Pyropheophorbide-a/(001) TiO2 Nanocomposites with Enhanced Charge Separation and O2 Adsorption for High-Efficiency Visible-Light Degradation of Ametryn
Abstract
:1. Introduction
2. Results
2.1. Structural Characterization
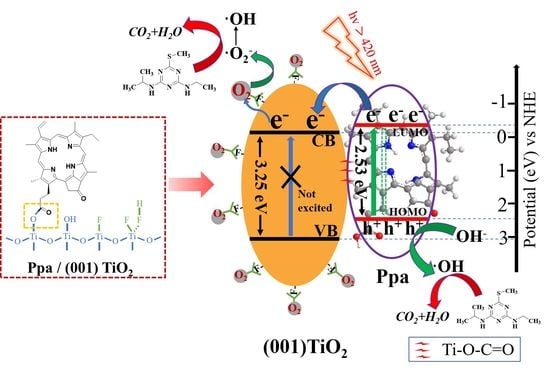
2.2. Photogenerated Charge Separation
2.3. Photocatalytic Performance
2.4. Photocatalytic Mechanism
2.5. Degradation Pathways
2.6. Mechanism Discussion
3. Materials and Methods
3.1. Materials
3.2. Preparation of 001T
3.3. Synthesis of xPpa/001T
3.4. Characterizations
3.5. Evaluation of •OH
3.6. Photocatalytic Degradation Experiments
3.7. Analytical Methods
3.8. Scavenging Trapping Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Navaratna, D.; Shu, L.; Baskaran, K.; Jegatheesan, V. Treatment of ametryn in wastewater by a hybrid MBR system: A lab-scale study. Water Sci. Technol. 2012, 66, 1317–1324. [Google Scholar] [CrossRef] [PubMed]
- Navaratna, D.; Elliman, J.; Cooper, A.; Shu, L.; Baskaran, K.; Jegatheesan, V. Impact of herbicide Ametryn on microbial communities in mixed liquor of a membrane bioreactor (MBR). Bioresour. Technol. 2012, 113, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Directive 2006/118/EC of the European Parliament and of the Council of 12 December 2006 on the Protection of Groundwater against Pollution and Deterioration; 2006C. Available online: http://extwprlegs1.fao.org/docs/pdf/eur68257.pdf (accessed on 25 August 2022).
- U.S. Environmental Protection Agency (USEPA). Amentryn Health Advisory. Office of Drinking Water, U.S. Environmental Protdction Agency, August. 1988. Available online: https://www.epa.gov/gliclearinghouse/human-health-water-ingestion-only-fact-sheet-ametryn-human-health-water-ingestion (accessed on 25 August 2022).
- Scheel, G.L.; Tarley, C.R.T. Fp HPLC-DAD. Microchem. J. 2017, 133, 650–657. [Google Scholar] [CrossRef]
- Yang, H.; Wei, H.; Hu, L.; Liu, H.; Yang, L.; Au, C.; Yi, B. Mechanism for the photocatalytic transformation of s-triazine herbicides by radical ·OH radicals over TiO2. Chem. Eng. J. 2016, 300, 209–216. [Google Scholar] [CrossRef]
- Ali, I.; AL-Othman, Z.A.; Alwarthan, A. Green synthesis of functionalized iron nano particles and molecular liquid phase adsorption of ametryn from water. J. Mol. Liq. 2016, 221, 1168–1174. [Google Scholar] [CrossRef]
- Szewczyk, R.; Kuśmierska, A.; Bernat, P. Ametryn removal by Metarhizium brunneum: Biodegradation pathway proposal and metabolic background revealed. Chemosphere 2018, 190, 174–183. [Google Scholar] [CrossRef]
- Sangami, S.; Manu, B. Fenton’s treatment of actual agriculture runoff water containing herbicides. Water Sci. Technol. 2017, 75, 451–461. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, M.; Wang, Z.F.; Chen, H.Y.; Chen, Y.; Murakami, N.; Ohno, T. Synthesis of anatase TiO2 with exposed {001} and {101} facets and photocatalytic activity. Rare Met. 2019, 38, 287–291. [Google Scholar] [CrossRef]
- Li, L.; Hu, Z.; Yu, J.C. On-Demand Synthesis of H2O2 by Water Oxidation for Sustainable Resource Production and Organic Pollutant Degradation. Angew. Chem. 2020, 132, 20719–20725. [Google Scholar] [CrossRef]
- Chen, S.; Yan, R.; Zhang, X.; Hu, K.; Li, Z.; Humayun, M.; Qu, Y.; Jing, L. Photogenerated electron modulation to dominantly induce efficient 2, 4-dichlorophenol degradation on BiOBr nanoplates with different phosphate modification. Appl. Catal. B 2017, 209, 320–328. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Huang, J.; Li, S.; Meng, A.; Li, Z. Interfacial chemical bond and internal electric field modulated Z-scheme Sv-ZnIn2S4/MoSe2 photocatalyst for efficient hydrogen evolution. Nat. Commun. 2021, 12, 4112. [Google Scholar] [CrossRef] [PubMed]
- Siwawannapong, K.; Zhang, R.; Lei, H.; Jin, Q.; Tang, W.; Dong, Z.; Lai, R.; Liu, Z.; Kamkaew, A.; Cheng, L. Ultra-small pyropheophorbide-a nanodots for near-infrared fluorescence/photoacoustic imaging-guided photodynamic therapy. Theranostics 2020, 10, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, X.; Yu, W.; Zeng, J.; Shi, Y.; Lin, S.; Huang, G.; Zhang, L.; Wang, H.; Kong, Z.; et al. Photocatalytic study of a novel crystal facets sensitive heterojunction between Sb8O11Cl2 and anatase TiO2 with different exposed facets. Dye. Pigm. 2019, 160, 530–539. [Google Scholar] [CrossRef]
- Byrne, C.; Subramanian, G.; Pillai, S.C. Recent advances in photocatalysis for environmental applications. J. Environ. Chem. Eng. 2018, 6, 3531–3555. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Yu, P.Y.; Mao, S.S. Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef]
- Fröschl, T.; Hörmann, U.; Kubiak, P.; Kučerová, G.; Pfanzelt, M.; Weiss, C.K.; Behm, R.J.; Husing, N.; Kaiser, U.; Landfester, K.; et al. High surface area crystalline titanium dioxide: Potential and limits in electrochemical energy storage and catalysis. Chem. Soc. Rev. 2012, 41, 5313–5360. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Wang, J.; Jia, M.; Yang, F.; Andriamitantsoa, R.S.; Huang, X.; Dong, W.; Wang, G. Construction of TiO2 nanosheets/tetra (4-carboxyphenyl) porphyrin hybrids for efficient visible-light photoreduction of CO2. Chem. Eng. J. 2019, 374, 684–693. [Google Scholar] [CrossRef]
- Luan, Y.; Jing, L.; Xie, Y.; Sun, X.; Feng, Y.; Fu, H. Exceptional photocatalytic activity of 001-facet-exposed TiO2 mainly depending on enhanced adsorbed oxygen by residual hydrogen fluoride. ACS Catal. 2013, 3, 1378–1385. [Google Scholar] [CrossRef]
- Liu, Y.; He, M.; Guo, R.; Fang, Z.; Kang, S.; Ma, Z.; Dong, M.; Wang, W.; Cui, L. Ultrastable metal-free near-infrared-driven photocatalysts for H2 production based on protonated 2D g-C3N4 sensitized with Chlorin e6. Appl. Catal. B 2020, 260, 118137. [Google Scholar] [CrossRef]
- Phongamwong, T.; Chareonpanich, M.; Limtrakul, J. Role of chlorophyll in Spirulina on photocatalytic activityof CO2 reduction under visible light over modified N-dopedTiO2 photocatalysts. Appl. Catal. B Environ. 2015, 168, 114–124. [Google Scholar] [CrossRef]
- Yang, C.; Wang, X.; Ma, W.; Wang, Z.; Tan, G.; Fang, W.; Jin, Y. Improving the photodynamic therapy of pyropheophorbide a through the combination of hypoxia-sensitive molecule and infrared light-excited d-TiO2-X nanoparticles. J. Porphyr. Phthalocyanines 2022, 26, 31–43. [Google Scholar] [CrossRef]
- Wang, Z.; Song, Q.; Zhu, L.; Zhao, C.; Ma, H. Synthesis, Characterization of NR@SiO2/PNIPAm-co-Ppa Composite Nanogel and Study On Its Application in Photodynamic Therapy. J. Fluoresc. 2022, 32, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Cai, J.; Mao, J.; Li, S.; Shen, J.; Gao, S.; Huang, J.; Wang, X.; Parkin, I.P.; Lai, Y. Defective black Ti3+ self-doped TiO2 and reduced graphene oxide composite nanoparticles for boosting visible-light driven photocatalytic and photoelectrochemical activity. Appl. Surf. Sci. 2019, 467, 45–55. [Google Scholar] [CrossRef]
- Ma, X.Y.; Chen, Z.G.; Hartono, S.B.; Jiang, H.B.; Zou, J.; Qiao, S.Z.; Yang, H.G. Fabrication of uniform anatase TiO2 particles exposed by {001} facets. Chem. Commun. 2010, 46, 6608–6610. [Google Scholar] [CrossRef]
- Humayun, M.; Ullah, H.; Cao, J.; Pi, W.; Yuan, Y.; Ali, S.; Tahir, A.A.; Yue, P.; Khan, A.; Zheng, Z.; et al. Experimental and DFT studies of Au deposition over WO3/g-C3N4 Z-scheme heterojunction. Nanomicro Lett. 2019, 12, 7. [Google Scholar] [CrossRef]
- Humayun, M.; Zada, A.; Li, Z.; Xie, M.; Zhang, X.; Qu, Y.; Raziq, F.; Jing, L. Enhanced visible-light activities of porous BiFeO3 by coupling with nanocrystalline TiO2 and mechanism. Appl. Catal. B 2016, 180, 219–226. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, H.; Li, S.; Wang, Z.; Wu, X.; Yan, R.; Geng, F.; Mu, W.; Jin, Y. A Multifunctional Nanoplatform Based on Fenton-like and Russell Reactions of Cu, Mn Bimetallic Ions Synergistically Enhanced ROS Stress for Improved Chemodynamic Therapy. ACS Biomater. Sci. Eng. 2022, 8, 1354–1366. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.; Ma, W.; Wu, X.; Fang, W.; Guo, C.; Jin, Y. Construction of a nanotheranostic system Zr-MOF@ PPa/AF@ PEG for improved photodynamic therapy effects based on the PDT-oxygen consumption and hypoxia sensitive chemotherapeutic drug. J. Photochem. Photobiol. B Biol. 2021, 222, 112274. [Google Scholar] [CrossRef]
- Shi, Y.; Yan, Z.; Xu, Y.; Tian, T.; Zhang, J.; Pang, J.; Peng, X.; Zhang, Q.; Shao, M.; Tan, W.; et al. Visible-light-driven AgBr–TiO2-Palygorskite photocatalyst with excellent photocatalytic activity for tetracycline hydrochloride. J. Clean. Prod. 2020, 277, 124021. [Google Scholar] [CrossRef]
- Hu, K.; Li, R.; Ye, C.; Wang, A.; Wei, W.; Hu, D.; Qiu, R.; Yan, K. Facile synthesis of Z-scheme composite of TiO2 nanorod/g-C3N4 nanosheet efficient for photocatalytic degradation of ciprofloxacin. J. Clean. Prod. 2020, 253, 120055. [Google Scholar] [CrossRef]
- Bian, J.; Qu, Y.; Zhang, X.; Sun, N.; Tang, D.; Jing, L. Dimension-matched plasmonic Au/TiO2/BiVO4 nanocomposites as efficient wide-visible-light photocatalysts to convert CO2 and mechanistic insights. J. Mater. Chem. A 2018, 6, 11838–11845. [Google Scholar] [CrossRef]
- Xie, Z.; Feng, Y.; Wang, F.; Chen, D.; Zhang, Q.; Zeng, Y.; Lv, W.; Liu, G. Construction of carbon dots modified MoO3/g-C3N4 Z-scheme photocatalyst with enhanced visible-light photocatalytic activity for the degradation of tetracycline. Appl. Catal. B 2018, 229, 96–104. [Google Scholar] [CrossRef]
- Xiao, Y.; Tian, G.; Chen, Y.; Zhang, X.; Fu, H.; Fu, H. Exceptional visible-light photoelectrocatalytic activity of In2O3/In2S3/CdS ternary stereoscopic porous heterostructure film for the degradation of persistent 4-fluoro-3-methylphenol. Appl. Catal. B 2018, 225, 477–486. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Yan, R.; Humayun, M.; Zhang, H.; Qu, Y.; Jin, Y. Pyropheophorbide-a/(001) TiO2 Nanocomposites with Enhanced Charge Separation and O2 Adsorption for High-Efficiency Visible-Light Degradation of Ametryn. Molecules 2022, 27, 5576. https://doi.org/10.3390/molecules27175576
Liu S, Yan R, Humayun M, Zhang H, Qu Y, Jin Y. Pyropheophorbide-a/(001) TiO2 Nanocomposites with Enhanced Charge Separation and O2 Adsorption for High-Efficiency Visible-Light Degradation of Ametryn. Molecules. 2022; 27(17):5576. https://doi.org/10.3390/molecules27175576
Chicago/Turabian StyleLiu, Songtao, Rui Yan, Muhammad Humayun, Huanli Zhang, Yang Qu, and Yingxue Jin. 2022. "Pyropheophorbide-a/(001) TiO2 Nanocomposites with Enhanced Charge Separation and O2 Adsorption for High-Efficiency Visible-Light Degradation of Ametryn" Molecules 27, no. 17: 5576. https://doi.org/10.3390/molecules27175576