BCI-215, a Dual-Specificity Phosphatase Inhibitor, Reduces UVB-Induced Pigmentation in Human Skin by Activating Mitogen-Activated Protein Kinase Pathways
Abstract
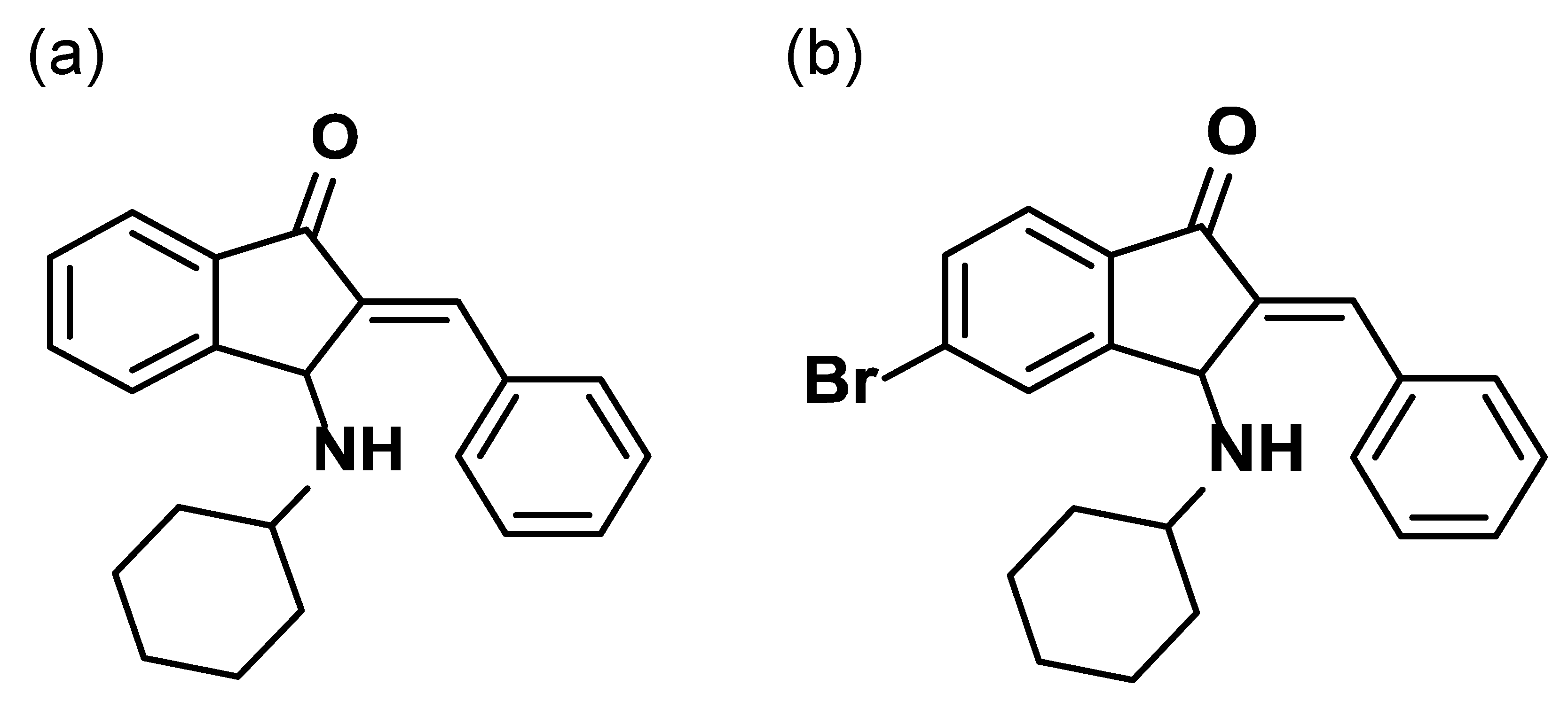
:1. Introduction
2. Results
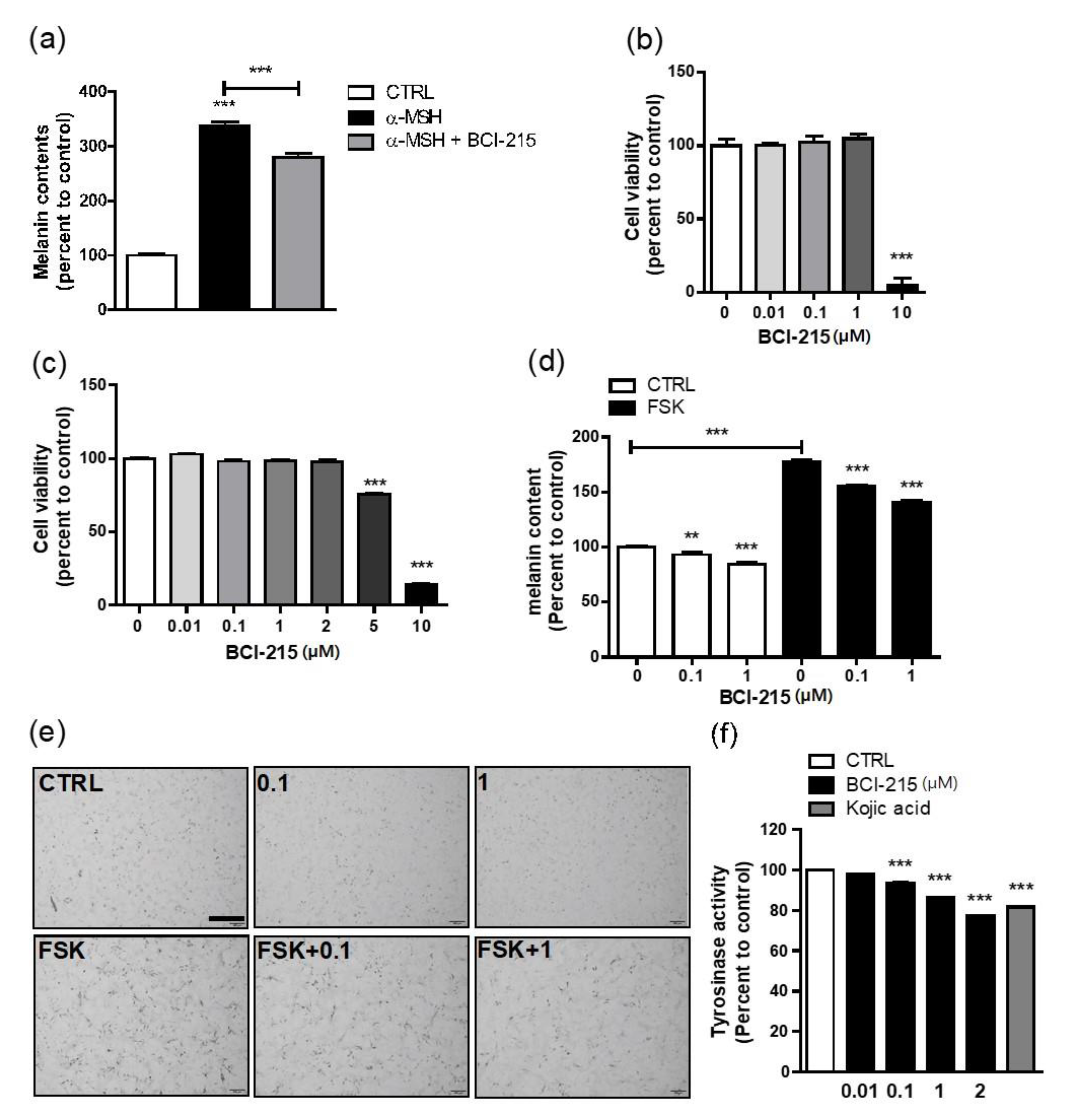
2.1. BCI-215 Suppresses Melanin Content, Which Was Increased by cAMP
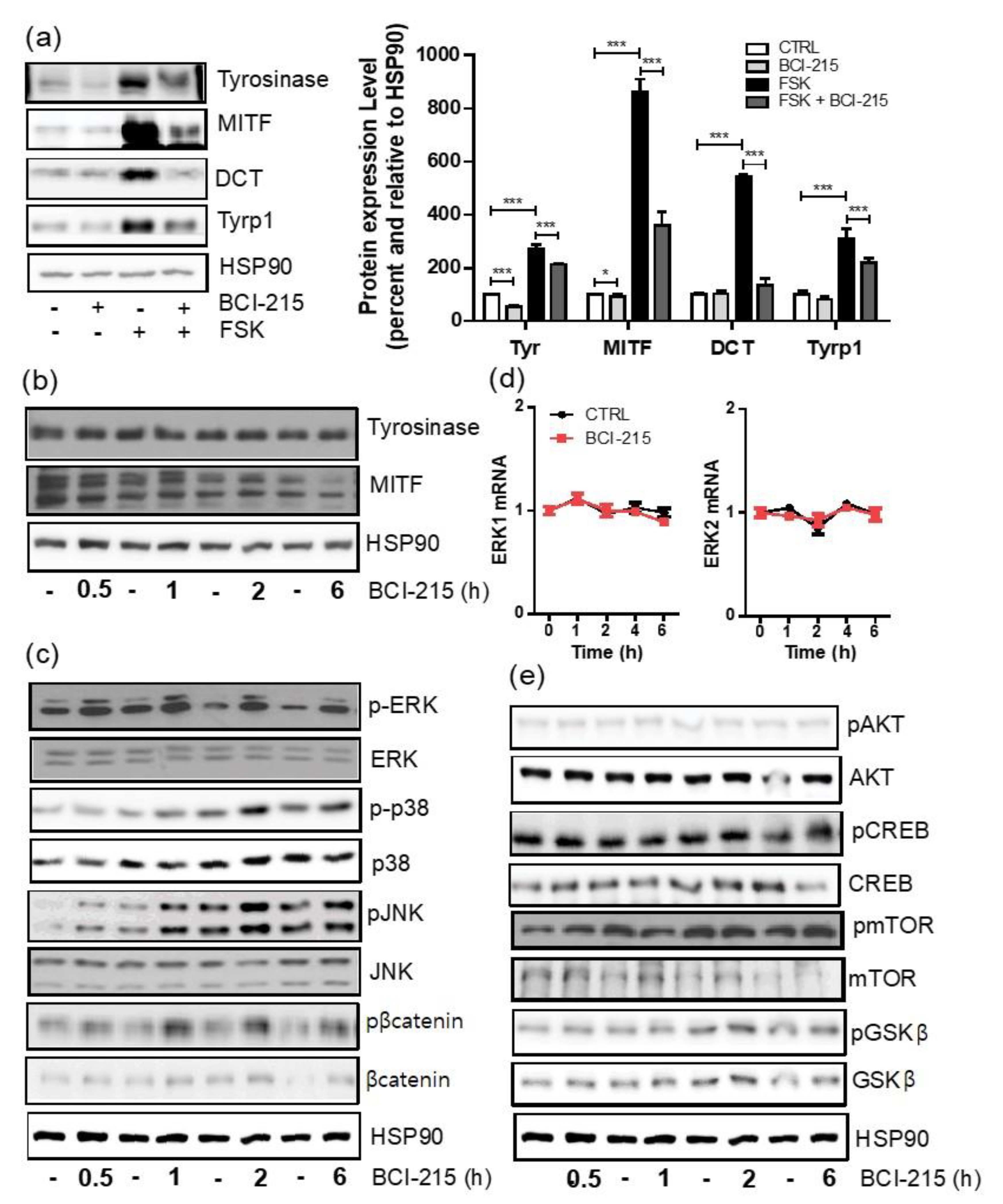
2.2. BCI-215 Does Not Downregulate the Transcription of MITF and Its Downstream Melanogenic Genes in a 24 h Frame
2.3. BCI-215 Downregulates the MITF Protein and Downstream Melanogenic Proteins
2.4. BCI-215 Induces the Activation of All MAPKs, Including ERK, JNK, and p38 in Mel-Ab Cells
2.5. BCI-215 Effectively Reduces Melanogenesis in Human Keratinocyte-Cocultured Melanocytes and Ex Vivo Human Skin
3. Discussion
4. Materials and Methods
4.1. Compounds and Cell Culture Reagents
4.2. Cell Lines and Cell Culture
4.3. Quantitative Real-Time PCR
4.4. Western Blotting and Antibodies
4.5. Cell Treatments and Cytotoxicity
4.6. Melanin Content Measurements
4.7. Cellular Tyrosinase Activity
4.8. Ex Vivo Human Skin Culture and Melanin Index of Human Skin
4.9. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, Y.H.; Kim, D.; Hong, A.R.; Kim, J.H.; Yoo, H.; Kim, J.; Kim, I.; Kang, S.W.; Chang, S.E.; Song, Y. Therapeutic Potential of Rottlerin for Skin Hyperpigmentary Disorders by Inhibiting the Transcriptional Activity of CREB-Regulated Transcription Coactivators. J. Investig. Dermatol. 2019, 139, 2359–2367.e2. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.; Lee, H.R.; Kim, K.H.; Kim, M.A.; Bang, S.; Kang, Y.H.; Kim, W.H.; Song, Y.; Chang, S.E. CRTC3, a sensor and key regulator for melanogenesis, as a tunable therapeutic target for pigmentary disorders. Theranostics 2021, 11, 9918–9936. [Google Scholar] [CrossRef] [PubMed]
- Bastonini, E.; Kovacs, D.; Picardo, M. Skin Pigmentation and Pigmentary Disorders: Focus on Epidermal/Dermal Cross-Talk. Ann. Dermatol. 2016, 28, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Hyter, S.; Coleman, D.J.; Ganguli-Indra, G.; Merrill, G.F.; Ma, S.; Yanagisawa, M.; Indra, A.K. Endothelin-1 is a transcriptional target of p53 in epidermal keratinocytes and regulates ultraviolet-induced melanocyte homeostasis. Pigment Cell Melanoma Res. 2013, 26, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Shibata, T.; Kwon, S.; Park, T.J.; Kang, H.Y. Ultraviolet-irradiated endothelial cells secrete stem cell factor and induce epidermal pigmentation. Sci. Rep. 2018, 8, 4235. [Google Scholar] [CrossRef]
- Choi, M.E.; Yoo, H.; Lee, H.R.; Moon, I.J.; Lee, W.J.; Song, Y.; Chang, S.E. Carvedilol, an Adrenergic Blocker, Suppresses Melanin Synthesis by Inhibiting the cAMP/CREB Signaling Pathway in Human Melanocytes and Ex Vivo Human Skin Culture. Int. J. Mol. Sci. 2020, 21, 8796. [Google Scholar] [CrossRef]
- Lee, H.R.; Jung, J.M.; Seo, J.Y.; Chang, S.E.; Song, Y. Anti-melanogenic property of ginsenoside Rf from Panax ginseng via inhibition of CREB/MITF pathway in melanocytes and ex vivo human skin. J. Ginseng. Res. 2021, 45, 555–564. [Google Scholar] [CrossRef]
- Levy, C.; Khaled, M.; Fisher, D.E. MITF: Master regulator of melanocyte development and melanoma oncogene. Trends Mol. Med. 2006, 12, 406–414. [Google Scholar] [CrossRef]
- Roskoski, R., Jr. ERK1/2 MAP kinases: Structure, function, and regulation. Pharmacol. Res. 2012, 66, 105–143. [Google Scholar] [CrossRef]
- Hemesath, T.J.; Price, E.R.; Takemoto, C.; Badalian, T.; Fisher, D.E. MAP kinase links the transcription factor Microphthalmia to c-Kit signalling in melanocytes. Nature 1998, 391, 298–301. [Google Scholar] [CrossRef]
- Wortzel, I.; Seger, R. The ERK Cascade: Distinct Functions within Various Subcellular Organelles. Genes Cancer 2011, 2, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Hong, A.R.; Kim, Y.H.; Yoo, H.; Kang, S.W.; Chang, S.E.; Song, Y. JNK suppresses melanogenesis by interfering with CREB-regulated transcription coactivator 3-dependent MITF expression. Theranostics 2020, 10, 4017–4029. [Google Scholar] [CrossRef] [PubMed]
- Jeffrey, K.L.; Camps, M.; Rommel, C.; Mackay, C.R. Targeting dual-specificity phosphatases: Manipulating MAP kinase signalling and immune responses. Nat. Rev. Drug Discov. 2007, 6, 391–403. [Google Scholar] [CrossRef]
- Bermudez, O.; Jouandin, P.; Rottier, J.; Bourcier, C.; Pages, G.; Gimond, C. Post-transcriptional regulation of the DUSP6/MKP-3 phosphatase by MEK/ERK signaling and hypoxia. J. Cell Physiol. 2011, 226, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Hemesath, T.J.; Takemoto, C.M.; Horstmann, M.A.; Wells, A.G.; Price, E.R.; Fisher, D.Z.; Fisher, D.E. c-Kit triggers dual phosphorylations, which couple activation and degradation of the essential melanocyte factor Mi. Genes Dev. 2000, 14, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.J.; O’Hare, K.B.; Allen, J.C. Selective cytotoxicity of hydroquinone for melanocyte-derived cells is mediated by tyrosinase activity but independent of melanin content. Pigment Cell Res. 1988, 1, 386–389. [Google Scholar] [CrossRef]
- Garcia-Gavin, J.; Gonzalez-Vilas, D.; Fernandez-Redondo, V.; Toribio, J. Pigmented contact dermatitis due to kojic acid. A paradoxical side effect of a skin lightener. Contact Dermat. 2010, 62, 63–64. [Google Scholar] [CrossRef]
- Lajis, A.F.; Hamid, M.; Ariff, A.B. Depigmenting effect of Kojic acid esters in hyperpigmented B16F1 melanoma cells. J. Biomed. Biotechnol. 2012, 2012, 952452. [Google Scholar] [CrossRef]
- Chaowattanapanit, S.; Silpa-Archa, N.; Kohli, I.; Lim, H.W.; Hamzavi, I. Postinflammatory hyperpigmentation: A comprehensive overview: Treatment options and prevention. J. Am. Acad. Dermatol. 2017, 77, 607–621. [Google Scholar] [CrossRef]
- Kwon, S.H.; Na, J.I.; Huh, C.H.; Park, K.C. A Clinical and Biochemical Evaluation of a Temperature-Controlled Continuous Non-Invasive Radiofrequency Device for the Treatment of Melasma. Ann. Dermatol. 2021, 33, 522–530. [Google Scholar] [CrossRef]
- Mysore, V.; Anitha, B.; Hosthota, A. Successful treatment of laser induced hypopigmentation with narrowband ultraviolet B targeted phototherapy. J. Cutan. Aesthet. Surg. 2013, 6, 117–119. [Google Scholar]
- Saleem, M.D.; Oussedik, E.; Picardo, M.; Schoch, J.J. Acquired disorders with hypopigmentation: A clinical approach to diagnosis and treatment. J. Am. Acad. Dermatol. 2019, 80, 1233–1250.e10. [Google Scholar] [CrossRef] [PubMed]
- White, C.; Miller, R. A Literature Review Investigating the Use of Topical Janus Kinase Inhibitors for the Treatment of Vitiligo. J. Clin. Aesthet. Dermatol. 2022, 15, 20–25. [Google Scholar] [PubMed]
- Yang, H.J.; Lee, W.J.; Lee, M.W.; Choi, J.H.; Chang, S.E. A Case of New-Onset Vitiligo in a Healthy Volunteer After Administration of Adalimumab. Ann. Dermatol. 2021, 33, 474–476. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.L.; Lapadat, R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 2002, 298, 1911–1912. [Google Scholar] [CrossRef]
- Jung, J.M.; Kim, S.Y.; Lee, W.J.; Hwang, J.S.; Chang, S.E. Dopamine D4 receptor antagonist inhibits melanogenesis through transcriptional downregulation of MITF via ERK signalling. Exp. Dermatol. 2016, 25, 325–328. [Google Scholar] [CrossRef]
- Fisher, D.E. Microphthalmia: A signal responsive transcriptional regulator in development. Pigment Cell Res. 2000, 13 (Suppl. S8), 145–149. [Google Scholar] [CrossRef]
- Grabarek, B.O.; Dabala, M.; Kasela, T.; Gralewski, M.; Gladysz, D. Changes in the Expression Pattern of DUSP1-7 and miRNA Regulating their Expression in the Keratinocytes Treated with LPS and Adalimumab. Curr. Pharm. Biotechnol. 2022, 23, 873–881. [Google Scholar] [CrossRef]
- Phillips, M.A.; Qin, Q.; Hu, Q.; Zhao, B.; Rice, R.H. Arsenite suppression of BMP signaling in human keratinocytes. Toxicol. Appl. Pharmacol. 2013, 269, 290–296. [Google Scholar] [CrossRef]
- Sanovic, R.; Krammer, B.; Grumboeck, S.; Verwanger, T. Time-resolved gene expression profiling of human squamous cell carcinoma cells during the apoptosis process induced by photodynamic treatment with hypericin. Int. J. Oncol. 2009, 35, 921–939. [Google Scholar]
- Picozza, M.; Avitabile, D.; Magenta, A. Monocyte dysfunction induced by low density lipoprotein occurs via a DUSP-1/p38 MAPK signaling impairment. Int. J. Cardiol. 2018, 255, 166–167. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.B.; Khanna, S.; Raut, S.K.; Sharma, S.; Sharma, R.; Khullar, M. DUSP-1 gene expression is not regulated by promoter methylation in diabetes-associated cardiac hypertrophy. Ther. Adv. Cardiovasc. Dis. 2017, 11, 147–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, H.S.; Park, K.C.; Kim, D.S. PP2A and DUSP6 are involved in sphingosylphosphorylcholine-induced hypopigmentation. Mol. Cell Biochem. 2012, 367, 43–49. [Google Scholar] [CrossRef]
- Groom, L.A.; Sneddon, A.A.; Alessi, D.R.; Dowd, S.; Keyse, S.M. Differential regulation of the MAP, SAP and RK/p38 kinases by Pyst1, a novel cytosolic dual-specificity phosphatase. EMBO J. 1996, 15, 3621–3632. [Google Scholar] [CrossRef] [PubMed]
- Hoppstadter, J.; Ammit, A.J. Role of Dual-Specificity Phosphatase 1 in Glucocorticoid-Driven Anti-inflammatory Responses. Front. Immunol. 2019, 10, 1446. [Google Scholar] [CrossRef]
- Molina, G.; Vogt, A.; Bakan, A.; Dai, W.; de Oliveira, P.Q.; Znosko, W.; Smithgall, T.E.; Bahar, I.; Lazo, J.S.; Day, B.W.; et al. Zebrafish chemical screening reveals an inhibitor of Dusp6 that expands cardiac cell lineages. Nat. Chem. Biol. 2009, 5, 680–687. [Google Scholar] [CrossRef]
- Korotchenko, V.N.; Saydmohammed, M.; Vollmer, L.L.; Bakan, A.; Sheetz, K.; Debiec, K.T.; Greene, K.A.; Agliori, C.S.; Bahar, I.; Day, B.W.; et al. In vivo structure-activity relationship studies support allosteric targeting of a dual specificity phosphatase. ChemBioChem 2014, 15, 1436–1445. [Google Scholar] [CrossRef]
- Kaltenmeier, C.T.; Vollmer, L.L.; Vernetti, L.A.; Caprio, L.; Davis, K.; Korotchenko, V.N.; Day, B.W.; Tsang, M.; Hulkower, K.I.; Lotze, M.T.; et al. A Tumor Cell-Selective Inhibitor of Mitogen-Activated Protein Kinase Phosphatases Sensitizes Breast Cancer Cells to Lymphokine-Activated Killer Cell Activity. J. Pharmacol. Exp. Ther. 2017, 361, 39–50. [Google Scholar] [CrossRef]
- Zhu, P.Y.; Yin, W.H.; Wang, M.R.; Dang, Y.Y.; Ye, X.Y. Andrographolide suppresses melanin synthesis through Akt/GSK3beta/beta-catenin signal pathway. J. Dermatol. Sci. 2015, 79, 74–83. [Google Scholar] [CrossRef]
- Zang, D.; Niu, C.; Aisa, H.A. Amine derivatives of furocoumarin induce melanogenesis by activating Akt/GSK-3beta/beta-catenin signal pathway. Drug Des. Devel. Ther. 2019, 13, 623–632. [Google Scholar] [CrossRef] [Green Version]


| Name | Forward | Reverse |
|---|---|---|
| GAPDH | CATCACTGCCACCCAGAAGACTG | ATGCCAGTGAGTTCCCGTTCAG |
| MITF | GGGATGCCTTGTTTATGGTG | CACCGCAGACCACTTAGTCC |
| Tyrosinase | TTATGCGATGGAACACCTGA | GAGCGGTATGAAAGGAACCA |
| Tyrp1 | CCCCTAGCCTATATCTCCCT | TACCATCGTGGGGATAATGG |
| DCT | CTTTGCAACCGGGAAGAACG | CCGACTAATCAGCGTTGGGT |
| OCA2 | ATAGTGAGCAGGGAGGCTGT | ACTGATGGGCCAGCAAAAGA |
| ERK1 | CCTGCTGGACCGGATGTTA | TGAGCCAGCCTTCCTCTAC |
| ERK2 | GGAGCAGTATTATGACCCAAGTGA | TCGTCCACTCCATGTCAAACT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.H.; Choi, M.E.; An, H.; Moon, J.W.; Yeo, H.J.; Song, Y.; Chang, S.E. BCI-215, a Dual-Specificity Phosphatase Inhibitor, Reduces UVB-Induced Pigmentation in Human Skin by Activating Mitogen-Activated Protein Kinase Pathways. Molecules 2022, 27, 5449. https://doi.org/10.3390/molecules27175449
Lee JH, Choi ME, An H, Moon JW, Yeo HJ, Song Y, Chang SE. BCI-215, a Dual-Specificity Phosphatase Inhibitor, Reduces UVB-Induced Pigmentation in Human Skin by Activating Mitogen-Activated Protein Kinase Pathways. Molecules. 2022; 27(17):5449. https://doi.org/10.3390/molecules27175449
Chicago/Turabian StyleLee, Jeong Hyeon, Myoung Eun Choi, Hongchan An, Ju Won Moon, Hye Jin Yeo, Youngsup Song, and Sung Eun Chang. 2022. "BCI-215, a Dual-Specificity Phosphatase Inhibitor, Reduces UVB-Induced Pigmentation in Human Skin by Activating Mitogen-Activated Protein Kinase Pathways" Molecules 27, no. 17: 5449. https://doi.org/10.3390/molecules27175449






