Sublimation and Diffusion Kinetics of 2,4,6-Trinitrotoluene (TNT) Single Crystals by Atomic Force Microscopy (AFM)
Abstract
:1. Introduction
2. Theory
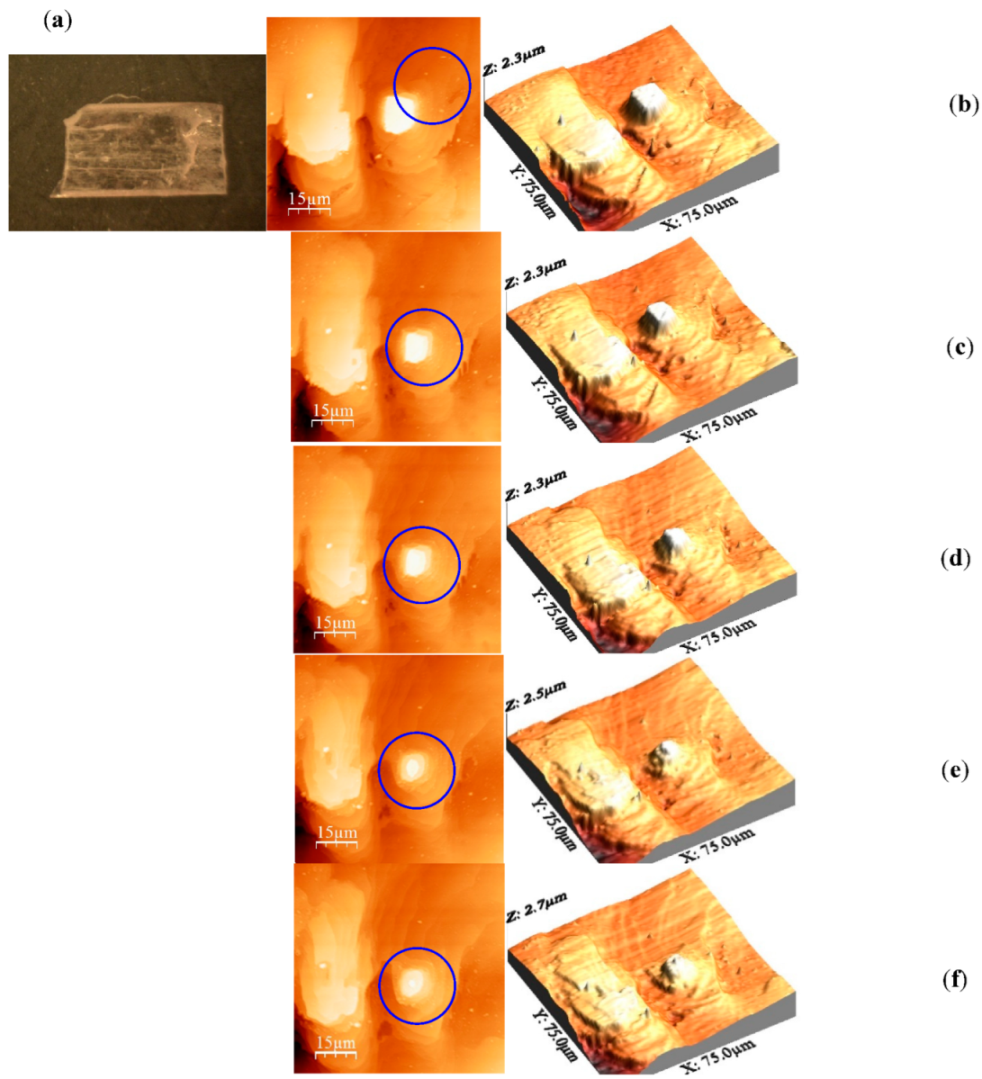
3. Results and Discussion
| Diffusion Coefficient (D) | Sublimation Rates | ||
|---|---|---|---|
| AFM | TGA [24] | AFM | |
| T (°C) | D (m2/s) | D (m2/s) | ng/s |
| 55 | 4.23 × 10−6 | 5.78 × 10−6 | 0.00015 |
| 60 | 4.31 × 10−6 | 5.79 × 10−6 | 0.00199 |
| 65 | 4.39 × 10−6 | 5.81 × 10−6 | 0.00231 |
| 70 | 4.48 × 10−6 | 5.82 × 10−6 | 0.00285 |
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Czarnecki, J.; Sestak, J. Practical thermogravimetry. J. Therm. Anal. Calorim. 2000, 60, 759–778. [Google Scholar] [CrossRef]
- Hajimirsadeghis, S.S.; Teimouri, M.R.; Rahimi-Nasrabadi, M.; Dehghanpour, S. Non-isothermal kinetic study of the thermal decomposition of N-{bis[benzyl(methyl)amino]phosphoryl}-2,2-dichloroacetamide and N-{bis[dibenzylamino]phosphoryl}-2,2-dichloroacetamide. J. Therm. Anal. Calorim. 2009, 98, 463–468. [Google Scholar] [CrossRef]
- Hobbs, M.L.; Nakos, J.T.; Brady, P.D. Response of a glass/phenolic composite to high temperatures. J. Therm. Anal. Calorim. 2011, 103, 543–553. [Google Scholar] [CrossRef]
- Gershanik, A.P.; Zeiri, Y. Sublimation rate of TNT microcrystals in air. J. Phys. Chem. A 2010, 114, 12403–12410. [Google Scholar] [CrossRef]
- Mu, R.; Ueda, A.; Liu, Y.C.; Wu, M.; Henderson, D.O.; Lareau, R.T.; Chamberlain, R.T. Effects of interfacial interaction potential on the sublimation rates of TNT films on a silica surface examined by QCM and AFM techniques. Surf. Sci. 2003, 530, L293–L296. [Google Scholar] [CrossRef]
- Pitchimani, R.; Burnham, A.K.; Weeks, B.L. Quantitative thermodynamic analysis of sublimation rates using an atomic force microscope. J. Phys. Chem. B 2007, 11, 9182–9185. [Google Scholar] [CrossRef]
- Burnham, A.K.; Qiu, S.R.; Pitchimani, R.; Weeks, B.L. Comparison of kinetic parameters of single crystal pentaerythritol tetranitrate using atomic force microscopy and thermogravimetric analysis: Implications on coarsening mechanisms. J. Appl. Phys. 2009, 105, 104312. [Google Scholar] [CrossRef]
- Hikal, W.M.; Paden, J.T.; Weeks, B.L. Simple Method for Determining the Vapor Pressure of Materials Using UV-Absorbance Spectroscopy. J. Phys. Chem. B 2011, 115, 13287–13291. [Google Scholar] [CrossRef]
- Langmuir, I. The vapor pressure of metallic tungsten. Phys. Rev. 1913, 2, 329–342. [Google Scholar] [CrossRef]
- Kunz, R.R.; Gregory, K.E.; Aernecke, M.J.; Clark, M.L.; Ostrinskaya, A.; Fountain, A.W. Fate Dynamics of Environmentally Exposed Explosive Traces. J. Phys. Chem. A 2012, 116, 3611–3624. [Google Scholar] [CrossRef]
- Liu, B.Y.H.; Yoo, S.H.; Davies, J.P.; Gresham, G.L.; Hallowell, S.F. Development of particles standards for testing explosives detection systems—Characterization of the adhesion forces between Composition-4 particles and polyethylene. Proc. SPIE 1994, 2276, 45–55. [Google Scholar]
- Gershanik, A.P.; Zeiri, Y. Sublimation Rate of Energetic Materials in Air: RDX and PETN. Propell. Explos. Pyrotech. 2012, 37, 207–214. [Google Scholar] [CrossRef]
- Gray, A.; Mathews, B.B.; MacRobert, T.M. A Treatise on Bessel Functions and Their Applications to Physics. Macmillan and Co., Ltd.: London, UK, 1931. [Google Scholar]
- Hikal, W.M.; Burnham, A.K.; Weeks, B.L. Simultaneous determination of diffusion and sublimation kinetics at nanoscale: Pentaerythritol tetranitrate. Appl. Phys. Lett. 2013, 102, 16304–16306. [Google Scholar] [CrossRef]
- Hikal, W.M.; Weeks, B.L. Determination of sublimation rate of 2,4,6-trinitrotoluene (TNT) nano thin films using UV-absorbance spectroscopy. J. Therm. Anal. Calorim. 2012, 110, 560–595. [Google Scholar] [CrossRef]
- Leggett, D.C. Vapor pressure of 2,4,6-trinitrotoluene by a gas chromatographic headspace technique. J. Chromatogr. 1977, 133, 83–90. [Google Scholar] [CrossRef]
- Cundall, R.B.; Palmer, T.F.; Wood, C.E.C. Vapour pressure measurements on some organic high explosives. J. Chem. Soc. Faraday Trans. 1978, 74, 1339–1345. [Google Scholar] [CrossRef]
- Lenchitz, C.; Velicky, R.W. Vapor pressure and heat of sublimation of three nitrotoluenes. J. Chem. Eng. Data 1970, 15, 401–403. [Google Scholar] [CrossRef]
- Edwards, G.T. The vapour pressure of 2:4:6-trinitrotoluene. Trans. Faraday Soc. 1950, 46, 423–427. [Google Scholar] [CrossRef]
- Zeman, S. Analysis and prediction of the Arrhenius parameters of low temperature thermolysis of nitroamines by means of the 15N NMR spectroscopy. Thermochim. Acta 1999, 333, 121–129. [Google Scholar]
- Pella, P.E. Measurement of the vapor pressure of tnt, 2,4-dnt, and egdn. J. Chem. Thermodyn. 1977, 9, 301–305. [Google Scholar] [CrossRef]
- Oxley, J.C.; Smith, J.L.; Shinde, K.; Moran, J. Determination of the Vapor Density of Triacetone Triperoxide (TATP) Using A Gas Chromatography Headspace Technique. Propell. Explos. Pyrotech. 2005, 30, 127–130. [Google Scholar] [CrossRef]
- Lee, Y.J.; Weeks, B.L. Investigation of Size-Dependent Sublimation Kinetics of 2,4,6-Trinitrotoluene (TNT) Micro-Islands Using In Situ Atomic Force Microscopy. Molecules 2019, 24, 1895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenblatt, D.H.; Burrows, E.P.; Mitchell, W.R.; Parmer, D.L. The Handbook of Environmental Chemistry. Hutzinger, O., Ed.; Springer: Berlin/Heidelberg, Germany, 1989; pp. 195–234. [Google Scholar]
- McKone, T.E.; Daniels, J.I. Estimating human exposure through multiple pathways from air, water, and soil. Regul. Toxicol. Pharmacol. 1991, 13, 36–61. [Google Scholar] [CrossRef]
- Phelan, J.M.; Webb, S.W. Environmental Fate and Transport of Chemical Signatures from Buried Landmines-Screening Model Formulation and Initial Simulations; Report Sandia SAND97-1426.US-741; Sandia National Laboratories: Albuquerque, NM, USA, 1997.
- Hikal, W.M.; Weeks, B.L. Sublimation kinetics and diffusion coefficients of TNT, PETN, and RDX in air by thermogravimetry. Talanta 2014, 125, 24–28. [Google Scholar]
- Abramoff, M.D.; Magalhaes, P.J.; Ram, S.J. Image Processing with ImageJ. Biophotonics Int. 2004, 11, 26–42. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hikal, W.M.; Bhattacharia, S.K.; Vaughn, M.W.; Weeks, B.L. Sublimation and Diffusion Kinetics of 2,4,6-Trinitrotoluene (TNT) Single Crystals by Atomic Force Microscopy (AFM). Molecules 2022, 27, 5482. https://doi.org/10.3390/molecules27175482
Hikal WM, Bhattacharia SK, Vaughn MW, Weeks BL. Sublimation and Diffusion Kinetics of 2,4,6-Trinitrotoluene (TNT) Single Crystals by Atomic Force Microscopy (AFM). Molecules. 2022; 27(17):5482. https://doi.org/10.3390/molecules27175482
Chicago/Turabian StyleHikal, Walid M., Sanjoy K. Bhattacharia, Mark W. Vaughn, and Brandon L. Weeks. 2022. "Sublimation and Diffusion Kinetics of 2,4,6-Trinitrotoluene (TNT) Single Crystals by Atomic Force Microscopy (AFM)" Molecules 27, no. 17: 5482. https://doi.org/10.3390/molecules27175482





