Multimodal Imaging and Phototherapy of Cancer and Bacterial Infection by Graphene and Related Nanocomposites
Abstract
:1. Introduction
2. Preparation of Graphene Nanocomposites
2.1. Graphene Oxide
2.2. Reduced Graphene Oxide
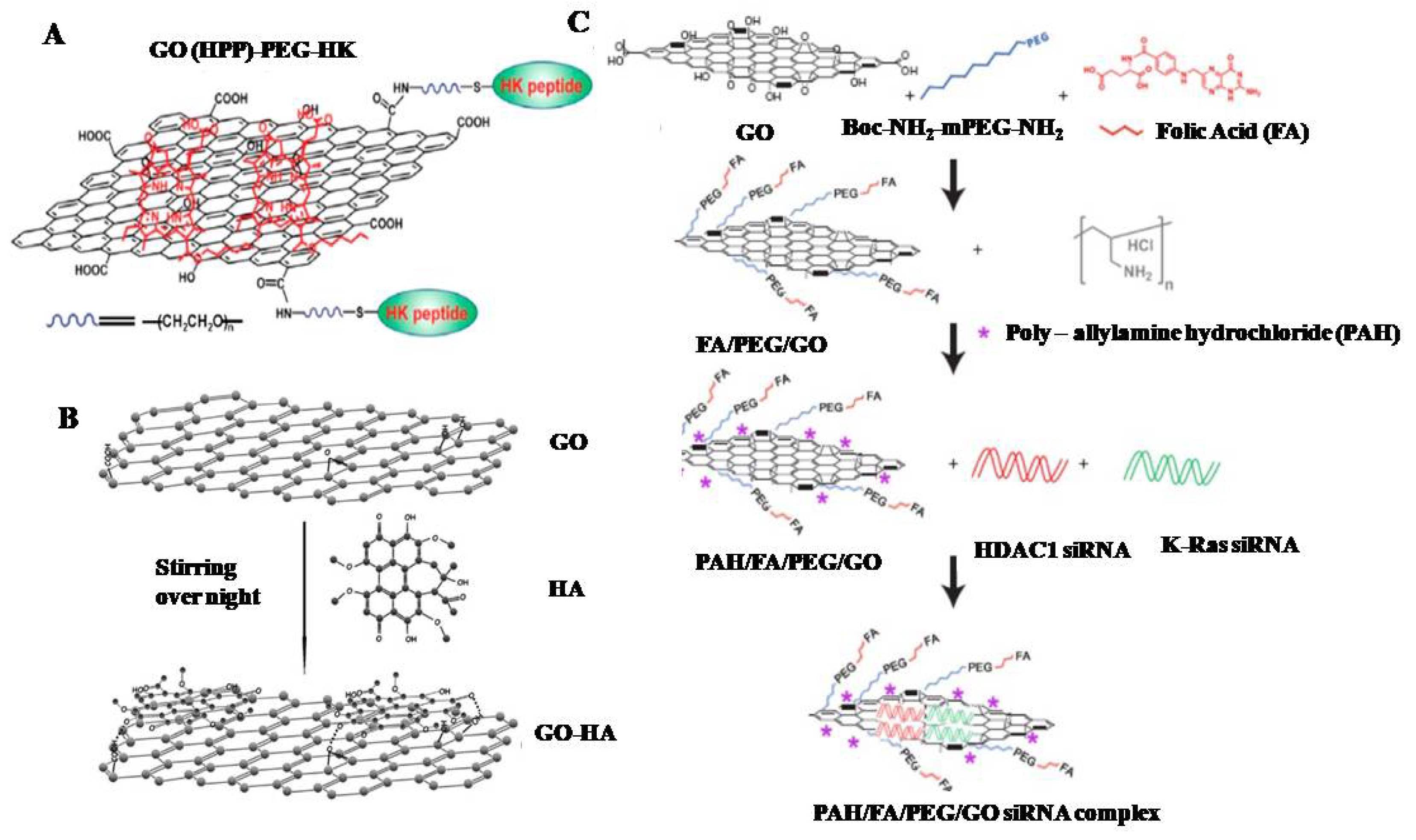
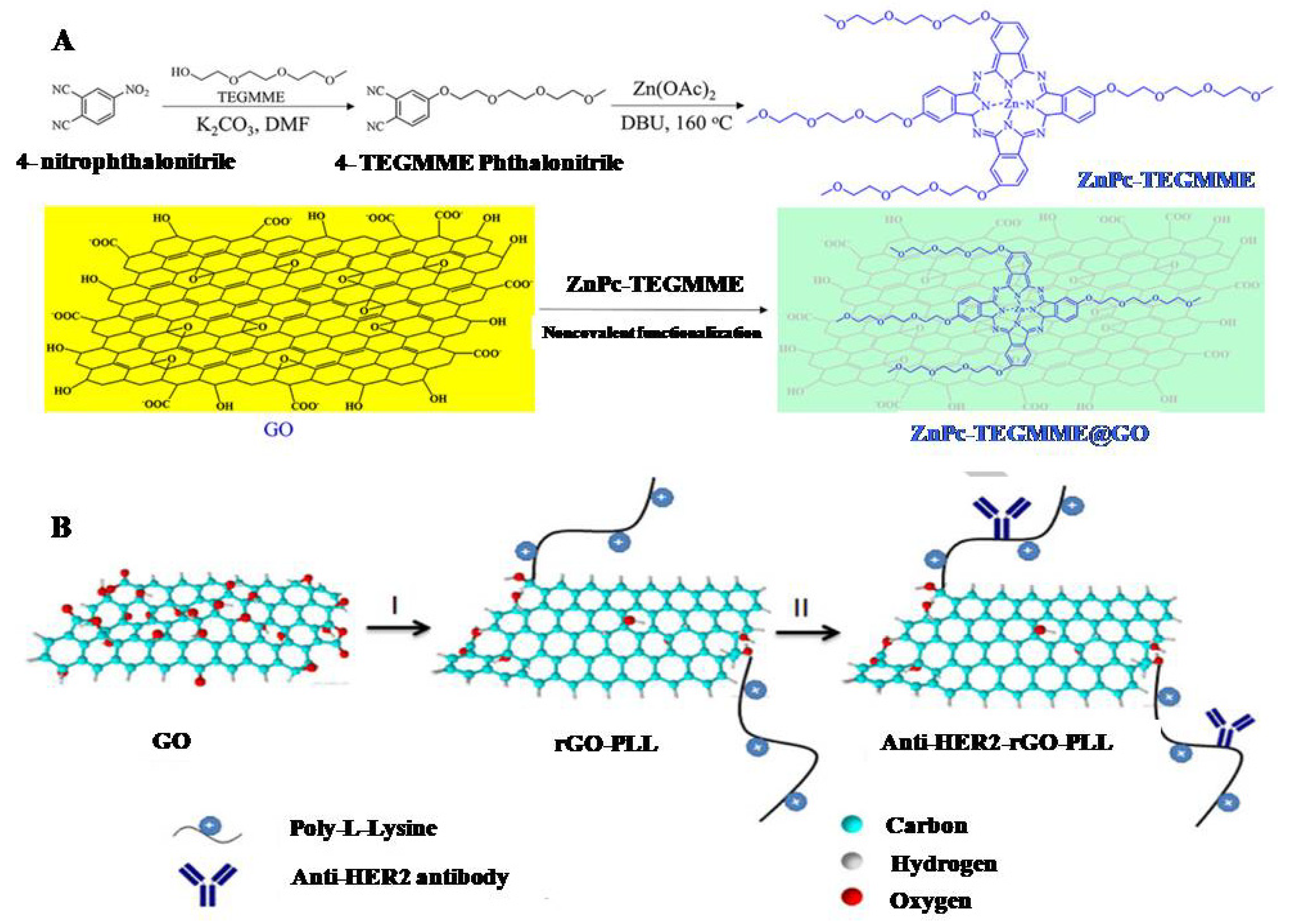
2.3. Functionalization
3. Graphene Nanocomposite Theranostics for Multimodal Imaging Guided Phototherapy
3.1. CLSM for Imaging Guided Therapy
3.2. MRI for Imaging Guided Therapy
3.3. CT for Imaging Guided Therapy
3.4. PET for Imaging Guided Therapy
3.5. PAI for Imaging Guided Therapy
3.6. Raman for Imaging Guided Therapy
3.7. ToF-SIMS for Cellular Imaging and Guided Cancer Therapy
3.8. Guided Phototherapy of Bacteria
3.9. Comparison among GNCs
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Editorial: Reflecting on 20 years of progress. Nat. Rev. Cancer 2021, 21, 605. [CrossRef] [PubMed]
- Knopman, D.S.; Amieva, H.; Petersen, R.C.; Chételat, G.; Holtzman, D.M.; Hyman, B.T.; Nixon, R.A.; Jones, D.T. Alzheimer disease. Nat. Rev. Dis. Primers 2021, 7, 33. [Google Scholar] [CrossRef]
- Flora, G.D.; Nayak, M.K. A Brief Review of Cardiovascular Diseases. Associated Risk Factors and Current Treatment Regimes. Curr. Pharm. Des. 2019, 25, 4063–4084. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.E.; Mahmud, A.S.; Miller, I.F.; Rajeev, M.; Rasambainarivo, F.; Rice, B.L.; Takahashi, S.; Tatem, A.J.; Wagner, C.E.; Wang, L.F.; et al. Infectious disease in an era of global change. Nat. Rev. Microbiol. 2022, 20, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Salata, O.V. Applications of nanoparticles in biology and medicine. J. Nanobiotechnol. 2004, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Mooney, D.J. Biomaterials and emerging anticancer therapeutics: Engineering the microenvironment. Nat. Rev. Cancer 2016, 16, 56–66. [Google Scholar] [CrossRef]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Roy, I.; Yang, C.; Prasad, P.N. Nanochemistry and Nanomedicine for Nanoparticle-based Diagnostics and Therapy. Chem. Rev. 2016, 116, 2826–2885. [Google Scholar] [CrossRef]
- Soares, S.; Sousa, J.; Pais, A.; Vitorino, C. Nanomedicine: Principles, Properties, and Regulatory Issues. Front. Chem. 2018, 6, 360. [Google Scholar] [CrossRef]
- Rakhshandehroo, T.; Smith, B.R.; Glockner, H.J.; Rashidian, M.; Pandit-Taskar, N. Molecular Immune Targeted Imaging of Tumour Microenvironment. Nanotheranostics 2022, 6, 286. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, W.; Zhu, G.; Xie, J.; Chen, X. Rethinking cancer nanotheranostics. Nat. Rev. Mater. 2017, 2, 17024. [Google Scholar] [CrossRef]
- Robson, A.L.; Dastoor, P.C.; Flynn, J.; Palmer, W.; Martin, A.; Smith, D.W.; Woldu, A.; Hua, S. Advantages and Limitations of Current Imaging Techniques for Characterizing Liposome Morphology. Front. Pharmacol. 2018, 9, 80. [Google Scholar] [CrossRef]
- Man, F.; Lammers, T.; de Rosales, R.T.M. Imaging Nanomedicine-Based Drug Delivery: A Review of Clinical Studies. Mol. Imaging Biol. 2018, 20, 683–695. [Google Scholar] [CrossRef]
- Adeel, M.; Duzagac, F.; Canzonieri, V.; Rizzolio, F. Self-therapeutic nanomaterials for cancer therapy: A review. ACS Appl. Nano Mater. 2020, 3, 4962–4971. [Google Scholar] [CrossRef]
- Chabner, B.A., Jr.; Roberts, T.G. Chemotherapy and the war on cancer. Nat. Rev. Cancer 2005, 5, 65–72. [Google Scholar] [CrossRef]
- Akkın, S.; Varan, G.; Bilensoy, E. A review on cancer immunotherapy and applications of nanotechnology to chemoimmunotherapy of different cancers. Molecules 2021, 11, 3382. [Google Scholar] [CrossRef]
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef]
- Bulaklak, K.; Gersbach, C.A. The once and future gene therapy. Nat. Commun. 2020, 11, 5820. [Google Scholar] [CrossRef]
- Altun, İ.; Sonkaya, A. The most common side effects experienced by patients were receiving first cycle of chemotherapy. Iran. J. Public Health 2018, 47, 1218–1219. [Google Scholar]
- Nurgali, K.; Jagoe, R.T.; Abalo, R. Editorial: Adverse effects of cancer chemotherapy: Anything new to improve tolerance and reduce sequelae? Front. Pharmacol. 2018, 9, 245. [Google Scholar] [CrossRef]
- Schirrmacher, V. From chemotherapy to biological therapy: A review of novel concepts to reduce the side effects of systemic cancer treatment (Review). Int. J. Oncol. 2019, 54, 407–419. [Google Scholar]
- Amjad, M.T.; Chidharla, A.; Kasi, A. Cancer Chemotherapy; StatPearls Publishing: Treasure Island, CA, USA, 2022. [Google Scholar]
- Shi, H.; Sadler, P.J. How promising is phototherapy for cancer? Br. J. Cancer 2020, 123, 871–873. [Google Scholar] [CrossRef]
- Chitgupi, U.; Qin, Y.; Lovell, J.F. Targeted nanomaterials for phototherapy. Nanotheranostics 2017, 1, 38–58. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, Y.; Li, X.; Zhao, Y.; Li, M.; Jiang, W.; Tang, X.; Dou, J.; Lu, L.; Wang, F.; et al. Near-Infrared II phototherapy induces deep tissue immunogenic cell death and potentiates cancer immunotherapy. ACS Nano 2019, 13, 11967–11980. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, S.; Cheng, G.; Yu, P.; Chang, J. Light-responsive nanomaterials for cancer therapy. Engineering 2022, 13, 18–30. [Google Scholar] [CrossRef]
- Albanese, A.; Tang, P.S.; Chan, W.C.W. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu. Rev. Biomed. Eng. 2012, 14, 1–16. [Google Scholar] [CrossRef]
- Renero-Lecuna, C.; Iturrioz-Rodríguez, N.; González-Lavado, E.; Padín-González, E.; Navarro-Palomares, E.; Valdivia-Fernández, L.; García-Hevia, L.; Fanarraga, M.L. Effect of size, shape, and composition on the interaction of different nanomaterials with Hela cells. Hindawi J. Nanomater. 2019, 11, 7518482. [Google Scholar] [CrossRef]
- Raza, M.A.; Kanwal, Z.; Rauf, A.; Sabri, A.N.; Riaz, S.; Naseem, S. Size- and shape-dependent antibacterial studies of silver nanoparticles synthesized by wet chemical routes. Nanomaterials 2016, 6, 74. [Google Scholar] [CrossRef]
- Garg, B.; Sung, C.H.; Ling, Y.C. Graphene-based nanomaterials as molecular imaging agents. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 737–758. [Google Scholar] [CrossRef]
- Mao, H.Y.; Laurent, S.; Chen, W.; Akhavan, O.; Imani, M.; Ashkarran, A.A.; Mahmoudi, M. Graphene: Promises, facts, opportunities, and challenges in nanomedicine. Chem. Rev. 2013, 113, 3407–3424. [Google Scholar] [CrossRef]
- Gollavelli, G.; Ling, Y.C. Chapter 21 Ultrathin graphene structure, fabrication and characterization for clinical diagnosis applications. In Smart Nanodevices for Point-of-Care Applications; SuvardhanKanchi, S., Chokkareddy, R., Rezakazemi, M., Eds.; CRC Press: Boca Raton, FL, USA, 2022; pp. 263–280. [Google Scholar]
- Yin, P.T.; Shah, S.; Chhowalla, M.; Lee, K.B. Design, synthesis, and characterization of graphene-nanoparticle hybrid materials for bioapplications. Chem. Rev. 2015, 115, 2483–2531. [Google Scholar] [CrossRef]
- Markovic, Z.M.; Harhaji-Trajkovic, L.M.; Todorovic-Markovic, B.M.; Kepić, D.P.; Arsikin, K.M.; Jovanović, S.P.; Pantovic, A.C.; Dramićanin, M.D.; Trajkovic, V.S. In vitro comparison of the photothermal anticancer activity of graphene nanoparticles and carbon nanotubes. Biomaterials 2011, 32, 1121–1129. [Google Scholar] [CrossRef]
- Edwards, R.S.; Coleman, K.S. Graphene synthesis: Relationship to applications. Nanoscale 2013, 5, 38–51. [Google Scholar] [CrossRef]
- Tang, L.; Li, X.; Ji, R.; Teng, K.S.; Tai, G.; Ye, J.; Wei, C.; Lau, S.P. Bottom-up synthesis of large-scale graphene oxide nanosheets. J. Mater. Chem. 2012, 22, 5676–5683. [Google Scholar] [CrossRef]
- Eskiizmir, G.; Baskın, Y.; Yapıcı, K. Chapter 9 Graphene-based nanomaterials in cancer treatment and diagnosis. In Fullerens, Graphenes and Nanotubes: A Pharmaceutical Approach; Grumezescu, A.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 331–374. [Google Scholar]
- Shamaila, S.; Sajjad, A.K.L.; Iqbal, A. Modifications in development of graphene oxide synthetic routes. Chem. Eng. J. 2016, 294, 458–477. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.; Lu, W.; Tour, M.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Naeem, H.; Ajmal, M.; Muntha, S.; Ambreen, J.; Siddiq, M. Synthesis and characterization of graphene oxide sheets integrated with gold nanoparticles and their applications to adsorptive removal and catalytic reduction of water contaminants. RSC Adv. 2018, 8, 3599–3610. [Google Scholar] [CrossRef]
- Wu, S.; Zhao, X.; Cui, Z. Cytotoxicity of graphene oxide and graphene oxide loaded with doxorubicin on human multiple myeloma cells. Int. J. Nanomed. 2014, 9, 1413–1421. [Google Scholar]
- Yu, X.; Gao, D.; Gao, L.; Lai, J.; Zhang, C.; Zhao, Y.; Zhong, L.; Jia, B.; Wang, F.; Chen, X.; et al. Inhibiting Metastasis and Preventing Tumor Relapse by Triggering Host Immunity with Tumor-Targeted Photodynamic Therapy Using Photosensitizer-Loaded Functional Nanographenes. ACS Nano 2017, 11, 10147–10158. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, W.; Tang, J.; Zhou, J.H.; Jiang, H.J.; Shen, J. Graphene oxide noncovalent photosensitizer and its anticancer activity in vitro. Chem. Eur. J. 2011, 17, 12084–12091. [Google Scholar] [CrossRef]
- Yin, F.; Hu, K.; Chen, Y. SiRNA Delivery with PEGylated Graphene Oxide Nanosheets for Combined Photothermal and Genetherapy for Pancreatic Cancer. Theranostics 2017, 7, 1133–1148. [Google Scholar] [CrossRef] [PubMed]
- Narayanaswamy, V.; Obaidat, I.M.; Kamzin, A.S.; Latiyan, S.; Jain, S.; Kumar, H.; Srivastava, C.; Alaabed, S.; Issa, B. Synthesis of Graphene Oxide-Fe3O4 Based Nanocomposites Using the Mechanochemical Method and In Vitro Magnetic Hyperthermia. Int. J. Mol. Sci. 2019, 20, 3368. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.W.; Lu, Y.J.; Lin, K.J.; Hsu, S.C.; Huang, C.Y.; She, S.H.; Liu, H.L.; Lin, C.W.; Xiao, M.C.; Wey, S.P.; et al. Biomaterials EGRF conjugated PEGylated nanographene oxide for targeted chemotherapy and photothermal therapy. Biomaterials 2013, 34, 7204–7214. [Google Scholar] [CrossRef]
- Miao, W.; Shim, G.; Lee, S.; Lee, S.; Choe, Y.S.; Oh, Y.K. Safety and tumor tissue accumulation of pegylated graphene oxide nanosheets for co-delivery of anticancer drug and photosensitizer. Biomaterials 2013, 34, 3402–3410. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, S.; Li, Y.; Wang, M.; Shi, P.; Huang, X. Covalent functionalization of graphene oxide with biocompatible poly(ethylene glycol) for delivery of paclitaxel. ACS Appl. Mater. Interfaces 2014, 6, 17268–17276. [Google Scholar] [CrossRef]
- Yue, L.; Wang, J.; Dai, Z.; Hu, Z.; Chen, X.; Qi, Y.; Zheng, X.; Yu, D. pH-Responsive, Self-Sacrificial Nanotheranostic Agent for Potential n Vivo and in Vitro Dual Modal MRI/CT Imaging, Real-Time, and in Situ Monitoring of Cancer Therapy. Bioconjug. Chem. 2017, 28, 400–409. [Google Scholar] [CrossRef]
- Liu, J.; Yuan, X.; Deng, L.; Yin, Z.; Tian, X.; Bhattacharyya, S. Graphene oxide activated by 980 nm laser for cascading two-photon photodynamic therapy and photothermal therapy against breast cancer. Appl. Mater. Today 2020, 20, 100665. [Google Scholar] [CrossRef]
- Suciu, M.; Porav, S.; Radu, T. Photodynamic effect of light emitting diodes on E. coli and human skin cells induced by a graphene-based ternary composite. J. Photochem. Photobiol. B Biol. 2021, 223, 12298. [Google Scholar] [CrossRef]
- Panda, S.; Rout, T.K.; Prusty, A.D.; Ajayan, P.M.; Nayak, S. Electron transfer directed antibacterial properties of graphene oxide on metals. Adv. Mater. 2018, 30, 1702149. [Google Scholar] [CrossRef]
- Nagaraj, E.; Shanmugam, P.; Karuppannan, K.; Chinnasamy, T.; Venugopal, S. The biosynthesis of a graphene oxide-based zinc oxide nanocomposite using Dalbergia latifolia leaf extract and its biological applications. New J. Chem. 2020, 44, 2166–2179. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, B.; Dong, W.; Zhong, Y.; Zhang, X.; Gong, Y.; Zhan, R.; Xing, M.; Zhang, J.; Luo, G.; et al. A dual-targeted platform based on graphene for synergistic chemo-photothermal therapy against multidrug-resistant Gram-negative bacteria and their biofilms. Chem. Eng. J. 2020, 393, 124595. [Google Scholar] [CrossRef]
- Kumar, M.; Thakur, M.; Bahadur, R.; Kaku, T. Preparation of graphene oxide-graphene quantum dots hybrid and its application in cancer theranostics. Mater. Sci. Eng. C 2019, 103, 109774. [Google Scholar]
- Mej, I.E.; Santos, C.M.; Rodrigues, D.F. Toxicity of a polymer-graphene oxide composite against bacterial planktonic. Nanoscale 2012, 4, 4746–4756. [Google Scholar]
- Qiu, J.; Liu, L.; Zhu, H.; Liu, X. Bioactive Materials Combination types between graphene oxide and substrate affect the antibacterial activity. Bioact. Mater. 2018, 3, 341–346. [Google Scholar] [CrossRef]
- Liu, T.; Tong, L.; Lv, N.; Ge, X.; Fu, Q.; Gao, S.; Ma, Q.; Song, J. Two-Stage Size Decrease and Enhanced Photoacoustic Performance of Stimuli-Responsive Polymer-Gold Nanorod Assembly for Increased Tumour Penetration. Adv. Funct. Mater. 2019, 29, 1806429. [Google Scholar] [CrossRef]
- Pei, S.; Cheng, H.M. The reduction of graphene oxide. Carbon 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Mathew, T.; Sree, R.A.; Aishwarya, S.; Kounaina, K.; Patil, A.G.; Satapathy, P.; Hudeda, S.P.; More, S.S.; Muthucheliyan, K.; Kumar, T.N.; et al. Graphene-based functional nanomaterials for biomedical and bioanalysis applications. FlatChem 2020, 23, 00184. [Google Scholar] [CrossRef]
- Gurunathan, S.; Han, J.; Kim, J.H. A novel functional molecule for the green synthesis of graphene. Colloids Surf. B Biointerfaces 2013, 111, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Zaharie-butucel, D.; Potara, M.; Suarasan, S.; Licarete, E.; Astilean, S. Efficient combined near-infrared-triggered therapy: Phototherapy over chemotherapy in chitosan-reduced graphene oxide-IR820 dye-doxorubicin nanoplatforms. J. Colloid Interface Sci. 2019, 552, 218–229. [Google Scholar] [CrossRef]
- Lee, S.; Kim, S.Y. Gold Nanorod/Reduced Graphene Oxide Composite Nanocarriers for Near-Infrared-Induced Cancer Therapy and Photoacoustic Imaging. ACS Appl. Nano Mater. 2021, 4, 11849–11860. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, H.; Zhang, F.; Wang, X.; Liu, X.; Zhang, Y. Polydopamine doped reduced graphene oxide/mesoporous silica nanosheets for chemo-photothermal and enhanced photothermal therapy. Mater. Sci. Eng. C 2019, 96, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Han, J.W.; Kim, E.S.; Park, J.H.; Kim, J.H. Reduction of graphene oxide by resveratrol: A novel and simple biological method for the synthesis of an effective anticancer nanotherapeutic molecule. Int. J. Nanomed. 2015, 2015, 2951–2969. [Google Scholar] [CrossRef] [PubMed]
- Amina, M.; Al Musayeib, N.M.; Alarfaj, N.A.; El-tohamy, M.F.; Al-hamoud, G.A. Facile multifunctional-mode of fabricated biocompatible human serum albumin/reduced graphene oxide/Cladophora glomerata nanoparticles for bacteriostatic phototherapy, bacterial tracking and antioxidant potential. Nanotechnology 2021, 32, 315301. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Wu, X.; Xing, Y.; Lilak, S.; Wu, M.; Zhao, J.X. Enhanced synergetic antibacterial activity by a reduce graphene oxide/Ag nanocomposite through the photothermal effect. J. Colloids Surf. B Biointerfaces 2020, 185, 110616. [Google Scholar] [CrossRef]
- Yang, Z.; Hao, X.; Chen, S.; Ma, Z.; Wang, W.; Wang, C.; Yue, L.; Sun, H.; Shao, Q.; Murugadoss, V.; et al. Long-term antibacterial stable reduced graphene oxide nanocomposites loaded with cuprous oxide nanoparticles. J. Colloid Interface Sci. 2019, 533, 13–23. [Google Scholar] [CrossRef]
- Li, Y.; Xu, X.; Liu, X.; Zheng, Y.; Chen, D.F.; Yeung, K.W.K.; Chiu, Z.; Li, Z.; Liang, Y.; Zhu, S.; et al. Photoelectrons Mediating Angiogenesis and Immunotherapy through Heterojunction Film for Noninvasive Disinfection. Adv. Sci. 2020, 7, 2000023. [Google Scholar] [CrossRef]
- Gollavelli, G.; Ling, Y.C. Magnetic and fluorescent graphene for dual modal imaging and single light induced photothermal and photodynamic therapy of cancer cells. Biomaterials 2014, 35, 4499–4507. [Google Scholar] [CrossRef]
- Choi, S.Y.; Baek, S.H.; Chang, S.J.; Song, Y.; Rafique, R.; Lee, K.T.; Park, T.J. Synthesis of upconversion nanoparticles conjugated with graphene oxide quantum dots and their use against cancer cell imaging and photodynamic therapy. Biosens. Bioelectron. 2017, 93, 267–273. [Google Scholar] [CrossRef]
- Gu, Y.; Guo, Y.; Wang, C.; Xu, J.; Wu, J.; Kirk, T.B.; Ma, D.; Xue, W. A polyamidoamne dendrimer functionalized graphene oxide for DOX and MMP-9 shRNA plasmid co-delivery. Mater. Sci. Eng. C 2017, 70, 572–585. [Google Scholar] [CrossRef]
- Yang, H.W.; Huang, C.Y.; Lin, C.W. Gadolinium-functionalized nanographene oxide for combined drug and microRNA delivery and magnetic resonance imaging. Biomaterials 2014, 35, 6534–6542. [Google Scholar] [CrossRef]
- Wu, C.; He, Q.; Zhu, A.; Li, D.; Xu, M.; Yang, H.; Liu, Y. Synergistic anticancer activity of photo- and chemoresponsive nanoformulation based on polylysine-functionalized graphene. ACS Appl. Mater Interfaces 2014, 6, 21615–21623. [Google Scholar] [CrossRef]
- Yang, H.; Bremner, D.H.; Tao, L.; Li, H.; Hu, J.; Zhu, L. Carboxymethyl chitosan-mediated synthesis of hyaluronic acid-targeted graphene oxide for cancer drug delivery. Carbohydr. Polym. 2016, 135, 72–78. [Google Scholar] [CrossRef]
- Mei, L.; Shi, Y.; Cao, F.; Liu, X.; Li, X.M.; Xu, Z.; Miao, Z. PEGylated phthalocyanine-functionalized graphene oxide with ultrahigh-efficient photothermal performance for triple-mode antibacterial therapy. ACS Biomater. Sci. Eng. 2021, 7, 2638–2648. [Google Scholar] [CrossRef]
- Zheng, X.T.; Ma, X.Q.; Li, C.M. Highly efficient nuclear delivery of anti-cancer drugs using a bio-functionalized reduced graphene oxide. J. Colloid Interface Sci. 2016, 467, 35–42. [Google Scholar] [CrossRef]
- Sun, B.; Wu, J.; Cui, S.; Zhu, H.; An, W.; Fu, Q.; Shao, C.; Yao, A.; Chen, B.; Shi, D. In situ synthesis of graphene oxide/gold nanorods theranostic hybrids for efficient tumor computed tomography imaging and photothermal therapy. Nano Res. 2017, 10, 37–48. [Google Scholar] [CrossRef]
- Lima-Sousa, R.; de Melo-Diogo, D.; Alves, C.G.; Costa, E.C.; Ferreira, P.; Louro, R.O.; Correia, I.J. Hyaluronic acid functionalized green reduced graphene oxide for targeted cancer photothermal therapy. Carbohydr. Polym. 2018, 200, 93–99. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, M.; Zhang, Z.; Ren, G.; Liu, Y.; Wu, S.; Shen, J. Facile synthesis of ZnO QDs@GO-CS hydrogel for synergetic antibacterial applications and enhanced wound healing. Chem. Eng. J. 2019, 378, 122043. [Google Scholar] [CrossRef]
- Siddique, S.; Chow, J.C.L. Application of Nanomaterials in Biomedical Imaging and Cancer Therapy. Nanomaterials 2020, 10, 1700. [Google Scholar] [CrossRef]
- Kunjachan, S.; Ehling, J.; Storm, G.; Kiessling, F.; Lammers, T. Noninvasive imaging of nanomedicines and nanotheranostics: Principles, progress, and prospects. Chem. Rev. 2015, 14, 10907–10937. [Google Scholar] [CrossRef]
- Lim, H.; Lee, S.Y.; Moon, D.W.; Kim, J.Y. Preparation of cellular samples using graphene cover and air-plasma treatment for time-of-flight secondary ion mass spectrometry imaging. RSC Adv. 2019, 9, 28432–28438. [Google Scholar] [CrossRef]
- Lim, H.; Lee, S.Y.; Park, Y.; Jin, H.; Seo, D.; Jang, Y.H.; Moon, D.W. Mass spectrometry imaging of untreated wet cell membranes in solution using single-layer Graphene. Nat. Methods 2021, 18, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.Y.; Zheng, Y.; Tan, C.P.; Sun, J.H.; Zhang, W.; Ji, L.N.; Mao, Z.W. Graphene oxide decorated with Ru(II)-polyethylene glycol complex for lysosome-targeted imaging and photodynamic/photothermal therapy. ACS Appl. Mater. Interfaces 2017, 9, 6761–6771. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Wang, C.; Feng, L.; Kai, Y.; Liu, Z. Functional Nanomaterials for Phototherapies of Cancer. Chem. Rev. 2014, 114, 10869–10939. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Gao, J.; Wang, S.; Wang, Y.; Liu, D.; Wang, J. Quantum dots in cell imaging and their safety issues. J. Mater. Chem. B 2021, 9, 5765–5779. [Google Scholar] [CrossRef]
- Boisselier, E.; Astruc, D. Gold nanoparticles in nanomedicine: Preparations, imaging, diagnostics, therapies and toxicity. Chem. Soc. Rev. 2009, 38, 1759–1782. [Google Scholar] [CrossRef]
- Si, P.; Razmi, N.; Nur, O.; Solanki, S.; Pandey, C.M.; Gupta, R.K.; Malhotra, B.D.; Willander, M.; Zerda, A. Gold nanomaterials for optical biosensing and bioimaging. Nanoscale Adv. 2021, 3, 2679–2698. [Google Scholar] [CrossRef]
- Alavi, M.; Jabari, E.; Jabbari, E. Functionalized carbon-based nanomaterials and quantum dots with antibacterial activity: A review. Expert Rev. Anti Infect. Ther. 2021, 19, 35–44. [Google Scholar] [CrossRef]
- Liu, Z.; Robinson, J.T.; Sun, X.M.; Dai, H. PEGylated nanographene oxide for delivery of water-insoluble cancer drugs. J. Am. Chem. Soc. 2008, 130, 10876–10877. [Google Scholar] [CrossRef]
- Sun, X.; Liu, Z.; Welsher, K.; Welsher, K.; Robinson, J.T.; Goodwin, A.; Zaric, S.; Dai, H. Nano-graphene oxide for cellular imaging and drug delivery. Nano Res. 2008, 1, 203–212. [Google Scholar] [CrossRef]
- Peng, C.; Hu, W.; Zhou, Y.; Fan, C.; Huang, Q. Intracellular imaging with a graphene-based fluorescent probe. Small 2010, 2, 1686–1692. [Google Scholar] [CrossRef]
- Li, J.L.; Tang, B.; Yuan, B.; Sun, L.; Wang, X.G. A review of optical imaging and therapy using nanosized graphene and graphene oxide. Biomaterials 2013, 34, 9519–9534. [Google Scholar] [CrossRef]
- Gollavelli, G.; Ling, Y.C. Multi-functional graphene as an in vitro and in vivo imaging probe. Biomaterials 2012, 33, 2532–2545. [Google Scholar] [CrossRef]
- Sinha, M.; Gollavelli, G.; Ling, Y.C. Exploring the photothermal hot spots of graphene in the first and second biological window to inactivate cancer cells and pathogens. RSC Adv. 2016, 6, 63859–63866. [Google Scholar] [CrossRef]
- Choi, H.W.; Lim, J.H.; Kim, C.W.; Lee, E.; Kim, J.M.; Chang, K.; Chung, B.G. Near-Infrared Light-triggered generation of reactive oxygen species and induction of local hyperthermia from indocyanine green encapsulated mesoporous silica-coated graphene oxide for colorectal cancer therapy. Antioxidants 2022, 11, 174. [Google Scholar] [CrossRef]
- Molkenova, A.; Atabaev, T.S.; Hong, S.W.; Mao, C.; Han, D.W.; Kim, K.S. Designing inorganic nanoparticles into computed tomography and magnetic resonance (CT/MR) imaging-guidable photomedicines. Mater. Today Nano 2022, 18, 100187. [Google Scholar] [CrossRef]
- Younis, M.R.; He, G.; Lin, J.; Huang, P. Recent Advances on Graphene Quantum Dots for Bioimaging Applications. Front. Chem. 2020, 8, 424. [Google Scholar] [CrossRef]
- Gulati, S.; Mansi; Vijayan, S.; Kumar, S.; Agarwal, V.; Harikumar, B.; Varma, R.S. Magnetic nanocarriers adorned on graphene: Promising contrast-enhancing agents with state-of-the-art performance in magnetic resonance imaging (MRI) and theranostics. Mater. Adv. 2022, 3, 2971–2989. [Google Scholar] [CrossRef]
- Mohanta, Z.; Gaonkar, S.K.; Kumar, M.; Saini, J.; Tiwari, V.; Srivastava, C.; Atreya, H.S. Influence of oxidation degree of graphene oxide on its nuclear relaxivity and contrast in MRI. ACS Omega 2020, 5, 22131–22139. [Google Scholar] [CrossRef]
- Enayati, M.; Nemati, A.; Zarrabi, A.; Shokrgozar, M.A. Reduced graphene oxide: An alternative for magnetic resonance imaging contrast agent. Mater. Lett. 2018, 233, 363–366. [Google Scholar] [CrossRef]
- Hu, Y.H. The first magnetic-nanoparticle-free carbon-based contrast agent of magnetic-resonance imaging fluorinated graphene oxide. Small 2014, 10, 1451–1452. [Google Scholar] [CrossRef]
- Wang, H.; Revia, R.; Wang, K.; Kant, R.J.; Mu, Q.; Gai, Z.; Hong, K.; Zhang, M. Paramagnetic properties of metal-free boron-doped graphene quantum dots and their application for safe magnetic resonance imaging. Adv. Mater. 2017, 29, 1605416. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Cao, Y.; Chong, Y.; Ma, Y.; Zhang, H.; Deng, Z.; Hu, C.; Zhang, Z. Graphene oxide based theranostic platform for t1-weighted magnetic resonance imaging and drug delivery. ACS Appl. Mater. Interfaces 2013, 5, 13325–13332. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Liu, X.; Xu, Z.; Ren, H.; Deng, B.; Tang, M.; Lu, L.; Fu, X.; Peng, H.; Liu, Z.; et al. Graphene encapsulated copper microwires as highly MRI compatible neural electrodes. Nano Lett. 2016, 16, 7731–7738. [Google Scholar] [CrossRef] [PubMed]
- Cao, T.; Zhou, X.; Zheng, Y.; Sun, Y.; Zhang, J.; Chen, W.; Zhang, J.; Zhou, Z.; Yang, S. Chelator-free conjugation of 99mTc and Gd3+ to PEGylated nanographene oxide for 3 dual-modality SPECT/MR imaging of lymph nodes. ACS Appl. Mater. Interfaces 2017, 9, 42612–42621. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Hu, L.; Ma, X.; Ye, S.; Cheng, L.; Shi, X.; Li, C.; Li, Y.; Liu, Z. Multimodal imaging guided photothermal therapy using functionalized graphene nanosheets anchored with magnetic nanoparticles. Adv. Mater. 2012, 24, 11868–11872. [Google Scholar] [CrossRef]
- Lin, C.H.; Chen, Y.C.; Huang, P.I. Preparation of multifunctional dopamine-coated zerovalent iron/reduced graphene oxide for targeted phototheragnosis in breast cancer. Nanomaterials 2020, 10, 1957. [Google Scholar] [CrossRef]
- Sadighian, S.; Bayat, N.; Najaflou, S.; Kermanian, M.; Hamidi, M. Preparation of graphene oxide/Fe3O4 nanocomposite as a potential magnetic nanocarrier and MRI contrast agent. Chem. Sel. 2021, 6, 2862–2868. [Google Scholar]
- Peng, E.; Choo, E.S.G.; Chandrasekharan, P.; Yang, C.T.; Ding, J.; Chuang, K.H.; Xue, J.M. Synthesis of manganese ferrite/graphene oxide nanocomposites for biomedical applications. Small 2012, 8, 3620–3630. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, S.; Li, H.; Yuan, Y.; Zhang, Z.; Xie, J.; Hwang, D.W.; Zhang, A.; Liu, M.; Zhou, X. Engineered paramagnetic graphene quantum dots with enhanced relaxivity for tumour imaging. Nano Lett. 2019, 9, 441–448. [Google Scholar] [CrossRef]
- Ding, H.; Wang, D.; Sadat, A.; Li, Z.; Hu, X.; Xu, M.; Morais, P.C.; Ge, B.; Sun, S.; Ge, J.; et al. Single-atom gadolinium anchored on graphene quantum dots as a magnetic resonance signal amplifier. ACS Appl. Bio Mater. 2021, 4, 2798–2809. [Google Scholar] [CrossRef]
- Lalwani, G.; Sundararaj, J.L.; Schaefer, K.; Button, T.; Sitharaman, B. Synthesis, characterization, In vitro phantom imaging, and cytotoxicity of a novel graphene-based multimodal magnetic resonance imaging-x-ray computed tomography contrast agent. J. Mater. Chem. B 2014, 2, 3519–3530. [Google Scholar] [CrossRef]
- Badrigilan, S.; Shaabani, B.; Aghaji, N.G.; Mesbahi, A. Graphene quantum dots-coated bismuth nanoparticles for improved CT imaging and photothermal performance. Int. J. Nanosci. 2020, 19, 1850043. [Google Scholar]
- Li, W.M.; Wei, D.M.; Wushouer, A.; Cao, S.D.; Zhao, T.T.; Yu, D.X.; Lei, D.P. Discovery and Validation of a CT-Based Radiomic Signature for Preoperative Prediction of Early Recurrence in Hypopharyngeal Carcinoma. BioMed Res. Int. 2020, 2020, 4340521. [Google Scholar] [CrossRef]
- Chang, X.; Zhang, M.; Wang, C.; Zhang, J.; Wu, H.; Yang, S. Graphene oxide/BaHoF5/PEG nanocomposite for dual-modal imaging and heat shock protein inhibitor-sensitized tumour photothermal therapy. Carbon 2020, 158, 372–385. [Google Scholar] [CrossRef]
- Bi, H.; He, F.; Dai, Y.; Xu, J.; Dong, Y.; Yang, D.; Gai, S.; Li, L.; Li, C.; Yang, P. Quad-model imaging-guided high-efficiency phototherapy based on upconversion nanoparticles and ZnFe2O4 integrated graphene oxide. Inorg. Chem. 2018, 57, 9988–9998. [Google Scholar] [CrossRef]
- Mirrahimi, M.; Alamzadeh, Z.; Beik, J.; Sarikhani, A.; Mousavi, M.; Irajirad, R.; Khani, T.; Davani, E.S.; Farashahi, A.; Ardakani, T.S.; et al. A 2D nanotheranostic platform based on graphene oxide and phase-change materials for bimodal CT/MR imaging, NIR-activated drug release, and synergistic thermo-chemotherapy. Nanotheranostics 2022, 6, 350–364. [Google Scholar] [CrossRef]
- Hong, H.; Yang, K.; Zhang, Y.; Engle, J.W.; Feng, L.; Yang, Y.; Tapas, R.; Goel, N.S.; Bean, J.; Theuer, C.P.; et al. In vivo targeting and imaging of tumour vasculature with radiolabeled, antibody-conjugated nanographene. ACS Nano 2012, 6, 2361–2370. [Google Scholar] [CrossRef] [Green Version]
- Hong, H.; Zhang, Y.; Engle, J.W.; Nayak, T.R.; Theuer, C.P.; Nickles, R.J.; Barnhart, T.E.; Cai, W. In vivo targeting and positron emission tomography imaging of tumour vasculature with 66Ga-labeled nano-graphene. Biomaterials 2012, 33, 4147–4156. [Google Scholar] [CrossRef]
- Shi, S.; Yang, K.; Hong, H.; Valdovinos, H.F.; Nayak, T.R.; Zhang, Y.; Theuer, C.P.; Barnhart, T.E.; Liu, Z.; Cai, W. Tumour vasculature targeting and imaging in living mice with reduced graphene oxide. Biomaterials 2013, 34, 3002–3009. [Google Scholar] [CrossRef]
- Ugalde, A.F.; Sandoval, S.; Pulagam, K.R.; Juan, A.M.; Laromaine, A.; Llop, J.; Tobias, G.; Núñez, R. Radiolabeled obaltabis (dicarbollide) anion-graphene oxide nanocomposites for in vivo bioimaging and boron delivery. ACS Appl. Nano Mater. 2021, 4, 1613–1625. [Google Scholar] [CrossRef]
- Binte, A.; Balasundaram, G.; Moothanchery, M.; Dinish, U.S.; Renzhe, B.; Ntziachristos, V.; Olivo, M. A review of clinical photoacoustic imaging: Current and future trends. Photoacoustics 2019, 16, 100–144. [Google Scholar]
- Han, S.H. Review of photoacoustic imaging for imaging-guided spinal surgery. Neurospine 2018, 15, 306–322. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Zhang, Y.; Lin, J.; Huang, P. Nanomaterials for photoacoustic imaging in the second near-infrared window. Biomater. Sci. 2019, 7, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Lalwani, G.; Cai, X.; Nie, L.; Wang, L.V. Balaji Sitharaman. Graphene-based contrast agents for photoacoustic and thermoacoustic tomography. Photoacoustics 2013, 1, 62–67. [Google Scholar] [CrossRef]
- Patel, M.A.; Yang, H.; Chiu, P.L.; Mastrogiovanni, D.D.T.; Flach, C.R.; Savaram, K.; Gomez, L.; Hemnarine, A.; Mendelsohn, R.; Garfunkel, E.; et al. Direct Production of graphene nanosheets for near infrared photoacoustic imaging. ACS Nano 2013, 7, 8147–8157. [Google Scholar] [CrossRef]
- Wang, Y.W.; Fu, Y.Y.; Peng, Q.; Guo, S.S.; Liu, G.; Li, J.; Yang, H.H.; Chen, G.N. Dye-enhanced graphene oxide for photothermal therapy and photoacoustic imaging. J. Mater. Chem. B 2013, 1, 5762–5767. [Google Scholar] [CrossRef]
- Toumia, Y.; Domenici, F.; Orlanducci, S.; Mura, F.; Grishenkov, D.; Trochet, P.; Lacerenza, S.; Bordi, F.; Paradossi, G. Graphene meets microbubbles: A superior contrast agent for photoacoustic imaging. ACS Appl. Mater. Interfaces 2016, 8, 16465–16475. [Google Scholar] [CrossRef]
- Jun, S.W.; Manivasagan, P.; Kwon, J.; Nguyen, V.T.; Mondal, S.; Ly, C.D.; Lee, J.; Kang, J.H.; Kim, C.S.; Oh, J. Folic acid-conjugated chitosan-functionalized graphene oxide for highly efficient photoacoustic imaging-guided tumour-targeted photothermal therapy. Int. J. Biol. Macromol. 2020, 155, 961–971. [Google Scholar] [CrossRef]
- Jia, X.; Xu, W.; Ye, Z.; Wang, Y.; Dong, Q.; Wang, E.; Li, D.; Wang, J. Functionalized graphene@goldnanostar/lipid for pancreatic cancer gene and photothermal synergistic therapy under photoacoustic/photothermal imaging dual-modal guidance. Small 2020, 16, e2003707. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, X.; Huang, T.; Song, J.; Wang, Y. A Sandwich nanostructure of gold nanoparticle coated reduced graphene oxide for photoacoustic imaging-guided photothermal therapy in the second NIR window. Front. Bioeng. Biotechnol. 2020, 8, 655. [Google Scholar] [CrossRef]
- El-Mashtoly, S.F.; Gerwert, K. Diagnostics and Therapy Assessment Using Label-Free Raman Imaging. Anal. Chem. 2022, 94, 120–142. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, Q.; Gao, D.; Luo, D.; Niu, Y.; Yang, J.; Li, Y. Graphene oxide as a multifunctional platform for Raman and fluorescence imaging of cells. Small 2015, 11, 3000–3005. [Google Scholar] [CrossRef]
- Liu, Z.; Guo, Z.; Zhong, H.; Qin, X.; Wan, M.; Yang, B. Graphene oxide based surface-enhanced Raman scattering probes for cancer cell imaging. Phys. Chem. Chem. Phys. 2013, 15, 2961–2966. [Google Scholar] [CrossRef]
- Deng, L.; Li, Q.; Yang, Y.; Omar, H.; Tang, N.; Zhang, J.; Nie, Z.; Khashab, N.M. “Two-step” Raman imaging technique to guide chemo-photothermal cancer therapy. Chem. Eur. J. 2015, 21, 17274–17281. [Google Scholar] [CrossRef]
- Chen, Y.W.; Liu, T.Y.; Chen, P.J.; Chang, P.H.; Chen, S.Y. A high-sensitivity and low-power theranostic nanosystem for cell SERS imaging and selectively photothermal therapy using anti-egfr-conjugated reduced graphene oxide/mesoporous Silica/AuNPs nanosheets. Small 2016, 12, 1458–1468. [Google Scholar] [CrossRef]
- Yang, L.; Kim, T.H.; Cho, H.Y.; Luo, J.; Lee, J.M.; Chueng, S.T.D.; Hou, Y.; Yin, P.T.T.; Han, J.; Kim, J.H.; et al. Hybrid graphene-gold nanoparticle-based nucleic acid conjugates for cancer-specific multimodal imaging and combined therapeutics. Adv. Funct. Mater. 2020, 31, 2006918. [Google Scholar] [CrossRef]
- Cai, L.; Sheng, L.; Xia, M.; Li, Z.; Zhang, S.; Zhang, X.; Chen, H. Graphene oxide as a novel evenly continuous phase matrix for TOF-SIMS. J. Am. Soc. Mass Spectrom. 2016, 28, 399–408. [Google Scholar] [CrossRef]
- Colliver, T.L.; Brummel, C.L.; Pacholski, M.L.; Swanek, F.D.; Ewing, A.G.; Winograd, N. Atomic and molecular imaging at the single-cell level with TOF-SIMS. Anal. Chem. 1997, 69, 2225–2231. [Google Scholar] [CrossRef]
- Lee, T.G.; Park, J.W.; Shon, H.K.; Moon, D.W.; Choi, W.W.; Li, K.; Chung, J.H. Biochemical imaging of tissues by SIMS for biomedical applications. Appl. Surf. Sci. 2008, 255, 1241–1248. [Google Scholar] [CrossRef]
- Belu, A.M.; Davies, M.C.; Newton, J.M.; Patel, N. TOF-SIMS characterization and imaging of controlled-release drug delivery systems. Anal. Chem. 2000, 72, 5625–5638. [Google Scholar] [CrossRef]
- Lee, P.L.; Chen, B.C.; Gollavelli, G.; Shen, S.Y.; Yin, Y.S.; Lei, S.L.; Jhang, C.L.; Lee, W.R.; Ling, Y.C. Development and validation of TOF-SIMS and CLSM imaging method for cytotoxicity study of ZnO nanoparticles in HaCaT cells. J. Hazard. Mater. 2014, 277, 3012. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Lim, H.; Moon, D.W. Mass spectrometry imaging of small molecules from live cells and tissues using nanomaterials. Surf. Interface Anal. 2022, 54, 381–388. [Google Scholar] [CrossRef]
- Li, H.W.; Hua1, X.; Long, Y.T. Graphene quantum dots enhanced ToF-SIMS for single-cell imaging. Anal. Bioanal. Chem. 2019, 411, 4025–4030. [Google Scholar] [CrossRef] [PubMed]
- Roope, L.S.J.; Smith, R.D.; Pouwels, K.B.; Buchanan, J.; Abel, L.; Eibich, P.; Butler, C.C.; Tan, P.S.; Walker, A.S.; Robotham, J.V.; et al. The challenge of antimicrobial resistance: What economics can contribute. Science 2019, 364, eaau4679. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial Biofilms: A Common Cause of Persistent Infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef]
- Qian, W.; Yan, C.; He, D.; Yu, X.; Yuan, L.; Liu, M.; Luo, G.; Deng, J. pH-triggered charge-reversible of glycol chitosan conjugated carboxyl graphene for enhancing photothermal ablation of focal infection. Acta Biomater. 2018, 69, 256–264. [Google Scholar] [CrossRef]
- Wu, M.C.; Deokar, A.R.; Liao, J.H.; Shih, P.Y.; Ling, Y.C. Graphene-based photothermal agent for rapid and effective killing of bacteria. ACS Nano 2013, 7, 1281–1290. [Google Scholar] [CrossRef]
- Gollavelli, G.; Chang, C.C.; Ling, Y.C. Facile synthesis of smart magnetic graphene for safe drinking water: Heavy metal removal and disinfection control. ACS Sustain. Chem. Eng. 2013, 1, 462–472. [Google Scholar] [CrossRef]
- Ghule, K.; Ghule, A.V.; Chen, B.J.; Ling, Y.C. Preparation and characterization of ZnO nanoparticles coated paper and its antibacterial activity study. Green Chem. 2006, 8, 1034–1041. [Google Scholar] [CrossRef]
- Deokar, A.R.; Lin, L.Y.; Chang, C.C.; Ling, Y.C. Single-walled carbon nanotube coated antibacterial paper: Preparation and mechanistic study. J. Mater. Chem. B 2013, 20, 2639–2646. [Google Scholar] [CrossRef]
- Deokar, A.R.; Madhulika, S.; Ganesh, G.; Ling, Y.C. Chapter 3 Antimicrobial Perspectives for Graphene-Based Nanomaterial. In Graphene Science Handbook; Applications and Industrialization; Aliofkhazraei, M., Ali, N., Milne, W.I., Ozkan, G.S., Mitura, S., Gervasoni, J.L., Eds.; CRC Press: Boca Raton, FL, USA, 2016; Volume 6, pp. 27–40. [Google Scholar]
- Bhaskar, G.; Ling, Y.C. Chapter 10 Richness of Graphene-Based Materials in Biomimetic Applications. In Graphene Science Handbook; Applications and Industrialization; Aliofkhazraei, M., Ali, N., Milne, W.I., Ozkan, G.S., Mitura, S., Gervasoni, J.L., Eds.; CRC Press: Boca Raton, FL, USA, 2016; Volume 6, pp. 125–142. [Google Scholar]
- Bhaskar, G.; Ling, Y.C. Chapter 14 Carbon-based nanomaterials as nanozymes. In Carbon Nanomaterials Sourcebook: Nanoparticles, Nanocapsules, Nanofibers, Nanoporous Structures, and Nanocomposites; Sattler, B.K., Ed.; CRC Press: Boca Raton, FL, USA, 2016; Volume 2, pp. 309–333. [Google Scholar]
- Wen, Y.J.; Yan, L.Y.; Ling, Y.C. The designing strategies of graphene-based peroxidase mimetic materials. Sci. China Chem. 2018, 61, 266–275. [Google Scholar] [CrossRef]
- Feng, W.J.; Wang, Z.K. Biomedical applications of chitosan-graphene oxide nanocomposites. iScience 2022, 25, 103629. [Google Scholar] [CrossRef]
- Sharma, H.; Mondal, S. Functionalized Graphene Oxide for Chemotherapeutic Drug Delivery and Cancer Treatment: A Promising Material in Nanomedicine. Int. J. Mol. Sci. 2020, 21, 6280. [Google Scholar] [CrossRef]
- Manzano, M.; Vallet-Regí, M. Mesoporous Silica Nanoparticles for Drug Delivery. Adv. Funct. Mater. 2020, 30, 1902634. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, S.; Xu, Y.; Shi, X.; Zhang, M.; Huang, Y.; Liang, Y.; Chen, Y.; Ji, W.; Kim, J.R.; et al. Engineering of hollow polymeric nanosphere-supported imidazolium-based ionic liquids with enhanced antimicrobial activities. Nano Res. 2022, 15, 5556–5568. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, W.; Lu, Y.; Xu, Y.; Wang, C.; Yu, D.G.; Kim, I. Recent Advances in Poly (α-L-glutamic acid)-Based Nanomaterials for Drug Delivery. Biomolecules 2022, 12, 636. [Google Scholar] [CrossRef]
- Tang, Y.; Varyambath, A.; Ding, Y.; Chen, B.; Huang, X.; Zhang, Y.; Yu, D.G.; Kim, I.; Song, W. Porous organic polymers for drug delivery: Hierarchical pore structures, variable morphologies, and biological properties. Biomater. Sci. 2022; advance article. [Google Scholar]
- Zhang, Y.; Kim, I.; Lu, Y.; Xu, Y.; Yu, D.G.; Song, W. Intelligent poly (l-histidine)-based nanovehicles for controlled drug delivery. J. Control. Release 2022, 10, 963–982. [Google Scholar] [CrossRef]
- Chen, H.; Chen, Z.; Yang, H.; Wen, L.; Yi, Z.; Zhou, Z.; Dai, B.; Zhang, J.; Wue, X.; Wu, P. Multi-mode surface plasmon resonance absorber based on dart-type single-layer graphene. RSC Adv. 2022, 12, 7821. [Google Scholar] [CrossRef]
- Cai, L.; Zhang, Z.; Xiao, H.; Chena, S.; Fua, J. An eco-friendly imprinted polymer based on graphene quantum dots for fluorescent detection of p-nitroaniline. RSC Adv. 2019, 9, 41383. [Google Scholar] [CrossRef]
- Tang, N.; Li, Y.; Chen, F.; Han, Z. In situ fabrication of a direct Z-scheme photocatalyst by immobilizing CdS quantum dots in the channels of graphene-hybridized and supported mesoporous titanium nanocrystals for high photocatalytic performance under visible light. RSC Adv. 2018, 8, 42233. [Google Scholar] [CrossRef] [Green Version]















| No | GNCs | Imaging | Cell/Animal Model | Light and Power |
|---|---|---|---|---|
| Time (min) | Therapy | Dose | Ref. No | |
| 1 | MFG-SiNC4 | CLSM and MRI | HeLa cells/Zebrafish | Tungsten halogen lamp, 1 W/cm2, 775 nm |
| 20 | PTT/PDT | 100 µg/mL | [70,95] | |
| 2 | MS-RGO-ICG-PEG-FA | CLSM and IVIS | CT-26 cells/mice | 808 nm laser, 2.0 W/cm2 |
| 10 | PTT/PDT | 100 µg/mL | [97] | |
| 3 | GO (99mTc− and Gd-usNGO-PEG) | MRI and SPECT/CT | Lymph nodes | - |
| - | - | - | [107] | |
| 4 | RGO-IONP-PEG | CLSM, MRI and PAT | 4T1 tumor cells/mice | 808 nm laser, 0.5 W cm2 |
| 5 | PTT/PDT | 2 mg/mL | [108] | |
| 5 | ZnFe2O4/UCNPs | UCL, CT, MRI, PAT. | U14 cells/mice | 980 nm laser, 0.8 W/cm2 |
| 15 | PTT/PDT | 250 μg/mL | [118] | |
| 6 | DOX-NCs | CT and MRI | CT26 cells/mice | 808 nm laser, 0.7 W/cm2 |
| 15 | Chemo and PTT | 20 µg/mL | [119] | |
| 7 | 64Cu-NOTA-GO-TRC105 | CT/PET | 4T1 tumor cells/mice | - |
| - | - | - | [120] | |
| 8 | rGADA-KrasI | PAI/PT | Pancreatic cancer cells/mice | 808 nm laser, 1.2 W/cm2 |
| 10 | PTT and gene therapy | 0.6 mg/mL | [132] | |
| 9 | anti-EGFR-PEG-rGO@CPSS-Au-R6G | Optical microscope/CLSM/SERS | A549 cells | 808 nm laser, 0.5 W/cm2 |
| 5 | PTT | 100 µg/mL | [138] | |
| 10 | Single layer graphene | ToF-SIMS | A549 cells | - |
| - | - | - | [84] | |
| 11 | GQDs | ToF-SIMS | MCF-7 cell | - |
| - | - | - | [146] | |
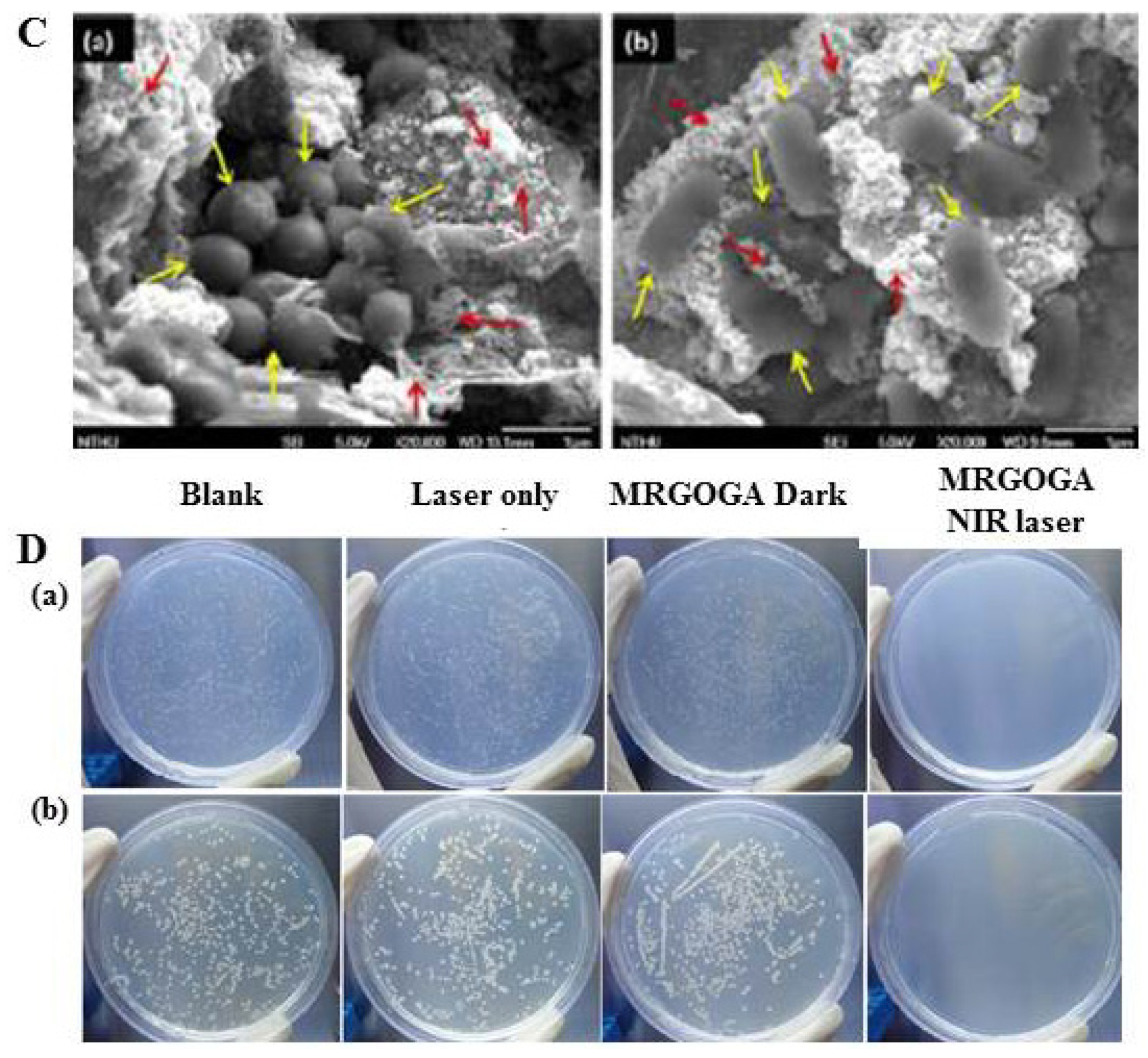
| 12 | MRGOGA | SEM and CLSM | S. aureus and E. coli | 808 nm laser, 1.2 W/cm2 |
| 10 | PTT | 80 μg/mL | [150] | |
| 13 | ZnPc-TEGMME@GO | SEM/Thermographic imaging | S. aureus and E. coli | 450 nm and 680 nm lasers, 0.0142 W/cm2 |
| 10 | PTT/PDT | 50 μg/mL | [76] | |
| 14 | RGO-PAA | CLSM | HeLa cells/S. aureus and E. coli | 808 nm/1064 nm laser, 0.4 mW/cm2 |
| 10 | PTT | 3 mg/mL | [96] | |
| 15 | Chitosan with ZnOQDs@GO | SEM/Thermographic imaging | S. aureus and E. coli | 808 nm laser, 2 W/cm2 |
| 6 | PTT | 500 μg/mL | [158] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gollavelli, G.; Ghule, A.V.; Ling, Y.-C. Multimodal Imaging and Phototherapy of Cancer and Bacterial Infection by Graphene and Related Nanocomposites. Molecules 2022, 27, 5588. https://doi.org/10.3390/molecules27175588
Gollavelli G, Ghule AV, Ling Y-C. Multimodal Imaging and Phototherapy of Cancer and Bacterial Infection by Graphene and Related Nanocomposites. Molecules. 2022; 27(17):5588. https://doi.org/10.3390/molecules27175588
Chicago/Turabian StyleGollavelli, Ganesh, Anil V. Ghule, and Yong-Chien Ling. 2022. "Multimodal Imaging and Phototherapy of Cancer and Bacterial Infection by Graphene and Related Nanocomposites" Molecules 27, no. 17: 5588. https://doi.org/10.3390/molecules27175588






