Antioxidant Capacity of Herzegovinian Wildflowers Evaluated by UV–VIS and Cyclic Voltammetry Analysis
Abstract
:1. Introduction
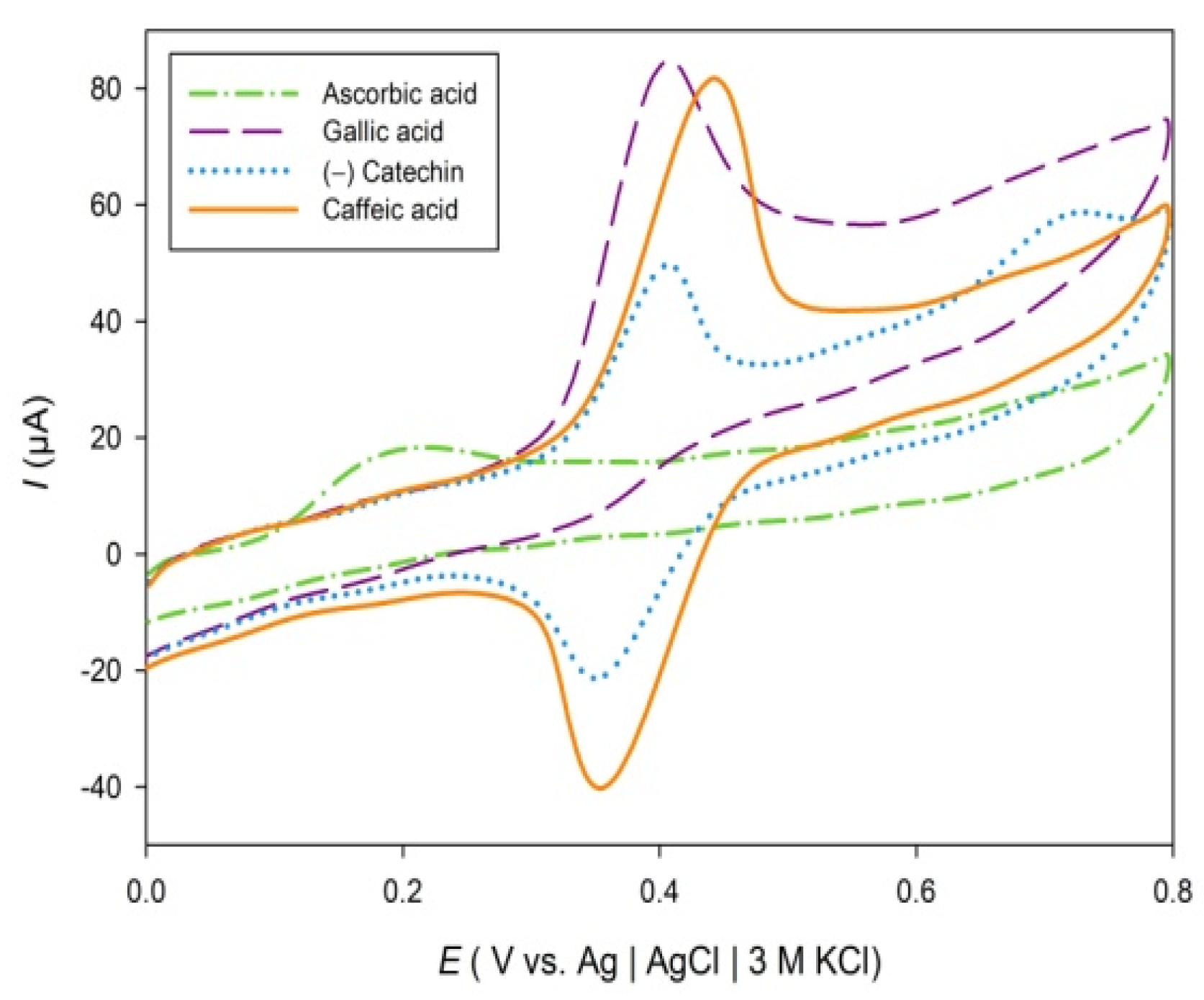
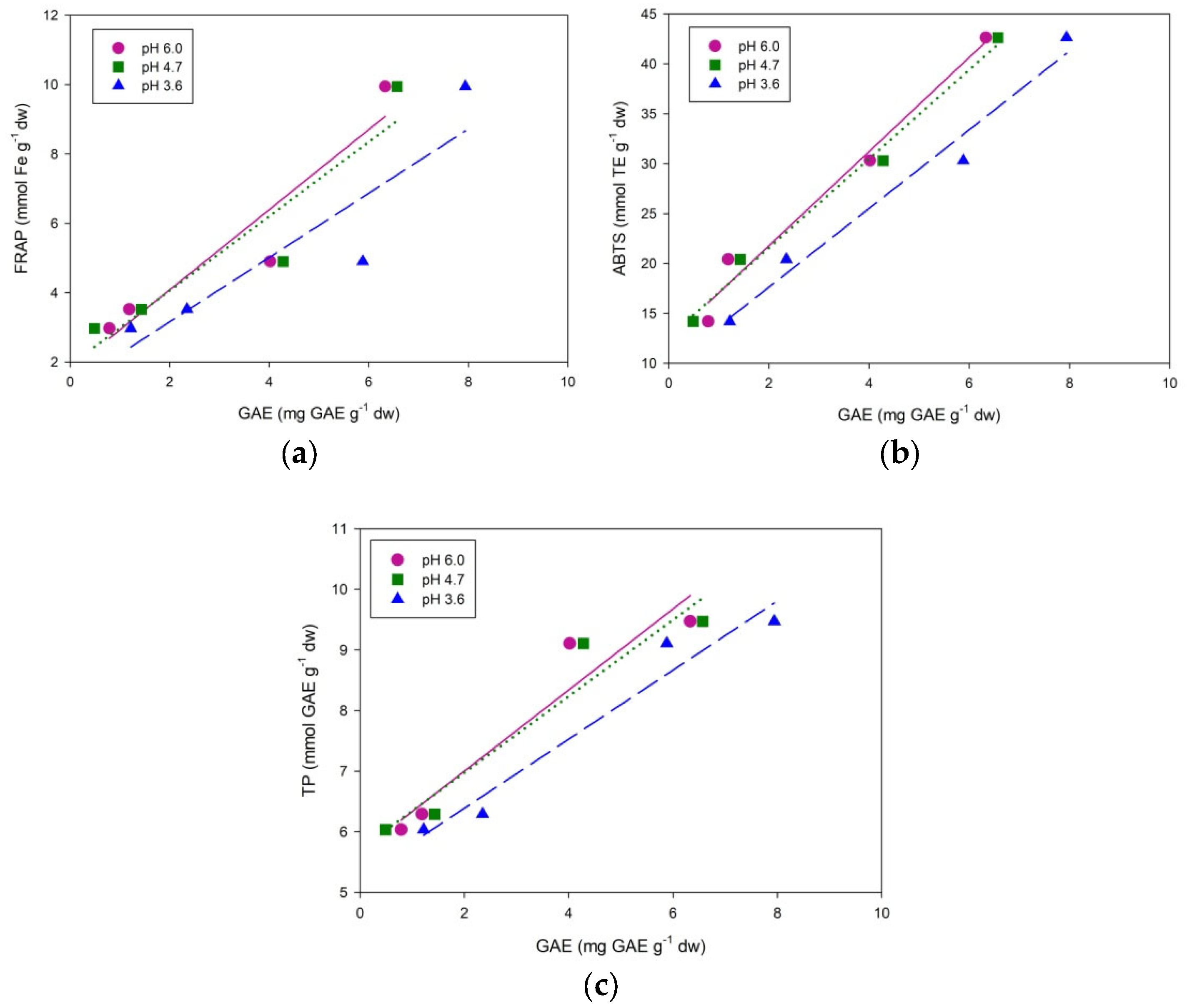
2. Results and Discussion
3. Materials and Methods
3.1. Reagents
3.2. Sample Preparation
3.3. Instruments
3.4. Electrochemical Determination of Antioxidant Capacity
3.5. Antioxidative Assays
3.6. Total Polyphenolic Content (TP), Total Flavonoid Content (TF), and Total Condensed Tannins (TT)
3.7. Antioxidant Composite Index (ACI)
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Jones, D.P. Radical-free biology of oxidative stress. Am. J. Physiol. Cell Physiol. 2008, 295, C849–C868. [Google Scholar] [CrossRef] [PubMed]
- Arroyave-Ospina, J.C.; Wu, Z.; Geng, Y.; Moshage, H. Role of Oxidative Stress in the Pathogenesis of Non-Alcoholic Fatty Liver Disease: Implications for Prevention and Therapy. Antioxidants 2021, 10, 174. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative eustress: On constant alert for redox homeostasis. Redox Biol. 2021, 41, 101867. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.; Umar, S.; Khan, N.A.; Corpas, F.J. Nitric oxide and hydrogen sulfide coordinately reduce glucose sensitivity and decrease oxidative stress via ascorbate-glutathione cycle in heat-stressed wheat (Triticum aestivum L.) plants. Antioxidants 2021, 10, 108. [Google Scholar] [CrossRef]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic Stress and Reactive Oxygen Species: Generation, Signaling, and Defense Mechanisms. Antioxidants 2021, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Andrés Juan, C.; Pérez de Lastra, J.M.; Plou Gasca, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Singh, T.G.; Garg, N.; Dhiman, S.; Gupta, S.; Rahman, M.H.; Najda, A.; Walasek-Janusz, M.; Kamel, M.; Albadrani, G.M.; et al. Dysbiosis and Alzheimer’s Disease: A Role for Chronic Stress? Biomolecules 2021, 11, 678. [Google Scholar] [CrossRef]
- Nikolaou, P.E.; Efentakis, P.; Abu Qourah, F.; Femminò, S.; Makridakis, M.; Kanaki, Z.; Varela, A.; Tsoumani, M.; Davos, C.H.; Dimitriou, C.A. Chronic empagliflozin treatment reduces myocardial infarct size in nondiabetic mice through STAT-3-mediated protection on microvascular endothelial cells and reduction of oxidative stress. Antioxid. Redox Signal. 2021, 34, 551–571. [Google Scholar] [CrossRef]
- Bhattarai, K.R.; Riaz, T.A.; Kim, H.R.; Chae, H.J. The aftermath of the interplay between the endoplasmic reticulum stress response and redox signaling. Exp. Mol. Med. 2021, 53, 151–167. [Google Scholar] [CrossRef]
- Rashki Ghaleno, L.; Alizadeh, A.; Drevet, J.R.; Shahverdi, A.; Valojerdi, M.R. Oxidation of Sperm DNA and Male Infertility. Antioxidants 2021, 10, 97. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J. Oxygen: Boon yet bane—Introducing oxygen toxicity and reactive species. Free Radicals Biol. Med. 2015, 5, 1–29. [Google Scholar]
- Rice-Evans, C.A.; Miller, N.J. Antioxidant activities of flavonoids as bioactive components of food. Biochem. Soc. Trans. 1996, 24, 790–795. [Google Scholar] [CrossRef]
- Proteggente, A.R.; Pannala, A.S.; Paganga, G.; Van Buren, L.; Wagner, E.; Wiseman, S.; Van De Put, F.; Dacombe, C.; Rice-Evans, C.A. The antioxidant activity of regularly consumed fruit and vegetables reflects their phenolic and vitamin C composition. Free Radic. Res. 2002, 36, 217–233. [Google Scholar] [CrossRef]
- Tsai, P.-J.; McIntosh, J.; Pearce, P.; Camden, B.; Jordan, B.R. Anthocyanin and antioxidant capacity in Roselle (Hibiscus sabdariffa L.) extract. Food Res. Int. 2002, 35, 351–356. [Google Scholar] [CrossRef]
- Yang, L.; Gou, Y.; Zhao, T.; Zhao, J.-l.; Li, F.; Zhang, B.; Wu, X. Antioxidant capacity of extracts from calyx fruits of roselle (Hibiscus sabdariffa L.). Afr. J. Biotechnol. 2012, 11, 4063–4068. [Google Scholar] [CrossRef]
- Sharma, V.; Anderson, D.; Dhawan, A. Zinc oxide nanoparticles induce oxidative DNA damage and ROS-triggered mitochondria mediated apoptosis in human liver cells (HepG2). Apoptosis Int. J. Program. Cell Death 2012, 17, 852–870. [Google Scholar] [CrossRef]
- Itoh, Y.; Iida, S.; Tamura, S.; Nagashima, R.; Shiraki, K.; Goto, T.; Hibino, K.; Ide, S.; Maeshima, K. 1,6-hexanediol rapidly immobilizes and condenses chromatin in living human cells. Life Sci. Alliance 2021, 4, e202001005. [Google Scholar] [CrossRef]
- Gowen, A.; Shahjin, F.; Chand, S.; Odegaard, K.E.; Yelamanchili, S.V. Mesenchymal Stem Cell-Derived Extracellular Vesicles: Challenges in Clinical Applications. Front. Cell Dev. Biol. 2020, 8, 149. [Google Scholar] [CrossRef]
- Meng, W.; He, C.; Hao, Y.; Wang, L.; Li, L.; Zhu, G. Prospects and challenges of extracellular vesicle-based drug delivery system: Considering cell source. Drug Deliv. 2020, 27, 585–598. [Google Scholar] [CrossRef]
- Park, S.K.; Hyun, S.H.; In, G.; Park, C.K.; Kwak, Y.S.; Jang, Y.J.; Kim, B.; Kim, J.H.; Han, C.K. The antioxidant activities of Korean Red Ginseng (Panax ginseng) and ginsenosides: A systemic review through in vivo and clinical trials. J. Ginseng Res. 2021, 45, 41–47. [Google Scholar] [CrossRef]
- Iakovleva, K.; Kurzeev, S.; Stepanova, E.; Fedorova, T.; Kuznetsov, B.; Koroleva, O. Characterization of plant phenolic compounds by cyclic voltammetry. Appl. Biochem. Microbiol. 2007, 43, 661–668. [Google Scholar] [CrossRef]
- Hajimahmoodi, M.; Aliabadipoor, M.; Moghaddam, G.; Sadeghi, N.; Oveisi, M.; Jannat, B. Evaluation of in vitro antioxidant activities of lemon juice for safety assessment. Am. J. Food Technol. 2012, 7, 708–714. [Google Scholar] [CrossRef]
- Redžić, S.; Ferrier, J. The Use of Wild Plants for Human Nutrition during a War: Eastern Bosnia (Western Balkans), Ethnobotany and Biocultural Diversities in the Balkans: Perspectives on Sustainable Rural Development and Reconciliation; Pieroni, A., Quave, C.L., Eds.; Springer: New York, NY, USA, 2014; pp. 149–183. [Google Scholar] [CrossRef]
- Swaffield, J.; Roberts, S.C. Exposure to Cues of Harsh or Safe Environmental Conditions Alters Food Preferences. Evol. Psychol. Sci. 2015, 1, 69–76. [Google Scholar] [CrossRef]
- Maier, S.U.; Makwana, A.B.; Hare, T.A. Acute Stress Impairs Self-Control in Goal-Directed Choice by Altering Multiple Functional Connections within the Brain’s Decision Circuits. Neuron 2015, 87, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R. Role of addiction and stress neurobiology on food intake and obesity. Biol. Psychol. 2018, 131, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Ćorić, N.; Jurić, A.; Karlovic, A. The Impact of the COVID-19 Pandemic Outbreak on Eating and Lifestyle Habits of Adolescents in Bosnia and Herzegovina: A Cross-sectional Study. Prepr. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Redzic, S. Wild medicinal plants and their usage in traditional human therapy (Southern Bosnia and Herzegovina, W. Balkan). J. Med. Plants Res. 2010, 4, 1003–1027. [Google Scholar] [CrossRef]
- Mustafa, B.; Hajdari, A. Medical Ethnobotanical Studies in Kosovo. In Ethnobotany and Biocultural Diversities in the Balkans: Perspectives on Sustainable Rural Development and Reconciliation; Pieroni, A., Quave, C.L., Eds.; Springer: New York, NY, USA, 2014; pp. 113–136. ISBN 978-1-4939-1492-0. [Google Scholar]
- Nedelcheva, A.; Draganov, S. Bulgarian Medical Ethnobotany: The Power of Plants in Pragmatic and Poetic Frames. In Ethnobotany and Biocultural Diversities in the Balkans: Perspectives on Sustainable Rural Development and Reconciliation; Pieroni, A., Quave, C.L., Eds.; Springer: New York, NY, USA, 2014; pp. 45–67. ISBN 978-1-4939-1492-0. [Google Scholar]
- Rexhepi, B.; Mustafa, B.; Hajdari, A.; Rushidi-Rexhepi, J.; Quave, C.L.; Pieroni, A. Cross-Cultural Ethnobotany of the Sharr Mountains (Northwestern Macedonia). In Ethnobotany and Biocultural Diversities in the Balkans: Perspectives on Sustainable Rural Development and Reconciliation; Pieroni, A., Quave, C.L., Eds.; Springer: New York, NY, USA, 2014; pp. 67–87. ISBN 978-1-4939-1492-0. [Google Scholar]
- Jarić, S.; Mitrović, M.; Pavlović, P. An Ethnobotanical and Ethnomedicinal Study on the Use of Wild Medicinal Plants in Rural Areas of Serbia. In Ethnobotany and Biocultural Diversities in the Balkans: Perspectives on Sustainable Rural Development and Reconciliation; Pieroni, A., Quave, C.L., Eds.; Springer: New York, NY, USA, 2014; pp. 87–113. ISBN 978-1-4939-1492-0. [Google Scholar]
- Dajić Stevanović, Z.; Petrović, M.; Aćić, S. Ethnobotanical Knowledge and Traditional Use of Plants in Serbia in Relation to Sustainable Rural Development. In Ethnobotany and Biocultural Diversities in the Balkans: Perspectives on Sustainable Rural Development and Reconciliation; Pieroni, A., Quave, C.L., Eds.; Springer: New York, NY, USA, 2014; pp. 229–253. ISBN 978-1-4939-1492-0. [Google Scholar]
- Łuczaj, Ł.; Dolina, K.; Fressel, N.; Perković, S. Wild Food and Medicinal Plants Used in the Mountainous: Wild Food Plants of Dalmatia (Croatia). In Ethnobotany and Biocultural Diversities in the Balkans: Perspectives on Sustainable Rural Development and Reconciliation; Pieroni, A., Quave, C.L., Eds.; Springer: New York, NY, USA, 2014; pp. 137–149. ISBN 978-1-4939-1492-0. [Google Scholar]
- Pieroni, A.; Quave, C.L. Albanian North, Northeast, and East: A Comparison. In Ethnobotany and Biocultural Diversities in the Balkans: Perspectives on Sustainable Rural Development and Reconciliation; Pieroni, A., Quave, C.L., Eds.; Springer: New York, NY, USA, 2014; pp. 183–197. ISBN 978-1-4939-1492-0. [Google Scholar]
- Menković, N.; Šavikin, K.; Zdunić, G.; Milosavljević, S.; Živković, J. Medicinal Plants in Northern Montenegro: Traditional Knowledge, Quality, and Resources Knowledge, Quality, and Resources. In Ethnobotany and Biocultural Diversities in the Balkans: Perspectives on Sustainable Rural Development and Reconciliation; Pieroni, A., Quave, C.L., Eds.; Springer: New York, NY, USA, 2014; pp. 197–229. ISBN 978-1-4939-1492-0. [Google Scholar]
- Šiljković, Ž.; Rimanić, A. Geographic Aspects of Medicinal Plants Organic Growing in Croatia. Geoadria 2005, 10, 53–68. [Google Scholar] [CrossRef]
- Łuczaj, Ł.; Jug-Dujaković, M.; Dolina, K.; Jeričević, M.; Vitasović-Kosić, I. Insular Pharmacopoeias: Ethnobotanical Characteristics of Medicinal Plants Used on the Adriatic Islands. Front. Pharmacol. 2021, 12, 623070. [Google Scholar] [CrossRef]
- Łuczaj, Ł.; Jug-Dujaković, M.; Dolina, K.; Vitasović-Kosić, I. Plants in alcoholic beverages on the Croatian islands, with special reference to rakija travarica. J. Ethnobiol. Ethnomed. 2019, 15, 51. [Google Scholar] [CrossRef]
- Redzic, S.; Basic, H.; Barudanovic, S. Models of organic certification in herbal sector of Western Balkan. Planta Med. 2009, 75, SL68. [Google Scholar] [CrossRef]
- Živković, J.; Ilić, M.; Šavikin, K.; Zdunić, G.; Ilić, A.; Stojković, D. Traditional Use of Medicinal Plants in South-Eastern Serbia (Pčinja District): Ethnopharmacological Investigation on the Current Status and Comparison with Half a Century Old Data. Front. Pharmacol. 2020, 11, 1020. [Google Scholar] [CrossRef]
- Živković, J.; Ilić, M.; Zdunić, G.; Jovanović-Lješković, N.; Menković, N.; Šavikin, K. Traditional use of medicinal plants in Jablanica district (South-Eastern Serbia): Ethnobotanical survey and comparison with scientific data. Genet. Resour. Crop. Evol. 2021, 68, 1655–1674. [Google Scholar] [CrossRef]
- Šavikin, K.; Zdunić, G.; Menković, N.; Živković, J.; Ćujić, N.; Tereščenko, M.; Bigović, D. Ethnobotanical study on traditional use of medicinal plants in South-Western Serbia, Zlatibor district. J. Ethnopharmacol. 2013, 146, 803–810. [Google Scholar] [CrossRef]
- Prazina, N.; Redzic, S.; Tuka, M. The use of wild medicinal plants in the traditional therapy of respiratory diseases in high mountain region of W. Balkan. Planta Med. 2011, 77, PF4. [Google Scholar] [CrossRef]
- Tucakov, J. Lečenje Biljem, 1st ed.; Izdavačka Radna Organizacija “RAD”: Belgrade, Serbia, 1984; pp. 16–21, 503. [Google Scholar]
- Weiss, M.G. Cultural models of diarrheal illness: Conceptual framework and review. Soc. Sci. Med. 1988, 27, 5–16. [Google Scholar] [CrossRef]
- Padumanonda, T.; Phontree, K. Total Phenolic, Total Flavonoid, Total Condensed Tannin Contents and Antimicrobial Activity Against Diarrheal Bacteria of the Bark and Fruit of Terminalia nigrovenulosa Pierre ex Laness. Sci. Technol. Asia 2021, 26, 162–169. [Google Scholar] [CrossRef]
- Nikolić, T. (2015—Onwards): Flora Croatica Baza Podataka, Faculty of Science and Mathematics, University of Zagreb. Available online: https://hirc.botanic.hr/fcd/beta/Herbar/57947 (accessed on 1 July 2022).
- Bogdanović, S.; Britvec, M.; Ljubičić, I.; Dujmović Purgar, D.; Vitasović Kosić, I. Herbarium ZAGR of the Faculty of Agriculture (Zagreb, Croatia). Agric. Conspec. Sci. 2016, 81, 1–5. [Google Scholar]
- Barina, Z.; Somogyi, G.; Pifkó, D.; Rakaj, M. Checklist of vascular plants of Albania. Phytotaxa 2018, 378, 1–339. [Google Scholar] [CrossRef]
- Lohitkarn, J.; Hemwech, P.; Chantiwas, R.; Jariyaboon, M. The Role of Cassava Leaf Extract as Green Inhibitor for Controlling Corrosion and Scale Problems in Cooling Water Systems. J. Fail. Anal. Prev 2021, 21, 847–860. [Google Scholar] [CrossRef]
- Pilic, Z.; Martinovic, I.; Pavlinovic, M.; Zlatic, G. Effect of Helichrysum italicum on the Electrochemical Corrosion Behaviour of Iron in Simulated Acid Rain Solution. Croat. Chem. Acta 2019, 92, 79–86. [Google Scholar] [CrossRef]
- Lahhit, N.; Bouyanzer, A.; Desjobert, J.; Hammouti, B.; Salghi, R.; Costa, J.; Jama, C.; Bentiss, F.; Majidi, L. Fennel (Foeniculum vulgare) essential oil as green corrosion inhibitor of carbon steel in hydrochloric acid solution. Port. Electrochim. Acta 2011, 29, 127–138. [Google Scholar] [CrossRef]
- Muthukrishnan, P.; Jeyaprabha, B.; Prakash, P. Mild steel corrosion inhibition by aqueous extract of Hyptis Suaveolens leaves. Int. J. Ind. Chem. 2014, 5, 5. [Google Scholar] [CrossRef]
- Pilić, Z.; Martinović, I.; Zlatić, G. Electrochemical behaviour of iron in simulated acid rain in presence of Achillea millefolium L. Int. J. Electrochem. Sci. 2018, 13, 5151–5163. [Google Scholar] [CrossRef]
- Chevion, S.; Roberts, M.A.; Chevion, M. The use of cyclic voltammetry for the evaluation of antioxidant capacity. Free Radic. Biol. Med. 2000, 28, 860–870. [Google Scholar] [CrossRef]
- Chevion, S.; Chevion, M.; Chock, P.B.; Beecher, G.R. Antioxidant capacity of edible plants: Extraction protocol and direct evaluation by cyclic voltammetry. J. Med. Food 1999, 2, 1–10. [Google Scholar] [CrossRef]
- Campanella, L.; Martini, E.; Rita, G.; Tomassetti, M. Antioxidant capacity of dry vegetal extracts checked by voltammetric method. J. Food Agric. Environ. 2006, 4, 135. [Google Scholar]
- Photinon, K.; Chalermchart, Y.; Khanongnuch, C.; Wang, S.-H.; Liu, C.-C. A thick-film sensor as a novel device for determination of polyphenols and their antioxidant capacity in white wine. Sensors 2010, 10, 1670–1678. [Google Scholar] [CrossRef]
- Nikolic, M.; Pavlovic, A.; Mitic, S.; Tosic, S.; Mitic, M.; Kalicanin, B.; Manojlovic, D.; Stankovic, D. Use of cyclic voltammetry to determine the antioxidant capacity of berry fruits: Correlation with spectrophotometric assays. Eur. J. Hortic. Sci. 2019, 84, 152–160. [Google Scholar] [CrossRef]
- El-Hawary, S.S.; Taher, M.A.; Amin, E.; AbouZid, S.F.; Mohammed, R. Genus Tabebuia: A comprehensive review journey from past achievements to future perspectives. Arab. J. Chem. 2021, 14, 103046. [Google Scholar] [CrossRef]
- Beykaya, M.; Samancı, A.E.T.; Samancı, T.; Önder, E.Y.; Uzun, E.M.; Tosun, F. Investigation of Nutritional and Antioxidant Properties of Anatolian Bee Bread. J. Apic. Sci. 2021, 65, 255–263. [Google Scholar] [CrossRef]
- Bendiab, H.C.; Djebli, N.; Kara, Y.; Uçar, M.; Kolayli, S. An Investigation of Algerian Dates (Phoenix dactylifera L.); Antioxidant, Anti-inflammatory Properties and Phenolic Compositons, H. Emir. J. Food Agric. 2021, 33, 629–638. [Google Scholar] [CrossRef]
- Jovanović, A.A.; Djordjević, V.B.; Petrović, P.M.; Pljevljakušić, D.S.; Zdunić, G.M.; Šavikin, K.P.; Bugarski, B.M. The influence of different extraction conditions on polyphenol content, antioxidant and antimicrobial activities of wild thyme. J. Appl. Res. Med. Aroma 2021, 25, 100328. [Google Scholar] [CrossRef]
- Barbasz, A.; Oćwieja, M.; Piergies, N.; Duraczyńska, D.; Nowak, A. Antioxidant-modulated cytotoxicity of silver nanoparticles. J. Appl. Toxicol. 2021, 41, 1863–1878. [Google Scholar] [CrossRef]
- Carbonara, T.; Pascale, R.; Argentieri, M.P.; Papadia, P.; Fanizzi, F.P.; Villanova, L.; Avato, P. Phytochemical analysis of a herbal tea from Artemisia annua L. J. Pharm. Biomed. Anal. 2012, 62, 79–86. [Google Scholar] [CrossRef]
- Hotta, H.; Ueda, M.; Nagano, S.; Tsujino, Y.; Koyama, J.; Osakai, T. Mechanistic study of the oxidation of caffeic acid by digital simulation of cyclic voltammograms. Anal. Biochem. 2002, 303, 66–72. [Google Scholar] [CrossRef]
- Trabelsi, S.K.; Tahar, N.B.; Abdelhedi, R. Electrochemical behavior of caffeic acid. Electrochim. Acta 2004, 49, 1647–1654. [Google Scholar] [CrossRef]
- Boualem, M.; Mokhtar, M.; Saiah, F.; Benourad, F.; Bouhadiba, R.; Berkani, A. Identification of Mentha piperita L. and Ricinus communis L. polyphenols by HPLC-DAD-ESI-MS and evaluation of their insecticidal properties against Aphis spiraecola P. South Asian J. Exp. Biol. 2017, 7, 28–34. [Google Scholar] [CrossRef]
- Ivanescu, B.; Vlase, L.; Corciova, A.; Lazar, M. HPLC-DAD-MS study of polyphenols from Artemisia absinthium, A. annua, and A. vulgaris. Chem. Nat. Compd. 2010, 46, 468–470. [Google Scholar] [CrossRef]
- Piljac-Žegarac, J.; Valek, L.; Stipčević, T.; Martinez, S. Electrochemical determination of antioxidant capacity of fruit tea infusions. Food Chem. 2010, 121, 820–825. [Google Scholar] [CrossRef]
- Hadjmohammadi, M.; Karimiyan, H.; Sharifi, V. Hollow fibre-based liquid phase microextraction combined with high-performance liquid chromatography for the analysis of flavonoids in Echinophora platyloba DC. and Mentha piperita. Food Chem. 2013, 141, 731–735. [Google Scholar] [CrossRef]
- Kilmartin, P.A.; Zou, H.; Waterhouse, A.L. A cyclic voltammetry method suitable for characterizing antioxidant properties of wine and wine phenolics. J. Agric. Food Chem. 2001, 49, 1957–1965. [Google Scholar] [CrossRef]
- Xhanari, K.; Finšgar, M.; Knez Hrnčič, M.; Maver, U.; Knez, Ž.; Seiti, B. Green corrosion inhibitors for aluminium and its alloys: A review. RSC Adv. 2017, 7, 27299–27330. [Google Scholar] [CrossRef]
- Kovačević, N.; Kokalj, A. The relation between adsorption bonding and corrosion inhibition of azole molecules on copper. Corros. Sci. 2013, 73, 7–17. [Google Scholar] [CrossRef]
- Ko, X.; Sharma, S. Adsorption and Self-Assembly of Surfactants on Metal-Water Interfaces. J. Phys. Chem. B 2017, 121, 10364–10370. [Google Scholar] [CrossRef]
- Shiwei Guo, S.; Ma, J.; Xing, Y.; Xu, Y.; Jin, X.; Yan, S.; Shi, B. Artemisia annua L. aqueous extract as an alternative to antibiotics improving growth performance and antioxidant function in broilers. Ital. J. Anim. Sci. 2020, 19, 399–409. [Google Scholar] [CrossRef]
- Kaoudoune, C.; Benchikh, F.; Benabdallah, H.; Loucif, K.; Mehlous, S.; Amira, S. Gastroprotective effect and in vitro Antioxidant Activities of the Aqueous Extract from Artemisia absinthium L. Aerial Parts. JDDT 2020, 10, 153–156. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Thiruvengadam, M.; Chung, I.-M.; Nagella, P. Polyphenol composition and antioxidant activity from the vegetable plant Artemisia absinthium L. Aust. J. Crop. Sci. 2013, 7, 1921–1926. [Google Scholar]
- Kaurinovic, B.; Popovic, M.; Vlaisavljevic, S.; Trivic, S. Antioxidant Capacity of Ocimum basilicum L. and Origanum vulgare L. Extracts. Molecules 2011, 16, 7401–7414. [Google Scholar] [CrossRef]
- Dorman, D.H.J.; Koşar, M.; Kahlos, K.; Holm, Y.; Hiltunen, R. Antioxidant Properties and Composition of Aqueous Extracts from Mentha Species, Hybrids, Varieties, and Cultivars. J. Agric. Food Chem. 2003, 51, 4563–4569. [Google Scholar] [CrossRef]
- Council of Europe. European Pharmacopoeia, 5th ed.; Council of Europe: Strasbourg, France, 2005; pp. 4099–4117. [Google Scholar]
- The Plant List Version 1.1. 2013. Available online: http://www.theplantlist.org/ (accessed on 1 July 2022).
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Li, H.-B.; Cheng, K.-W.; Wong, C.-C.; Fan, K.-W.; Chen, F.; Jiang, Y. Evaluation of antioxidant capacity and total phenolic content of different fractions of selected microalgae. Food Chem. 2007, 102, 771–776. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Broadhurst, R.B.; Jones, W.T. Analysis of condensed tannins using acidified vanillin. J. Sci. Food Agric. 1978, 48, 788–794. [Google Scholar] [CrossRef]
- Seeram, N.P.; Aviram, M.; Zhang, Y.; Henning, S.M.; Feng, L.; Dreher, M.; Heber, D. Comparison of antioxidant potency of commonly consumed polyphenol-rich beverages in the United States. J. Agric. Food. Chem. 2008, 56, 1415–1422. [Google Scholar] [CrossRef]
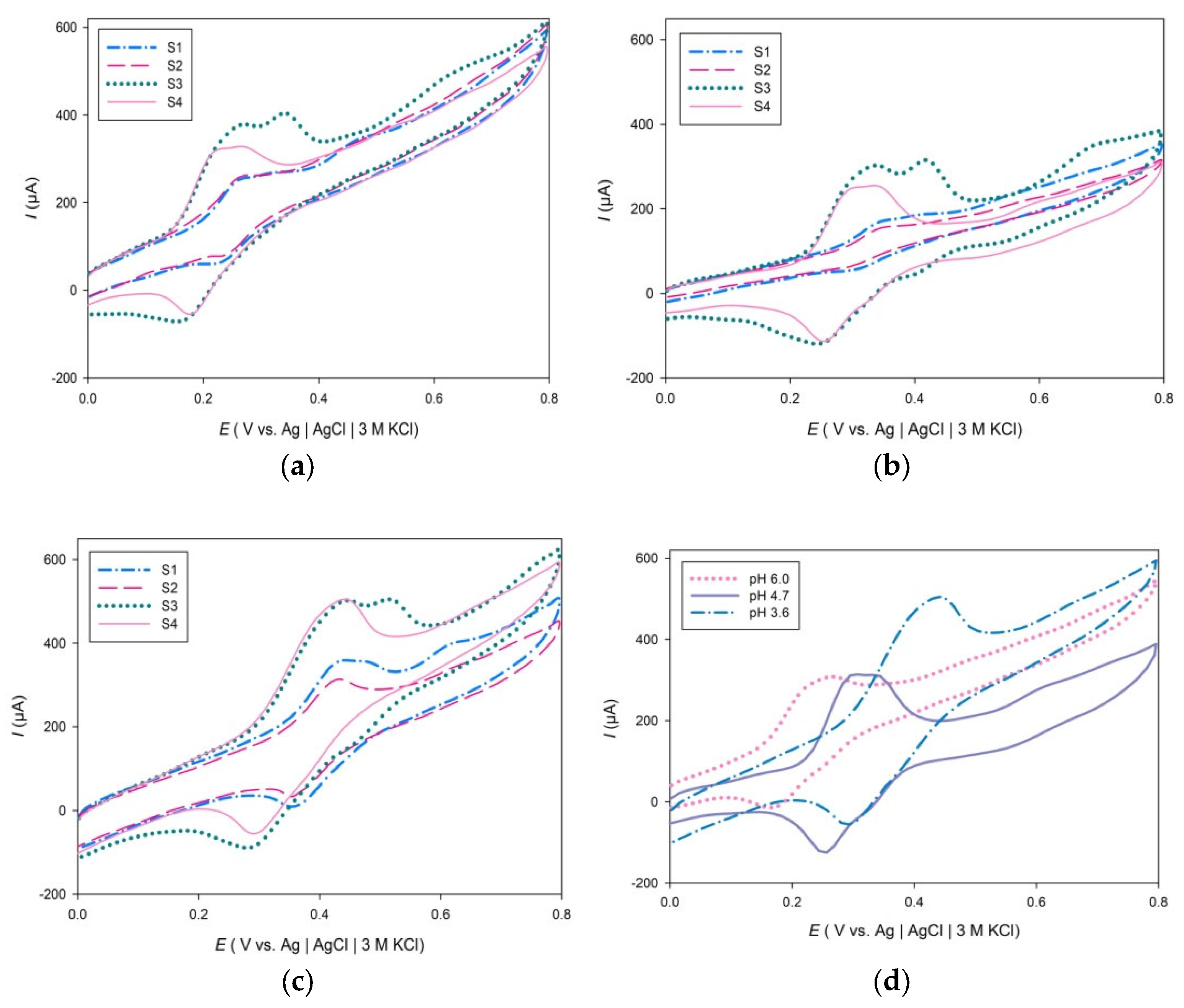
| Herbs | pH | Ep (V) | Ip, (µA cm−2) | Q (µC) | GAE (mg GA g−1 dw) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | ||||
| S1 | 6.0 | 0.288 | - | 0.471 | - | 262.52 | - | 343.51 | - | 5.18 | 1.19 |
| S2 | 0.268 | - | - | - | 262.63 | - | - | - | 3.59 | 0.79 | |
| S3 | 0.266 | 0.342 | - | 0.620 | 384.55 | 403.12 | - | 490.27 | 25.47 | 6.33 | |
| S4 | 0.239 | - | - | - | 327.85 | - | - | - | 12.51 | 4.02 | |
| S1 | 4.7 | 0.366 | - | 0.554 | - | 343.51 | - | 233.58 | - | 6.52 | 1.43 |
| S2 | 0.344 | - | - | - | 154.03 | - | - | - | 2.55 | 0.49 | |
| S3 | 0.341 | 0.417 | - | 0.696 | 305.72 | 315.54 | - | 356.15 | 28.26 | 6.57 | |
| S4 | 0.314 | - | - | 0.617 | 256.26 | - | - | 223.64 | 18.59 | 4.28 | |
| S1 | 3.6 | 0.447 | - | 0.622 | - | 358.09 | - | 400.10 | - | 11.83 | 2.35 |
| S2 | 0.429 | - | - | - | 313.40 | - | - | - | 7.56 | 1.22 | |
| S3 | 0.437 | 0.517 | - | - | 498.15 | 505.39 | - | - | 32.84 | 7.94 | |
| S4 | 0.430 | - | - | - | 500.53 | - | - | - | 25.11 | 5.88 | |
| Herbs | FRAP mmoL Fe g−1 dw | ABTS mmoL TE g−1 dw | Total Phenols mmoL GAE g−1 dw | Total Flavonoids mg CE g−1 dw | Total Tannins mg CE g−1 dw |
|---|---|---|---|---|---|
| S1 | 3.5218 ± 0.312 | 20.4013 ± 0.802 | 6.2903 ± 0.052 | 20.922 ± 0.019 | 86.538 ± 0.046 |
| S2 | 2.9702 ± 0.168 | 14.1842 ± 0.294 | 6.0343 ± 0.089 | 13.923 ± 0.079 | 119.230 ± 0.008 |
| S3 | 9.9418 ± 0.568 | 42.6217 ± 0.615 | 9.4720 ± 0.052 | 253.191 ± 0.015 | 90.384 ± 0.062 |
| S4 | 4.9008 ± 0.316 | 30.3026 ± 0.939 | 9.1063 ± 0.089 | 82.978 ± 0.077 | 69.231 ± 0.013 |
| Herbs | ABTS Index | FRAP Index | Q Index | ACI | ||
|---|---|---|---|---|---|---|
| pH 3.6 | pH 4.7 | pH 6.0 | ||||
| S1 | 47.87 | 35.42 | 36.04 | 23.10 | 20.34 | 32.5 |
| S2 | 33.28 | 29.88 | 23.04 | 9.02 | 14.10 | 21.9 |
| S3 | 100 | 100 | 100 | 100 | 100 | 100 |
| S4 | 71.10 | 49.29 | 76.46 | 65.78 | 49.14 | 56.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zlatić, G.; Arapović, A.; Martinović, I.; Martinović Bevanda, A.; Bošković, P.; Prkić, A.; Paut, A.; Vukušić, T. Antioxidant Capacity of Herzegovinian Wildflowers Evaluated by UV–VIS and Cyclic Voltammetry Analysis. Molecules 2022, 27, 5466. https://doi.org/10.3390/molecules27175466
Zlatić G, Arapović A, Martinović I, Martinović Bevanda A, Bošković P, Prkić A, Paut A, Vukušić T. Antioxidant Capacity of Herzegovinian Wildflowers Evaluated by UV–VIS and Cyclic Voltammetry Analysis. Molecules. 2022; 27(17):5466. https://doi.org/10.3390/molecules27175466
Chicago/Turabian StyleZlatić, Gloria, Anamarija Arapović, Ivana Martinović, Anita Martinović Bevanda, Perica Bošković, Ante Prkić, Andrea Paut, and Tina Vukušić. 2022. "Antioxidant Capacity of Herzegovinian Wildflowers Evaluated by UV–VIS and Cyclic Voltammetry Analysis" Molecules 27, no. 17: 5466. https://doi.org/10.3390/molecules27175466







