Multivalent Pyrrolidine Iminosugars: Synthesis and Biological Relevance
Abstract
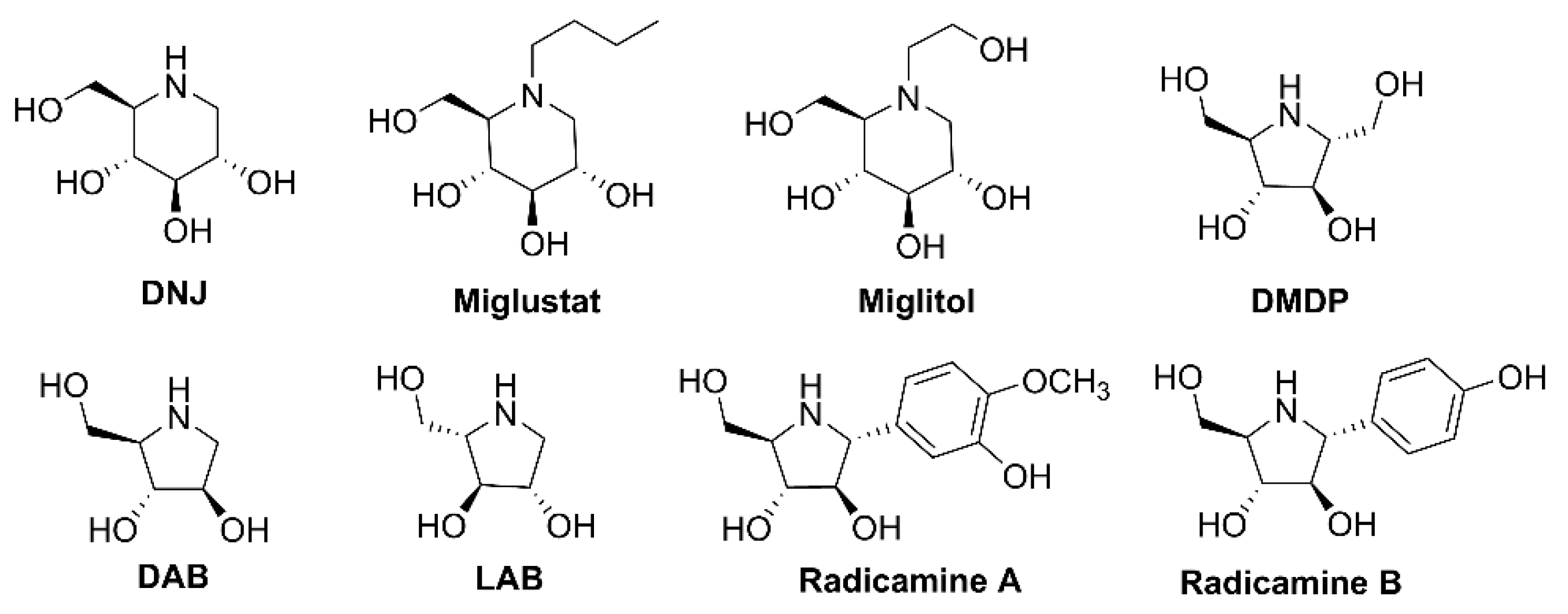
:1. Introduction
2. Syntheses of Multivalent Pyrrolidine Iminosugars
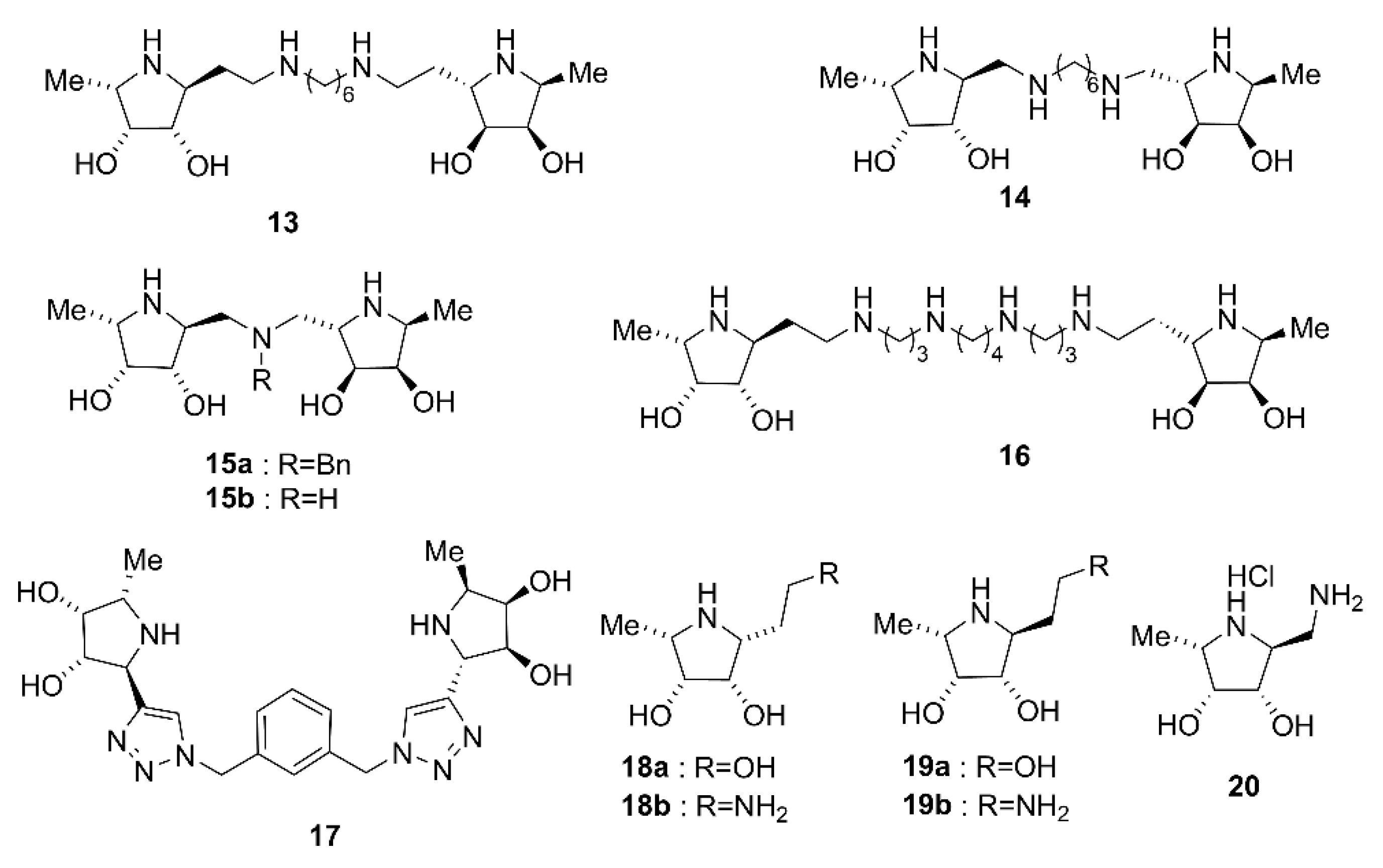
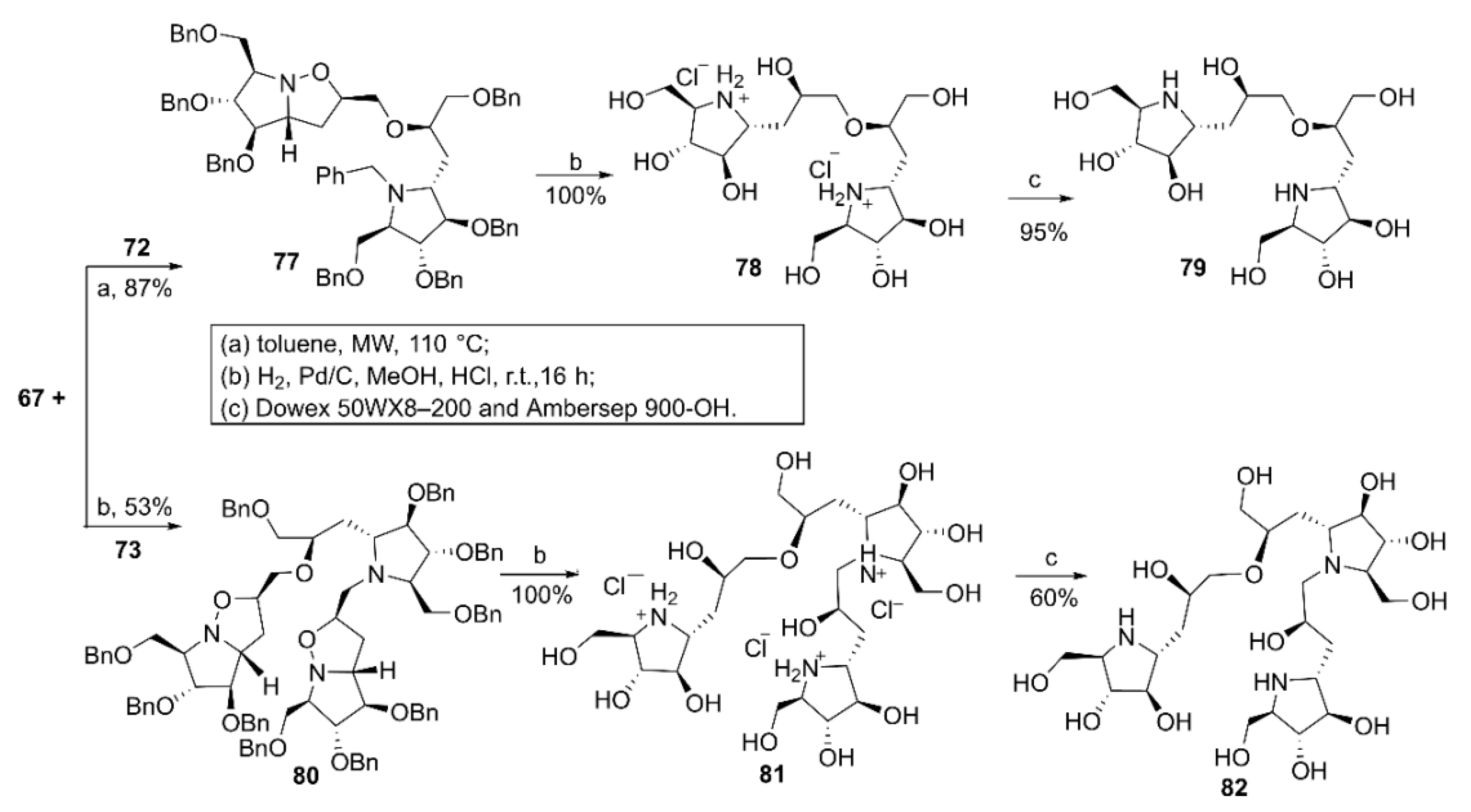
2.1. Synthesis of Di- and Trivalent Iminosugars
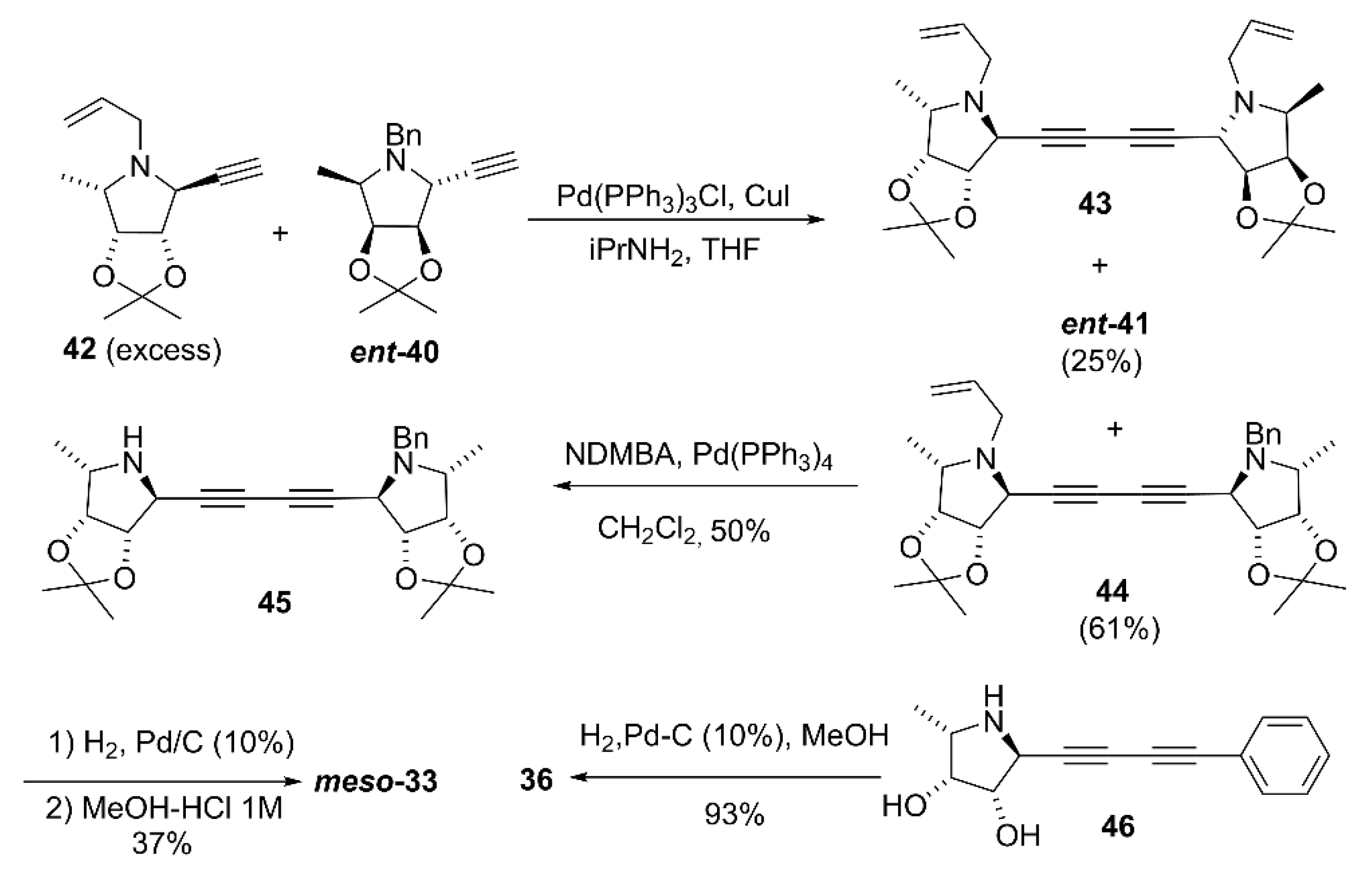
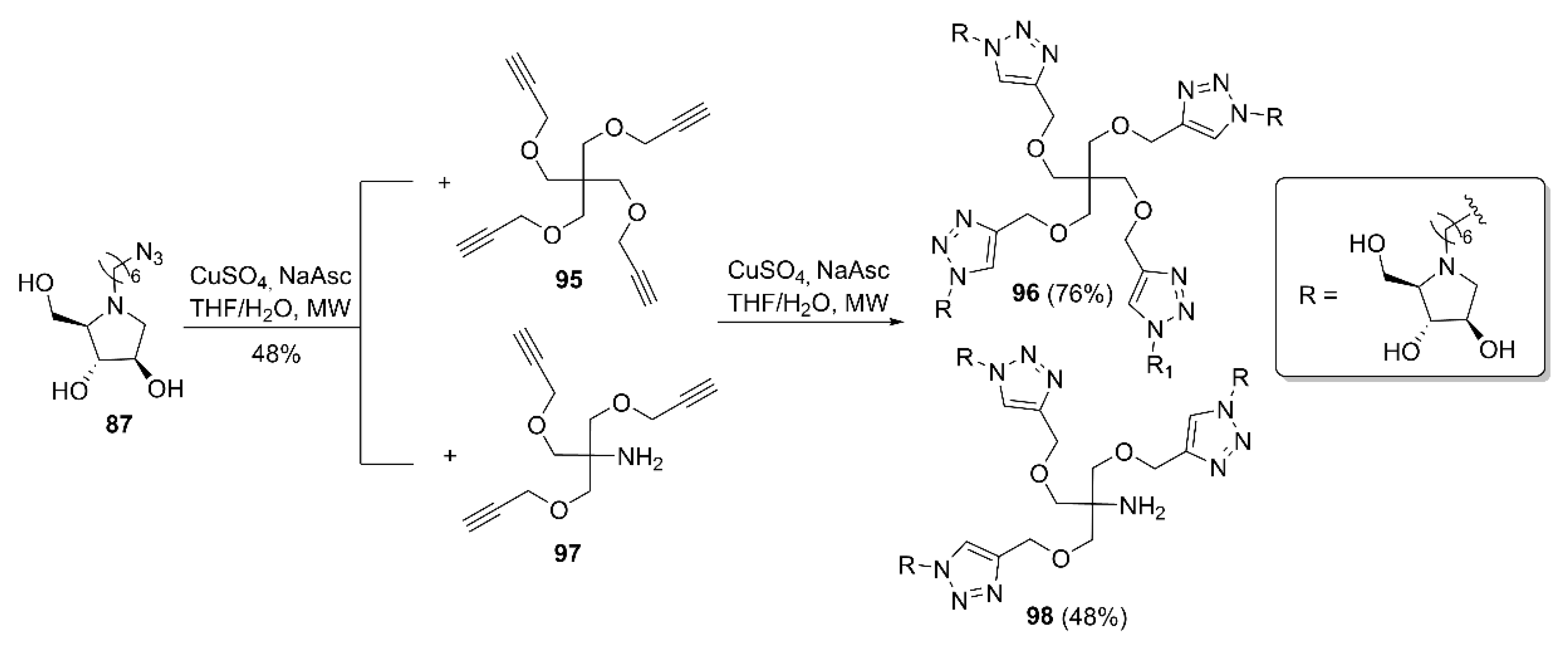
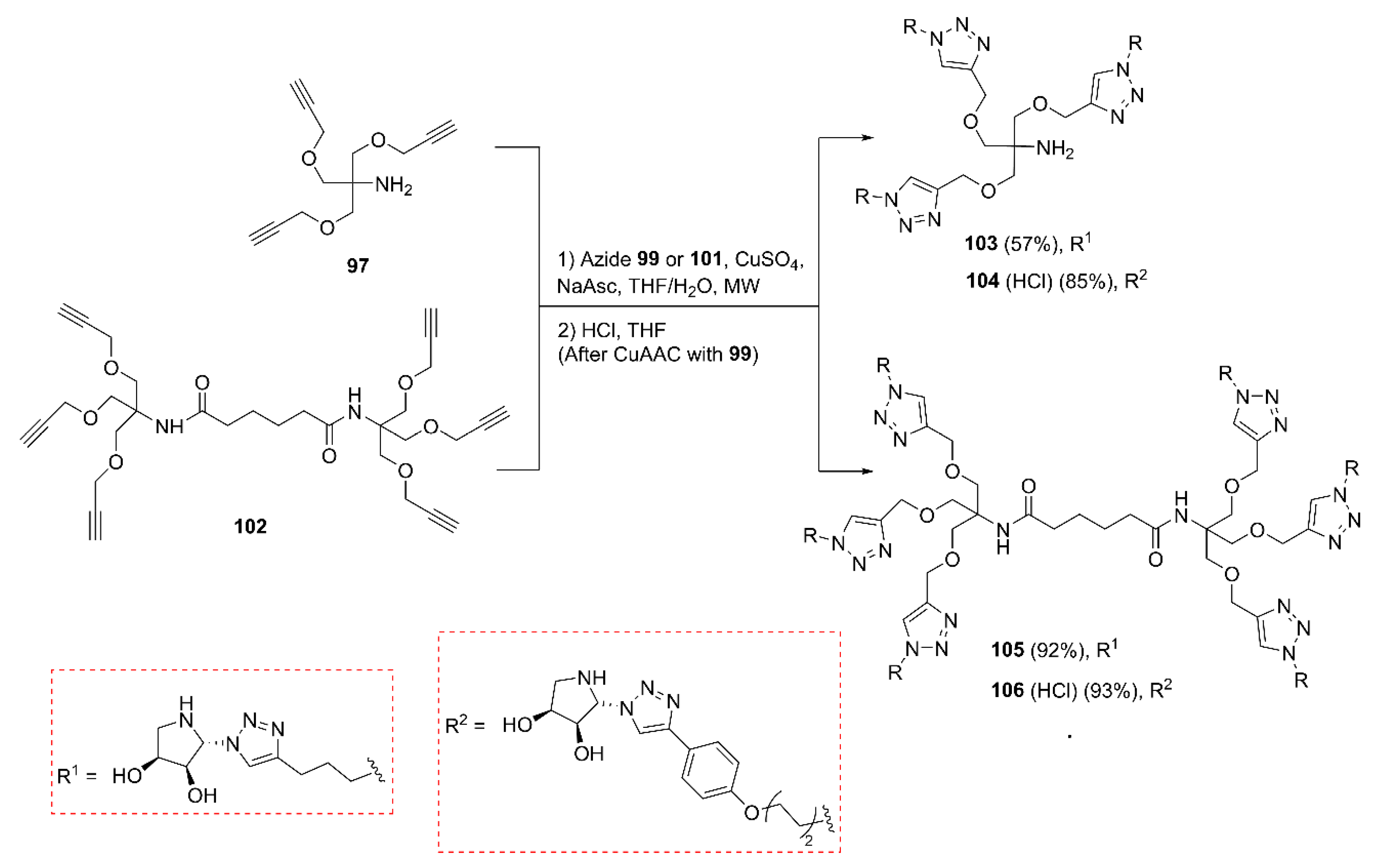
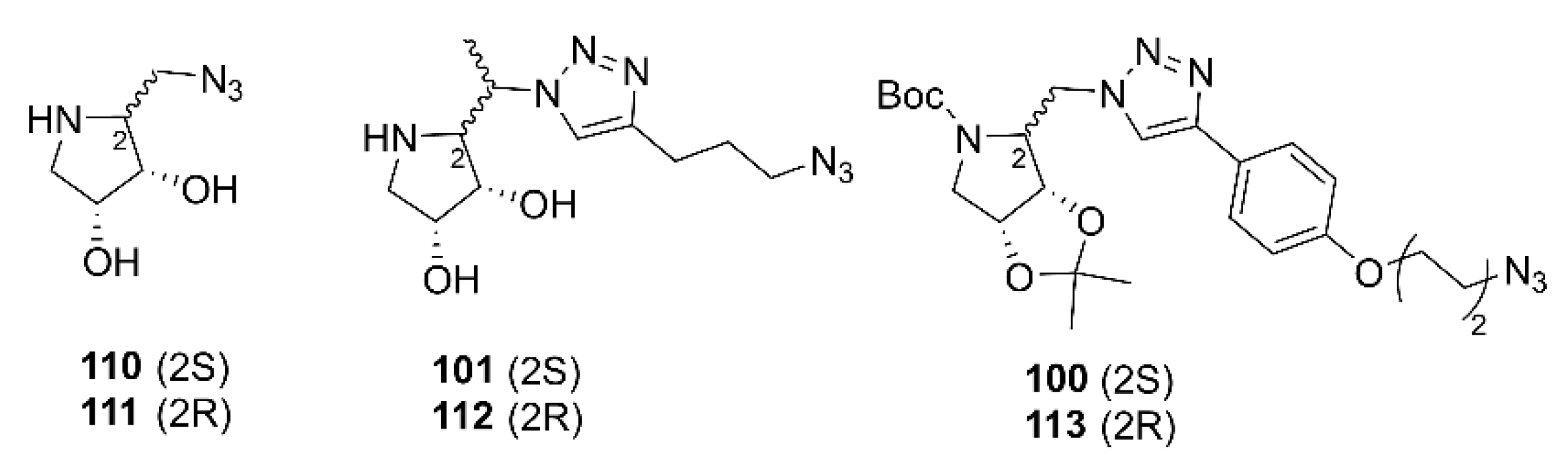
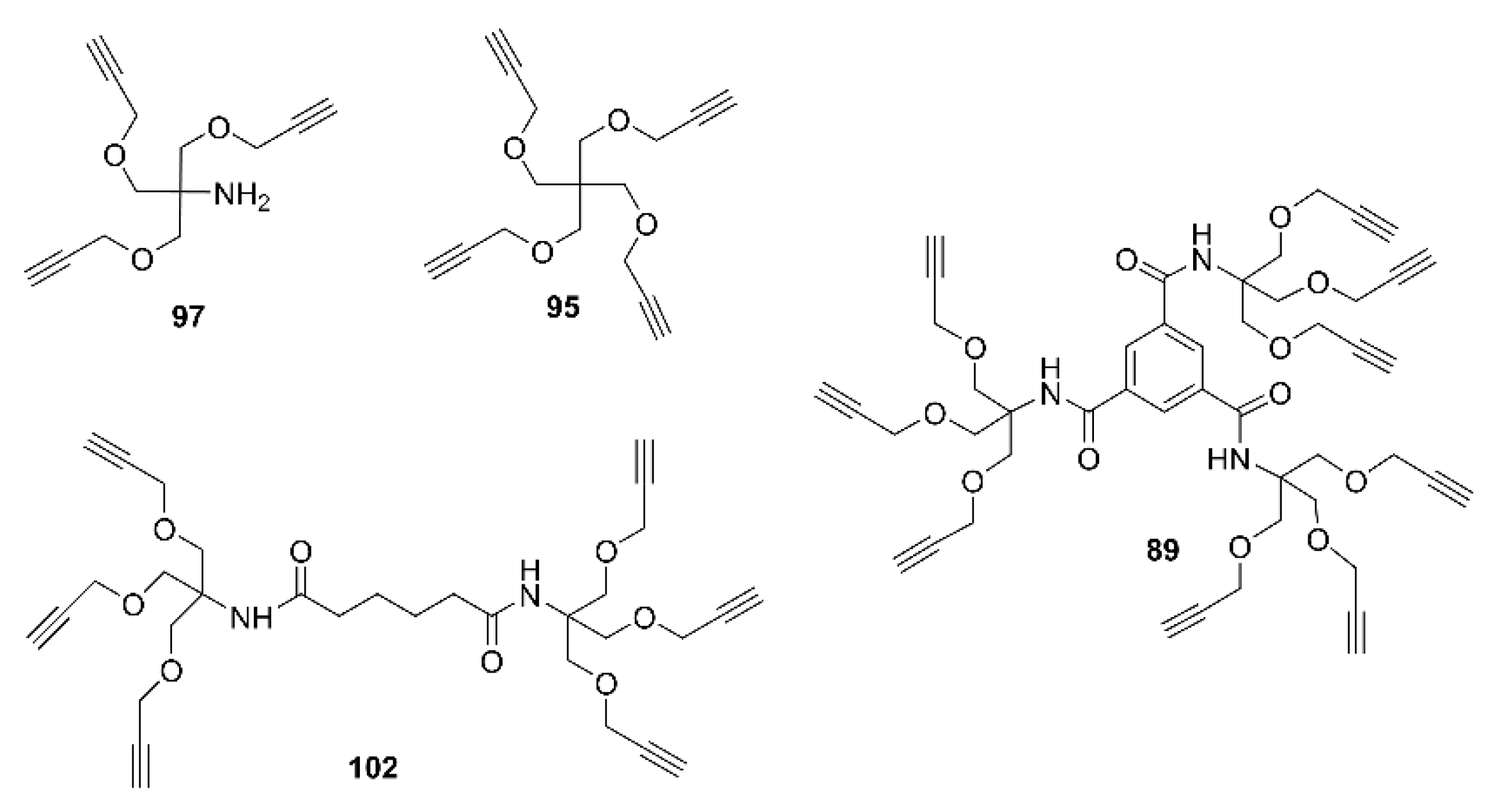
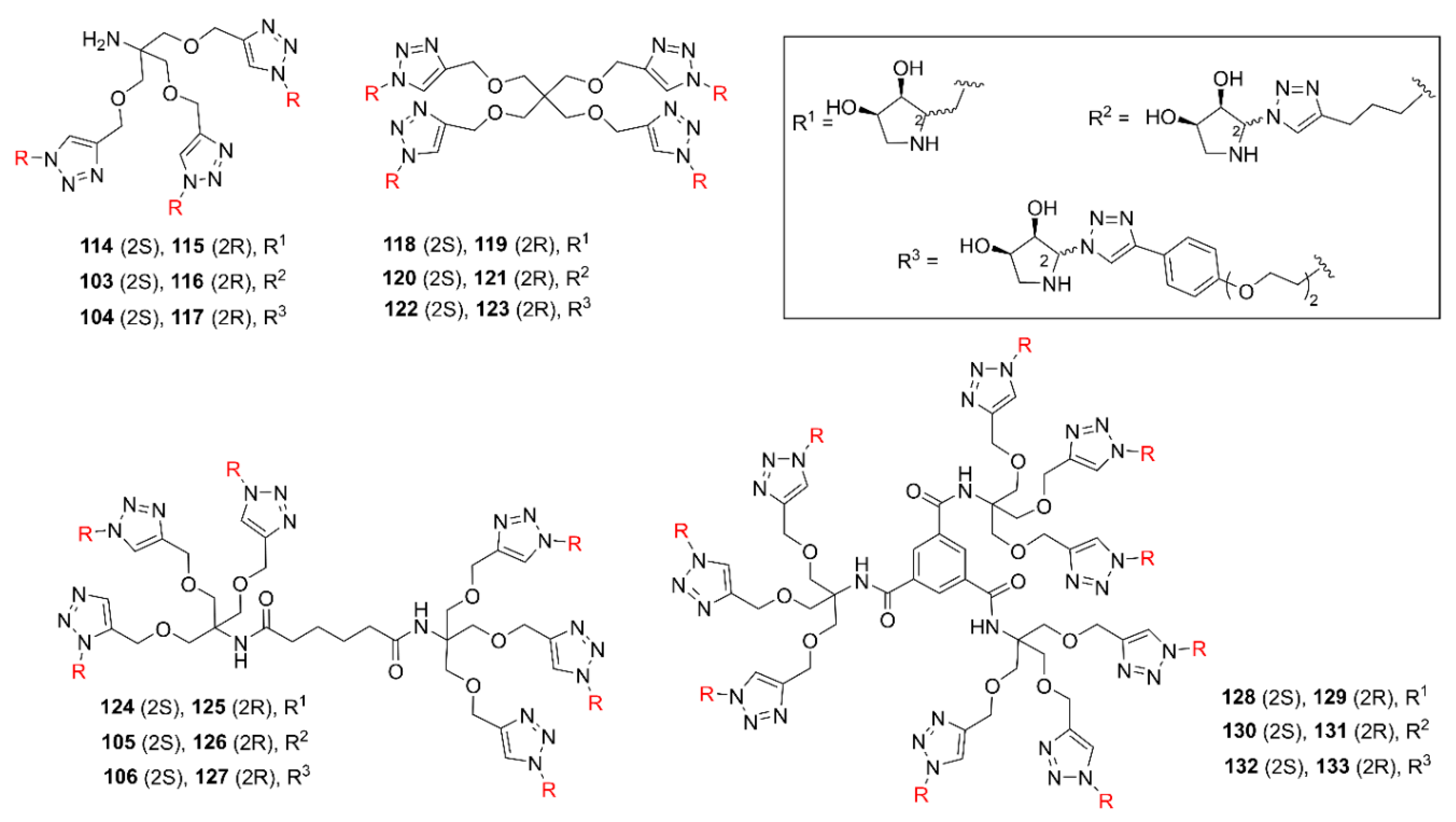
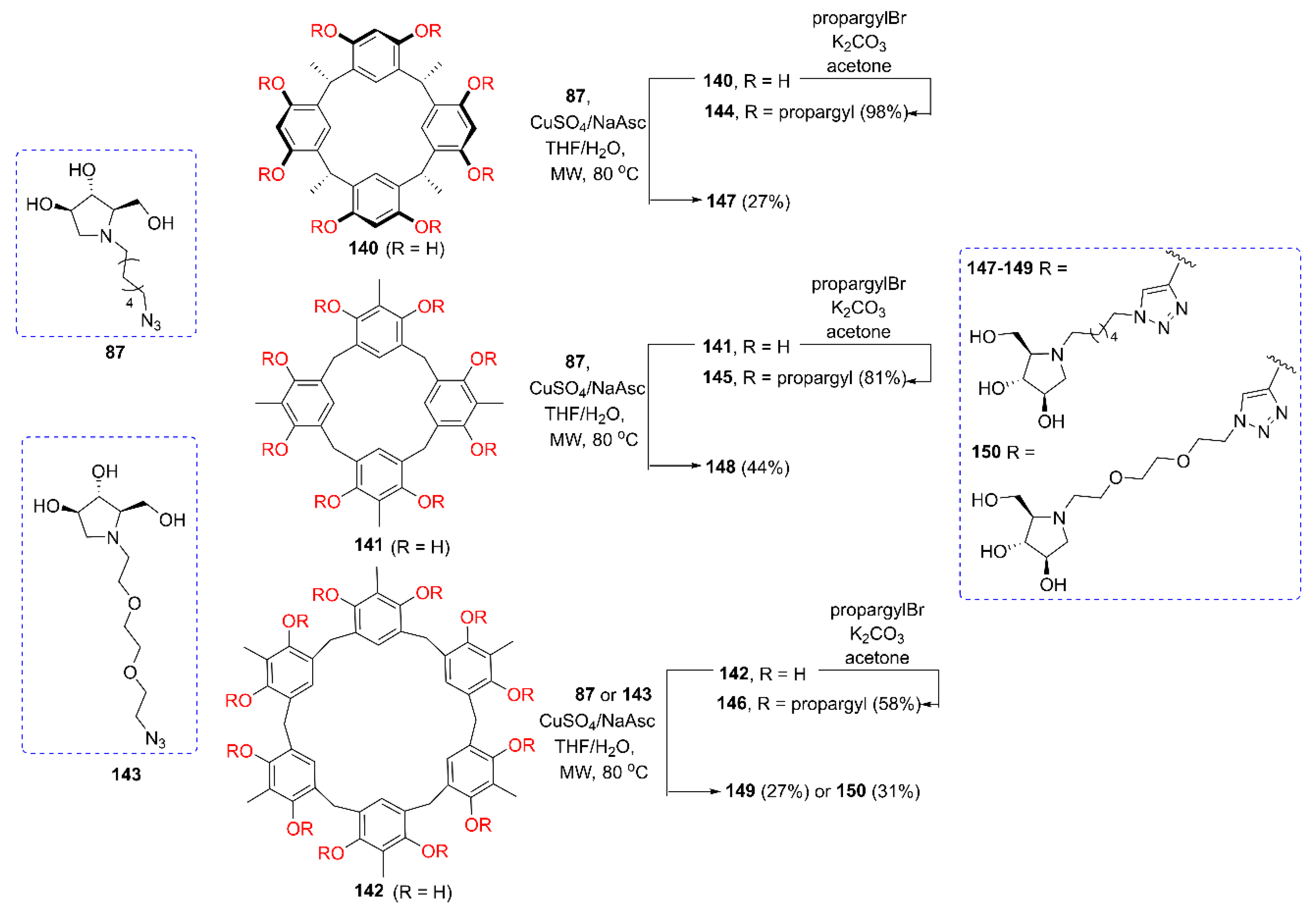
2.2. Synthesis of Multivalent Iminosugars
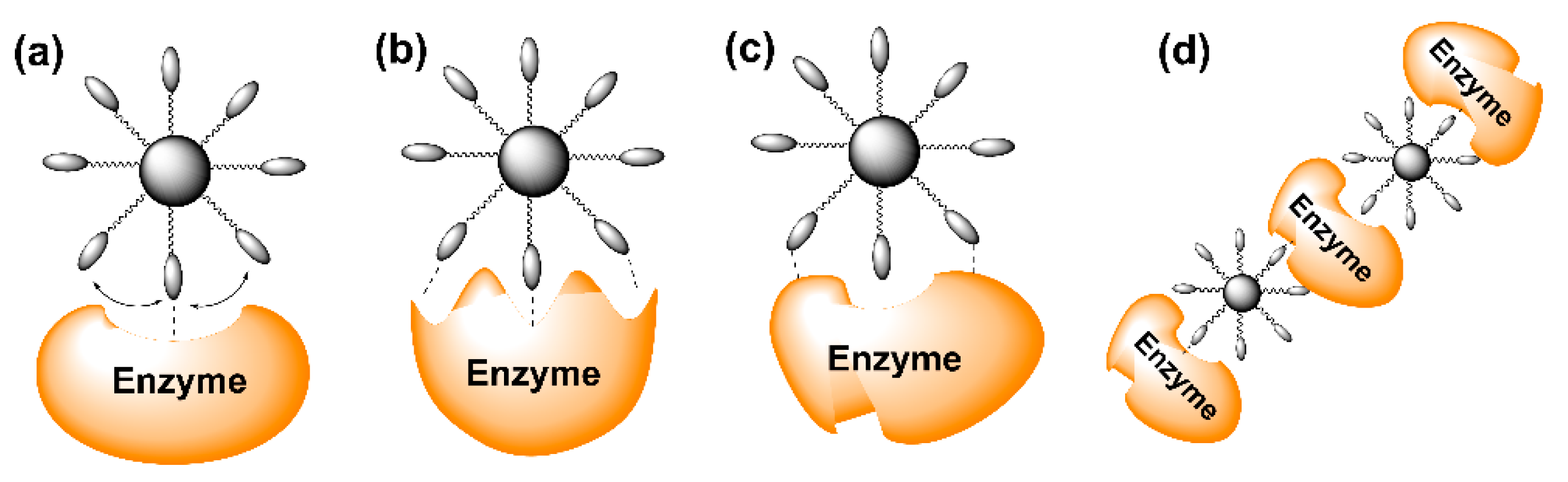
3. Biological Activity of Multivalent Pyrrolidine Iminosugars
3.1. Inhibition of α-Fucosidases
3.2. Inhibition of α-Mannosidases
3.3. Inhibition of Other Disease-Related Glycosidases
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Zamoner, L.O.B.; Aragão-Leoneti, V.; Carvalho, I. Iminosugars: Effects of stereochemistry, ring size, and n-substituents on glucosidase activities. Pharmaceuticals 2019, 12, 108. [Google Scholar] [CrossRef] [PubMed]
- Ferhati, X.; Matassini, C.; Fabbrini, M.G.; Goti, A.; Morrone, A.; Cardona, F.; Moreno-Vargas, A.J.; Paoli, P. Dual targeting of ptp1b and glucosidases with new bifunctional iminosugar inhibitors to address type 2 diabetes. Bioorganic Chem. 2019, 87, 534–549. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, F.; Su, L.; Wang, M.; Jia, T.; Xu, X.; Li, X.; Wei, C.; Luo, C.; Chen, S.; et al. Design and synthesis of novel benzimidazole-iminosugars linked a substituted phenyl group and their inhibitory activities against β-glucosidase. Bioorganic Chem. 2022, 127, 106016. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Hou, L.; Meng, A.; Han, G.; Zhang, W.; Jiang, S. Design, synthesis and bioactivity evaluation of galf mimics as antitubercular agents. Carbohydr. Res. 2016, 429, 135–142. [Google Scholar] [CrossRef]
- Liu, C.; Kang, H.; Wightman, R.H.; Jiang, S. Stereoselective synthesis of a novel galf-disaccharide mimic: β-D-galactofuranosyl-(1-5)-β-D-galactofuranosyl motif of mycobacterial cell walls. Tetrahedron Lett. 2013, 54, 1192–1194. [Google Scholar] [CrossRef]
- Evans DeWald, L.; Starr, C.; Butters, T.; Treston, A.; Warfield, K.L. Iminosugars: A host-targeted approach to combat flaviviridae infections. Antivir. Res. 2020, 184, 104881. [Google Scholar] [CrossRef]
- Conforti, I.; Marra, A. Iminosugars as glycosyltransferase inhibitors. Org. Biomol. Chem. 2021, 19, 5439–5475. [Google Scholar] [CrossRef]
- Huonnic, K.; Linclau, B. The synthesis and glycoside formation of polyfluorinated carbohydrates. Chem. Rev. 2022. [Google Scholar] [CrossRef]
- Uhrig, M.L.; Mora Flores, E.W.; Postigo, A. Approaches to the synthesis of perfluoroalkyl-modified carbohydrates and derivatives: Thiosugars, iminosugars, and tetrahydro(thio)pyrans. Chem. Eur. J. 2021, 27, 7813–7825. [Google Scholar] [CrossRef]
- Compain, P.; Martin, O.R. Iminosugars: From Synthesis to Therapeutic Applications; John Wiley & Sons Ltd.: Chichester, UK, 2007; ISBN 978-0-470-03391-3. [Google Scholar]
- Stutz, A.E. Iminosugars as Glycosidase Inhibitors: Nojirimycin and Beyond; Wiley-VCH: Chichester, Germany, 1999; ISBN 3-527-29544-5. [Google Scholar]
- Wang, H.; Shen, Y.; Zhao, L.; Ye, Y. Deoxynojirimycin and its derivatives: A mini review of the literature. Curr. Med. Chem. 2021, 28, 628–643. [Google Scholar] [CrossRef]
- Matassini, C.; Warren, J.; Wang, B.; Goti, A.; Cardona, F.; Morrone, A.; Bols, M. Imino- and azasugar protonation inside human acid β-glucosidase, the enzyme that is defective in gaucher disease. Angew. Chem. Int. Ed. 2020, 59, 10466–10469. [Google Scholar] [CrossRef] [PubMed]
- Iftikhar, M.; Lu, Y.; Zhou, M. An overview of therapeutic potential of N-alkylated 1-deoxynojirimycin congeners. Carbohydr. Res. 2021, 504, 108317. [Google Scholar] [CrossRef] [PubMed]
- Kar, S.; Sanderson, H.; Roy, K.; Benfenati, E.; Leszczynski, J. Green chemistry in the synthesis of pharmaceuticals. Chem. Rev. 2022, 122, 3637–3710. [Google Scholar] [CrossRef] [PubMed]
- Simone, M.I.; Wood, A.; Campkin, D.; Kiefel, M.J.; Houston, T.A. Recent results from non-basic glycosidase inhibitors: How structural diversity can inform general strategies for improving inhibition potency. Eur. J. Med. Chem. 2022, 235, 114282. [Google Scholar] [CrossRef]
- Ye, X.-S.; Sun, F.; Liu, M.; Li, Q.; Wang, Y.; Zhang, G.; Zhang, L.-H.; Zhang, X.-L. Synthetic iminosugar derivatives as new potential immunosuppressive agents. J. Med. Chem. 2005, 48, 3688–3691. [Google Scholar] [CrossRef]
- Wang, G.-N.; Xiong, Y.-L.; Ye, J.; Zhang, L.-H.; Ye, X.-S. Synthetic N-alkylated iminosugars as new potential immunosuppressive agents. ACS Med. Chem. Lett. 2011, 2, 682–686. [Google Scholar] [CrossRef]
- Yu, W.; Gill, T.; Wang, L.; Du, Y.; Ye, H.; Qu, X.; Guo, J.-T.; Cuconati, A.; Zhao, K.; Block, T.M.; et al. Design, synthesis, and biological evaluation of N-alkylated deoxynojirimycin (DNJ) derivatives for the treatment of dengue virus infection. J. Med. Chem. 2012, 55, 6061–6075. [Google Scholar] [CrossRef]
- Du, Y.; Ye, H.; Gill, T.; Wang, L.; Guo, F.; Cuconati, A.; Guo, J.-T.; Block, T.M.; Chang, J.; Xu, X. N-alkyldeoxynojirimycin derivatives with novel terminal tertiary amide substitution for treatment of bovine viral diarrhea virus (BVDV), dengue, and tacaribe virus infections. Bioorganic Med. Chem. Lett. 2013, 23, 2172–2176. [Google Scholar] [CrossRef]
- Cipolla, L.; Sgambato, A.; Forcella, M.; Fusi, P.; Parenti, P.; Cardona, F.; Bini, D. N-bridged 1-deoxynojirimycin dimers as selective insect trehalase inhibitors. Carbohydr. Res. 2014, 389, 46–49. [Google Scholar] [CrossRef]
- Welter, A.; Jadot, J.; Dardenne, G.; Marlier, M.; Casimir, J. 2,5-dihydroxymethyl 3,4-dihydroxypyrrolidine dans les feuilles de derris elliptica. Phytochemistry 1976, 15, 747–749. [Google Scholar] [CrossRef]
- Pinto, A.J.; Stewart, D.; van Rooijen, N.; Morahan, P.S. Selective depletion of liver and splenic macrophages using liposomes encapsulating the drug dichloromethylene diphosphonate: Effects on antimicrobial resistance. J. Leukoc. Biol. 1991, 49, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Shimadate, Y.; Kato, A.; Li, Y.-X.; Jia, Y.-M.; Fleet, G.W.J.; Yu, C.-Y. Synthesis of pyrrolidine monocyclic analogues of pochonicine and its stereoisomers: Pursuit of simplified structures and potent β-N-acetylhexosaminidase inhibition. Molecules 2020, 25, 1498. [Google Scholar] [CrossRef] [PubMed]
- Martínez, R.F.; Jenkinson, S.F.; Nakagawa, S.; Kato, A.; Wormald, M.R.; Fleet, G.W.J.; Hollinshead, J.; Nash, R.J. Isolation from stevia rebaudiana of DMDP acetic acid, a novel iminosugar amino acid: Synthesis and glycosidase inhibition profile of glycine and β-alanine pyrrolidine amino acids. Amino Acids 2019, 51, 991–998. [Google Scholar] [CrossRef]
- Nash, R.J.; Arthur Bell, E.; Michael Williams, J. 2-hydroxymethyl-3,4-dihydroxypyrrolidine in fruits of angylocalyx boutiqueanus. Phytochemistry 1985, 24, 1620–1622. [Google Scholar] [CrossRef]
- Wang, J.-Z.; Cheng, B.; Kato, A.; Kise, M.; Shimadate, Y.; Jia, Y.-M.; Li, Y.-X.; Fleet, G.W.J.; Yu, C.-Y. Design, synthesis and glycosidase inhibition of C-4 branched LAB and DAB derivatives. Eur. J. Med. Chem. 2022, 233, 114230. [Google Scholar] [CrossRef]
- Kato, A.; Hayashi, E.; Miyauchi, S.; Adachi, I.; Imahori, T.; Natori, Y.; Yoshimura, Y.; Nash, R.J.; Shimaoka, H.; Nakagome, I.; et al. A-L-C-butyl-1,4-dideoxy-1,4-imino-L-arabinitol as a second-generation iminosugar-based oral α-glucosidase inhibitor for improving postprandial hyperglycemia. J. Med. Chem. 2012, 55, 10347–10362. [Google Scholar] [CrossRef]
- Trapero, A.; Llebaria, A. A prospect for pyrrolidine iminosugars as antidiabetic α-glucosidase inhibitors. J. Med. Chem. 2012, 55, 10345–10346. [Google Scholar] [CrossRef]
- Mena-Barragán, T.; García-Moreno, M.I.; Nanba, E.; Higaki, K.; Concia, A.L.; Clapés, P.; García Fernández, J.M.; Ortiz Mellet, C. Inhibitor versus chaperone behaviour of D-fagomine, DAB and LAB sp2-iminosugar conjugates against glycosidases: A structure–activity relationship study in Gaucher fibroblasts. Eur. J. Med. Chem. 2016, 121, 880–891. [Google Scholar] [CrossRef]
- Tsou, E.-L.; Chen, S.-Y.; Yang, M.-H.; Wang, S.-C.; Cheng, T.-R.R.; Cheng, W.-C. Synthesis and biological evaluation of a 2-aryl polyhydroxylated pyrrolidine alkaloid-based library. Biorg. Med. Chem. 2008, 16, 10198–10204. [Google Scholar] [CrossRef]
- Li, Y.-X.; Iwaki, R.; Kato, A.; Jia, Y.-M.; Fleet, G.W.J.; Zhao, X.; Xiao, M.; Yu, C.-Y. Fluorinated radicamine A and B: Synthesis and glycosidase inhibition. Eur. J. Org. Chem. 2016, 2016, 1429–1438. [Google Scholar] [CrossRef]
- Liu, C.; Gao, J.; Yang, G.; Wightman, H.R.; Jiang, S. Enantiospecific synthesis of (-)-radicamine B. Lett. Org. Chem. 2007, 4, 556–558. [Google Scholar] [CrossRef]
- Nash, R.J.; Kato, A.; Yu, C.-Y.; Fleet, G.W.J. Iminosugars as therapeutic agents: Recent advances and promising trends. Future Med. Chem. 2011, 3, 1513–1521. [Google Scholar] [CrossRef] [PubMed]
- Chennaiah, A.; Dahiya, A.; Dubbu, S.; Vankar, Y.D. A stereoselective synthesis of an imino glycal: Application in the synthesis of (–)-1-epi-adenophorine and a homoimindosugar. Eur. J. Org. Chem. 2018, 2018, 6574–6581. [Google Scholar] [CrossRef]
- Chennaiah, A.; Bhowmick, S.; Vankar, Y.D. Conversion of glycals into vicinal-1,2-diazides and 1,2-(or 2,1)-azidoacetates using hypervalent iodine reagents. Application in the synthesis of N-glycopeptides, pseudo-trisaccharides and an iminosugar. RSC Adv. 2017, 7, 41755–41762. [Google Scholar] [CrossRef]
- Verma, A.K.; Dubbu, S.; Chennaiah, A.; Vankar, Y.D. Synthesis of di- and trihydroxy proline derivatives from D-glycals: Application in the synthesis of polysubstituted pyrrolizidines and bioactive C-aryl/alkyl pyrrolidines. Carbohydr. Res. 2019, 475, 48–55. [Google Scholar] [CrossRef]
- Horne, G.; Wilson, F.X.; Tinsley, J.; Williams, D.H.; Storer, R. Iminosugars past, present and future: Medicines for tomorrow. Drug Discovery Today 2011, 16, 107–118. [Google Scholar] [CrossRef]
- Ghani, U. Re-exploring promising α-glucosidase inhibitors for potential development into oral anti-diabetic drugs: Finding needle in the haystack. Eur. J. Med. Chem. 2015, 103, 133–162. [Google Scholar] [CrossRef]
- Singh, A.; Mhlongo, N.; Soliman, E.S.M. Anti-cancer glycosidase inhibitors from natural products: A computational and molecular modelling perspective. Anticancer Agents Med. Chem. 2015, 15, 933–946. [Google Scholar] [CrossRef]
- Vanni, C.; Bodlenner, A.; Marradi, M.; Schneider, J.P.; Ramirez, M.d.l.A.; Moya, S.; Goti, A.; Cardona, F.; Compain, P.; Matassini, C. Hybrid multivalent jack bean α-mannosidase inhibitors: The first example of gold nanoparticles decorated with deoxynojirimycin inhitopes. Molecules 2021, 26, 5864. [Google Scholar] [CrossRef]
- Brissonnet, Y.; Ladevèze, S.; Tezé, D.; Fabre, E.; Deniaud, D.; Daligault, F.; Tellier, C.; Šesták, S.; Remaud-Simeon, M.; Potocki-Veronese, G.; et al. Polymeric iminosugars improve the activity of carbohydrate-processing enzymes. Bioconjugate Chem. 2015, 26, 766–772. [Google Scholar] [CrossRef]
- González-Cuesta, M.; Ortiz Mellet, C.; García Fernández, J.M. Carbohydrate supramolecular chemistry: Beyond the multivalent effect. Chem. Commun. 2020, 56, 5207–5222. [Google Scholar] [CrossRef] [PubMed]
- Lepage, M.L.; Schneider, J.P.; Bodlenner, A.; Meli, A.; De Riccardis, F.; Schmitt, M.; Tarnus, C.; Nguyen-Huynh, N.-T.; Francois, Y.-N.; Leize-Wagner, E.; et al. Iminosugar-cyclopeptoid conjugates raise multivalent effect in glycosidase inhibition at unprecedented high levels. Chem. Eur. J. 2016, 22, 5151–5155. [Google Scholar] [CrossRef] [PubMed]
- Brissonnet, Y.; Ortiz Mellet, C.; Morandat, S.; Garcia Moreno, M.I.; Deniaud, D.; Matthews, S.E.; Vidal, S.; Sestak, S.; El Kirat, K.; Gouin, S.G. Topological effects and binding modes operating with multivalent iminosugar-based glycoclusters and mannosidases. J. Am. Chem. Soc. 2013, 135, 18427–18435. [Google Scholar] [CrossRef] [PubMed]
- Li, R.-F.; Yang, J.-X.; Liu, J.; Ai, G.-M.; Zhang, H.-Y.; Xu, L.-Y.; Chen, S.-B.; Zhang, H.-X.; Li, X.-L.; Cao, Z.-R.; et al. Positional isomeric effects on the optical properties, multivalent glycosidase inhibition effect, and hypoglycemic effect of perylene bisimide-deoxynojirimycin conjugates. J. Med. Chem. 2021, 64, 5863–5873. [Google Scholar] [CrossRef] [PubMed]
- Compain, P. Multivalent effect in glycosidase inhibition: The end of the beginning. Chem. Rec. 2020, 20, 10–22. [Google Scholar] [CrossRef]
- Howard, E.; Cousido-Siah, A.; Lepage, M.L.; Schneider, J.P.; Bodlenner, A.; Mitschler, A.; Meli, A.; Izzo, I.; Alvarez, H.A.; Podjarny, A.; et al. Structural basis of outstanding multivalent effects in jack bean α-mannosidase inhibition. Angew. Chem. Int. Ed. 2018, 57, 8002–8006. [Google Scholar] [CrossRef]
- Martínez-Bailén, M.; Carmona, A.T.; Cardona, F.; Matassini, C.; Goti, A.; Kubo, M.; Kato, A.; Robina, I.; Moreno-Vargas, A.J. Synthesis of multimeric pyrrolidine iminosugar inhibitors of human β-glucocerebrosidase and α-galactosidase a: First example of a multivalent enzyme activity enhancer for Fabry disease. Eur. J. Med. Chem. 2020, 192, 112173. [Google Scholar] [CrossRef]
- Johns, B.A.; Johnson, C.R. Scaffolded bis-azasugars: A dual warhead approach to glycosidase inhibition. Tetrahedron Lett. 1998, 39, 749–752. [Google Scholar] [CrossRef]
- Wennekes, T.; van den Berg, R.J.B.H.N.; Bonger, K.M.; Donker-Koopman, W.E.; Ghisaidoobe, A.; van der Marel, G.A.; Strijland, A.; Aerts, J.M.F.G.; Overkleeft, H.S. Synthesis and evaluation of dimeric lipophilic iminosugars as inhibitors of glucosylceramide metabolism. Tetrahedron Asymmetry 2009, 20, 836–846. [Google Scholar] [CrossRef]
- Lohse, A.; Jensen, K.B.; Lundgren, K.; Bols, M. Synthesis and deconvolution of the first combinatorial library of glycosidase inhibitors. Bioorganic Med. Chem. 1999, 7, 1965–1971. [Google Scholar] [CrossRef]
- Gestwicki, J.E.; Cairo, C.W.; Strong, L.E.; Oetjen, K.A.; Kiessling, L.L. Influencing receptor−ligand binding mechanisms with multivalent ligand architecture. J. Am. Chem. Soc. 2002, 124, 14922–14933. [Google Scholar] [CrossRef] [PubMed]
- Wittmann, V.; Pieters, R.J. Bridging lectin binding sites by multivalent carbohydrates. Chem. Soc. Rev. 2013, 42, 4492–4503. [Google Scholar] [CrossRef] [PubMed]
- Kiessling, L.L.; Gestwicki, J.E.; Strong, L.E. Synthetic multivalent ligands in the exploration of cell-surface interactions. Curr. Opin. Chem. Biol. 2000, 4, 696–703. [Google Scholar] [CrossRef]
- Tornøe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper(Ι)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A stepwise huisgen cycloaddition process: Copper(Ι)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Diot, J.; García-Moreno, M.I.; Gouin, S.G.; Ortiz Mellet, C.; Haupt, K.; Kovensky, J. Multivalent iminosugars to modulate affinity and selectivity for glycosidases. Org. Biomol. Chem. 2009, 7, 357–363. [Google Scholar] [CrossRef]
- Gouin, S.G. Multivalent inhibitors for carbohydrate-processing enzymes: Beyond the “lock-and-key” concept. Chem. Eur. J. 2014, 20, 11616–11628. [Google Scholar] [CrossRef]
- Compain, P.; Bodlenner, A. The multivalent effect in glycosidase inhibition: A new, rapidly emerging topic in glycoscience. Chembiochem 2014, 15, 1239–1251. [Google Scholar] [CrossRef]
- Zelli, R.; Bartolami, E.; Longevial, J.-F.; Bessin, Y.; Dumy, P.; Marra, A.; Ulrich, S. A metal-free synthetic approach to peptide-based iminosugar clusters as novel multivalent glycosidase inhibitors. RSC. Adv. 2016, 6, 2210–2216. [Google Scholar] [CrossRef]
- Matassini, C.; D’Adamio, G.; Vanni, C.; Goti, A.; Cardona, F. Studies for the multimerization of DAB-L-based iminosugars through iteration of the nitrone cycloaddition/ring-opening/allylation sequence. Eur. J. Org. Chem. 2019, 2019, 4897–4905. [Google Scholar] [CrossRef]
- Zamoner, L.O.B.; Aragao-Leoneti, V.; Mantoani, S.P.; Rugen, M.D.; Nepogodiev, S.A.; Field, R.A.; Carvalho, I. Cuaac click chemistry with N-propargyl 1,5-dideoxy-1,5-imino-D-gulitol and n-propargyl 1,6-dideoxy-1,6-imino-D-mannitol provides access to triazole-linked piperidine and azepane pseudo-disaccharide iminosugars displaying glycosidase inhibitory properties. Carbohydr. Res. 2016, 429, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Bonduelle, C.; Huang, J.; Mena-Barragán, T.; Ortiz Mellet, C.; Decroocq, C.; Etamé, E.; Heise, A.; Compain, P.; Lecommandoux, S. Iminosugar-based glycopolypeptides: Glycosidase inhibition with bioinspired glycoprotein analogue micellar self-assemblies. Chem. Commun. 2014, 50, 3350–3352. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-J.; Wang, K.-R.; Li, R.-F.; Yang, J.-X.; Li, M.; Zhang, H.-X.; Cao, Z.-R.; Li, X.-L. Synthesis, self-assembly behaviours and multivalent glycosidase inhibition effects of a deoxynojirimycin modified perylene bisimide derivative. J. Mater. Chem. B 2019, 7, 1270–1275. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-X.; Li, J.-J.; Yin, F.-Q.; Wang, G.-Y.; Wei, W.-T.; Li, X.-L.; Wang, K.-R. Multivalent glucosidase inhibitors based on perylene bisimide and iminosugar conjugates. Eur. J. Med. Chem. 2022, 241, 114621. [Google Scholar] [CrossRef] [PubMed]
- D’Adamio, G.; Matassini, C.; Parmeggiani, C.; Catarzi, S.; Morrone, A.; Goti, A.; Paoli, P.; Cardona, F. Evidence for a multivalent effect in inhibition of sulfatases involved in lysosomal storage disorders. RSC. Adv. 2016, 6, 64847–64851. [Google Scholar] [CrossRef]
- Della Sala, P.; Vanni, C.; Talotta, C.; Di Marino, L.; Matassini, C.; Goti, A.; Neri, P.; Šesták, S.; Cardona, F.; Gaeta, C. Multivalent resorcinarene clusters decorated with DAB-1 inhitopes: Targeting golgi α-mannosidase from drosophila melanogaster. Org. Chem. Front. 2021, 8, 6648–6656. [Google Scholar] [CrossRef]
- Pingitore, V.; Martinez-Bailen, M.; Carmona, A.T.; Meszaros, Z.; Kulik, N.; Slamova, K.; Kren, V.; Bojarova, P.; Robina, I.; Moreno-Vargas, A.J. Discovery of human hexosaminidase inhibitors by in situ screening of a library of mono- and divalent pyrrolidine iminosugars. Bioorganic Chem. 2022, 120, 105650. [Google Scholar] [CrossRef]
- Moreno-Clavijo, E.; Carmona, A.T.; Moreno-Vargas, A.J.; Molina, L.; Wright, D.W.; Davies, G.J.; Robina, I. Exploring a multivalent approach to α-L-fucosidase inhibition. Eur. J. Org. Chem. 2013, 2013, 7328–7336. [Google Scholar] [CrossRef]
- Prichard, K.; Campkin, D.; O’Brien, N.; Kato, A.; Fleet, G.W.J.; Simone, M.I. Biological activities of 3,4,5-trihydroxypiperidines and their N- and O-derivatives. Chem. Biol. Drug Des. 2018, 92, 1171–1197. [Google Scholar] [CrossRef]
- Dhara, D.; Dhara, A.; Bennett, J.; Murphy, P.V. Cyclisations and strategies for stereoselective synthesis of piperidine iminosugars. Chem. Rec. 2021, 21, 2958–2979. [Google Scholar] [CrossRef]
- Stocker, B.L.; Dangerfield, E.M.; Win-Mason, A.L.; Haslett, G.W.; Timmer, M.S.M. Recent developments in the synthesis of pyrrolidine-containing iminosugars. Eur. J. Org. Chem. 2010, 2010, 1615–1637. [Google Scholar] [CrossRef]
- Decroocq, C.; Rodríguez-Lucena, D.; Russo, V.; Mena Barragán, T.; Ortiz Mellet, C.; Compain, P. The multivalent effect in glycosidase inhibition: Probing the influence of architectural parameters with cyclodextrin-based iminosugar click clusters. Chem. Eur. J. 2011, 17, 13825–13831. [Google Scholar] [CrossRef]
- Cagnoni, A.J.; Varela, O.; Gouin, S.G.; Kovensky, J.; Uhrig, M.L. Synthesis of multivalent glycoclusters from L-thio-β-D-galactose and their inhibitory activity against the β-galactosidase from E. coli. J. Org. Chem. 2011, 76, 3064–3077. [Google Scholar] [CrossRef]
- Moreno-Clavijo, E.; Carmona, A.T.; Vera-Ayoso, Y.; Moreno-Vargas, A.J.; Bello, C.; Vogel, P.; Robina, I. Synthesis of novel pyrrolidine 3,4-diol derivatives as inhibitors of α-L-fucosidases. Org. Biomol. Chem. 2009, 7, 1192–1202. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Clavijo, E.; Carmona, A.T.; Moreno-Vargas, A.J.; Rodríguez-Carvajal, M.A.; Robina, I. Synthesis and inhibitory activities of novel C-3 substituted azafagomines: A new type of selective inhibitors of α-L-fucosidases. Bioorganic Med. Chem. 2010, 18, 4648–4660. [Google Scholar] [CrossRef] [PubMed]
- Robina, I.; Moreno-Vargas, A.J.; Fernández-Bolaños, J.G.; Fuentes, J.; Demange, R.; Vogel, P. New leads for selective inhibitors of α-L-fucosidases. Synthesis and glycosidase inhibitory activities of [(2R,3R,4R)-3,4-dihydroxypyrrolidin-2-yl]furan derivatives. Bioorganic Med. Chem. Lett. 2001, 11, 2555–2559. [Google Scholar] [CrossRef]
- Szczepanska, A.; Espartero, J.L.; Moreno-Vargas, A.J.; Carmona, A.T.; Robina, I.; Remmert, S.; Parish, C. Synthesis and conformational analysis of novel trimeric maleimide cross-linking reagents. J. Org. Chem. 2007, 72, 6776–6785. [Google Scholar] [CrossRef]
- Hottin, A.; Carrión-Jiménez, S.; Moreno-Clavijo, E.; Moreno-Vargas, A.J.; Carmona, A.T.; Robina, I.; Behr, J.-B. Expanding the library of divalent fucosidase inhibitors with polyamino and triazole-benzyl bridged bispyrrolidines. Org. Biomol. Chem. 2016, 14, 3212–3220. [Google Scholar] [CrossRef]
- Hottin, A.; Dubar, F.; Steenackers, A.; Delannoy, P.; Biot, C.; Behr, J.-B. Iminosugar–ferrocene conjugates as potential anticancer agents. Org. Biomol. Chem. 2012, 10, 5592–5597. [Google Scholar] [CrossRef]
- Bergeron-Brlek, M.; Goodwin-Tindall, J.; Cekic, N.; Roth, C.; Zandberg, W.F.; Shan, X.; Varghese, V.; Chan, S.; Davies, G.J.; Vocadlo, D.J.; et al. A convenient approach to stereoisomeric iminocyclitols: Generation of potent brain-permeable OGA inhibitors. Angew. Chem. Int. Ed. 2015, 54, 15429–15433. [Google Scholar] [CrossRef]
- Elías-Rodríguez, P.; Moreno-Clavijo, E.; Carmona, A.T.; Moreno-Vargas, A.J.; Robina, I. Rapid discovery of potent α-fucosidase inhibitors by in situ screening of a library of (pyrrolidin-2-yl)triazoles. Org. Biomol. Chem. 2014, 12, 5898–5904. [Google Scholar] [CrossRef] [PubMed]
- Hottin, A.; Wright, D.W.; Moreno-Clavijo, E.; Moreno-Vargas, A.J.; Davies, G.J.; Behr, J.-B. Exploring the divalent effect in fucosidase inhibition with stereoisomeric pyrrolidine dimers. Org. Biomol. Chem. 2016, 14, 4718–4727. [Google Scholar] [CrossRef]
- Wong, C.-H.; Provencher, L.; Porco, J.A.; Jung, S.-H.; Wang, Y.-F.; Chen, L.; Wang, R.; Steensma, D.H. Synthesis and evaluation of homoaza sugars as glycosidase inhibitors. J. Org. Chem. 1995, 60, 1492–1501. [Google Scholar] [CrossRef]
- Srihari, P.; Prem Kumar, B.; Subbarayudu, K.; Yadav, J.S. A convergent approach for the total synthesis of (−)-synrotolide diacetate. Tetrahedron Lett. 2007, 48, 6977–6981. [Google Scholar] [CrossRef]
- Rao, M.V.; Rao, B.V.; Ramesh, B. Total synthesis of AI-77-B. Tetrahedron Lett. 2014, 55, 5921–5924. [Google Scholar] [CrossRef]
- Hottin, A.; Wright, D.W.; Davies, G.J.; Behr, J.-B. Exploiting the hydrophobic terrain in fucosidases with aryl-substituted pyrrolidine iminosugars. Chembiochem 2015, 16, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Carmona, A.T.; Carrion-Jimenez, S.; Pingitore, V.; Moreno-Clavijo, E.; Robina, I.; Moreno-Vargas, A.J. Harnessing pyrrolidine iminosugars into dimeric structures for the rapid discovery of divalent glycosidase inhibitors. Eur. J. Med. Chem. 2018, 151, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Huisgen, R.; Szeimies, G.; Möbius, L. 1.3-dipolare cycloadditionen, XXXII. Kinetik der additionen organischer azide an cc-mehrfachbindungen. Chem. Ber. 1967, 100, 2494–2507. [Google Scholar] [CrossRef]
- Huisgen, R. 1,3-dipolar cycloadditions. Past and future. Angew. Chem. Int. Ed. 1963, 2, 565–598. [Google Scholar] [CrossRef]
- Moreno-Vargas, A.J.; Demange, R.; Fuentes, J.; Robina, I.; Vogel, P. Synthesis of [(2S,3S,4R)-3,4-dihydroxypyrrolidin-2-yl]-5-methylfuran-4-carboxylic acid derivatives: New leads as selective β-galactosidase inhibitors. Bioorganic Med. Chem. Lett. 2002, 12, 2335–2339. [Google Scholar] [CrossRef]
- Moreno-Vargas, A.J.; Robina, I.; Demange, R.; Vogel, P. Synthesis and glycosidase inhibitory activities of 5-(1′,4′-dideoxy-1′,4′-imino-D-erythrosyl)-2-methyl-3-furoic acid (=5-[(3S,4R)-3,4-dihydroxypyrrolidin-2-yl]-2-methylfuran-3-carboxylic acid) derivatives: New leads as selective α-L-fucosidase and β-galactosidase inhibitors. Helv. Chim. Acta 2003, 86, 1894–1913. [Google Scholar]
- Aucagne, V.; Valverde, I.E.; Marceau, P.; Galibert, M.; Dendane, N.; Delmas, A.F. Towards the simplification of protein synthesis: Iterative solid-supported ligations with concomitant purifications. Angew. Chem. Int. Ed. 2012, 51, 11320–11324. [Google Scholar] [CrossRef]
- Liu, J.; Shikhman, A.R.; Lotz, M.K.; Wong, C.-H. Hexosaminidase inhibitors as new drug candidates for the therapy of osteoarthritis. Chem. Biol. 2001, 8, 701–711. [Google Scholar] [CrossRef]
- Tsou, E.-L.; Yeh, Y.-T.; Liang, P.-H.; Cheng, W.-C. A convenient approach toward the synthesis of enantiopure isomers of DMDP and ADMDP. Tetrahedron 2009, 65, 93–100. [Google Scholar] [CrossRef]
- Shaikh, T.M.; Sudalai, A. Enantioselective synthesis of (+)-α-conhydrine and (–)-sedamine by L-proline-catalysed α-aminooxylation. Eur. J. Org. Chem. 2010, 2010, 3437–3444. [Google Scholar] [CrossRef]
- Ornelas, C.; Broichhagen, J.; Weck, M. Strain-promoted alkyne azide cycloaddition for the functionalization of poly(amide)-based dendrons and dendrimers. J. Am. Chem. Soc. 2010, 132, 3923–3931. [Google Scholar] [CrossRef] [PubMed]
- Martella, D.; D’Adamio, G.; Parmeggiani, C.; Cardona, F.; Moreno-Clavijo, E.; Robina, I.; Goti, A. Cycloadditions of sugar-derived nitrones targeting polyhydroxylated indolizidines. Eur. J. Org. Chem. 2016, 2016, 1588–1598. [Google Scholar] [CrossRef]
- Ilardi, E.A.; Stivala, C.E.; Zakarian, A. Hexafluoroisopropanol as a unique solvent for stereoselective iododesilylation of vinylsilanes. Org. Lett. 2008, 10, 1727–1730. [Google Scholar] [CrossRef]
- Ishikawa, T.; Tajima, Y.; Fukui, M.; Saito, S. Synthesis and asymmetric [3 + 2] cycloaddition reactions of chiral cyclic nitrone: A novel system providing maximal facial bias for both nitrone and dipolarophile. Angew. Chem. Int. Ed. 1996, 35, 1863–1864. [Google Scholar] [CrossRef]
- Tamayo, J.A.; Franco, F.; Lo Re, D.; Sánchez-Cantalejo, F. Synthesis of pentahydroxylated pyrrolizidines and indolizidines. J. Org. Chem. 2009, 74, 5679–5682. [Google Scholar] [CrossRef]
- Merino, P.; Delso, I.; Tejero, T.; Cardona, F.; Marradi, M.; Faggi, E.; Parmeggiani, C.; Goti, A. Nucleophilic additions to cyclic nitrones en route to iminocyclitols—total syntheses of DMDP, 6-deoxy-DMDP, DAB-1, CYB-3, nectrisine, and radicamine B. Eur. J. Org. Chem. 2008, 2008, 2929–2947. [Google Scholar] [CrossRef]
- Parmeggiani, C.; Catarzi, S.; Matassini, C.; D’Adamio, G.; Morrone, A.; Goti, A.; Paoli, P.; Cardona, F. Human acid β-glucosidase inhibition by carbohydrate derived iminosugars: Towards new pharmacological chaperones for gaucher disease. Chembiochem 2015, 16, 2054–2064. [Google Scholar] [CrossRef] [PubMed]
- Coutrot, F.; Busseron, E. Controlling the chair conformation of a mannopyranose in a large-amplitude [2]rotaxane molecular machine. Chem. Eur. J. 2009, 15, 5186–5190. [Google Scholar] [CrossRef] [PubMed]
- Chabre, Y.M.; Giguère, D.; Blanchard, B.; Rodrigue, J.; Rocheleau, S.; Neault, M.; Rauthu, S.; Papadopoulos, A.; Arnold, A.A.; Imberty, A.; et al. Combining glycomimetic and multivalent strategies toward designing potent bacterial lectin inhibitors. Chem. Eur. J. 2011, 17, 6545–6562. [Google Scholar] [CrossRef]
- Mirabella, S.; D’Adamio, G.; Matassini, C.; Goti, A.; Delgado, S.; Gimeno, A.; Robina, I.; Moreno-Vargas, A.J.; Šesták, S.; Jiménez-Barbero, J.; et al. Mechanistic insight into the binding of multivalent pyrrolidines to α-mannosidases. Chem. Eur. J. 2017, 23, 14585–14596. [Google Scholar] [CrossRef]
- Martínez-Bailén, M.; Jiménez-Ortega, E.; Carmona, A.T.; Robina, I.; Sanz-Aparicio, J.; Talens-Perales, D.; Polaina, J.; Matassini, C.; Cardona, F.; Moreno-Vargas, A.J. Structural basis of the inhibition of gh1 β-glucosidases by multivalent pyrrolidine iminosugars. Bioorganic Chem. 2019, 89, 103026. [Google Scholar] [CrossRef]
- Veliks, J.; Seifert, H.M.; Frantz, D.K.; Klosterman, J.K.; Tseng, J.-C.; Linden, A.; Siegel, J.S. Towards the molecular borromean link with three unequal rings: Double-threaded ruthenium(II) ring-in-ring complexes. Org. Chem. Front. 2016, 3, 667–672. [Google Scholar] [CrossRef]
- Chabre, Y.M.; Contino-Pépin, C.; Placide, V.; Shiao, T.C.; Roy, R. Expeditive synthesis of glycodendrimer scaffolds based on versatile tris and mannoside derivatives. J. Org. Chem. 2008, 73, 5602–5605. [Google Scholar] [CrossRef]
- Martinez-Bailen, M.; Carmona, A.T.; Moreno-Clavijo, E.; Robina, I.; Ide, D.; Kato, A.; Moreno-Vargas, A.J. Tuning of β-glucosidase and α-galactosidase inhibition by generation and in situ screening of a library of pyrrolidine-triazole hybrid molecules. Eur. J. Med. Chem. 2017, 138, 532–542. [Google Scholar] [CrossRef]
- Martinez-Bailen, M.; Carmona, A.T.; Patterson-Orazem, A.C.; Lieberman, R.L.; Ide, D.; Kubo, M.; Kato, A.; Robina, I.; Moreno-Vargas, A.J. Exploring substituent diversity on pyrrolidine-aryltriazole iminosugars: Structural basis of β-glucocerebrosidase inhibition. Bioorganic Chem. 2019, 86, 652–664. [Google Scholar] [CrossRef]
- Tunstad, L.M.; Tucker, J.A.; Dalcanale, E.; Weiser, J.; Bryant, J.A.; Sherman, J.C.; Helgeson, R.C.; Knobler, C.B.; Cram, D.J. Host-guest complexation. 48. Octol building blocks for cavitands and carcerands. J. Org. Chem. 1989, 54, 1305–1312. [Google Scholar] [CrossRef]
- Gaeta, C.; Della Sala, P.; Talotta, C.; De Rosa, M.; Soriente, A.; Brancatelli, G.; Geremia, S.; Neri, P. A tetrasulfate-resorcin [6]arene cavitand as the host for organic ammonium guests. Org. Chem. Front. 2016, 3, 1276–1280. [Google Scholar] [CrossRef]
- Brancatelli, G.; Geremia, S.; Gaeta, C.; Della Sala, P.; Talotta, C.; De Rosa, M.; Neri, P. Solid-state assembly of a resorcin [6]arene in twin molecular capsules. CrystEngComm 2016, 18, 5045–5049. [Google Scholar] [CrossRef]
- Della Sala, P.; Gaeta, C.; Navarra, W.; Talotta, C.; De Rosa, M.; Brancatelli, G.; Geremia, S.; Capitelli, F.; Neri, P. Improved synthesis of larger resorcinarenes. J. Org. Chem. 2016, 81, 5726–5731. [Google Scholar] [CrossRef] [PubMed]
- Timmerman, P.; Verboom, W.; Reinhoudt, D.N. Resorcinarenes. Tetrahedron 1996, 52, 2663–2704. [Google Scholar] [CrossRef]
- Moloney, D.J.; Shair, L.H.; Lu, F.M.; Xia, J.; Locke, R.; Matta, K.L.; Haltiwanger, R.S. Mammalian notch1 is modified with two unusual forms of O-linked glycosylation found on epidermal growth factor-like modules. J. Biol. Chem. 2000, 275, 9604–9611. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.; Al-Shareffi, E.; Haltiwanger, R.S. Biological functions of fucose in mammals. Glycobiology 2017, 27, 601–618. [Google Scholar] [CrossRef]
- Listinsky, J.J.; Siegal, G.P.; Listinsky, C.M. A-l-fucose: A potentially critical molecule in pathologic processes including neoplasia. Am. J. Clin. Pathol. 1998, 110, 425–440. [Google Scholar] [CrossRef]
- Lopes, G.; Ho, C.K.; Liau, K.H.; Chung, A.; Cheow, P.; Chang, A.Y. Gefitinib in the adjuvant treatment of hepatocellular carcinoma: A pilot study by the singapore hepatocellular carcinoma consortium. J. Clin. Oncol. 2010, 28, 210. [Google Scholar] [CrossRef]
- Ayude, D.; de la Cadena, M.P.; Cordero, O.J.; Nogueira, M.; Ayude, J.; Fernández-Briera, A.; Rodríguez-Berrocal, F.J. Clinical interest of the combined use of serum CD26 and alpha-L-fucosidase in the early diagnosis of colorectal cancer. Dis. Markers 2004, 19, 834309. [Google Scholar] [CrossRef]
- Ayude, D.; Páez de la Cadena, M.; Martínez-Zorzano, V.S.; Fernández-Briera, A.; Rodríguez-Berrocal, F.J. Preoperative serum alpha-L-fucosidase activity as a prognostic marker in colorectal cancer. Oncology 2003, 64, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.-W.; Ho, C.-W.; Huang, H.-H.; Chang, S.-M.; Popat Shide, D.; Wang, Y.-T.; Wu, M.-S.; Chen, Y.-J.; Lin, C.-H. Role for α-L-fucosidase in the control of helicobacter pylori-infected gastric cancer cells. Proc. Natl. Acad. Sci. USA 2009, 106, 14581–14586. [Google Scholar] [CrossRef] [PubMed]
- Kotland, A.; Accadbled, F.; Robeyns, K.; Behr, J.-B. Synthesis and fucosidase inhibitory study of unnatural pyrrolidine alkaloid 4-epi-(+)-codonopsinine. J. Org. Chem. 2011, 76, 4094–4098. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Chang, C.-F.; Chen, J.S.-Y.; Wong, C.-H.; Lin, C.-H. Rapid diversity-oriented synthesis in microtiter plates for in situ screening: Discovery of potent and selective α-fucosidase inhibitors. Angew. Chem. Int. Ed. 2003, 42, 4661–4664. [Google Scholar] [CrossRef] [PubMed]
- Gnanesh Kumar, B.S.; Pohlentz, G.; Schulte, M.; Mormann, M.; Siva Kumar, N. Jack bean α-mannosidase: Amino acid sequencing and N-glycosylation analysis of a valuable glycomics tool. Glycobiology 2014, 24, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Daniel, P.F.; Winchester, B.; Warren, C.D. Mammalian α-mannosidases—multiple forms but a common purpose? Glycobiology 1994, 4, 551–566. [Google Scholar] [CrossRef]
- Berardi, A.C.; Manieri, P.; Ciraci, E.; Tribuzi, R.; Di Girolamo, I.; Cavalieri, C.; Isacchi, G.; Emiliani, C.; Bottazzo, G.; Orlacchio, A.; et al. Lysosomal glycohydrolase activities in dendritic cells: Is it a function of hematopoietic stem cells differentiation process? Blood 2004, 104, 4193. [Google Scholar] [CrossRef]
- Solanki, G.A.; Martin, K.W.; Theroux, M.C.; Lampe, C.; White, K.K.; Shediac, R.; Lampe, C.G.; Beck, M.; Mackenzie, W.G.; Hendriksz, C.J.; et al. Spinal involvement in mucopolysaccharidosis IVA (morquio-brailsford or morquio A syndrome): Presentation, diagnosis and management. J. Inherit. Metab. Dis. 2013, 36, 339–355. [Google Scholar] [CrossRef]
- Sanford, M.; Lo, J.H. Elosulfase alfa: First global approval. Drugs 2014, 74, 713–718. [Google Scholar] [CrossRef]
- Platt, F.M. Sphingolipid lysosomal storage disorders. Nature 2014, 510, 68–75. [Google Scholar] [CrossRef]








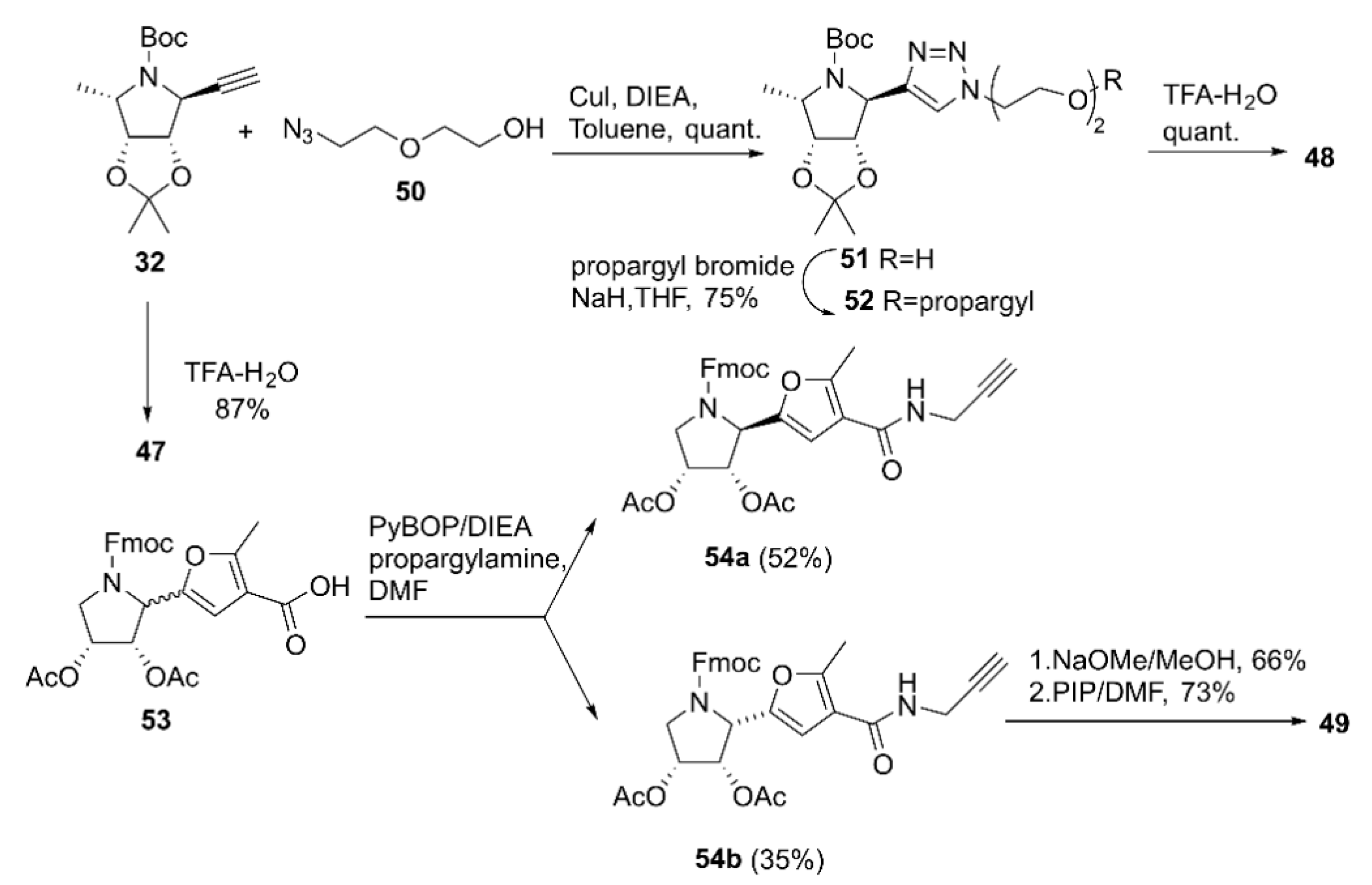
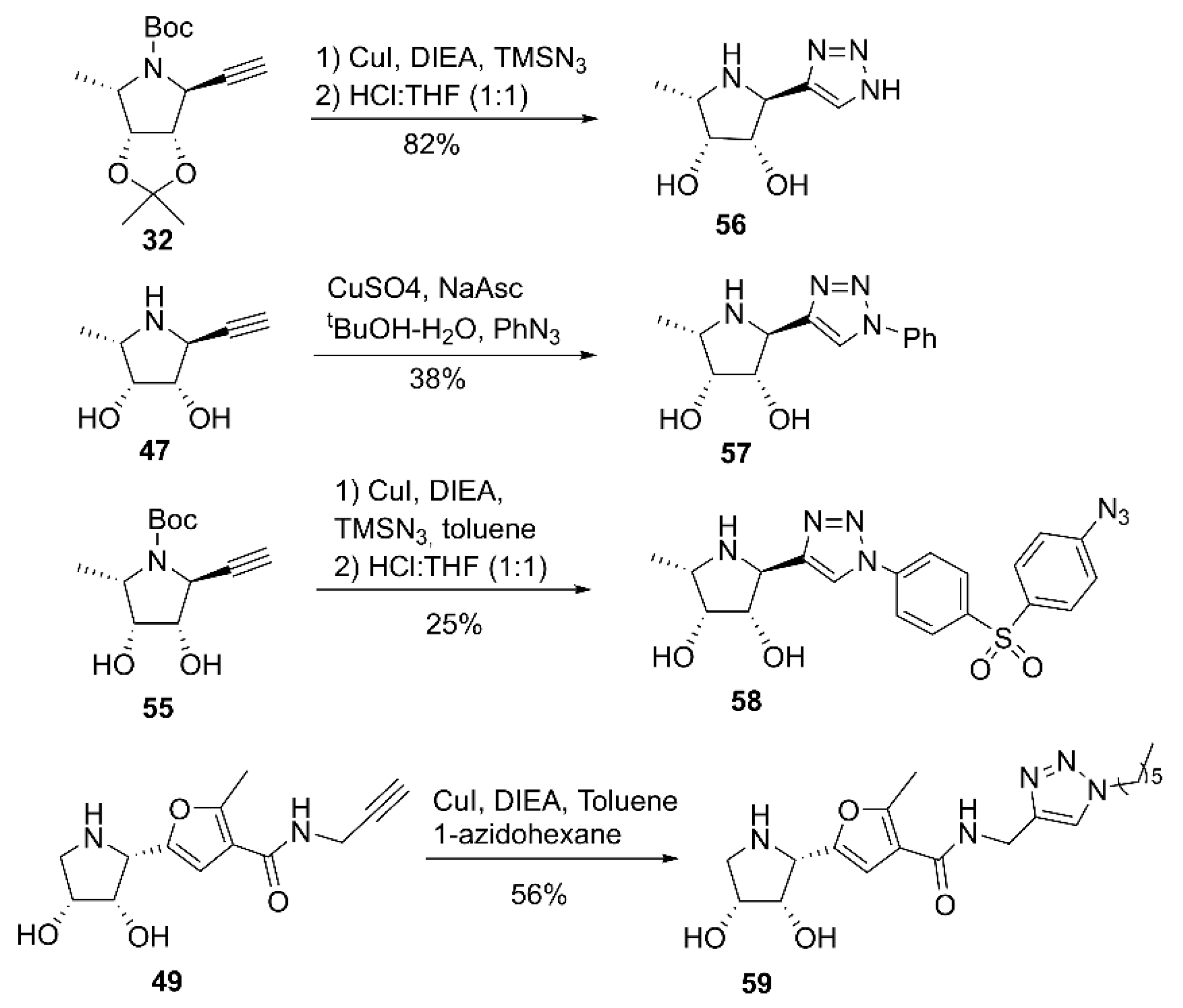

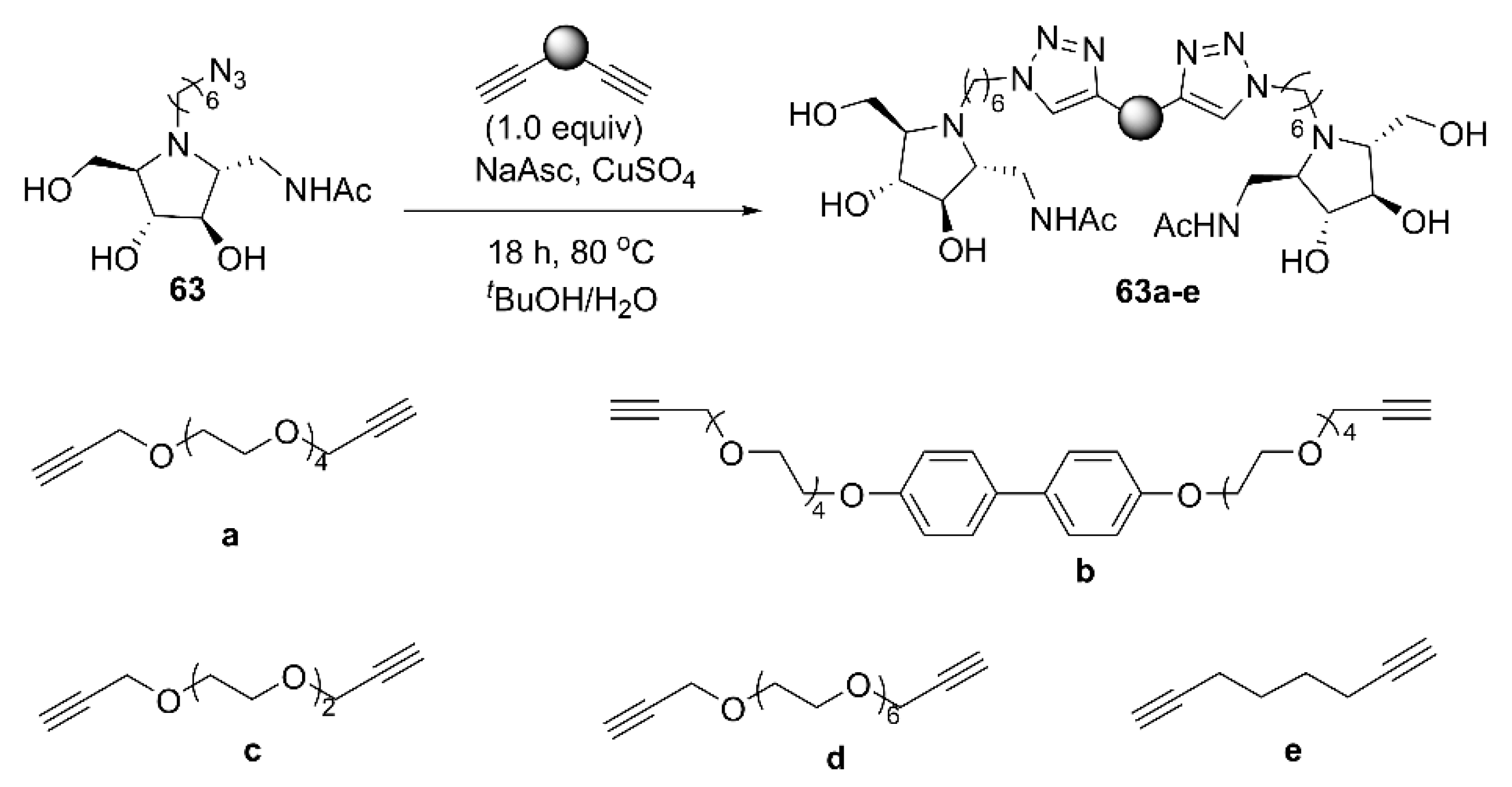
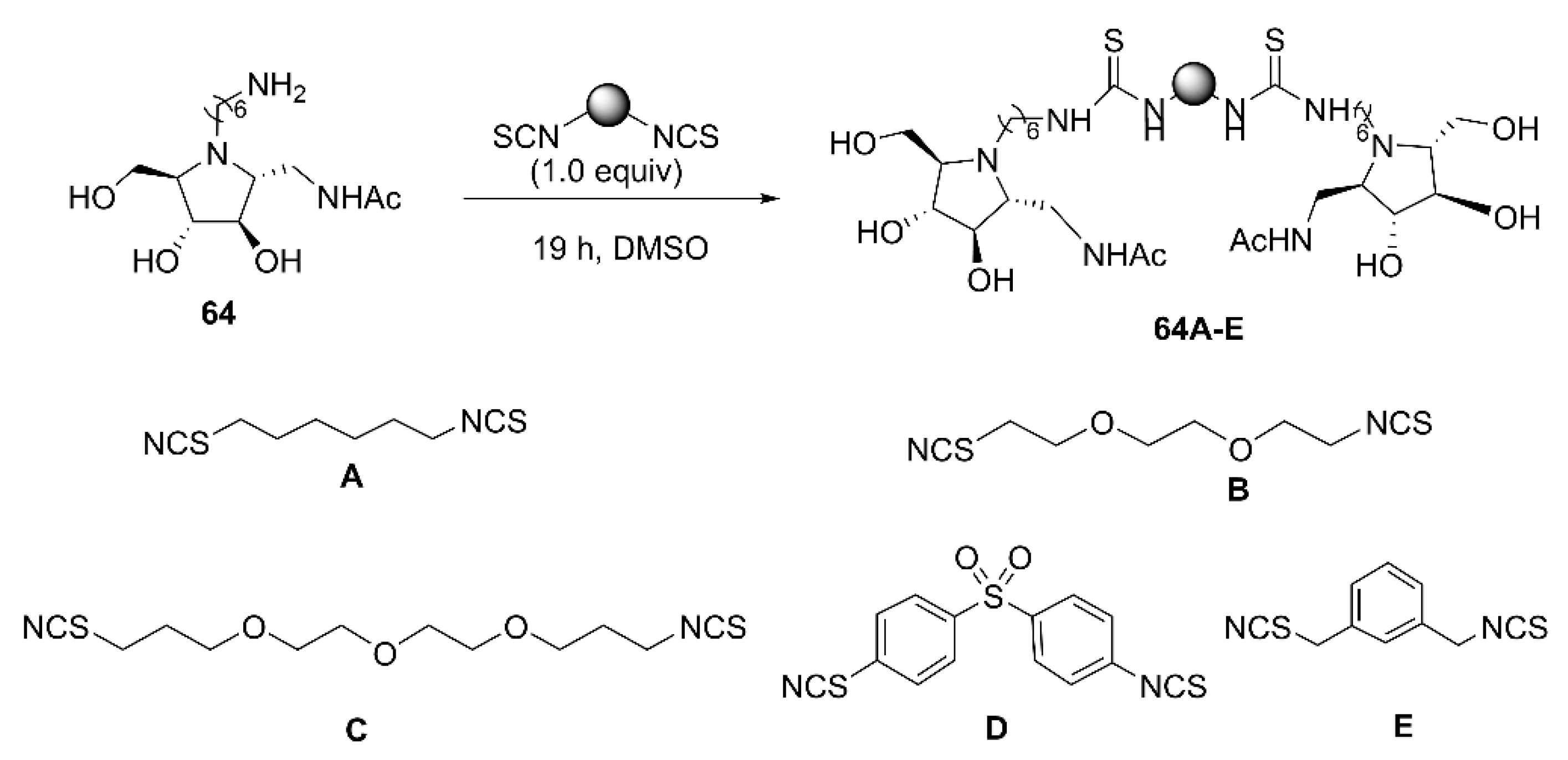







| Enzyme | Compound | Valency (n) | Ki a | IC50 b | Rp c | Rp/n d | Ref. |
|---|---|---|---|---|---|---|---|
| α-fucosidase e | 2 | 3 | 0.3 | 1.6 | 7.0 | 2.3 | [70] |
| “ | 3 | 3 | 0.4 | 3.8 | 5.3 | 1.8 | [70] |
| “ | 13 | 2 | - | 1.2 | 10.8 | 5.4 | [80] |
| “ | 17 | 2 | 0.0037 | 0.074 | 4.1 | 2.1 | [80] |
| “ | 33 | 2 | 0.023 | 0.108 | 7.8 | 3.9 | [84] |
| “ | 47i | 2 | 0.48 × 10−3 | 0.15 × 10−3 | - | - | [89] |
| α-mannosidase f | 83 | 9 | - | 0.095 | 13,684 | 1520 | [107] |
| “ | 147 | 8 | - | 5.3 | 245 | 31 | [68] |
| “ | 148 | 8 | - | 14.8 | 88 | 11 | [68] |
| “ | 149 | 12 | - | 1.2 | 1083 | 90 | [68] |
| “ | 150 | 12 | - | 10.5 | 124 | 10 | [68] |
| Golgi α-mannosidase IIb g | 147 | 8 | -- | 3.7 | 47 | 6 | [68] |
| “ | 148 | 8 | 5.3 | 33 | 4.1 | [68] | |
| “ | 149 | 12 | - | 0.7 | 250 | 21 | [68] |
| “ | 150 | 12 | - | 28.5 | 6 | 0.5 | [68] |
| N-acetylgalactosamine-6-sulfatase h | 83 | 9 | - | 47 | 83 | 9.2 | [67] |
| 84 | 9 | - | 85 | 59 | 6.5 | [67] | |
| iduronate-2-sulfatase i | 83 | 9 | - | 140 | 23 | 2.5 | [67] |
| “ | 84 | 9 | - | 31 | 177 | 19.7 | [67] |
| β-N-acetylhexosaminidase j | 64D | 2 | 168 | - | 1.9 | 0.96 | [69] |
| β-N-acetylglucosaminidase k | 64D | 2 | 0.0061 | - | 7.8 | 3.9 | [69] |
| α-galactosidase A l | 133 | 9 | 0.2 | 1.2 | 378 | 42 | [49] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Xiao, J.; Meng, A.; Liu, C. Multivalent Pyrrolidine Iminosugars: Synthesis and Biological Relevance. Molecules 2022, 27, 5420. https://doi.org/10.3390/molecules27175420
Wang Y, Xiao J, Meng A, Liu C. Multivalent Pyrrolidine Iminosugars: Synthesis and Biological Relevance. Molecules. 2022; 27(17):5420. https://doi.org/10.3390/molecules27175420
Chicago/Turabian StyleWang, Yali, Jian Xiao, Aiguo Meng, and Chunyan Liu. 2022. "Multivalent Pyrrolidine Iminosugars: Synthesis and Biological Relevance" Molecules 27, no. 17: 5420. https://doi.org/10.3390/molecules27175420
APA StyleWang, Y., Xiao, J., Meng, A., & Liu, C. (2022). Multivalent Pyrrolidine Iminosugars: Synthesis and Biological Relevance. Molecules, 27(17), 5420. https://doi.org/10.3390/molecules27175420






