Molecular Design, Preparation, and Characterization of Fluoro-Containing Polyimide Ultrafine Fibrous Membranes with High Whiteness, High Thermal Stability, and Good Hydrophobicity
Abstract
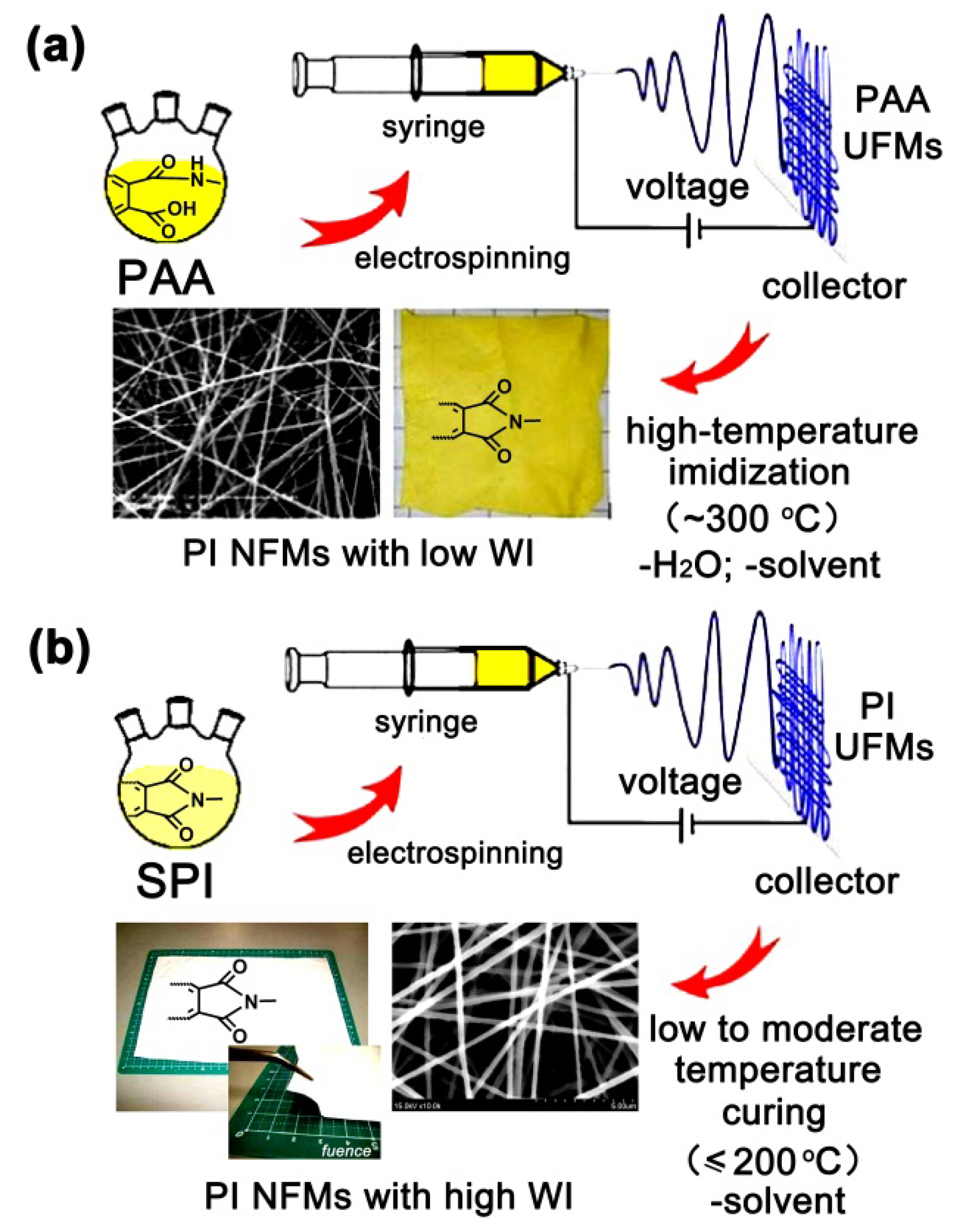
:1. Introduction
2. Results
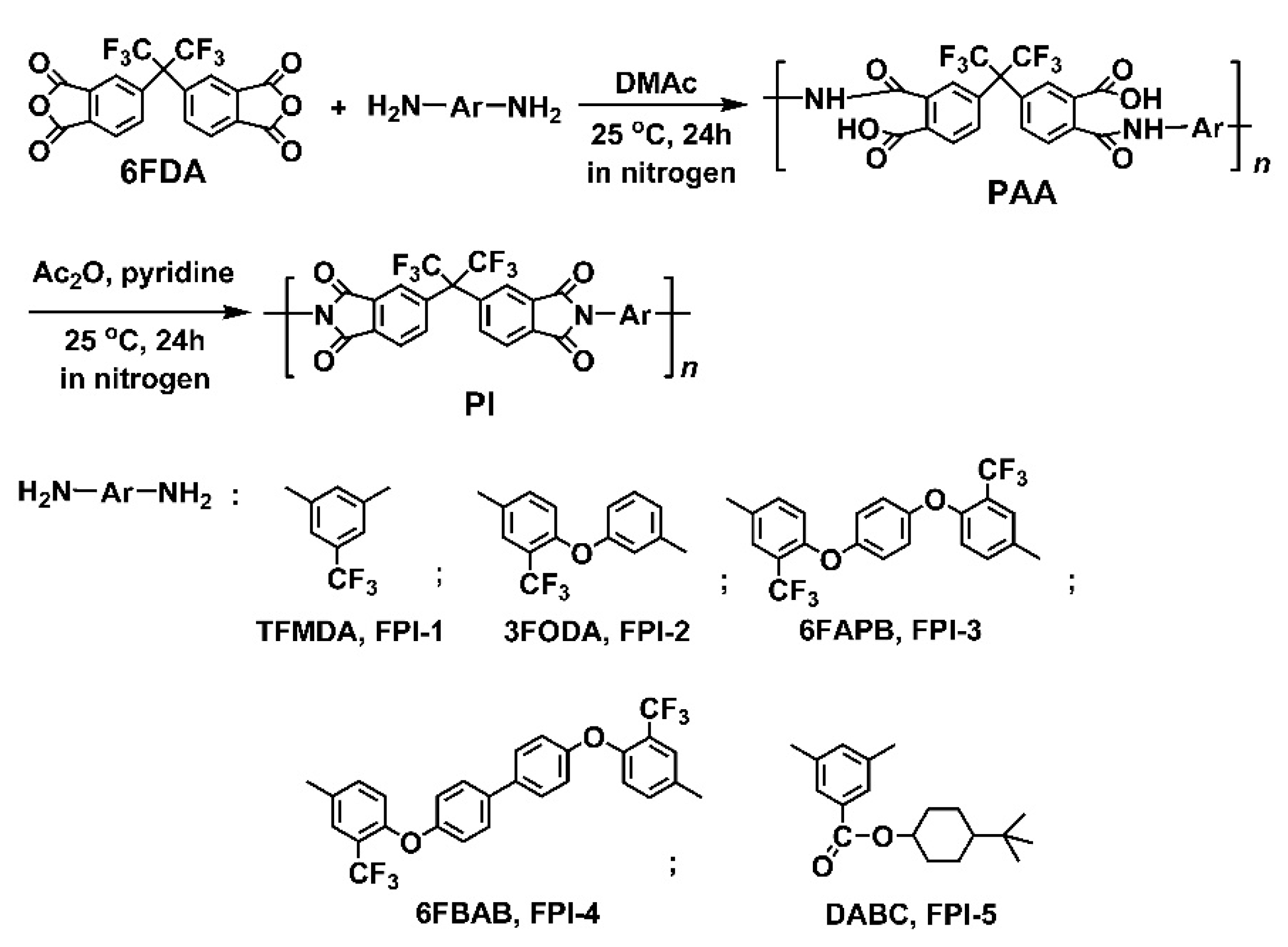
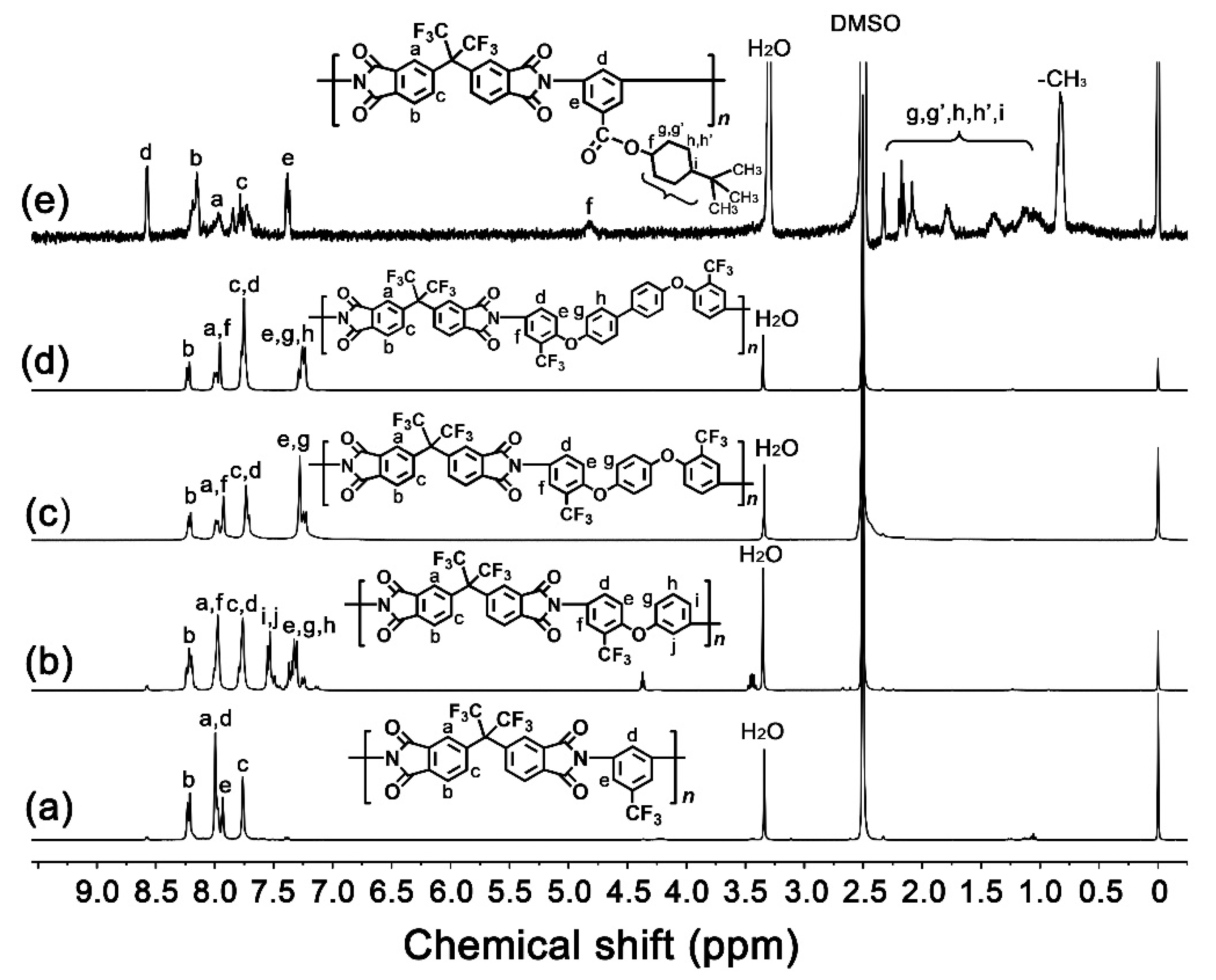
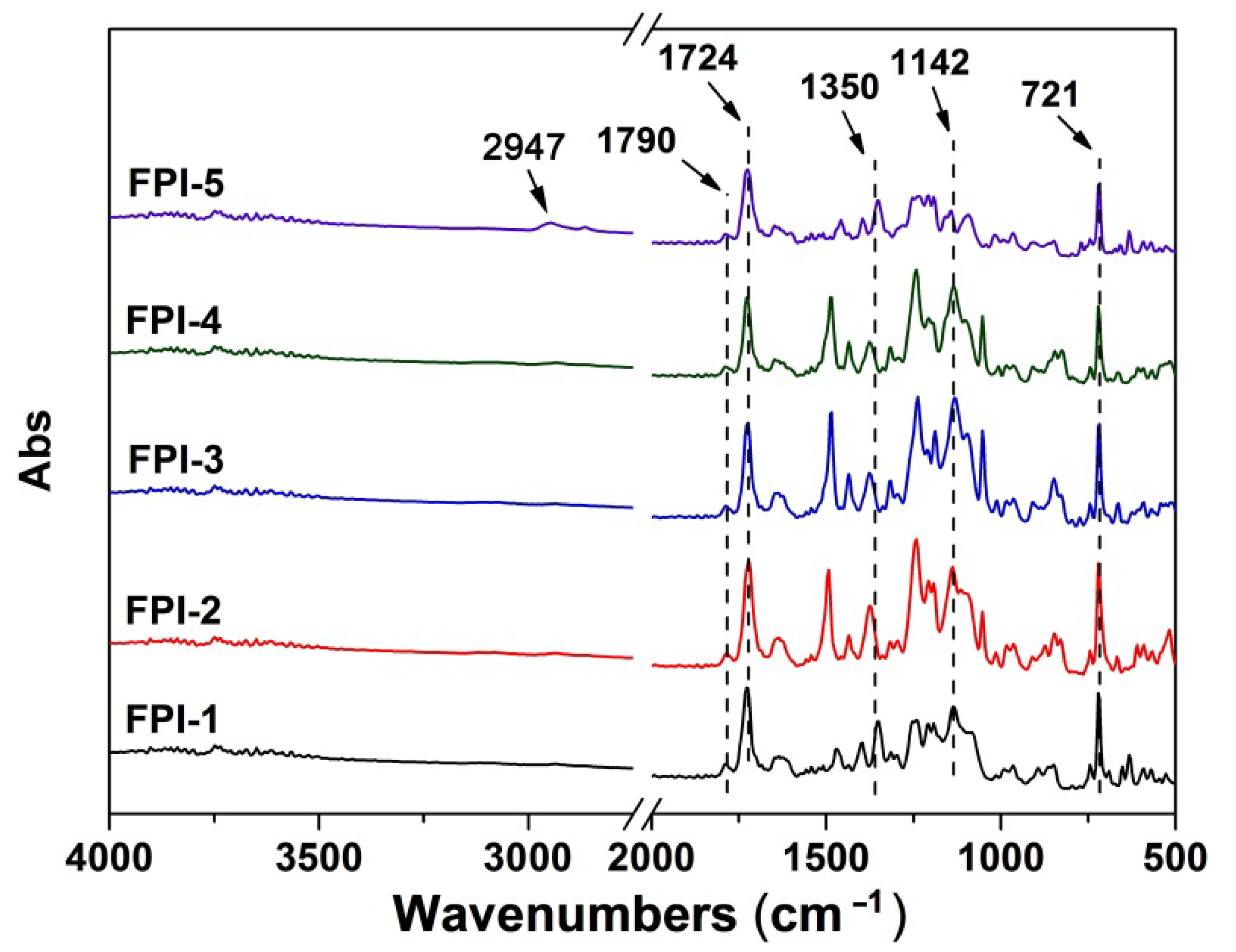
2.1. Structural Characterization
2.2. Optical Properties
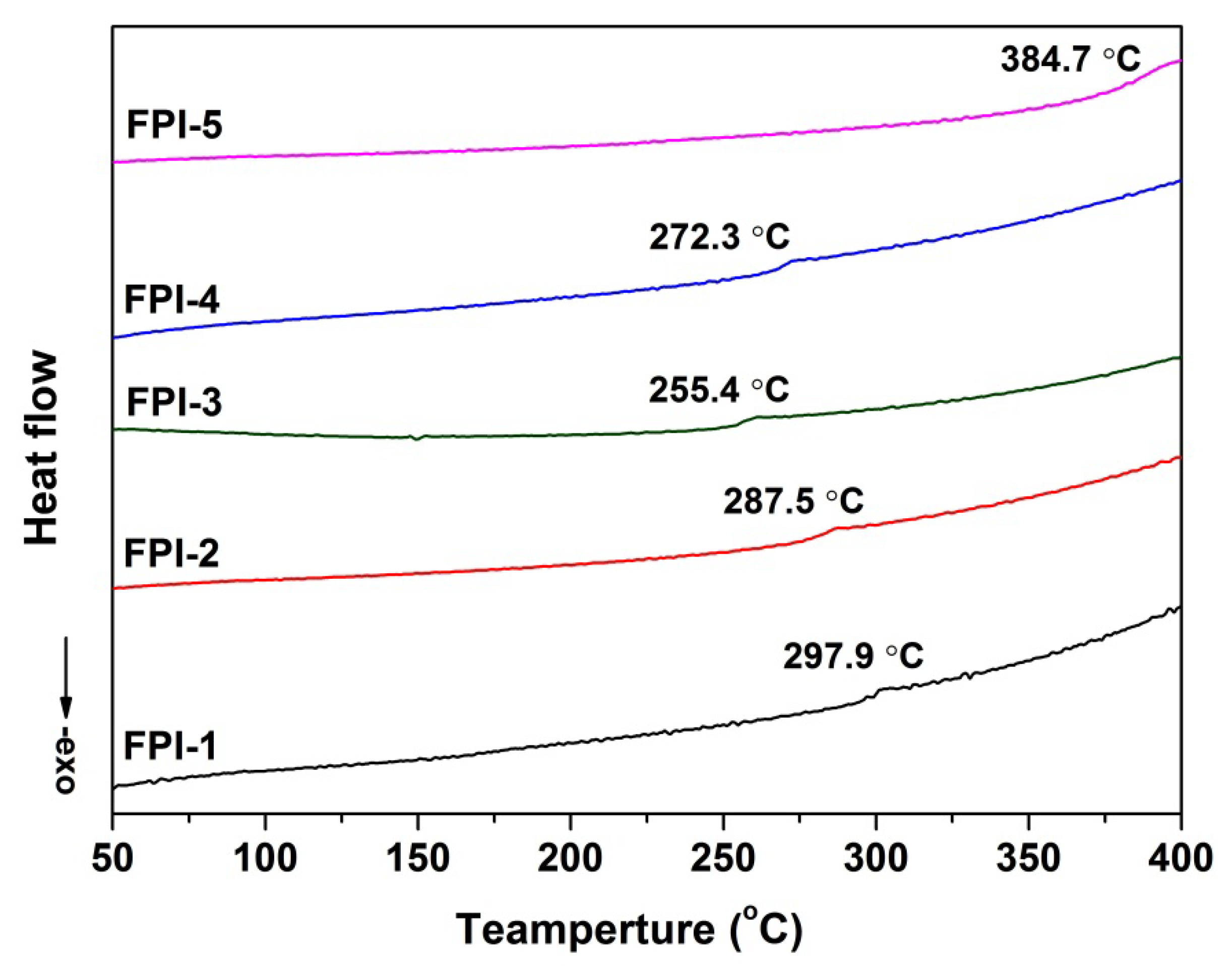
2.3. Thermal Properties
3. Materials and Methods
3.1. Materials
3.2. Measurements
3.3. Synthesis of 4′-tert-butyl-cyclohexyl-3,5-diaminobenzoate (DABC)
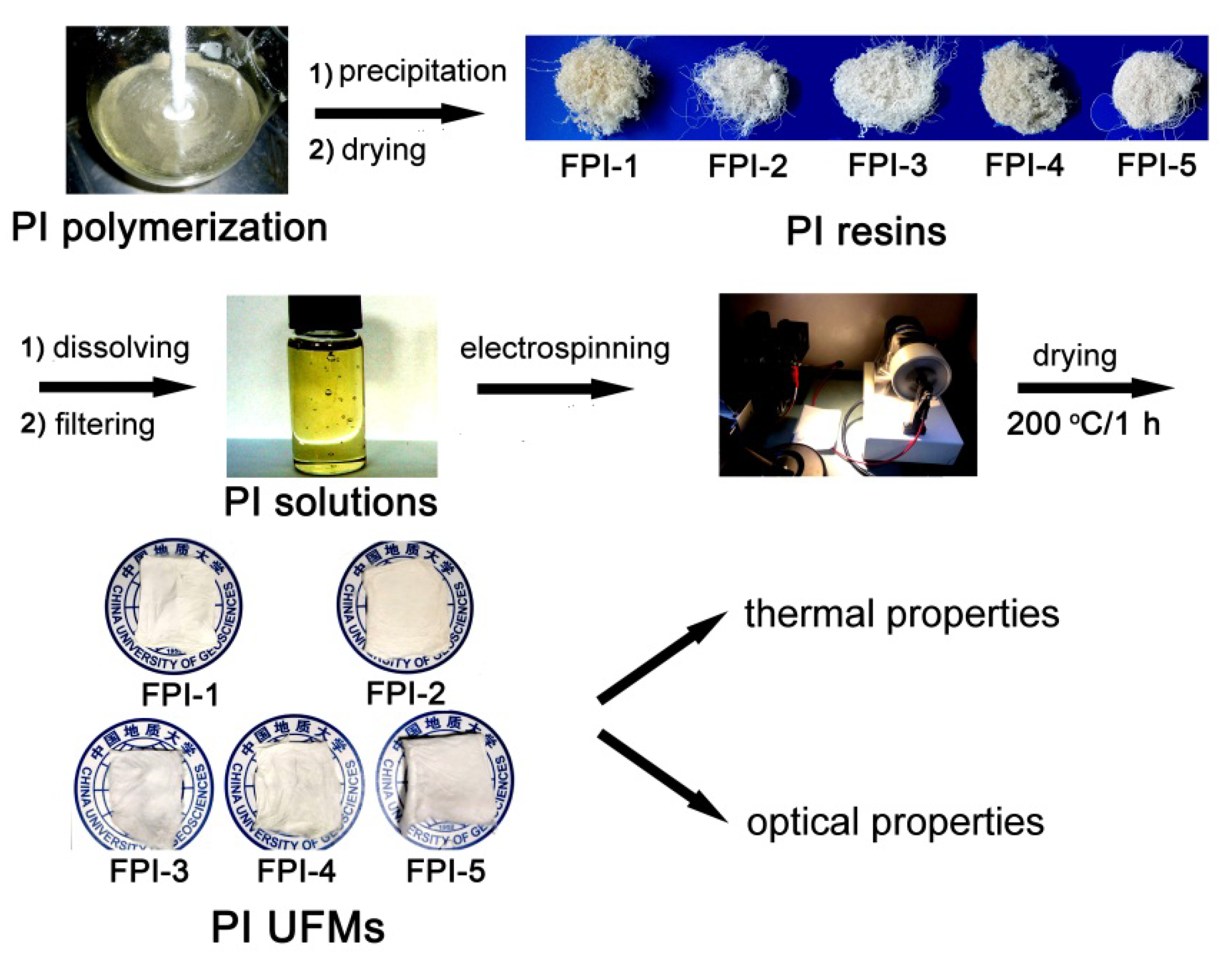
3.4. Preparation of PI Resins and Electrospun UFMs
3.5. Electrospinning Fabrication
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Liao, Y.; Loh, C.; Tian, M.; Wang, R.; Fane, A.G. Progress in electrospun polymeric nanofibrous membranes for water treatment: Fabrication, modification and applications. Prog. Polym. Sci. 2018, 77, 69–94. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, J.; Li, J.; Xiao, C.; Xiao, H.; Yang, H.; Zhuang, X.; Chen, X. Electrospun polymer biomaterials. Prog. Polym. Sci. 2019, 90, 1–34. [Google Scholar] [CrossRef]
- Ahmed, F.E.; Lalia, B.S.; Hashaikeh, R. A review on electrospinning for membrane fabrication: Challenges and applications. Desalination 2015, 356, 15–30. [Google Scholar] [CrossRef]
- Lu, T.; Cui, J.; Qu, Q.; Wang, Y.; Zhang, J.; Xiong, R.; Ma, W.; Huang, C. Multistructured electrospun nanofibers for air filtration: A review. ACS Appl. Mater. Interf. 2021, 13, 23293–23313. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xu, W.; Ding, Y.; Agarwal, S.; Greiner, A.; Duan, G. A review of smart electrospun fibers toward textiles. Compos. Commun. 2020, 22, 100506. [Google Scholar] [CrossRef]
- Guo, H.; Chen, Y.; Li, Y.; Zhou, W.; Xu, W.; Pang, L.; Fan, X.; Jiang, S. Electrospun fibrous materials and their applications for electromagnetic interference shielding: A review. Compos. Part A 2021, 143, 106309. [Google Scholar] [CrossRef]
- Yu, B.; Huang, Z.; Fang, D.; Yu, S.; Fu, T.; Tang, Y.; Li, Z. Biomimetic porous fluoropolymer films with brilliant whiteness by using polymerization-induced phase separation. Adv. Mater. Interf. 2022, 9, 2101485. [Google Scholar] [CrossRef]
- Tang, Y.; Liang, G.; Chen, J.; Yu, S.; Li, Z.; Rao, L.; Yu, B. Highly reflective nanofiber films based on electrospinning and their application on color uniformity and luminous efficacy improvement of white light-emitting diodes. Opt. Exp. 2017, 25, 20598–20611. [Google Scholar] [CrossRef]
- Li, X.; Zhou, J.; Quan, Z.; Wang, L.; Li, F.; Qin, X.; Yu, J. Light scattering tunability of nanofiber membrane for enhancing color yield. Dye. Pigment. 2021, 193, 109462. [Google Scholar] [CrossRef]
- Pedicini, A.; Farris, R.J. Thermally induced color change in electrospun fiber mats. J. Polym. Sci. Part B: Polym. Phys. 2004, 42, 752–757. [Google Scholar] [CrossRef]
- Yip, J.; Ng, S.P.; Wong, K.H. Brilliant whiteness surfaces from electrospun nanofiber webs. Text. Res. J. 2009, 79, 771–779. [Google Scholar] [CrossRef]
- Costoya, A.; Concheiro, A.; Alvarez-Lorenzo, C. Electrospun fibers of cyclodextrins and poly(cyclodextrins). Molecules 2017, 22, 230. [Google Scholar] [CrossRef]
- Xu, S.C.; Qin, C.C.; Yu, M.; Dong, R.H.; Yan, X.; Zhao, H.; Han, W.P.; Zhang, H.D.; Long, Y.Z. A battery-operated portable handheld electrospinning apparatus. Nanoscale 2015, 7, 12351–12355. [Google Scholar] [CrossRef]
- Wong, W.S.Y.; Li, M.; Nisbet, D.R.; Craig, V.S.J.; Wang, Z.; Tricoli, A. Mimosa origami: A nanostructure-enabled directional self-organization regime of materials. Sci. Adv. 2016, 2, e1600417. [Google Scholar] [CrossRef]
- Ge, J.C.; Choi, N.J. Fabrication of functional polyurethane/rare earth nanocomposite membranes by electrospinning and its VOCs absorption capacity from air. Nanomaterials 2017, 7, 60. [Google Scholar] [CrossRef]
- Ding, Y.; Hou, H.; Zhao, Y.; Zhu, Z.; Fong, H. Electrospun polyimide nanofibers and their applications. Prog. Polym. Sci. 2016, 61, 67–103. [Google Scholar] [CrossRef]
- Guo, C.Y.; Yin, L.M.; Liu, J.G.; Wang, X.K.; Zhang, N.; Qi, L.; Zhang, Y.; Wu, X.; Zhang, X.M. Electrospun polyphenylquinoxaline ultrafine non-woven fibrous membranes with excellent thermal and alkaline resistance: Preparation and characterization. Fibers Polym. 2019, 20, 2485–2492. [Google Scholar] [CrossRef]
- Yin, L.M.; Guo, C.Y.; Qi, L.; Wang, X.K.; Zhang, N.; Wu, L.; Liu, J.G. Electrospun polyphenylquinoxaline fibrous membrane: A versatile filtering medium for separation of highly alkaline aqueous red mud pollutant. J. Polym. Res. 2020, 27, 321. [Google Scholar] [CrossRef]
- Yin, L.M.; Yang, M.W.; Liu, Q.; Li, X.; Zhang, Y.; Zhi, X.X.; Qi, H.R.; Liu, J.G.; Lv, F.Z. Electrospun polyimide ultrafine non-woven fibrous membranes containing phenyl-substituted quinoxaline units with improved hydrolysis resistance for potential applications in high-temperature water environments. Fibers Polym. 2022, 23, 37–47. [Google Scholar] [CrossRef]
- Guo, C.Y.; Wang, Q.W.; Liu, J.G.; Qi, L.; Huangfu, M.G.; Wu, X.; Zhang, Y.; Zhang, X.M. Electrospun polyimide ultrafine non-woven fabrics with high whiteness and good thermal stability from organo-soluble semi-alicyclic polyimides: Preparation and properties. Express Polym. Lett. 2019, 13, 724–738. [Google Scholar] [CrossRef]
- Guo, C.Y.; Liu, J.G.; Yin, L.M.; Huangfu, M.G.; Zhang, Y.; Wu, X.; Zhang, X.M. Preparation and characterization of electrospun polyimide microfibrous mats with high whiteness and high thermal stability from organo-soluble polyimides containing rigid-rod moieties. Fibers Polym. 2018, 19, 1706–1714. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Wang, Q.; Wei, Q. Effects of imidization temperature on the structure and properties of electrospun polyimide nanofibers. J. Eng. Fibers Fabr. 2014, 9, 33–38. [Google Scholar] [CrossRef]
- Goponenko, A.V.; Hou, H.Q.; Dzenis, Y.A. Avoiding fusion of electrospun 3,3′,4,4′-biphenyltetracarboxylic dianhydride-4,4′-oxydianiline copolymer nanofibers during conversion to polyimide. Polymer 2011, 52, 3776–3782. [Google Scholar] [CrossRef]
- Guo, C.Y.; Wu, X.; Liu, J.G.; Zhang, Y.; Zhang, X.M. Preparation and properties of high-whiteness polyimide ultrafine fabrics by electrospinning from organo-soluble semi-alicyclic polyimide resins. J. Photopolym. Sci. Technol. 2018, 31, 27–36. [Google Scholar] [CrossRef]
- Qi, H.R.; Zhang, Y.; Zhi, X.X.; Qi, L.; Wu, H.; Wei, X.Y.; Liu, J.G. Preparation and properties of electrospun polyimide ultrafine fibrous mats with excellent heat-fusibility via hot-press procedure from organo-soluble polyimides containing phenolphthalein units. Fibers Polym. 2022, 23, 68–76. [Google Scholar] [CrossRef]
- Yi, B.; Zhao, Y.; Tian, E.; Li, J.; Ren, Y. High-performance polyimide nanofiber membranes prepared by electrospinning. High Perform. Polym. 2019, 31, 438–448. [Google Scholar] [CrossRef]
- Dhara, M.G.; Banerjee, S. Fluorinated high-performance polymers: Poly(arylene ether)s and aromatic polyimides containing trifluoromethyl groups. Prog. Polym. Sci. 2010, 35, 1022–1077. [Google Scholar] [CrossRef]
- Wang, R.; Cao, C.; Chung, T.S. A critical review on diffusivity and the characterization of diffusivity of 6FDA-6FpDA polyimide membranes for gas separation. J. Membr. Sci. 2002, 198, 259–271. [Google Scholar] [CrossRef]
- Ni, H.J.; Liu, J.G.; Wang, Z.H.; Yang, S.Y. A review on colorless and optically transparent polyimide films: Chemsitry, process and engineering applications. J. Ind. Eng. Chem. 2015, 28, 16–27. [Google Scholar] [CrossRef]
- Zhi, X.X.; Wang, H.S.; Wei, X.Y.; Zhang, Y.; An, Y.C.; Qi, H.R.; Liu, J.G. Electrospun semi-alicyclic polyimide nanofibrous membrane: High-reflectance and high-whiteness with superior thermal and ultraviolet radiation stability for potential applications in high-power UV-LEDs. Nanomaterials 2021, 11, 1977. [Google Scholar] [CrossRef]
- Gong, G.; Wu, J.; Zhao, Y.; Liu, J.; Jin, X.; Jiang, L. A novel fluorinated polyimide surface with petal effect produced by electrospinning. Soft Matter 2014, 10, 549–552. [Google Scholar]
- You, N.H.; Chueh, C.C.; Liu, C.L.; Ueda, M.; Chen, W.C. Synthesis and memory device characteristics of new sulfur donor containing polyimides. Macromolecules 2009, 42, 4456–4463. [Google Scholar] [CrossRef]
- Wang, K.L.; Liu, Y.L.; Shih, I.H.; Neoh, K.G.; Kang, E.T. Synthesis of polyimide containing triphenylamine-substituted triazole moieties for polymer memory applications. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 5790–5800. [Google Scholar] [CrossRef]
- Lasprilla-Botero, J.; Alcarez-Lainez, M.; Lagaron, J.M. The influence of electrospinning parameters and solvent selection on the morphology and diameter of polyimide nanofibers. Mater. Today Commun. 2018, 14, 1–9. [Google Scholar] [CrossRef]
- Estedlali, Z.; Aroujalian, A.; Salimi, P. Investigating performance of fabricated electrospun polyethersulfone/Zeolite Y composite nanofibers in concentrate lemon juice by the osmotic membrane distillation process. J. Food Proc. Eng. 2021, 44, e13829. [Google Scholar] [CrossRef]
- Tian, Y.; Zhu, S.; Di, Y.; Liu, H.; Yao, H.; Zhang, Y.; Guan, S. HOMO-controlled donor-acceptor contained polyimide for nonvolatile resistive memory device. Dye. Pigment. 2021, 186, 109020. [Google Scholar] [CrossRef]
- Cheng, S.X.; Chung, T.S.; Wang, R.; Vora, R.H. Gas-sorption properties of 6FDA-durene/1,4- phenylenediamine (pPDA) and 6FDA-durene/1,3-phenylenediamine (mPDA) copolyimides. J. Appl. Polym. Sci. 2003, 90, 2187–2193. [Google Scholar] [CrossRef]
- Tsuda, Y.; Kojima, M.; Oh, J.M. Soluble polyimides based on diaminobenzoic acid alkylester. Polym. J. 2006, 38, 1043–1054. [Google Scholar] [CrossRef]
- Li, P.; Liu, L.; Ding, L.; Lv, F.; Zhang, Y. Thermal and dielectric properties of electrospun fiber membranes from polyimides with different structural units. J. Appl. Polym. Sci. 2016, 133, 43081. [Google Scholar] [CrossRef]



| PI | [η]inh a (dL/g) | Molecular Weight | Solubility | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mn (×104 g/mol) | Mw (×104 g/mol) | PDI | NMP | DMAc | GBL | CPA | CHCl3 | ||
| FPI-1 | 0.84 | 5.37 | 10.56 | 1.65 | ++ | ++ | ++ | ++ | ++ |
| FPI-2 | 0.97 | 6.45 | 11.63 | 1.80 | ++ | ++ | ++ | ++ | ++ |
| FPI-3 | 1.13 | 10.12 | 20.48 | 2.02 | ++ | ++ | ++ | ++ | + |
| FPI-4 | 1.16 | 10.21 | 17.70 | 1.73 | ++ | ++ | ++ | ++ | + |
| FPI-5 | 1.22 | 28.70 | 47.47 | 1.65 | ++ | ++ | ++ | + | + |
| PI | dav (nm) | WCA (°) | Optical Properties | Thermal Properties | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| R457 (%) | L* | A* | B* | WI | T5% (°C) | Tmax (°C) | Rw700 (%) | Tg (°C) | |||
| FPI-1 | 360 | 134.5 ± 2.1 | 91.8 | 92.28 | −0.65 | 2.64 | 91.82 | 569.6 | 651.8 | 53.6 | 297.9 |
| FPI-2 | 665 | 130.5 ± 1.3 | 81.7 | 90.64 | 0.21 | 8.96 | 87.04 | 569.1 | 637.0 | 58.2 | 287.5 |
| FPI-3 | 1937 | 117.5 ± 2.2 | 88.1 | 90.16 | −1.10 | 2.65 | 89.75 | 562.2 | 629.7 | 53.9 | 255.4 |
| FPI-4 | 1037 | 105.5 ± 1.7 | 90.5 | 92.01 | −1.76 | 4.99 | 90.42 | 565.7 | 639.2 | 58.5 | 272.3 |
| FPI-5 | 1363 | 119.8 ± 1.8 | 80.5 | 93.25 | −0.44 | 2.21 | 92.88 | 510.5 | 519.5, 635.0 | 52.1 | 384.7 |
| PI-ref | 993 | 86.2 ± 2.6 | 37.3 | 84.65 | 1.62 | 37.96 | 59.02 | ND | ND | ND | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, Z.; Wang, H.-l.; Qi, H.-r.; Gao, Y.-s.; Wang, X.-l.; Zhi, X.-x.; Zhang, Y.; Ren, X.; Liu, J.-g. Molecular Design, Preparation, and Characterization of Fluoro-Containing Polyimide Ultrafine Fibrous Membranes with High Whiteness, High Thermal Stability, and Good Hydrophobicity. Molecules 2022, 27, 5447. https://doi.org/10.3390/molecules27175447
Pan Z, Wang H-l, Qi H-r, Gao Y-s, Wang X-l, Zhi X-x, Zhang Y, Ren X, Liu J-g. Molecular Design, Preparation, and Characterization of Fluoro-Containing Polyimide Ultrafine Fibrous Membranes with High Whiteness, High Thermal Stability, and Good Hydrophobicity. Molecules. 2022; 27(17):5447. https://doi.org/10.3390/molecules27175447
Chicago/Turabian StylePan, Zhen, Han-li Wang, Hao-ran Qi, Yan-shuang Gao, Xiao-lei Wang, Xin-xin Zhi, Yan Zhang, Xi Ren, and Jin-gang Liu. 2022. "Molecular Design, Preparation, and Characterization of Fluoro-Containing Polyimide Ultrafine Fibrous Membranes with High Whiteness, High Thermal Stability, and Good Hydrophobicity" Molecules 27, no. 17: 5447. https://doi.org/10.3390/molecules27175447







