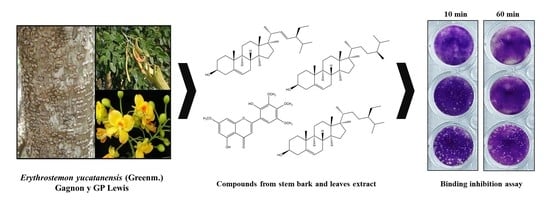
Bioassay-Guided Fractionation of Erythrostemon yucatanensis (Greenm.) Gagnon & GP Lewis Components with Anti-hemagglutinin Binding Activity against Influenza A/H1N1 Virus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Methanolic Extract Fractionation
2.3. Biological Assays
2.3.1. Cells and Viruses
2.3.2. Cytotoxicity Assay
2.3.3. Cytopathic Effect Reduction Assay
2.3.4. Hemagglutination Inhibition Assay
2.3.5. Plaque Assay
2.3.6. RNA Extraction and Quantitative RT-PCR (qRT-PCR)
2.3.7. Data Analysis and Interpretation
2.4. Bioassay-Guided Fractionation of Leaf Extracts
2.5. Bioassay-Guided Fractionation of Stem Bark Extracts
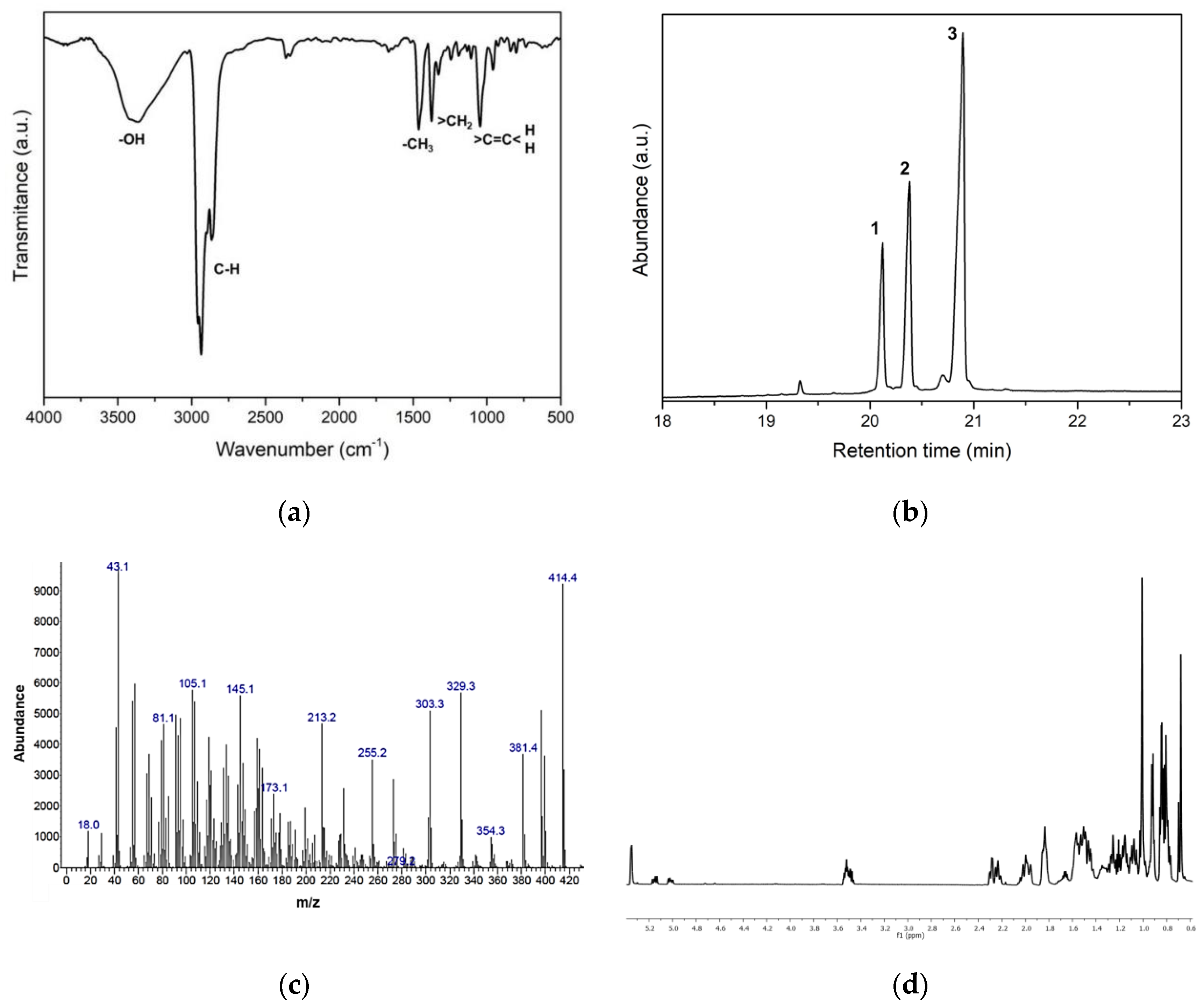
2.6. Characterization of Anti-HA Compounds in C4
2.6.1. Infrared Spectroscopy (FTIR-ATR)
2.6.2. Gas Chromatography-Mass Spectrometry (GC-MS)
2.6.3. NMR Structure Elucidation
3. Results
3.1. Antiviral Activity of Leaf, Root and Stem Bark Fractions of E. yucatanensis
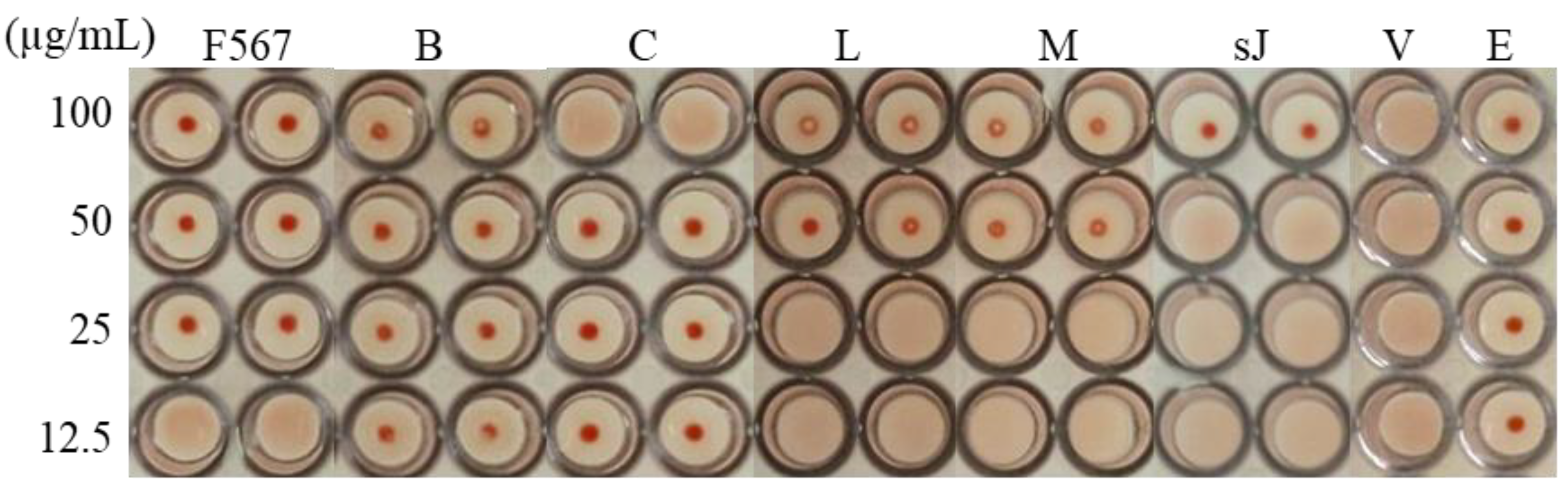
3.2. Leaf Components from E. yucatanensis with Anti-HA Activity
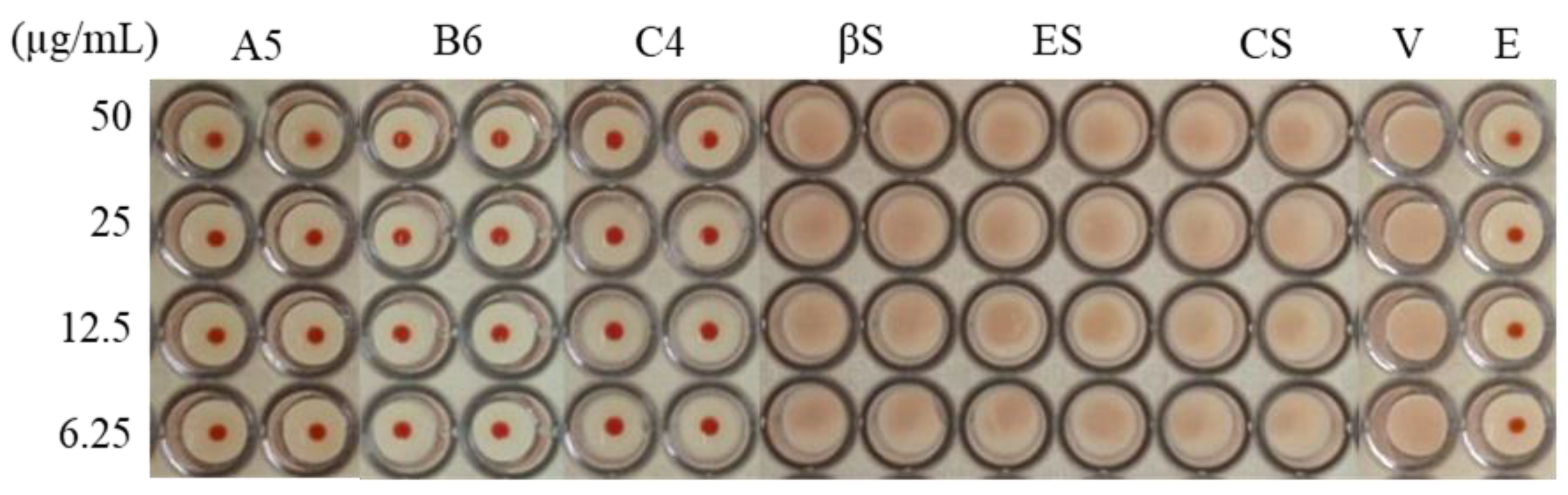
3.3. Stem Bark Fractions from E. yucatanensis with Anti-HA Activity
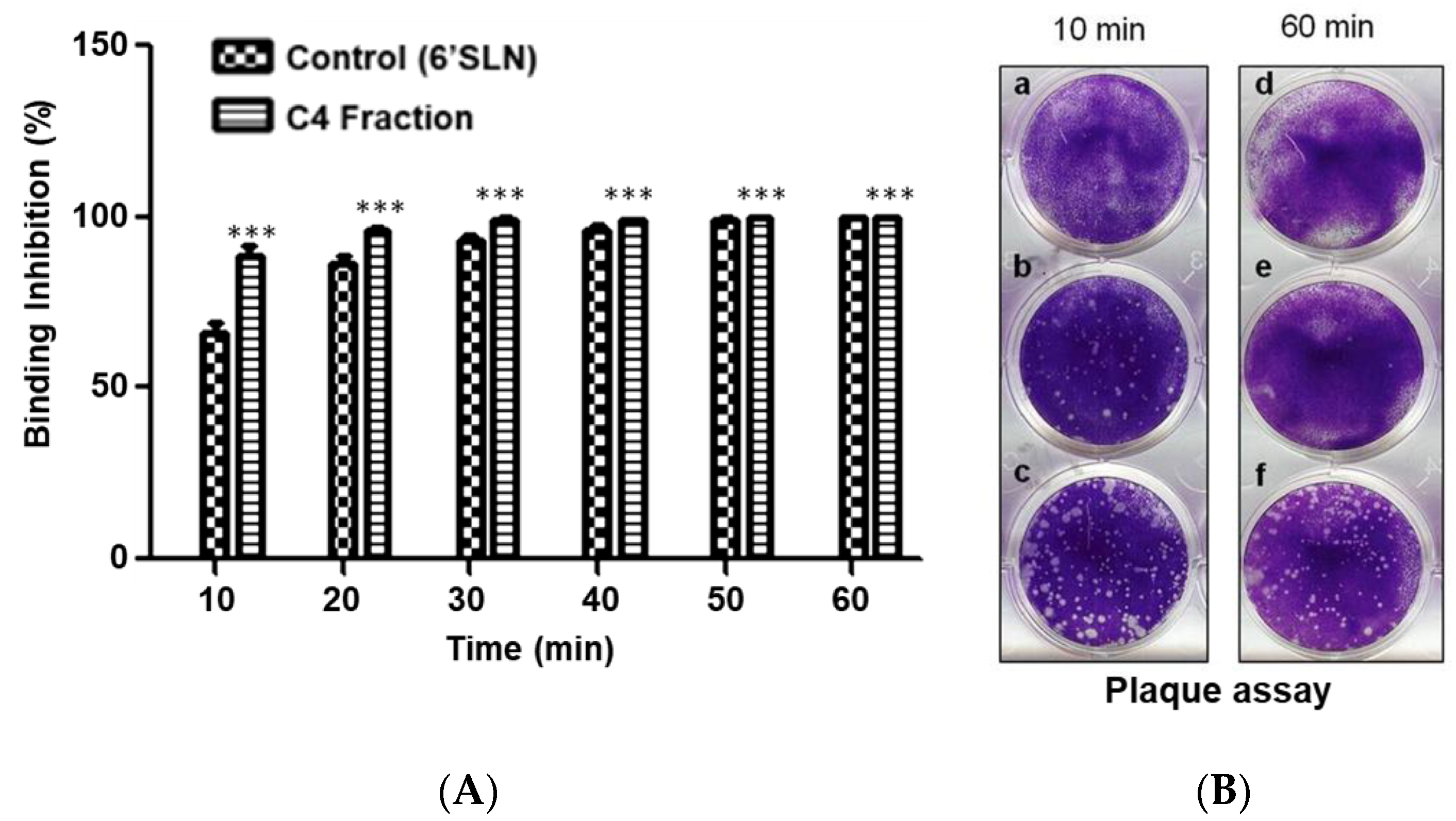
3.4. Inhibition of Virus HA Binding and Infection by Component C4 from the Stem Bark of E. yucatanensis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- González Mera, I.F.; González Falconí, D.E.; Morera Córdova, V. Secondary metabolites in plants: Main classes, phytochemical analysis and pharmacological activities. Bionatura 2019, 4, 1000–1009. [Google Scholar] [CrossRef]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef]
- Ahad, B.; Shahri, W.; Rasool, H.; Reshi, Z.A.; Rasool, S.; Hussain, T. Medicinal Plants and Herbal Drugs: An Overview. Med. Aromat. Plants 2021, 1–40. [Google Scholar]
- Harrington, W.N.; Kackos, C.M.; Webby, R.J. The evolution and future of influenza pandemic preparedness. Exp. Mol. Med. 2021, 53, 737–749. [Google Scholar] [CrossRef]
- Juárez-Méndez, M.T.; Borges-Argáez, R.; Ayora-Talavera, G.; Escalante-Rebolledo, S.E.; Escalante-Erosa, F.; Cáceres-Farfán, M. Diospyros anisandra phytochemical analysis and anti-hemagglutinin-neuraminidase activity on influenza AH1N1pdm09 virus. Nat. Prod. Res. 2021, 36, 2666–2672. [Google Scholar] [CrossRef] [PubMed]
- Karar, M.G.E.; Matei, M.F.; Jaiswal, R.; Illenberger, S.; Kuhnert, N. Neuraminidase inhibition of Dietary chlorogenic acids and derivatives–potential antivirals from dietary sources. Food Funct. 2016, 7, 2052–2059. [Google Scholar] [CrossRef] [PubMed]
- Ginex, T.; Luque, F.J. Searching for Effective Antiviral Small Molecules against Influenza A Virus: A Patent Review. Expert Opin. Ther. Pat. 2021, 31, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Wohlbold, T.J.; Krammer, F. In the shadow of hemagglutinin: A growing interest in influenza viral neuraminidase and its role as a vaccine antigen. Viruses 2014, 6, 2465–2494. [Google Scholar] [CrossRef]
- Rosado-Aguilar, J.A.; Rodríguez-Vivas, R.I.; Borges-Argaez, R.; Arjona-Cambranes, K.A. Acaricidal Activity of Havardia albicans and Caesalpinia gaumeri Methanolic Leaf Extracts on Rhipicephalus microplus and Its Toxicity to Laboratory Animals. Exp. Appl. Acarol. 2017, 71, 345–354. [Google Scholar] [CrossRef]
- Meiyanto, E.; Lestari, B.; Sugiyanto, R.N.; Jenie, R.I.; Utomo, R.Y.; Sasmito, E.; Murwanti, R. Caesalpinia sappan L. heartwood ethanolic extract exerts genotoxic inhibitory and cytotoxic effects. Orient. Pharm. Exp. Med. 2019, 19, 27–36. [Google Scholar] [CrossRef]
- Liu, A.L.; Shu, S.H.; Qin, H.L.; Lee, S.M.Y.; Wang, Y.T.; Du, G.H. In vitro anti-influenza viral activities of constituents from Caesalpinia sappan. Planta Med. 2009, 75, 337–339. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Patel, D.K.; Prasad, S.K.; Sairam, K.; Hemalatha, S. Antidiabetic activity of alcoholic root extract of Caesalpinia digyna in streptozotocin-nicotinamide induced diabetic rats. Asian Pac. J. Trop. Biomed. 2012, 2, S934–S940. [Google Scholar] [CrossRef]
- Rao, Y.K.; Fang, S.H.; Tzeng, Y.M. Anti-inflammatory activities of flavonoids isolated from Caesalpinia pulcherrima. J. Ethnopharmacol. 2005, 100, 249–253. [Google Scholar] [CrossRef]
- Chethana, K.R.; Sasidhar, B.S.; Naika, M.; Keri, R.S. Phytochemical composition of Caesalpinia crista extract as potential source for inhibiting cholinesterase and β-amyloid aggregation: Significance to Alzheimer’s disease. Asian Pac. J. Trop. Biomed. 2018, 8, 500. [Google Scholar]
- Wu, S.; Wu, Z.; Fu, C.; Wu, C.; Yuan, J.; Xian, X.; Gao, H. Simultaneous identification and analysis of cassane diterpenoids in Caesalpinia minax Hance by high-performance liquid chromatography with quadrupole time-of-flight mass spectrometry. J. Sep. Sci. 2015, 38, 4000–4013. [Google Scholar] [CrossRef]
- Gagnon, E.; Bruneau, A.; Hughes, C.E.; De Queiroz, L.P.; Lewis, G.P. A new generic system for the pantropical Caesalpinia group (Leguminosae). PhytoKeys 2016, 71, 1–160. [Google Scholar] [CrossRef]
- Zanin, J.L.B.; De Carvalho, B.A.; Salles Martineli, P.; Dos Santos, M.H.; Lago, J.H.G.; Sartorelli, P.; Viegas, C., Jr.; Soares, M.G. The genus Caesalpinia L. (Caesalpiniaceae): Phytochemical and pharmacological characteristics. Molecules 2012, 17, 7887–7902. [Google Scholar] [CrossRef]
- Quiroz-Carranza, J.; Orellana, R. Use and management of firewood in dwellings of six localities from Yucatan, Mexico. Madera Bosques 2010, 16, 47–67. [Google Scholar] [CrossRef]
- Méndez-González, M.E.; Torres-Avilez, W.M.; Dorantes-Euán, A.; Durán-García, R. Jardines medicinales en Yucatán: Una alternativa para la conservación de la flora medicinal de los mayas. Rev. Fitotec. Mex. 2014, 37, 97–106. [Google Scholar] [CrossRef]
- Borges-Argáez, R.; Chan-Balan, R.; Cetina-Montejo, L.; Ayora-Talavera, G.; Sansores-Peraza, P.; Gómez-Carballo, J.; Cáceres-Farfán, M. In vitro evaluation of anthraquinones from Aloe vera (Aloe barbadensis Miller) roots and several derivatives against strains of influenza virus. Ind. Crops Prod. 2019, 132, 468–475. [Google Scholar] [CrossRef]
- Cetina-Montejo, L.; Ayora-Talavera, G.; Borges-Argáez, R. Zeylanone epoxide isolated from Diospyros anisandra stem bark inhibits influenza virus in vitro. Arch. Virol. 2019, 164, 1543–1552. [Google Scholar] [CrossRef]
- Borges-Argáez, R.; Peña-Rodríguez, L.M.; Waterman, P.G. Flavonoids from two Lonchocarpus species of the Yucatan Peninsula. Phytochemistry 2002, 60, 533–540. [Google Scholar] [CrossRef]
- Ayora-Talavera, G.; Cetina-Montejo, L.; Matos-Patrón, A.; Romero-Beltrán, L. Hemagglutinin variants of influenza A (H1N1) pdm09 virus with reduced affinity for sialic acid receptors. Arch. Virol. 2014, 159, 1207–1211. [Google Scholar] [CrossRef]
- Hoffmann, E.; Neumann, G.; Kawaoka, Y.; Hobom, G.; Webster, R.G. A DNA transfection system for generation of influenza A virus from eight plasmids. Proc. Natl. Acad. Sci. USA 2000, 97, 6108–6113. [Google Scholar] [CrossRef]
- Hoffmann, E.; Stech, J.; Guan, Y.; Webster, R.G.; Perez, D.R. Universal primer set for the full-length amplification of all influenza A viruses. Arch. Virol. 2001, 146, 2275–2289. [Google Scholar] [CrossRef]
- De Graaf, M.; Fouchier, R.A. Role of receptor binding specificity in influenza A virus transmission and pathogenesis. EMBO J. 2014, 33, 823–841. [Google Scholar] [CrossRef]
- Dou, D.; Revol, R.; Östbye, H.; Wang, H.; Daniels, R. Influenza A virus cell entry, replication, virion assembly and movement. Front. Immunol. 2018, 9, 1581. [Google Scholar] [CrossRef]
- Heida, R.; Bhide, Y.C.; Gasbarri, M.; Kocabiyik, Ö.; Stellacci, F.; Huckriede, A.L.W.; Hinrichs, W.L.J.; Frijlink, H.W. Advances in the development of entry inhibitors for sialic-acid-targeting viruses. Drug Discov. Today 2021, 26, 122–137. [Google Scholar] [CrossRef]
- Tuchinda, P.; Saiai, A.; Pohmakotr, M.; Yoosook, C.; Kasisit, J.; Napaswat, C.; Santisuk, T.; Reutrakul, V. Anti-HIV-1 cycloartanes from leaves and twigs of Gardenia thailandica. Planta Med. 2004, 70, 366–370. [Google Scholar] [CrossRef]
- Kongkum, N.; Tuchinda, P.; Pohmakotr, M.; Reutrakul, V.; Piyachaturawat, P.; Jariyawat, S.; Suksen, K.; Yoosook, C.; Kasisit, J.; Napaswad, C. DNA topoisomerase IIα inhibitory and anti-HIV-1 flavones from leaves and twigs of Gardenia carinata. Fitoterapia 2012, 83, 368–372. [Google Scholar] [CrossRef]
- Prasad, A.; Muthamilarasan, M.; Prasad, M. Synergistic antiviral effects against SARS-CoV-2 by plant-based molecules. Plant Cell Rep. 2020, 39, 1109–1114. [Google Scholar] [CrossRef]
- Tan, Y.L.; Tan, K.S.W.; Chu, J.J.H.; Chow, V.T. Combination Treatment with Remdesivir and Ivermectin Exerts Highly Synergistic and Potent Antiviral Activity Against Murine Coronavirus Infection. Front. Cell. Infect. Microbiol. 2021, 11, 700502. [Google Scholar] [CrossRef] [PubMed]
- Pineda Rodríguez, M.; Hung Llamos, B.R.; García Delgado, B. Fitosteroles y Patentes: Sus Aplicaciones en la Industria Farmaceutica. Revista CENIC. Cienc. Biológicas 2005, 36. Available online: https://www.redalyc.org/articulo.oa?id=181220525018 (accessed on 7 July 2022).
- Villa-De la Torre, F.; Kinscherf, R.; Bonaterra, G.; Arana-Argaez, V.E.; Méndez-González, M.; Cáceres-Farfán, M.; Borges-Argáez, R. Anti-inflammatory and immunomodulatory effects of Critonia aromatisans leaves: Downregulation of pro-inflammatory cytokines. J. Ethnopharmacol. 2016, 190, 174–182. [Google Scholar] [CrossRef]
- Ashrafi, S.; Rahman, M.; Ahmed, P.; Alam, S.; Hossain, M. Prospective Asian plants with corroborated antiviral potentials: Position standing in recent years. Beni-Suef Univ. J. Basic Appl Sci. 2022, 11, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.H.; Segovia, J.; Sabbah, A.; Mgbemena, V.; Bose, S. Cholesterol-rich lipid rafts are required for release of infectious human respiratory syncytial virus particles. Virology 2012, 422, 205–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Fractions | CC50 | A/Yucatán/2370/09 | A/México/InDRE797/10 |
|---|---|---|---|
| (Plant part) | µg/mL | MOI:0.001 | MOI:0.01 |
| IC50 (SI) | IC50(SI) | ||
| µg/mL | µg/mL | ||
| A1 (leaves) | >100 | >25 (4.00) | >25 (4.00) |
| A2 (leaves) | 71.95 | <0.195 (368.97) | <0.195 (368.97) |
| A3 (roots) | 15.96 | >6.25 (2.55) | >6.25 (2.55) |
| A4 (roots) | 12.24 | >6.25 (1.95) | >6.25 (1.95) |
| A5 (stem bark) | 41.7 | 2.51(16.6) | 4.83 (8.63) |
| A6 (stem bark) | 50.47 | >25 (8.04) | <0.78 (64.70) |
| Samples | CC50 | A/Yucatán/2370/09 | |
|---|---|---|---|
| µg/mL | MOI:0.01 | ||
| IC50 | SI | ||
| µg/mL | CC50/IC50 | ||
| C4 (fraction) | 196.36 | 3.125 | 62.84 |
| β-sitosterol | >100 | >100 | <1 |
| Stigmasterol | >100 | >100 | <1 |
| Campesterol | >100 | >100 | <1 |
| Time | Number of Copies | % Inhibition | ||
|---|---|---|---|---|
| (min) | 6′SLN | C4 | 6′SLN | C4 |
| 10 | 685.25 | 1189.51 | 91.2 | 84.72 |
| 20 | 0 | 1193.4 | 100 | 84.67 |
| 30 | 0 | 1112.74 | 100 | 85.7 |
| 40 | 0 | 1277.16 | 100 | 83.59 |
| 50 | 0 | 1334.84 | 100 | 82.85 |
| 60 | 0 | 556.51 | 100 | 92.85 |
| * CV | 7780.06 | |||
| * CC | 0 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortiz-López, T.; Borges-Argáez, R.; Ayora-Talavera, G.; Canto-Ramírez, E.; Cetina-Montejo, L.; May-May, Á.; Escalante-Erosa, F.; Cáceres-Farfán, M. Bioassay-Guided Fractionation of Erythrostemon yucatanensis (Greenm.) Gagnon & GP Lewis Components with Anti-hemagglutinin Binding Activity against Influenza A/H1N1 Virus. Molecules 2022, 27, 5494. https://doi.org/10.3390/molecules27175494
Ortiz-López T, Borges-Argáez R, Ayora-Talavera G, Canto-Ramírez E, Cetina-Montejo L, May-May Á, Escalante-Erosa F, Cáceres-Farfán M. Bioassay-Guided Fractionation of Erythrostemon yucatanensis (Greenm.) Gagnon & GP Lewis Components with Anti-hemagglutinin Binding Activity against Influenza A/H1N1 Virus. Molecules. 2022; 27(17):5494. https://doi.org/10.3390/molecules27175494
Chicago/Turabian StyleOrtiz-López, Tania, Rocío Borges-Argáez, Guadalupe Ayora-Talavera, Ernesto Canto-Ramírez, Lisseth Cetina-Montejo, Ángel May-May, Fabiola Escalante-Erosa, and Mirbella Cáceres-Farfán. 2022. "Bioassay-Guided Fractionation of Erythrostemon yucatanensis (Greenm.) Gagnon & GP Lewis Components with Anti-hemagglutinin Binding Activity against Influenza A/H1N1 Virus" Molecules 27, no. 17: 5494. https://doi.org/10.3390/molecules27175494
APA StyleOrtiz-López, T., Borges-Argáez, R., Ayora-Talavera, G., Canto-Ramírez, E., Cetina-Montejo, L., May-May, Á., Escalante-Erosa, F., & Cáceres-Farfán, M. (2022). Bioassay-Guided Fractionation of Erythrostemon yucatanensis (Greenm.) Gagnon & GP Lewis Components with Anti-hemagglutinin Binding Activity against Influenza A/H1N1 Virus. Molecules, 27(17), 5494. https://doi.org/10.3390/molecules27175494