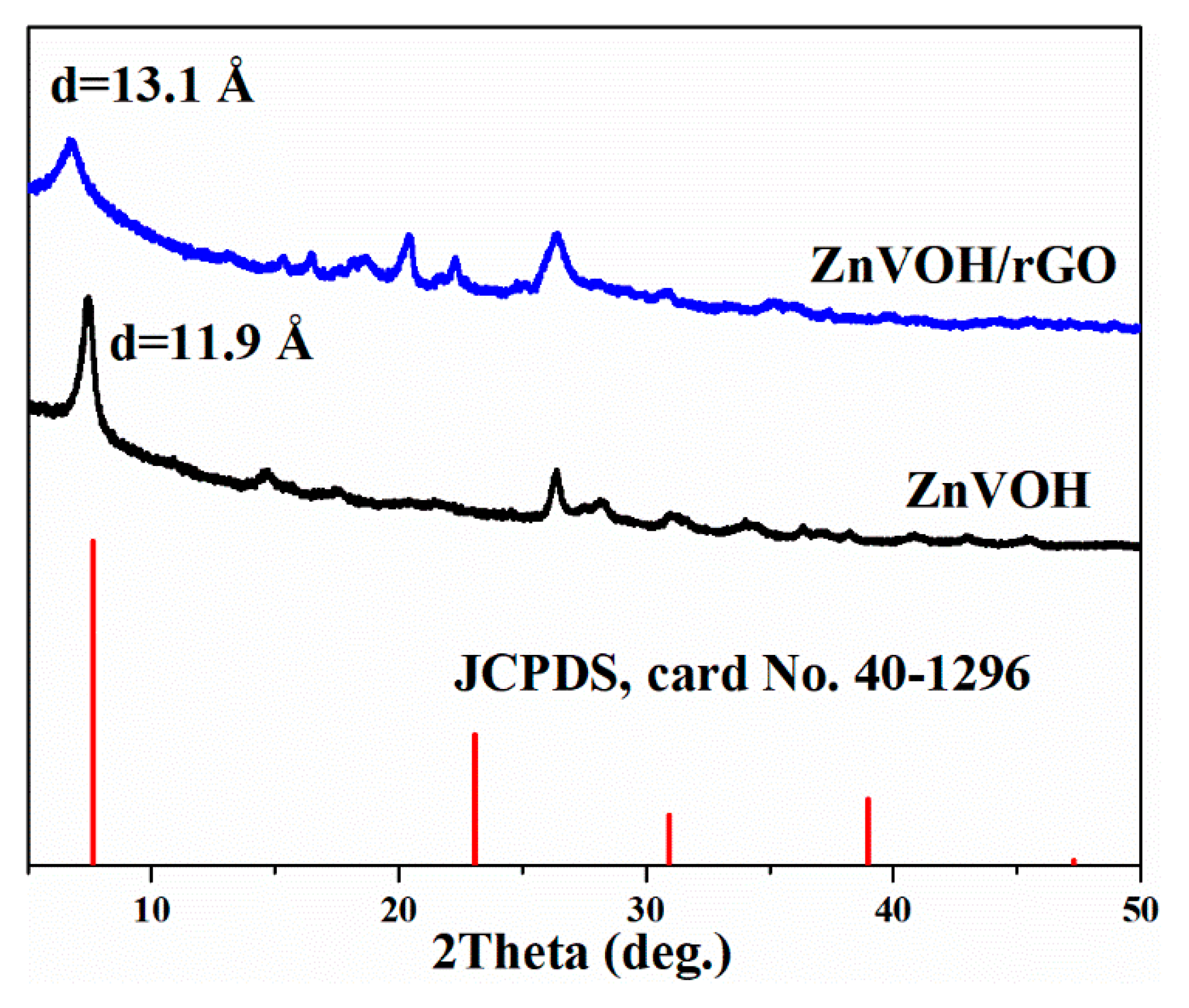
Figure 1 shows the XRD patterns of ZnVOH and ZnVOH/rGO. The characteristic peaks correspond to the standard pattern of V2O5·1.6H2O (JCPDS, card No. 40-1296) [39]. The main peaks of the four materials matched well with PDF#40-1296, but due to the intercalation of metal ions, the peak position of VOH will be shifted. These phenomena are reported in the previous literature [39]. The (001) peak with the lattice spacing of 13.1 Å is achieved from ZnVOH/rGO, which is larger than the value (11.9 Å) of ZnVOH. The increase of interlayer spacing not only expands the transport channel of zinc ions, but also weakens the electrostatic interaction with the main material. In order to determine the composition of the materials, we conducted the ICP test for the two materials. The result is shown in Tables S1 and S2. The Zn/V ratios in ZnVOH and ZnVOH/rGO are similar, both about 0.11:1. This is very consistent with the synthesis of ZnVOH/rGO compared with ZnVOH only the addition of rGO. From the test results, it is found that the ratio of Zn to V does not change greatly with the addition of rGO, but the addition of rGO greatly improves the layer spacing. This may be because the introduction of rGO makes more water molecules enter the layer between vanadium oxides. This result suggests the addition of GO can not only facilitate the fast Zn ion transfers but also improve the electrochemical conductivity of ZnVOH [40]. In our experiment, we also investigated the amount of rGO. However, the interlayer spacing of ZnVOH/rGO has changed little with the change of rGO.
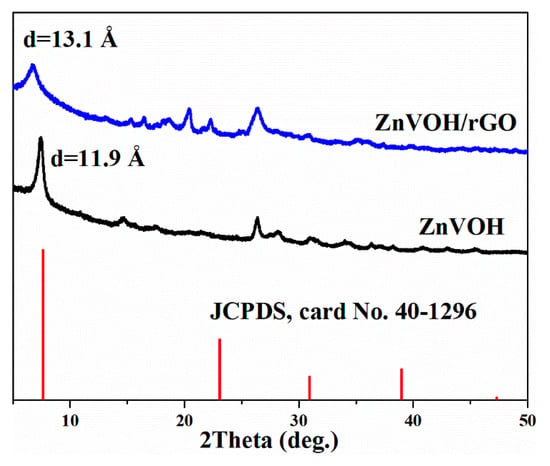
Figure 1.
XRD patterns of ZnVOH and ZnVOH/rGO.
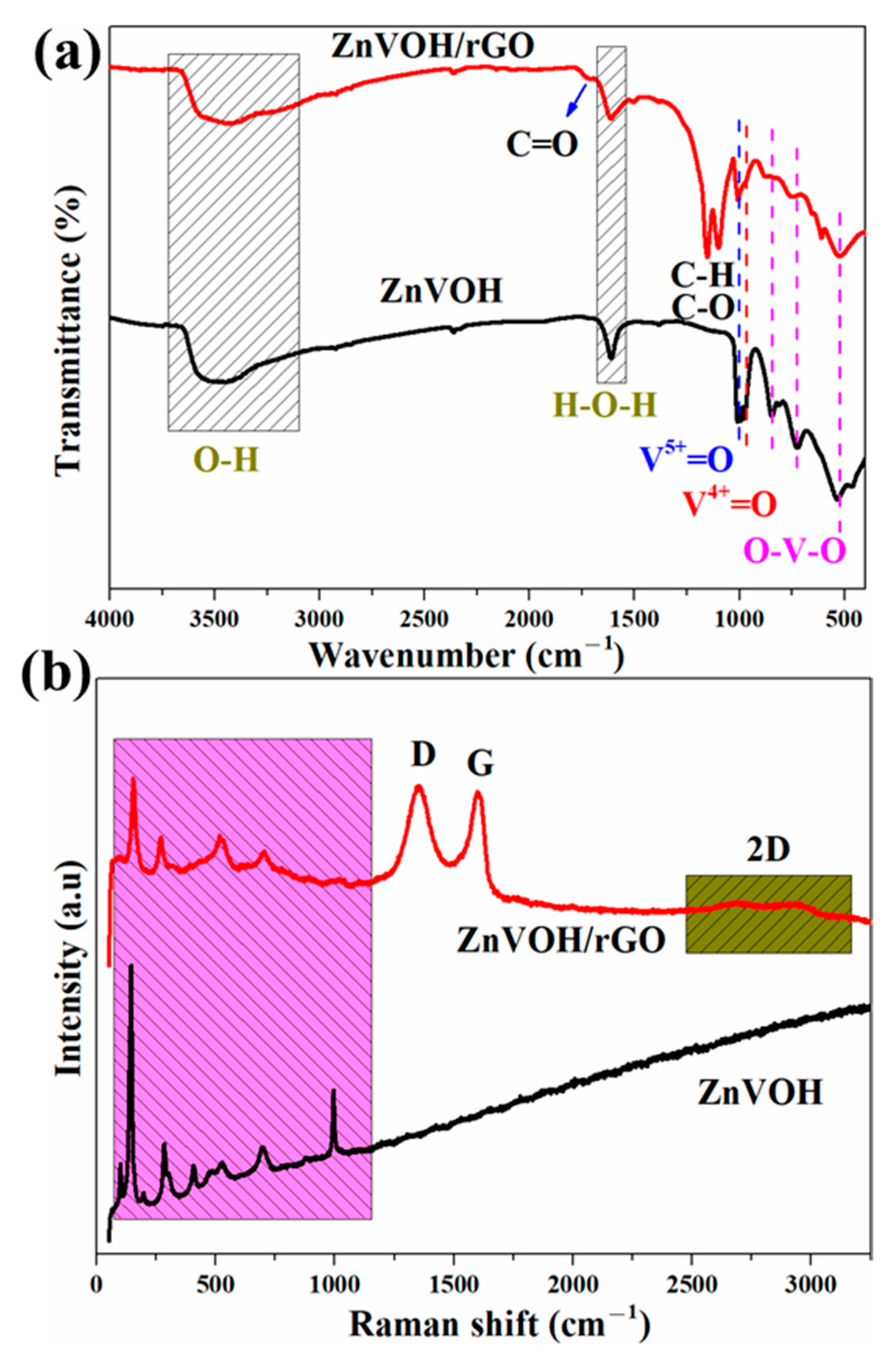
Figure 2a depicts FTIR spectra of ZnVOH and ZnVOH/rGO. The characteristic bands at 524, 728 and 836 cm−1 are assigned to the stretching vibrations of O-V-O. The bands at 995 and 1015 cm−1 are assigned to V4+ = O and V5+ = O, respectively [41]. Meanwhile, some emerged characteristic bands at 1095, 1147 and 1730 cm−1 are observed, which are attributed to the C-H, C-O and C = O bonds from rGO [42]. More structural details of ZnVOH and ZnVOH/rGO are provided by Raman spectra, as depicted in Figure 2b. The Raman peaks below 1000 cm−1 are assigned to (V2O2)n, V3-O, V-O-V and V = O from ZnVOH [43]. Figure 2b gives Raman spectra of ZnVOH and ZnVOH/rGO. In ZnVOH/rGO, two characteristic peaks at approximately 1340 and 1580 cm−1 observed, which are indexed to D-band and G-band, respectively. These two bands are related to defective carbon atoms (D-band) and ordered carbon atoms (G-band) [38]. The ratio of peak strength of ID/IG suggests the carbon defect density [38]. Materials with a larger ratio of ID/IG suggest a higher carbon defect density, and a better electrochemical performance [40]. The ID/IG value of ZnVOH/rGO is 1.02, which means that the composite has a relative higher carbon defect density. This is conducive to energy storage. Moreover, compared to the ZnVOH, the Raman spectrum of ZnVOH/rGO shows peaks at approximately 2700 cm−1, which is attributed to the second-order peak of the D peak (2D-band). This further demonstrates the existence of graphene layers [44]. The above results suggest the successful preparation of the ZnVOH/rGO composite.
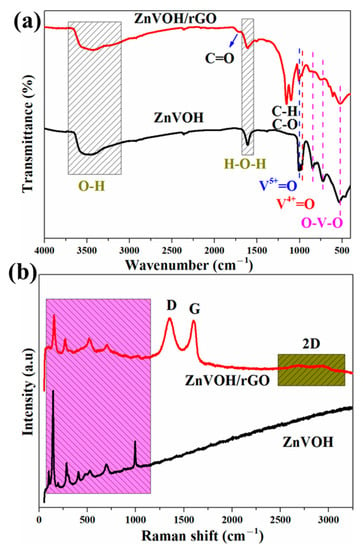
Figure 2.
FTIR (a) and Raman (b) spectra of ZnVOH and ZnVOH/rGO.
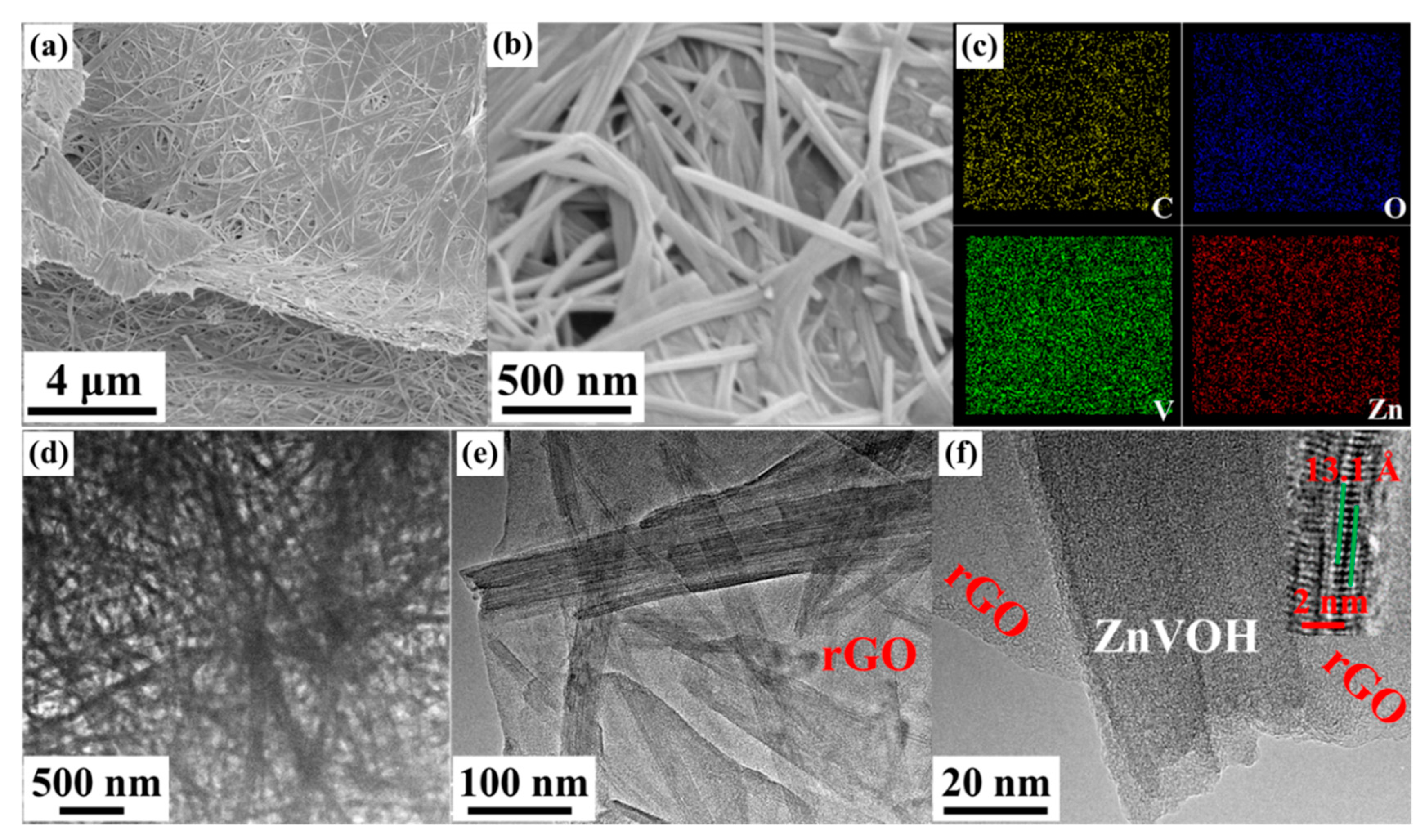
The morphologies of the samples were investigated by SEM and TEM. Figure 3 and Figure S1 show the morphologies of ZnVOH and ZnVOH/rGO, respectively. Both samples show uniform nanowires. As shown in Figure 3a,b, ZnVOH nanowires tightly stick to rGO sheets through chemical bonds or electrostatic attraction (ZnVOH nanowires were shown in Figure S1). The results suggest that ZnVOH nanowires and rGO sheets are dispersed well, endowing the electrical conductivity of ZnVOH nanowires. Furthermore, the element of ZnVOH/rGO is advocated by elemental mapping images, as shown in Figure 3c and Figure S2. The elements V, O, Zn and C are uniformly dispersed in ZnVOH/rGO, in line with the results of XRD, FTIR and Raman. We tested the quality of the ZnVOH/rGO before and after calcination, and the content of rGO was about 7.1%. Figure 3d–f represents the TEM images of ZnVOH/rGO, which also reveal that ZnVOH nanowires are densely anchored on rGO sheets, where the strong interaction with each other is formed. This architecture can effectively restrain the aggregation of rGO through π stacking interactions [45]. An HRTEM image inserted in Figure 3f reveals that the lattice distance of (001) plane is expanded to 13.1 Å, which is in line with XRD observation (Figure 1). All the above results confirm that ZnVOH/rGO with an expanded layer spacing is successfully synthesized. This feature not only facilitates fast kinetics and efficient ion-transfer, but also enhances structural stability and electrical conductivity [46].
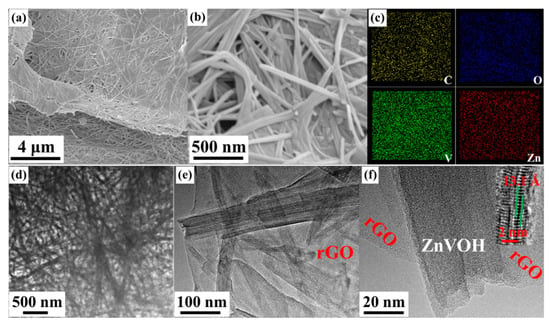
Figure 3.
Morphologies of ZnVOH/rGO: (a,b) SEM images; (c) elemental mapping images collected form Figure S2; (d–f) TEM images and a HRTEM image is inserted.
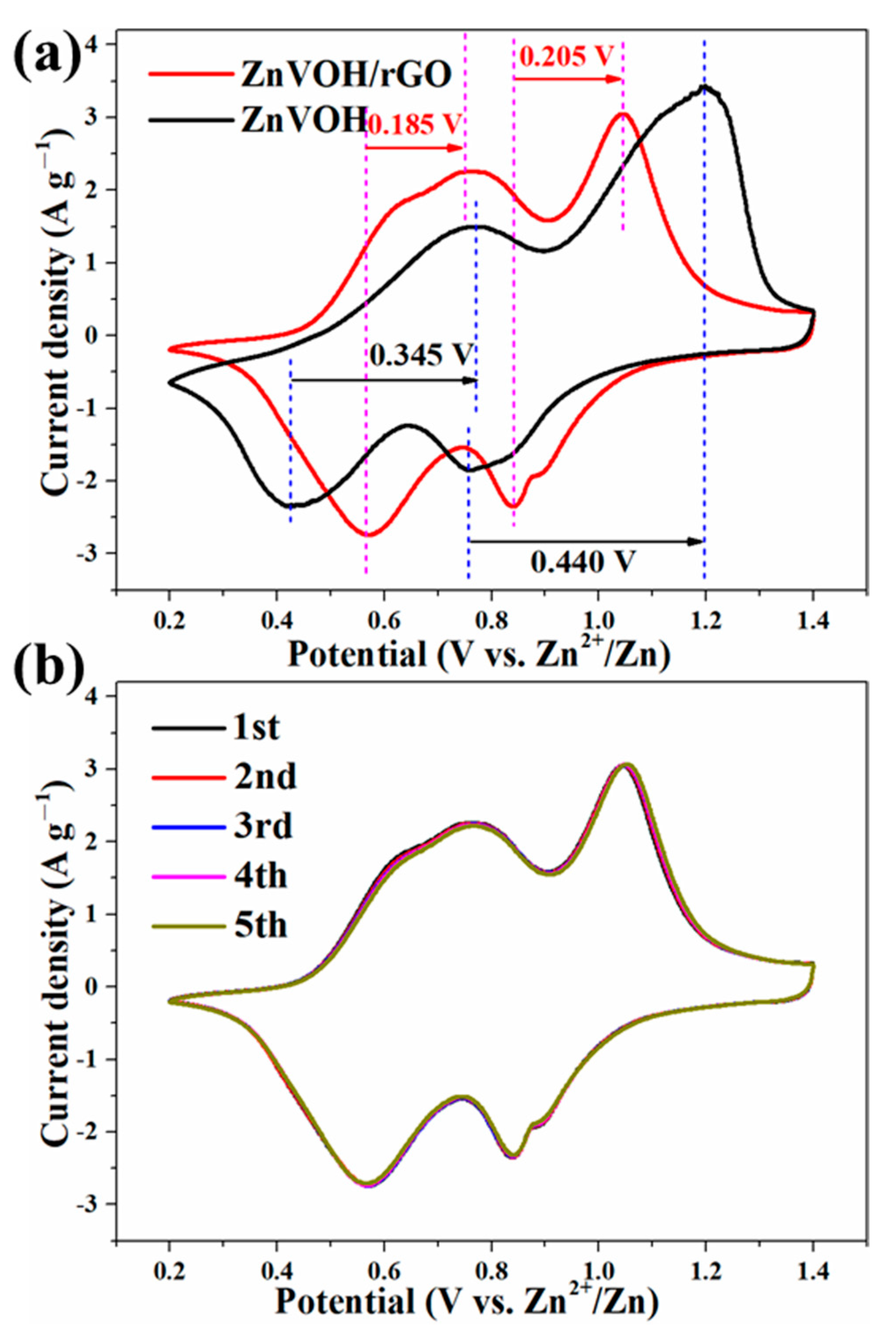
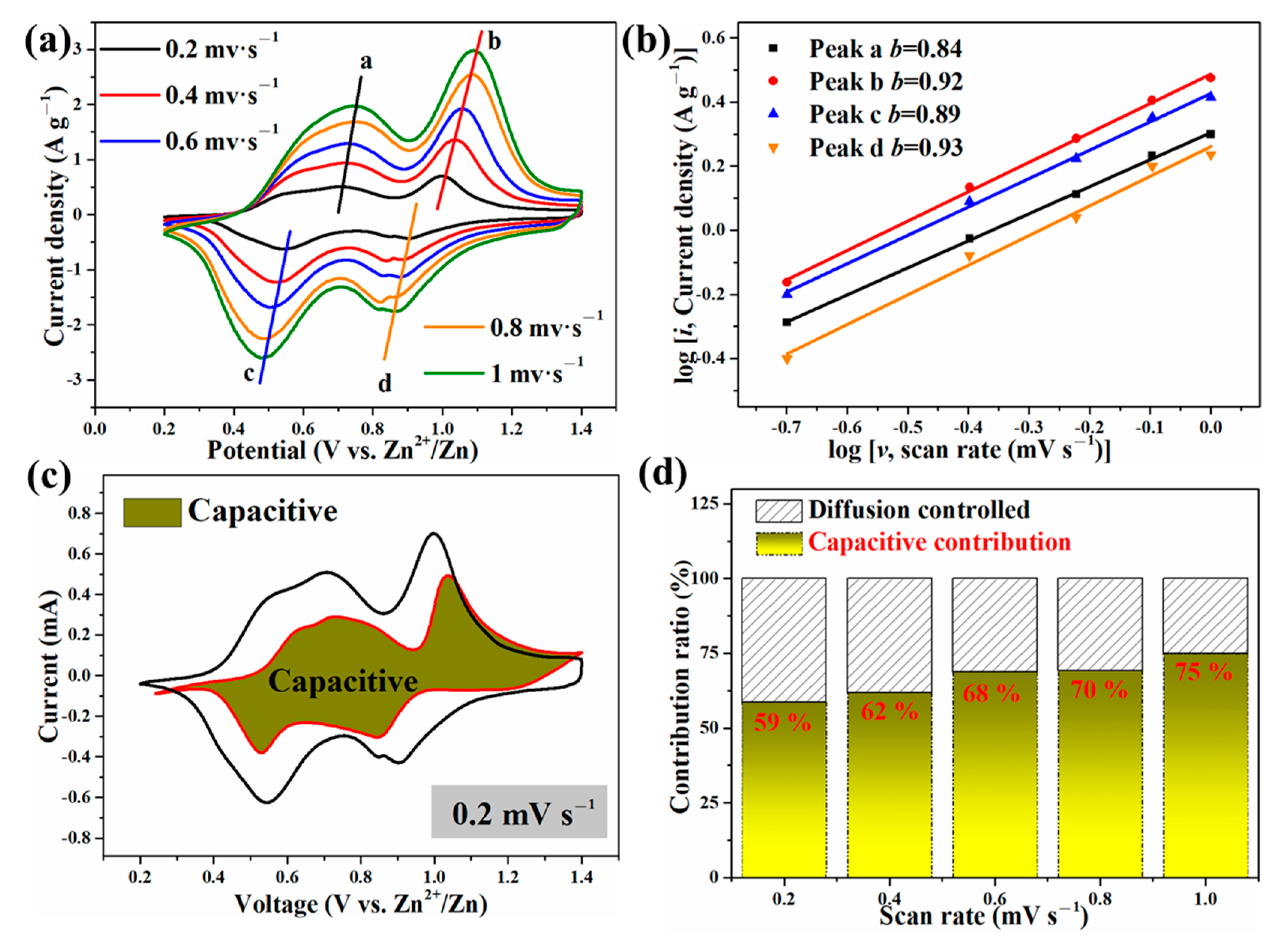
The Zn-ion storage performance of ZnVOH/rGO was tested (details in Supplementary Material). Figure 4a depicts CV curves of ZnVOH and ZnVOH/rGO at scan rate of 0.1 mV·s−1 in 0.2~1.4 V potential range. They have two pairs of redox peaks, and they are assigned to the reactions of V5+/V4+ and V4+/V3+, respectively. As for ZnVOH, these two pairs of redox peaks are located at 0.428/0.771 V and 0.760/1.201 V. However, they are shifted to 0.565/0.752 V and 0.843/1.045 V for ZnVOH/rGO. The potential gap of ZnVOH/rGO is much smaller than that of ZnVOH, revealing better redox reaction kinetics and faster ion diffusion leading to the smaller polarization. The above result suggests that the introduction of rGO is good for the redox reaction kinetics, and it also plays a role in promoting thermodynamics [47]. Figure 4b shows the CV curves of ZnVOH/rGO at the initial cycles. These curves are well overlapped, which indicates the excellently reversible Zn2+ (de)intercalation of ZnVOH/rGO. Furthermore, two couples of redox peaks in all CV curves (Figure 4) are indexed to ingress/egress of Zn2+, suggesting a multistep intercalation mechanism [29].
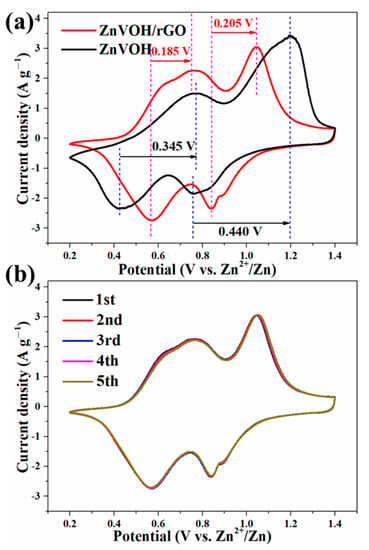
Figure 4.
(a) Comparative CV curves of ZnVOH and ZnVOH/rGO; (b) CV curves of ZnVOH/rGO at the first 5 cycles.
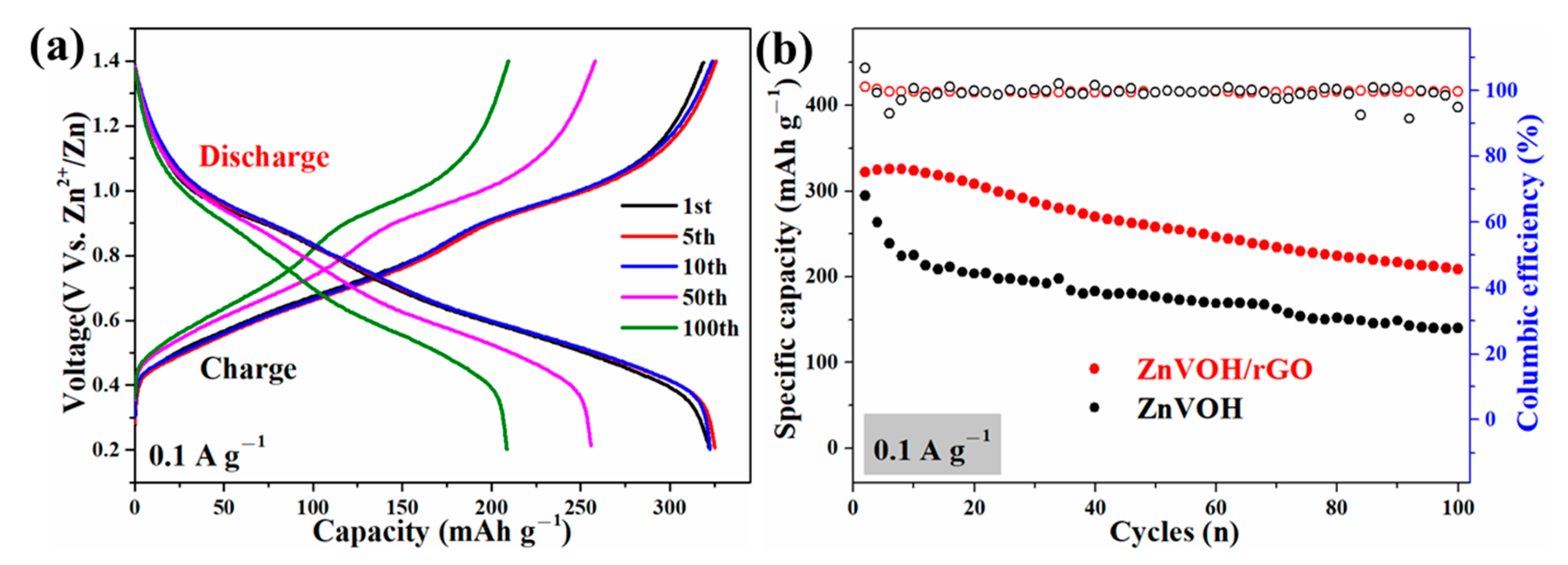
Figure 5 and Figure S3 represent GCD curves for different cycles and cycle performances of ZnVOH and ZnVOH/rGO at 0.1 and 4 A·g−1, respectively. There are two voltage plateaus in all GCD curves (Figure 5a and Figure S4a), which well coincide to the multistep intercalation process in CV curves. It is noted that in the ZnVOH/rGO composite we regard ZnVOH and rGO as a whole as the active material to calculate the electrochemical capacity. At 0.1 A·g−1 (Figure 5b), ZnVOH/rGO exhibits a specific capacity of 325 mAh·g−1 and this value still reach 210 mAh·g−1 after 100 cycles, whereas ZnVOH displays lower specific capacity and these two values of ZnVOH are 295 and 140 mAh·g−1. At 4 A·g−1 (Figure S4b), ZnVOH/rGO exhibits a capacity of 196 mAh·g−1 and it retains 161 mAh·g−1 after 1200 cycles, whereas ZnVOH exhibits a capacity of 145 and 126 mAh·g−1. Moreover, the coulombic efficiencies of ZnVOH/rGO are near 100%, which means that ZnVOH/rGO is more stable than ZnVOH (Figure 5b and Figure S4b). The electrochemical performance of the ZnVOH mixture electrode with the same amount of C (carbon black + C coming from rGO), and its electrochemical performance is far inferior to that of ZnVOH/rGO. The results show that the connection of rGO to ZnVOH is conducive to the rapid transfer of electrons [38].
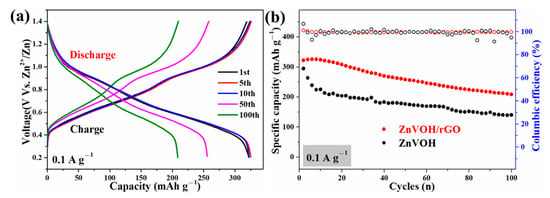
Figure 5.
(a) GCD curves of ZnVOH and ZnVOH/rGO; (b) cycle performance of ZnVOH and ZnVOH/rGO at 0.1 A·g−1.
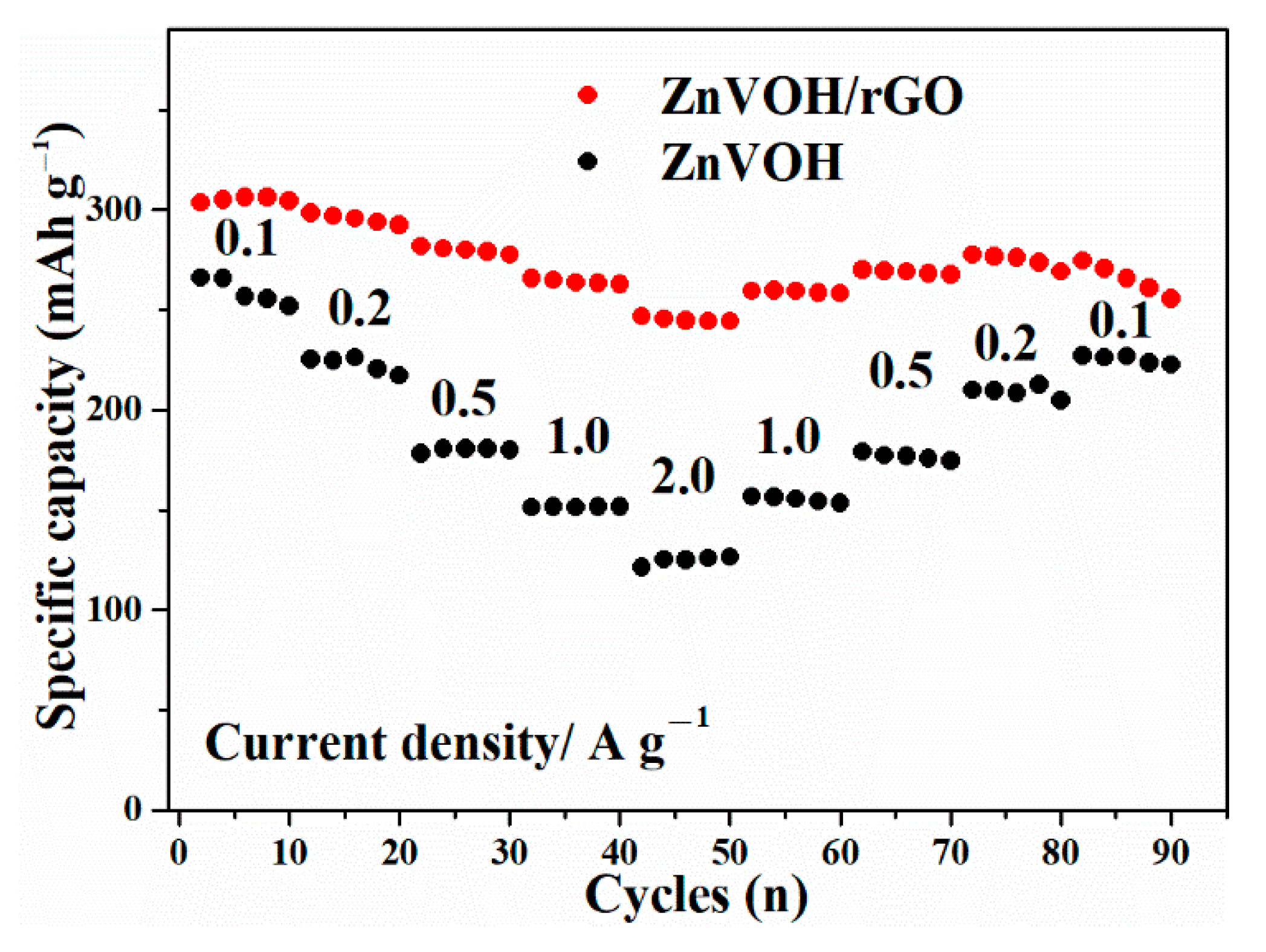
Figure 6 describes the rate performances of ZnVOH and ZnVOH/rGO at 0.1, 0.2, 0.5, 1 and 2 A·g−1 and then returns to 0.1 A·g−1. From the Figure 6, it is clearly seen that ZnVOH/rGO shows higher electrochemical properties compared to ZnVOH. In detail, at 0.1, 0.2, 0.5, 1 and 2 A·g−1, ZnVOH/rGO delivers capacities of 306, 298, 282, 265 and 246 mAh·g−1, respectively. This means, compared with the value at 0.1 A·g−1, the rate retention of ZnVOH/rGO is near 80%. For ZnVOH, this rate retention is approximately 48%. After the current density is back to 0.1 A·g−1, the capacities of ZnVOH/rGO and ZnVOH are 274 and 229 mAh·g−1, respectively, suggesting that these values return to 90% and 86% of the initial values. According to the mass of ZnVOH/rGO, the energy densities of Zn//ZnVOH/rGO cell are calculated to be 249 and 225 Wh·kg−1 at 0.1 and 2 A·g−1, respectively. The above findings reveal that ZnVOH/rGO exhibits much better electrochemical properties (specific capacity, rate and performances) than ZnVOH and VOH [38]. This shows that rGO enhances the rapid electron transport and boosts the electrical conductivity. Furthermore, the achieved performance of ZnVOH/rGO is superior to some recently reported cathodes for AZIBs, as listed in Table S1.
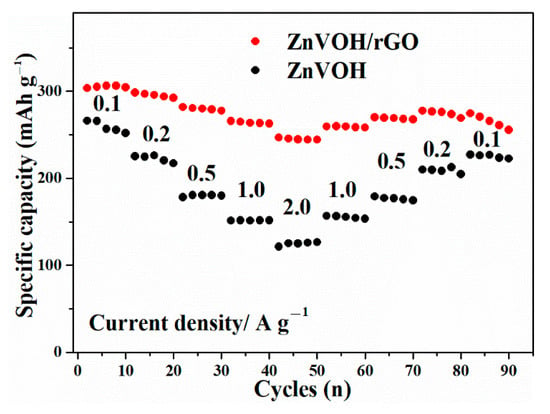
Figure 6.
The rate performances of ZnVOH and ZnVOH/rGO.
To better understand why the Zn//ZnVOH/rGO battery has good electrochemical properties, the electrochemical kinetics of Zn//ZnVOH/rGO cell was further studied by CV curves at the scan rage of 0.2 to 1.0 mV·s−1, as shown in Figure 7. The shapes of these shapes of curves are similar and the redox peaks display some shifts and become broader with the scan rate increasing (Figure 7a). The reason for such variation is owing to the polarization effect and can be used to study the detailed kinetic [48]. In general, the empirical power–law relationship between the peak current (i) and scanning rates (v) can be depicted in Equation (1):
i = aνb
log(i) = log(a) + blog(υ)
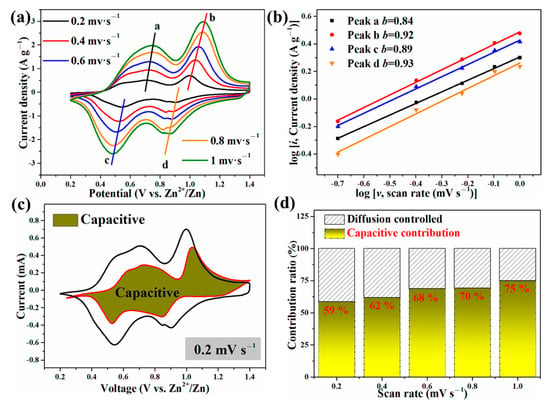
Figure 7.
The electrochemical kinetics of Zn//ZnVOH/rGO battery: (a) CV curves at 0.2~1 mV·s−1, (b) The relationship between log (sweep rate) and log (peak current); (c) A typical CV curve with capacitive contribution; (d) capacitive contributions at current densities.
In these two equations [49,50], a and b are parameters. b often ranges from 0.5 (diffusion behavior) to 1 (capacitive behavior) and is related to the charge storage mechanism. To obtain b values, the linear relation between log (i) and log (v) is calculated by Equation (2), as represented in Figure 7b. The b values of these four peaks are fitted to be 0.84, 0.92, 0.89 and 0.93, respectively. These b values are near 1, revealing that the capacitive process dominates [22].
The current (i = aυb) can be further separated into two parts. The ratio of capacitive behavior is acquired by Equations (3) and (4):
i = k1υ + k2υ1/2
i/υ1/2 = k1υ1/2 + k2
In these two equations, k1υ represents the capacitive behavior, and k2υ1/2 means the diffusion-controlled behavior. The contribution of capacitive behavior is quantitatively reflected by current response at typical voltages, and the representative result at 0.2 mV s−1 is shown in Figure 7c. The contribution rates of capacitive behavior are measured to be 59%, 62%, 68%, 70% and 75% at 0.2, 0.4, 0.6, 0.8 and 1 mV·s−1, respectively. This also reveals the major role of capacitive behavior (Figure 7d).
3. Materials and Methods
All materials are presented in the Supplementary Materials and were used as received. The GO solution was synthesized by the reported improved Hummer’s method [24]. To synthesize ZnVOH/rGO, 1 mmol V2O5 was dispersed into 36 mL H2O under vigorous magnetic stirring at room temperature. Then, 1 mL of H2O2 (30 wt.%) was slowly dropped into the above solution to obtain a clear orange-red solution. After that, 20 mmol ZnSO4 and 3.6 mL GO solution (10%) were added, in order. The obtained suspension was further vigorously stirred for 0.5 h. Last, the mixed suspension was sealed in a 50 mL Teflon-lined stainless-steel autoclave and maintained at 120 °C for 6 h. After reaction, it was cooled to the room temperature naturally, and dark-green precipitates were obtained by suction filtration and washed with H2O and freeze-dried for 48 h. For comparison, ZnVOH was prepared without GO solution.
The composition and structure of ZnVOH/rGO were characterized by X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), Raman spectroscopy, Energy-dispersive X-ray spectrometer (EDS) elemental mapping and inductive coupled plasma emission spectrometer (ICP). Morphology of ZnVOH/rGO was characterized by scanning/transmission electron microscopy (SEM/TEM). The details are represented in the Supplementary Materials.
The cathode was prepared by mixing 80 wt% ZnVOH/rGO, 10 wt% carbon black and 10 wt% polyvinylidene with the N-methyl-2-pyrrolidone as solvent. The slurry was coated onto the above slurry on a clean circular Ti foil (12 mm diameter). After the cathode was dried at 60 ℃ overnight, the 2032-typed AZIB were assembled with glass fiber as the separator, a circular Zn plate of the same dimensions as the anode and 3 mol L−1 [Zn(CF3SO3)2] as the electrolyte. The mass loading of the ZnVOH/rGO is about 2 mg. The electrochemical properties of Zn//ZnVOH/rGO batteries were characterized by cyclic voltammetry (CV), electrochemical impedance spectroscopy (EIS), galvanostatic/intermittent titration technique (GITT) and galvanostatic charge–discharge (GCD) in a potential range of 0.2~1.4 V (vs. Zn2+/Zn).
4. Conclusions
In summary, ZnVOH/rGO was successfully prepared by a facile hydrothermal route. The recombination with rGO can boost the Zn2+ storage of Zn2+-pre-intercalated VOH. ZnVOH/rGO exhibits much higher specific capacity, better cycle stability and rate performance than ZnVOH. The achieved performances of ZnVOH/rGO even exceed that of some state-of-the-art cathodes for AZIBs. This is mainly due to: (a) Zn2+ in a “pillar” structure expanding interlayer distance of VOH enhances efficient Zn2+ migration, (b) rGO improves the fast electron flow and (c) both materials stabilize the structure.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27175387/s1, Materials, materials characterizations and electrochemical characterizations. Figure S1: SEM images of ZnVOH. Figure S2: A SEM image of ZnVOH/rGO for collecting elemental mapping images. Figure S3: (a) Cycle performance and (b) GCD curves at 4 A·g−1 of ZnVOH and ZnVOH/rGO. Table S1: Atomic ratio of Zn and V in ZnVOH by ICP analysis. Table S2: Atomic ratio of Zn and V in ZnVOH/rGO by ICP analysis. Table S3: Comparison of the specific capacities between the previously reported cathode materials for AZIBs and this work [51,52,53,54,55,56,57,58,59,60,61,62].
Author Contributions
Conceptualization, M.H. and Y.Z.; methodology, Y.F.; software, Y.F.; validation, Y.F., X.Y., Z.F. and Y.Z.; formal analysis, Y.F., X.Y. and Z.F.; investigation, Y.F. and X.Y.; resources; data curation, Y.F. and X.Y.; writing—original draft preparation, Y.F.; writing—review and editing, M.H. and Y.Z.; visualization, Y.F.; supervision, M.H. and Y.Z.; project administration, Y.Z.; funding acquisition, Y.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No information.
Acknowledgments
This work was supported by the Large Instrument and Equipment Open Foundation of Dalian University of Technology.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
No information.
References
- Zhang, N.; Chen, X.; Yu, M.; Niu, Z.; Cheng, F.; Chen, J. Materials chemistry for rechargeable zinc-ion batteries. Chem. Soc. Rev. 2020, 49, 4203–4219. [Google Scholar] [CrossRef]
- Chen, X.; Wang, P.; Feng, Z.; Meng, C.; Zhang, Y. Conductive polymer intercalated vanadium oxide on carbon cloth for fast ammonium-ion storage in supercapacitor applications. Chem. Eng. J. 2022, 445, 136747. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, Y.; Jiang, H.; Dong, X.; Meng, C. Ammonium vanadium oxide framework with stable NH4+ aqueous storage for flexible quasi-solid-state supercapacitor. Chem. Eng. J. 2022, 427, 131548. [Google Scholar] [CrossRef]
- Yang, D.; Tan, H.; Rui, X.; Yu, Y. Electrode Materials for Rechargeable Zinc-Ion and Zinc-Air Batteries: Current Status and Future Perspectives. Electrochem. Energy Rev. 2019, 2, 395–427. [Google Scholar] [CrossRef]
- Feng, Z.; Sun, J.; Liu, Y.; Jiang, H.; Cui, M.; Hu, T.; Meng, C.; Zhang, Y. Engineering Interlayer Space of Vanadium Oxide by Pyridinesulfonic Acid-Assisted Intercalation of Polypyrrole Enables Enhanced Aqueous Zinc-Ion Storage. ACS Appl. Mater. Interfaces 2021, 13, 61154–61165. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, Y.; Jiang, H.; Sun, J.; Feng, Z.; Hu, T.; Meng, C.; Pan, Z. Synergistic engineering of oxygen-defect and heterojunction boosts Zn2+ (De)intercalation kinetics in vanadium oxide for high-performance zinc-ion batteries. Chem. Eng. J. 2022, 435, 134949. [Google Scholar] [CrossRef]
- Feng, Z.; Sun, J.; Liu, Y.; Jiang, H.; Hu, T.; Cui, M.; Tian, F.; Meng, C.; Zhang, Y. Polypyrrole-intercalation tuning lamellar structure of V2O5·nH2O boosts fast zinc-ion kinetics for aqueous zinc-ion battery. J. Power Sources 2022, 536, 231489. [Google Scholar] [CrossRef]
- Chao, D.; Zhou, W.; Xie, F.; Ye, C.; Li, H.; Jaroniec, M.; Qiao, S.-Z. Roadmap for advanced aqueous batteries: From design of materials to applications. Sci. Adv. 2020, 6, eaba4098. [Google Scholar] [CrossRef]
- Tie, Z.; Niu, Z. Design Strategies for High-Performance Aqueous Zn/Organic Batteries. Angew. Chem. Int. Ed. 2020, 59, 21293–21303. [Google Scholar] [CrossRef]
- Liu, S.; Zhu, H.; Zhang, B.; Li, G.; Zhu, H.; Ren, Y.; Geng, H.; Yang, Y.; Liu, Q.; Li, C.C. Tuning the Kinetics of Zinc-Ion Insertion/Extraction in V2O5 by In Situ Polyaniline Intercalation Enables Improved Aqueous Zinc-Ion Storage Performance. Adv. Mater. 2020, 32, 2001113. [Google Scholar] [CrossRef]
- Chen, S.; Li, K.; Hui, K.S.; Zhang, J. Regulation of Lamellar Structure of Vanadium Oxide via Polyaniline Intercalation for High-Performance Aqueous Zinc-Ion Battery. Adv. Funct. Mater. 2020, 30, 2003890. [Google Scholar] [CrossRef]
- Wan, F.; Zhou, X.; Lu, Y.; Niu, Z.; Chen, J. Energy Storage Chemistry in Aqueous Zinc Metal Batteries. ACS Energy Lett. 2020, 5, 3569–3590. [Google Scholar] [CrossRef]
- Xu, S.; Sun, M.; Wang, Q.; Wang, C. Recent progress in organic electrodes for zinc-ion batteries. J. Semicond. 2020, 41, 091704. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, Y.; Shi, L.; Wang, K.; Wang, B.; Li, L.; Ma, Y.; Li, Y.; Sun, Z.; Ali, W.; et al. An Overview and Future Perspectives of Rechargeable Zinc Batteries. Small 2020, 16, 2000730. [Google Scholar] [CrossRef] [PubMed]
- Xue, T.; Fan, H.J. From aqueous Zn-ion battery to Zn-MnO2 flow battery: A brief story. J. Energy Chem. 2021, 54, 194–201. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, Y.; Xu, L.; Gao, Z.; Zheng, J.; Wang, Q.; Meng, C.; Wang, J. Fabrication of (NH4)2V3O8 nanoparticles encapsulated in amorphous carbon for high capacity electrodes in aqueous zinc ion batteries. Chem. Eng. J. 2020, 382, 122844. [Google Scholar] [CrossRef]
- Liu, S.; Kang, L.; Kim, J.M.; Chun, Y.T.; Zhang, J.; Jun, S.C. Recent Advances in Vanadium-Based Aqueous Rechargeable Zinc-Ion Batteries. Adv. Energy Mater. 2020, 10, 2000477. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, Y.; Liu, Y.; Yang, J.; Xu, L.; Wang, P.; Gao, Z.; Zheng, J.; Meng, C.; Pan, Z. In situ grown 2D hydrated ammonium vanadate nanosheets on carbon cloth as a free-standing cathode for high-performance rechargeable Zn-ion batteries. J. Mater. Chem. A 2020, 8, 15130–15139. [Google Scholar] [CrossRef]
- Feng, Z.; Zhang, Y.; Zhao, Y.; Sun, J.; Liu, Y.; Jiang, H.; Cui, M.; Hu, T.; Meng, C. Dual intercalation of inorganics–organics for synergistically tuning the layer spacing of V2O5·nH2O to boost Zn2+ storage for aqueous zinc-ion batteries. Nanoscale 2022, 14, 8776–8788. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, L.; Jiang, H.; Liu, Y.; Meng, C. Polyaniline-expanded the Interlayer Spacing of Hydrated Vanadium Pentoxide by the Interface-intercalation for Aqueous Rechargeable Zn-ion Batteries. J. Colloid Interface Sci. 2021, 603, 641–650. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, Y.; Zheng, J.; Jiang, H.; Hu, T.; Meng, C. Ammonium ion intercalated hydrated vanadium pentoxide for advanced aqueous rechargeable Zn-ion batteries. Mater. Today Energy 2020, 18, 100509. [Google Scholar] [CrossRef]
- Yan, M.; He, P.; Chen, Y.; Wang, S.; Wei, Q.; Zhao, K.; Xu, X.; An, Q.; Shuang, Y.; Shao, Y.; et al. Water-Lubricated Intercalation in V2O5·nH2O for High-Capacity and High-Rate Aqueous Rechargeable Zinc Batteries. Adv. Mater. 2018, 30, 1703725. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Rui, X.; Zhang, Q.; Geng, H.; Gan, L.; Zhang, W.; Li, C.; Huang, S.; Yu, Y. Persistent zinc-ion storage in mass-produced V2O5 architectures. Nano Energy 2019, 60, 171–178. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, Y.; Liu, Y.; Jiang, H.; Dong, X.; Hu, T.; Meng, C. Hydrated vanadium pentoxide/reduced graphene oxide-polyvinyl alcohol (V2O5⋅nH2O/rGO-PVA) film as a binder-free electrode for solid-state Zn-ion batteries. J. Colloid Interface Sci. 2021, 587, 845–854. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, A.; Sun, J. Promise and challenge of vanadium-based cathodes for aqueous zinc-ion batteries. J. Energy Chem. 2021, 54, 655–667. [Google Scholar] [CrossRef]
- Xu, X.; Xiong, F.; Meng, J.; Wang, X.; Niu, C.; An, Q.; Mai, L. Vanadium-Based Nanomaterials: A Promising Family for Emerging Metal-Ion Batteries. Adv. Funct. Mater. 2020, 30, 1904398. [Google Scholar] [CrossRef]
- Ming, F.; Liang, H.; Lei, Y.; Kandambeth, S.; Eddaoudi, M.; Alshareef, H.N. Layered MgxV2O5·nH2O as Cathode Material for High-Performance Aqueous Zinc Ion Batteries. ACS Energy Lett. 2018, 3, 2602–2609. [Google Scholar] [CrossRef] [Green Version]
- Kundu, D.; Adams, B.D.; Duffort, V.; Vajargah, S.H.; Nazar, L.F. A high-capacity and long-life aqueous rechargeable zinc battery using a metal oxide intercalation cathode. Nat. Energy 2016, 1, 16119. [Google Scholar] [CrossRef]
- Xia, C.; Guo, J.; Li, P.; Zhang, X.; Alshareef, H.N. Highly Stable Aqueous Zinc-Ion Storage Using a Layered Calcium Vanadium Oxide Bronze Cathode. Angew. Chem. Int. Ed. 2018, 57, 3943–3948. [Google Scholar] [CrossRef]
- Yang, Y.; Tang, Y.; Liang, S.; Wu, Z.; Fang, G.; Cao, X.; Wang, C.; Lin, T.; Pan, A.; Zhou, J. Transition metal ion-preintercalated V2O5 as high-performance aqueous zinc-ion battery cathode with broad temperature adaptability. Nano Energy 2019, 61, 617–625. [Google Scholar] [CrossRef]
- Yang, Y.; Tang, Y.; Fang, G.; Shan, L.; Guo, J.; Zhang, W.; Wang, C.; Wang, L.; Zhou, J.; Liang, S. Li+ intercalated V2O5·nH2O with enlarged layer spacing and fast ion diffusion as an aqueous zinc-ion battery cathode. Energy Environ. Sci. 2018, 11, 3157–3162. [Google Scholar] [CrossRef]
- Wu, T.; Zhu, K.; Qin, C.; Huang, K. Unraveling the role of structural water in bilayer V2O5 during Zn2+-intercalation: Insights from DFT calculations. J. Mater. Chem. A 2019, 7, 5612–5620. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, J.; Zhang, L.; Li, J.; Wang, W.; Yang, Z.; Zhang, L.; Wang, Y.; Chen, J.; Huang, Y.; et al. Graphene-like Vanadium Oxygen Hydrate (VOH) Nanosheets Intercalated and Exfoliated by Polyaniline (PANI) for Aqueous Zinc-Ion Batteries (ZIBs). ACS Appl. Mater. Interfaces 2020, 12, 31564–31574. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhao, Y.; Liu, Y.; Jiang, H.; Huang, C.; Cui, M.; Hu, T.; Meng, C.; Zhang, Y. “Three-in-One” Strategy that Ensures V2O5·nH2O with Superior Zn2+ Storage by Simultaneous Protonated Polyaniline Intercalation and Encapsulation. Small Struct. 2022, 3, 2100212. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, Z.; Tian, D.; Hu, T.; Jiang, H.; Yang, J.; Sun, J.; Zheng, J.; Meng, C.; Zhang, Y. Employing “one for two” strategy to design polyaniline-intercalated hydrated vanadium oxide with expanded interlayer spacing for high-performance aqueous zinc-ion batteries. Chem. Eng. J. 2020, 399, 125842. [Google Scholar] [CrossRef]
- Mathew, V.; Sambandam, B.; Kim, S.; Kim, S.; Park, S.; Lee, S.; Alfaruqi, M.H.; Soundharrajan, V.; Islam, S.; Putro, D.Y.; et al. Manganese and Vanadium Oxide Cathodes for Aqueous Rechargeable Zinc-Ion Batteries: A Focused View on Performance, Mechanism, and Developments. ACS Energy Lett. 2020, 5, 2376–2400. [Google Scholar] [CrossRef]
- Pang, Q.; Sun, C.; Yu, Y.; Zhao, K.; Zhang, Z.; Voyles, P.M.; Chen, G.; Wei, Y.; Wang, X. H2V3O8 Nanowire/Graphene Electrodes for Aqueous Rechargeable Zinc Ion Batteries with High Rate Capability and Large Capacity. Adv. Energy Mater. 2018, 8, 1800144. [Google Scholar] [CrossRef]
- Hu, T.; Feng, Z.; Zhang, Y.; Liu, Y.; Sun, J.; Zheng, J.; Jiang, H.; Wang, P.; Dong, X.; Meng, C. “Double guarantee mechanism” of Ca2+-intercalation and rGO-integration ensures hydrated vanadium oxide with high performance for aqueous zinc-ion batteries. Inorg. Chem. Front. 2021, 8, 79–89. [Google Scholar] [CrossRef]
- Liu, C.; Neale, Z.; Zheng, J.; Jia, X.; Huang, J.; Yan, M.; Tian, M.; Wang, M.; Yang, J.; Cao, G. Expanded hydrated vanadate for high-performance aqueous zinc-ion batteries. Energy Environ. Sci. 2019, 12, 2273–2285. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Wang, S.; Zhou, F.; Das, P.; Sun, C.; Zheng, S.; Wu, Z.-S. 2D Amorphous V2O5/Graphene Heterostructures for High-Safety Aqueous Zn-Ion Batteries with Unprecedented Capacity and Ultrahigh Rate Capability. Adv. Energy Mater. 2020, 10, 2000081. [Google Scholar] [CrossRef]
- Wei, T.; Li, Q.; Yang, G.; Wang, C. Highly reversible and long-life cycling aqueous zinc-ion battery based on ultrathin (NH4)2V10O25·8H2O nanobelts. J. Mater. Chem. A 2018, 6, 20402–20410. [Google Scholar] [CrossRef]
- Sun, J.; Liu, Y.; Jiang, H.; Dong, X.; Hu, T.; Meng, C.; Zhang, Y. Mn2+ as the “spearhead” preventing the trap of Zn2+ in layered Mn2+ inserted hydrated vanadium pentoxide enables high rate capacity. J. Colloid Interface Sci. 2021, 602, 14–22. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Zhang, G.; Liao, X.; Yan, M.; Xu, X.; An, Q.; Liu, J.; Mai, L. Sodium Ion Stabilized Vanadium Oxide Nanowire Cathode for High-Performance Zinc-Ion Batteries. Adv. Energy Mater. 2018, 8, 1702463. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman Spectrum of Graphene and Graphene Layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khazaeli, A.; Godbille-Cardona, G.; Barz, D.P.J. A Novel Flexible Hybrid Battery–Supercapacitor Based on a Self-Assembled Vanadium-Graphene Hydrogel. Adv. Funct. Mater. 2020, 30, 1910738. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, M.; Hu, T.; Meng, C. 3D Interlaced Networks of VO(OH)2 Nanoflakes Wrapped with Graphene Oxide Nanosheets as Electrodes for Energy Storage Devices. ACS Appl. Nano Mater. 2019, 2, 2934–2945. [Google Scholar] [CrossRef]
- Zheng, J.; Liu, C.; Tian, M.; Jia, X.; Jahrman, E.P.; Seidler, G.T.; Zhang, S.; Liu, Y.; Zhang, Y.; Meng, C.; et al. Fast and reversible zinc ion intercalation in Al-ion modified hydrated vanadate. Nano Energy 2020, 70, 104519. [Google Scholar] [CrossRef]
- Islam, S.; Alfaruqi, M.H.; Putro, D.Y.; Soundharrajan, V.; Sambandam, B.; Jo, J.; Park, S.; Lee, S.; Mathew, V.; Kim, J. K+ intercalated V2O5 nanorods with exposed facets as advanced cathodes for high energy and high rate zinc-ion batteries. J. Mater. Chem. A 2019, 7, 20335–20347. [Google Scholar] [CrossRef]
- Zhang, N.; Jia, M.; Dong, Y.; Wang, Y.; Xu, J.; Liu, Y.; Jiao, L.; Cheng, F. Hydrated Layered Vanadium Oxide as a Highly Reversible Cathode for Rechargeable Aqueous Zinc Batteries. Adv. Funct. Mater. 2019, 29, 1807331. [Google Scholar] [CrossRef]
- Yong, B.; Ma, D.; Wang, Y.; Mi, H.; He, C.; Zhang, P. Understanding the Design Principles of Advanced Aqueous Zinc-Ion Battery Cathodes: From Transport Kinetics to Structural Engineering, and Future Perspectives. Adv. Energy Mater. 2020, 10, 2002354. [Google Scholar] [CrossRef]
- Wei, T.; Li, Q.; Yang, G.; Wang, C. An electrochemically induced bilayered structure facilitates long-life zinc storage of vanadium dioxide. J. Mater. Chem. A 2018, 6, 8006–8012. [Google Scholar] [CrossRef]
- Dai, X.; Wan, F.; Zhang, L.; Cao, H.; Niu, Z. Freestanding graphene/VO2 composite films for highly stable aqueous Zn-ion batteries with superior rate performance. Energy Storage Mater. 2018, 17, 143–150. [Google Scholar] [CrossRef]
- Chen, X.; Wang, L.; Li, H.; Cheng, F.; Chen, J. Porous V2O5 nanofibers as cathode materials for rechargeable aqueous zinc-ion batteries. J. Energy Chem. 2019, 38, 20–25. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Xi, B.; Ma, X.; Feng, Z.; Jia, Y.; Feng, J.; Qian, Y.; Xiong, S. Boosting Zinc-Ion Storage Capability by Effectively Suppressing Vanadium Dissolution Based on Robust Layered Barium Vanadate. Nano Lett. 2020, 20, 2899–2906. [Google Scholar] [CrossRef] [PubMed]
- Lan, B.; Tang, C.; Chen, L.; Zhang, W.; Tang, W.; Zuo, C.; Fu, X.; Dong, S.; An, Q.; Luo, P. FeVO4⋅nH2O@rGO nanocomposite as high performance cathode materials for aqueous Zn-ion batteries. Journal of Alloys and Compounds 2020, 818, 153372. [Google Scholar] [CrossRef]
- Chen, L.; Yang, Z.; Wu, J.; Chen, H.; Meng, J. Energy storage performance and mechanism of the novel copper pyrovanadate Cu3V2O7(OH)2·2H2O cathode for aqueous zinc ion batteries. Electrochimica Acta 2019, 330, 135347. [Google Scholar] [CrossRef]
- Li, S.; Chen, M.; Fang, G.; Shan, L.; Cao, X.; Huang, J.; Liang, S.; Zhou, J. Synthesis of polycrystalline K0.25V2O5 nanoparticles as cathode for aqueous zinc-ion battery. J. Alloys Compd. 2019, 801, 82–89. [Google Scholar] [CrossRef]
- Alfaruqi, M.H.; Mathew, V.; Song, J.; Kim, S.; Islam, S.; Pham, D.T.; Jo, J.; Kim, S.; Baboo, J.P.; Xiu, Z.; et al. Electrochemical Zinc Intercalation in Lithium Vanadium Oxide: A High-Capacity Zinc-Ion Battery Cathode. Chem. Mater. 2017, 29, 1684–1694. [Google Scholar] [CrossRef]
- Cai, Y.; Liu, F.; Luo, Z.; Fang, G.; Zhou, J.; Pan, A.; Liang, S. Pilotaxitic Na1.1V3O7.9 nanoribbons/graphene as high-performance sodium ion battery and aqueous zinc ion battery cathode. Energy Storage Mater. 2018, 13, 168–174. [Google Scholar] [CrossRef]
- Guo, X.; Fang, G.; Zhang, W.; Zhou, J.; Shan, L.; Wang, L.; Wang, C.; Lin, T.; Tang, Y.; Liang, S. Mechanistic Insights of Zn2+ Storage in Sodium Vanadates. Adv. Energy Mater. 2018, 8, 1801819. [Google Scholar] [CrossRef]
- Xia, C.; Guo, J.; Lei, Y.; Liang, H.; Zhao, C.; Alshareef, H.N. Rechargeable Aqueous Zinc-Ion Battery Based on Porous Framework Zinc Pyrovanadate Intercalation Cathode. Adv. Mater. 2020, 32, e1907798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chao, D.; Zhu, C.; Song, M.; Liang, P.; Zhang, X.; Tiep, N.H.; Zhao, H.; Wang, J.; Wang, R.; Zhang, H.; et al. A High-Rate and Stable Quasi-Solid-State Zinc-Ion Battery with Novel 2D Layered Zinc Orthovanadate Array. Adv. Mater. 2018, 30, e1803181. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).