Molecular Mechanism of Tanshinone against Prostate Cancer
Abstract
:1. Introduction
1.1. Current Status of PCa
1.2. The Basic Introduction of Tanshinone
1.3. Comparison of Main Components of Tanshinone
1.4. Tanshinone and PCa
2. Tanshinone as a Potential Anti-Cancer Agent for PCa
2.1. Tanshinone-Induced Stagnation of the PCa Cell Cycle
2.2. Tanshinone-Induced Apoptosis of PCa Cells
2.3. Tanshinone-Induced Motility Inhibition of PCa Cells
2.4. Tanshinone Maintains Gene Stability of PCa Cells
2.5. Tanshinone Reverses Multidrug Resistance in PCa
2.6. Tanshinone Changes the Metabolic Process of PCa
3. Molecular Targets of Tanshinone Action
3.1. Tanshinone and NF-κB
3.2. Tanshinone and AR
3.3. Tanshinone and mTOR
4. Dilemma of Clinical Application of Tanshinone
5. Conclusions and Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Pernar, C.H.; Ebot, E.M.; Wilson, K.M.; Mucci, L.A. The Epidemiology of Prostate Cancer. Cold Spring Harb. Perspect Med. 2018, 8, a030361. [Google Scholar] [CrossRef] [PubMed]
- Daniyal, M.; Siddiqui, Z.A.; Akram, M.; Asif, H.M.; Sultana, S.; Khan, A. Epidemiology, etiology, diagnosis and treatment of prostate cancer. Asian Pac. J. Cancer Prev. 2014, 15, 9575–9578. [Google Scholar] [CrossRef]
- Karantanos, T.; Corn, P.G.; Thompson, T.C. Prostate cancer progression after androgen deprivation therapy: Mechanisms of castrate resistance and novel therapeutic approaches. Oncogene 2013, 32, 5501–5511. [Google Scholar] [CrossRef]
- Cattrini, C.; Castro, E.; Lozano, R.; Zanardi, E.; Rubagotti, A.; Boccardo, F.; Olmos, D. Current Treatment Options for Metastatic Hormone-Sensitive Prostate Cancer. Cancers 2019, 11, 1355. [Google Scholar] [CrossRef]
- Singh, K.; Nassar, N.; Bachari, A.; Schanknecht, E.; Telukutla, S.; Zomer, R.; Piva, T.J.; Mantri, N. The Pathophysiology and the Therapeutic Potential of Cannabinoids in Prostate Cancer. Cancers 2021, 13, 4107. [Google Scholar] [CrossRef] [PubMed]
- Pang, H.; Wu, L.; Tang, Y.; Zhou, G.; Qu, C.; Duan, J.-A. Chemical Analysis of the Herbal Medicine Salviae miltiorrhizae Radix et Rhizoma (Danshen). Molecules 2016, 21, 51. [Google Scholar] [CrossRef]
- Jiang, Z.; Gao, W.; Huang, L. Tanshinones, Critical Pharmacological Components in Salvia miltiorrhiza. Front. Pharmacol. 2019, 10, 202. [Google Scholar] [CrossRef]
- Kai, G.; Xu, H.; Zhou, C.; Liao, P.; Xiao, J.; Luo, X.; You, L.; Zhang, L. Metabolic engineering tanshinone biosynthetic pathway in Salvia miltiorrhiza hairy root cultures. Metab. Eng. 2011, 13, 319–327. [Google Scholar] [CrossRef]
- Cao, W.; Wang, Y.; Shi, M.; Hao, X.; Zhao, W.; Wang, Y.; Ren, J.; Kai, G. Transcription Factor SmWRKY1 Positively Promotes the Biosynthesis of Tanshinones in Salvia miltiorrhiza. Front. Plant Sci. 2018, 9, 554. [Google Scholar] [CrossRef] [Green Version]
- Lai, Z.; He, J.; Zhou, C.; Zhao, H.; Cui, S. Tanshinones: An Update in the Medicinal Chemistry in Recent 5 Years. Curr. Med. Chem. 2021, 28, 2807–2827. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Han, B.; Zhou, Y.; Ren, J.; Cao, W.; Patel, G.; Kai, G.; Zhang, J. The Anticancer Properties of Tanshinones and the Pharmaco-logical Effects of Their Active Ingredients. Front Pharmacol. 2020, 11, 193. [Google Scholar] [CrossRef]
- Fang, Z.; Zhang, M.; Liu, J.-N.; Zhao, X.; Zhang, Y.-Q.; Fang, L. Tanshinone IIA: A Review of its Anticancer Effects. Front. Pharmacol. 2021, 11, 611087. [Google Scholar] [CrossRef]
- Ma, Y.; Cui, G.; Chen, T.; Ma, X.; Wang, R.; Jin, B.; Yang, J.; Kang, L.; Tang, J.; Lai, C.; et al. Expansion within the CYP71D subfamily drives the heterocyclization of tanshinones synthesis in Salvia miltiorrhiza. Nat. Commun. 2021, 12, 685. [Google Scholar] [CrossRef]
- Huang, X.; Jin, L.; Deng, H.; Wu, D.; Shen, Q.-K.; Quan, Z.-S.; Zhang, C.-H.; Guo, H.-Y. Research and Development of Natural Product Tanshinone I: Pharmacology, Total Synthesis, and Structure Modifications. Front. Pharmacol. 2022, 13, 920411. [Google Scholar] [CrossRef]
- Gao, H.; Sun, W.; Zhao, J.; Wu, X.; Lu, J.J.; Chen, X.; Xu, Q.M.; Khan, I.A.; Yang, S. Tanshinones and diethyl blechnics with an-ti-inflammatory and anti-cancer activities from Salvia miltiorrhiza Bunge (Danshen). Sci. Rep. 2016, 6, 33720. [Google Scholar] [CrossRef]
- Guo, J.; Ma, X.; Cai, Y.; Ma, Y.; Zhan, Z.; Zhou, Y.; Liu, W.; Guan, M.; Yang, J.; Cui, G.; et al. Cytochrome P450 promiscuity leads to a bifurcating biosynthetic pathway for tanshinones. New Phytol. 2016, 210, 525–534. [Google Scholar] [CrossRef]
- Zhao, J.-L.; Zhou, L.-G.; Wu, J.-Y. Effects of biotic and abiotic elicitors on cell growth and tanshinone accumulation in Salvia miltiorrhiza cell cultures. Appl. Microbiol. Biotechnol. 2010, 87, 137–144. [Google Scholar] [CrossRef]
- Song, M.; Hang, T.J.; Zhang, Z.; Chen, H.Y. Effects of the coexisting diterpenoid Tanshinones on the pharmacokinetics of Cryp-totanshinone and Tanshinone IIA in rat. Eur. J. Pharm. Sci. 2007, 32, 247–253. [Google Scholar] [CrossRef]
- Chen, F.; Li, L.; Tian, D.-D. Salvia miltiorrhiza Roots against Cardiovascular Disease: Consideration of Herb-Drug Interactions. BioMed Res. Int. 2017, 2017, 9868694. [Google Scholar]
- Hao, D.-C.; Xiao, P.-G. Impact of Drug Metabolism/Pharmacokinetics and their Relevance Upon Salviabased Drug Discovery. Curr. Drug Metab. 2017, 18, 1071–1084. [Google Scholar] [CrossRef]
- Sun, J.; Yang, M.; Han, J.; Wang, B.; Ma, X.; Xu, M.; Liu, P.; Guo, D. Profiling the metabolic difference of seven tanshinones using high-performance liquid chromatography/multi-stage mass spectrometry with data-dependent acquisition. Rapid Commun. Mass Spectrom. 2007, 21, 2211–2226. [Google Scholar] [CrossRef]
- Kim, D.H.; Paudel, P.; Yu, T.; Ngo, T.M.; Kim, J.A.; Jung, H.A.; Yokozawa, T.; Choi, J.S. Characterization of the inhibitory activity of natural tanshinones from Salvia miltiorrhiza roots on protein tyrosine phosphatase 1B. Chem. Interact. 2017, 278, 65–73. [Google Scholar] [CrossRef]
- Wang, L.; Liu, A.; Zhang, F.-L.; Yeung, J.H.; Li, X.-Q.; Cho, C.-H. Evaluation and SAR analysis of the cytotoxicity of tanshinones in colon cancer cells. Chin. J. Nat. Med. 2014, 12, 167–171. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, J.; Jin, L.; Song, T.; Wu, G.; Gao, J. Tanshinone IIA Interacts with DNA by Minor Groove-Binding. Biol. Pharm. Bull. 2008, 31, 2342–2345. [Google Scholar] [CrossRef]
- Li, Z.; Zou, J.; Cao, D.; Ma, X. Pharmacological basis of tanshinone and new insights into tanshinone as a multitarget natural product for multifaceted diseases. Biomed. Pharmacother. 2020, 130, 110599. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Zarrabi, A.; Orouei, S.; Saberifar, S.; Salami, S.; Hushmandi, K.; Najafi, M. Recent advances and future directions in anti-tumor activity of cryptotanshinone: A mechanistic review. Phytotherapy Res. 2021, 35, 155–179. [Google Scholar] [CrossRef]
- Wu, Y.H.; Wu, Y.R.; Li, B.; Yan, Z.Y. Cryptotanshinone: A review of its pharmacology activities and molecular mechanisms. Fitoterapia 2020, 145, 104633. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, C.; Li, J.; Lu, Y.; Li, H.; Zhuang, J.; Ren, X.; Wang, M.; Sun, C. Tanshinone IIA: New Perspective on the Anti-Tumor Mechanism of a Traditional Natural Medicine. Am. J. Chin. Med. 2022, 50, 209–239. [Google Scholar] [CrossRef]
- Gong, Y.; Li, Y.; Lu, Y.; Li, L.; Abdolmaleky, H.M.; Blackburn, G.L.; Zhou, J.-R. Bioactive tanshinones in Salvia miltiorrhiza inhibit the growth of prostate cancer cells in vitro and in mice. Int. J. Cancer 2011, 129, 1042–1052. [Google Scholar] [CrossRef]
- Lee, H.-J.; Jung, D.-B.; Sohn, E.J.; Kim, H.H.; Park, M.N.; Lew, J.-H.; Lee, S.-G.; Kim, B.; Kim, S.-H. Inhibition of Hypoxia Inducible Factor Alpha and Astrocyte-Elevated Gene-1 Mediates Cryptotanshinone Exerted Antitumor Activity in Hypoxic PC-3 Cells. Evidence-Based Complement. Altern. Med. 2012, 2012, 390957. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.Y.; Yang, Y.H.; Lin, Y.Y.; Kuan, F.C.; Lin, Y.S.; Lin, W.Y.; Tsai, M.Y.; Yang, J.J.; Cheng, Y.C.; Shu, L.H.; et al. Anti-cancer effect of danshen and dihydroisoTanshinone I on prostate cancer: Targeting the crosstalk between macro-phages and cancer cells via inhibition of the STAT3/CCL2 signaling pathway. Oncotarget 2017, 8, 40246–40263. [Google Scholar] [CrossRef]
- Termini, D.; Hartogh, D.J.D.; Jaglanian, A.; Tsiani, E. Curcumin against Prostate Cancer: Current Evidence. Biomolecules 2020, 10, 1536. [Google Scholar] [CrossRef]
- Won, S.H.; Lee, H.J.; Jeong, S.J.; Lü, J.; Kim, S.H. Activation of p53 signaling and inhibition of AR mediate Tanshinone IIA induced G1 arrest in LNCaP prostate cancer cells. Phytother Res. 2012, 26, 669–674. [Google Scholar] [CrossRef]
- Li, C.; Han, X.; Zhang, H.; Wu, J.; Li, B. The interplay between autophagy and apoptosis induced by tanshinone IIA in prostate cancer cells. Tumor Biol. 2016, 37, 7667–7674. [Google Scholar] [CrossRef]
- Hou, L.-L.; Xu, Q.-J.; Hu, G.-Q.; Xie, S.-Q. Synergistic antitumor effects of tanshinone II A in combination with cisplatin via apoptosis in the prostate cancer cells. Yao xue xue bao = Acta Pharm. Sin. 2013, 48, 675–679. (In Chinese) [Google Scholar]
- Yun, S.-M.; Jung, J.H.; Jeong, S.-J.; Sohn, E.J.; Kim, B.; Kim, S.-H. Tanshinone IIA Induces Autophagic Cell Death via Activation of AMPK and ERK and Inhibition of mTOR and p70 S6K in KBM-5 Leukemia Cells. Phytotherapy Res. 2014, 28, 458–464. [Google Scholar] [CrossRef]
- Won, S.-H.; Lee, H.-J.; Jeong, S.-J.; Lee, E.-O.; Jung, D.-B.; Shin, J.-M.; Kwon, T.-R.; Yun, S.-M.; Lee, M.-H.; Choi, S.-H.; et al. Tanshinone IIA Induces Mitochondria Dependent Apoptosis in Prostate Cancer Cells in Association with an Inhibition of Phosphoinositide 3-Kinase/AKT Pathway. Biol. Pharm. Bull. 2010, 33, 1828–1834. [Google Scholar] [CrossRef]
- Chiu, S.C.; Huang, S.Y.; Chen, S.P.; Su, C.C.; Chiu, T.L.; Pang, C.-Y. Tanshinone IIA inhibits human prostate cancer cells growth by induction of endoplasmic reticulum stress in vitro and in vivo. Prostate Cancer Prostatic Dis. 2013, 16, 315–322. [Google Scholar] [CrossRef]
- Zhang, Y.; Won, S.H.; Jiang, C.; Lee, H.J.; Jeong, S.J.; Lee, E.O.; Zhang, J.; Ye, M.; Kim, S.H.; Lü, J. Tanshinones from Chinese medicinal herb Danshen (Salvia miltiorrhiza Bunge) suppress prostate cancer growth and AR signaling. Pharm. Res. 2012, 29, 1595–1608. [Google Scholar] [CrossRef]
- Liu, W.; Zhou, J.; Geng, G.; Shi, Q.; Sauriol, F.; Wu, J.H. Antiandrogenic, Maspin Induction, and Antiprostate Cancer Activities of Tanshinone IIA and Its Novel Derivatives with Modification in Ring, A. J. Med. Chem. 2012, 55, 971–975. [Google Scholar] [CrossRef]
- Yu, J.; Li, S.; Zeng, X.; Song, J.; Hu, S.; Cheng, S.; Chen, C.; Luo, H.; Pan, W. Design, synthesis, and evaluation of proliferation inhibitory activity of novel L-shaped ortho-quinone analogs as anticancer agents. Bioorg. Chem. 2021, 117, 105383. [Google Scholar] [CrossRef]
- Yao, Y.; Li, H.-Z.; Qian, B.-J.; Liu, C.-M.; Zhang, J.-B.; Lin, M.-C. Crypotanshione reduces the expression of metadherin in DU145 prostate cancer cells. Zhonghua nan ke xue = Natl. J. Androl. 2015, 21, 782–787. (In Chinese) [Google Scholar]
- Wu, C.-Y.; Hsieh, C.-Y.; Huang, K.-E.; Chang, C.; Kang, H.-Y. Cryptotanshinone down-regulates androgen receptor signaling by modulating lysine-specific demethylase 1 function. Int. J. Cancer 2012, 131, 1423–1434. [Google Scholar] [CrossRef]
- Park, I.J.; Kim, M.J.; Park, O.J.; Park, M.G.; Choe, W.; Kang, I.; Kim, S.S.; Ha, J. Cryptotanshinone sensitizes DU145 prostate cancer cells to Fas(APO1/CD95)-mediated apoptosis through Bcl-2 and MAPK regulation. Cancer Lett. 2010, 298, 88–98. [Google Scholar] [CrossRef]
- Xu, D.; Lin, T.H.; Li, S.; Da, J.; Wen, X.Q.; Ding, J.; Chang, C.; Yeh, S. Cryptotanshinone suppresses AR-mediated growth in androgen dependent and castration resistant prostate cancer cells. Cancer Lett. 2012, 316, 11–22. [Google Scholar] [CrossRef]
- Zhang, Y.; Cabarcas, S.M.; Zheng, J.I.; Sun, L.; Mathews, L.A.; Zhang, X.; Lin, H.; Farrar, W.L. Cryptotanshinone targets tu-mor-initiating cells through down-regulation of stemness genes expression. Oncol Lett. 2016, 11, 3803–3812. [Google Scholar] [CrossRef]
- Chen, W.; Luo, Y.; Liu, L.; Zhou, H.; Xu, B.; Han, X.; Shen, T.; Liu, Z.; Lu, Y.; Huang, S. Cryptotanshinone inhibits cancer cell prolif-eration by suppressing Mammalian target of rapamycin-mediated cyclin D1 expression and Rb phosphorylation. Cancer Prev. Res. 2010, 3, 1015–1025. [Google Scholar] [CrossRef]
- Lin, T.H.; Lee, S.O.; Niu, Y.; Xu, D.; Liang, L.; Li, L.; Yeh, S.D.; Fujimoto, N.; Yeh, S.; Chang, C. Differential androgen deprivation therapies with anti-androgens casodex/bicalutamide or MDV3100/Enzalutamide versus anti-AR ASC-J9(R) Lead to promotion versus suppression of prostate cancer metastasis. J. Biol. Chem. 2013, 288, 19359–19369. [Google Scholar] [CrossRef]
- Kim, E.J.; Kim, S.Y.; Kim, S.-M.; Lee, M. A novel topoisomerase 2a inhibitor, cryptotanshinone, suppresses the growth of PC3 cells without apparent cytotoxicity. Toxicol. Appl. Pharmacol. 2017, 330, 84–92. [Google Scholar] [CrossRef]
- Shin, D.-S.; Kim, H.-N.; Shin, K.D.; Yoon, Y.J.; Kim, S.-J.; Han, D.C.; Kwon, B.-M. Cryptotanshinone Inhibits Constitutive Signal Transducer and Activator of Transcription 3 Function through Blocking the Dimerization in DU145 Prostate Cancer Cells. Cancer Res. 2009, 69, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.A.; Sohn, E.J.; Won, G.; Choi, J.U.; Jeong, M.; Kim, B.; Kim, M.J.; Kim, S.H. Upregulation of microRNA135a-3p and death receptor 5 plays a critical role in Tanshinone I sensitized prostate cancer cells to TRAIL induced apoptosis. Oncotarget 2014, 5, 5624–5636. [Google Scholar] [CrossRef] [PubMed]
- Chuang, M.-T.; Ho, F.-M.; Wu, C.-C.; Zhuang, S.-Y.; Lin, S.-Y.; Suk, F.-M.; Liang, Y.-C. 15,16-Dihydrotanshinone I, a Compound ofSalvia miltiorrhizaBunge, Induces Apoptosis through Inducing Endoplasmic Reticular Stress in Human Prostate Carcinoma Cells. Evidence-Based Complement. Altern. Med. 2011, 2011, 865435. [Google Scholar]
- Wang, M.; Zeng, X.; Li, S.; Sun, Z.; Yu, J.; Chen, C.; Shen, X.; Pan, W.; Luo, H. A Novel Tanshinone Analog Exerts Anti-Cancer Effects in Prostate Cancer by Inducing Cell Apoptosis, Arresting Cell Cycle at G2 Phase and Blocking Metastatic Ability. Int. J. Mol. Sci. 2019, 20, 4459. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.; Lin, T.-H.; Zhang, C.; Tsai, Y.-C.; Li, S.; Zhang, J.; Yin, M.; Yeh, S.; Chang, C. The selective inhibitory effect of a synthetic tanshinone derivative on prostate cancer cells. Prostate 2012, 72, 803–816. [Google Scholar] [CrossRef]
- Bae, W.J.; Choi, J.B.; Kim, K.S.; Ha, U.S.; Hong, S.H.; Lee, J.Y.; Hwang, T.-K.; Hwang, S.Y.; Wang, Z.-P.; Kim, S.W. Inhibition of Proliferation of Prostate Cancer Cell Line DU-145 in vitro and in vivo Using Salvia miltiorrhiza Bunge. Chin. J. Integr. Med. 2020, 26, 533–538. [Google Scholar] [CrossRef]
- Lee, J.; Choi, B.Y.; Keum, Y.-S. Acetonitrile extract of Salvia miltiorrhiza Radix exhibits growth-inhibitory effects on prostate cancer cells through the induction of cell cycle arrest and apoptosis. Oncol. Lett. 2017, 13, 2921–2928. [Google Scholar] [CrossRef]
- Qiu, S.; Granet, R.; Mbakidi, J.P.; Brégier, F.; Pouget, C.; Micallef, L.; Sothea-Ouk, T.; Leger, D.Y.; Liagre, B.; Chaleix, V.; et al. Delivery of Tanshinone IIA and α-mangostin from gold/PEI/cyclodextrin nanoparticle platform designed for prostate cancer chemo-therapy. Bioorg. Med. Chem. Lett. 2016, 26, 2503–2506. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, X.; Ravi, S.O.A.S.; Ramachandran, A.; Ibrahim, I.A.A.; Nassir, A.M.; Yao, J. Synthesis of silver nanoparticles (AgNPs) from leaf extract of Salvia miltiorrhiza and its anticancer potential in human prostate cancer LNCaP cell lines. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2846–2854. [Google Scholar] [CrossRef]
- Sun, G.; Sun, K.; Sun, J. Combination prostate cancer therapy: Prostate-specific membranes antigen targeted, pH-sensitive nanoparticles loaded with doxorubicin and tanshinone. Drug Deliv. 2021, 28, 1132–1140. [Google Scholar] [CrossRef]
- Bates, S.; Bonetta, L.; MacAllan, D.; Parry, D.; Holder, A.; Dickson, C.; Peters, G. CDK6 (PLSTIRE) and CDK4 (PSK-J3) are a distinct subset of the cyclin-dependent kinases that associate with cyclin D1. Oncogene 1994, 9, 71–79. [Google Scholar]
- Petroni, G.; Formenti, S.C.; Chen-Kiang, S.; Galluzzi, L. Immunomodulation by anticancer cell cycle inhibitors. Nat. Rev. Immunol. 2020, 20, 669–679. [Google Scholar] [CrossRef]
- Kato, J.; Matsushime, H.; Hiebert, S.W.; Ewen, M.E.; Sherr, C.J. Direct binding of cyclin D to the retinoblastoma gene product (pRb) and pRb phosphorylation by the cyclin D-dependent kinase CDK4. Genes Dev. 1993, 7, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Leone, G.W.; Wang, H. Cyclin D-CDK4/6 functions in cancer. Adv. Cancer Res. 2020, 148, 147–169. [Google Scholar] [PubMed]
- Hernández-Monge, J.; Rousset-Roman, A.B.; Medina-Medina, I.; Olivares-Illana, V. Dual function of MDM2 and MDMX toward the tumor suppressors p53 and RB. Genes Cancer 2016, 7, 278–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Sun, H.N.; Luo, Y.H.; Piao, X.J.; Wu, D.D.; Meng, L.Q.; Wang, Y.; Zhang, Y.; Wang, J.R.; Wang, H.; et al. Cryptotanshinone induces ROS-mediated apoptosis in human gastric cancer cells. Oncotarget 2017, 8, 115398–115412. [Google Scholar] [CrossRef]
- Pistritto, G.; Trisciuoglio, D.; Ceci, C.; Garufi, A.; D’Orazi, G. Apoptosis as anticancer mechanism: Function and dysfunction of its modulators and targeted therapeutic strategies. Aging 2016, 8, 603–619. [Google Scholar] [CrossRef]
- Goldar, S.; Khaniani, M.S.; Derakhshan, S.M.; Baradaran, B. Molecular Mechanisms of Apoptosis and Roles in Cancer Development and Treatment. Asian Pac. J. Cancer Prev. 2015, 16, 2129–2144. [Google Scholar] [CrossRef]
- Lambert, A.W.; Pattabiraman, D.R.; Weinberg, R.A. Emerging Biological Principles of Metastasis. Cell 2017, 168, 670–691. [Google Scholar] [CrossRef]
- Erdogan, B.; Webb, D.J. Cancer-associated fibroblasts modulate growth factor signaling and extracellular matrix remodeling to regulate tumor metastasis. Biochem. Soc. Trans. 2017, 45, 229–236. [Google Scholar] [CrossRef]
- Cai, T.; Santi, R.; Tamanini, I.; Galli, I.C.; Perletti, G.; Bjerklund Johansen, T.E.; Nesi, G. Current Knowledge of the Potential Links between Inflammation and Prostate Cancer. Int. J. Mol. Sci. 2019, 20, 3833. [Google Scholar] [CrossRef] [PubMed]
- Archer, M.; Dogra, N.; Kyprianou, N. Inflammation as a Driver of Prostate Cancer Metastasis and Therapeutic Resistance. Cancers 2020, 12, 2984. [Google Scholar] [CrossRef] [PubMed]
- Nikonova, A.S.; Astsaturov, I.; Serebriiskii, I.G.; Dunbrack, R.L., Jr.; Golemis, E.A. Aurora A kinase (AURKA) in normal and patho-logical cell division. Cell Mol. Life Sci. 2013, 70, 661–687. [Google Scholar] [CrossRef]
- Dhiman, G.; Srivastava, N.; Goyal, M.; Rakha, E.; Lothion-Roy, J.; Mongan, N.P.; Miftakhova, R.R.; Khaiboullina, S.; Rizvanov, A.A.; Baranwal, M. Metadherin: A Therapeutic Target in Multiple Cancers. Front. Oncol. 2019, 9, 349. [Google Scholar] [CrossRef] [PubMed]
- Manzoni, L.; Zucal, C.; Di Maio, D.; D’Agostino, V.G.; Thongon, N.; Bonomo, I.; Lal, P.; Miceli, M.; Baj, V.; Brambilla, M.; et al. Interfering with HuR–RNA Interaction: Design, Synthesis and Biological Characterization of Tanshinone Mimics as Novel, Effective HuR Inhibitors. J. Med. Chem. 2018, 61, 1483–1498. [Google Scholar] [CrossRef]
- D’Agostino, V.; Lal, P.; Mantelli, B.; Tiedje, C.; Zucal, C.; Thongon, N.; Gaestel, M.; Latorre, E.; Marinelli, L.; Seneci, P.; et al. Dihydrotanshinone-I interferes with the RNA-binding activity of HuR affecting its post-transcriptional function. Sci. Rep. 2015, 5, 16478. [Google Scholar] [CrossRef]
- Soares, J.; Keppler, B.R.; Wang, X.; Lee, K.-H.; Jarstfer, M.B. ortho-Quinone tanshinones directly inhibit telomerase through an oxidative mechanism mediated by hydrogen peroxide. Bioorg. Med. Chem. Lett. 2011, 21, 7474–7478. [Google Scholar] [CrossRef]
- Liu, X.-D.; Fan, R.-F.; Zhang, Y.; Yang, H.-Z.; Fang, Z.-G.; Guan, W.-B.; Lin, D.-J.; Xiao, R.-Z.; Huang, R.-W.; Huang, H.-Q.; et al. Down-Regulation of Telomerase Activity and Activation of Caspase-3 Are Responsible for Tanshinone I-Induced Apoptosis in Monocyte Leukemia Cells in Vitro. Int. J. Mol. Sci. 2010, 11, 2267–2280. [Google Scholar] [CrossRef]
- Zhang, S.; Duan, S.; Xie, Z.; Bao, W.; Xu, B.; Yang, W.; Zhou, L. Epigenetic Therapeutics Targeting NRF2/KEAP1 Signaling in Cancer Oxidative Stress. Front Pharmacol. 2022, 13, 924817. [Google Scholar] [CrossRef]
- Chen, W.; Lu, Y.; Chen, G.; Huang, S. Molecular evidence of cryptotanshinone for treatment and prevention of human cancer. Anti-Cancer Agents Med. Chem. 2013, 13, 979–987. [Google Scholar] [CrossRef]
- Tsegay, P.S.; Lai, Y.; Liu, Y. Replication Stress and Consequential Instability of the Genome and Epigenome. Molecules 2019, 24, 3870. [Google Scholar] [CrossRef]
- Budakoti, M.; Panwar, A.S.; Molpa, D.; Singh, R.K.; Büsselberg, D.; Mishra, A.P.; Coutinho, H.D.M.; Nigam, M. Micro-RNA: The dar-khorse of cancer. Cell Signal. 2021, 83, 109995. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Qureshi, M.Z.; Romero, M.A.; Khalid, S.; Aras, A.; Ozbey, U.; Farooqi, A.A. Regulation of signaling pathways by tanshinones in different cancers. Cell. Mol. Biol. 2017, 63, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Teo, M.Y.; Rathkopf, D.E.; Kantoff, P. Treatment of Advanced Prostate Cancer. Annu. Rev. Med. 2019, 70, 479–499. [Google Scholar] [CrossRef]
- Komura, K.; Sweeney, C.J.; Inamoto, T.; Ibuki, N.; Azuma, H.; Kantoff, P.W. Current treatment strategies for advanced prostate cancer. Int. J. Urol. 2018, 25, 220–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, T.; To, K.K.; Wang, L.; Zhang, L.; Lu, L.; Shen, J.; Chan, R.L.; Li, M.; Yeung, J.H.; Cho, C.H. Reversal of P-glycoprotein (P-gp) mediated multidrug resistance in colon cancer cells by cryptotanshinone and dihydrotanshinone of Salvia miltiorrhiza. Phytomedicine 2014, 21, 1264–1272. [Google Scholar] [CrossRef]
- Tian, Q.T.; Ding, C.Y.; Song, S.S.; Wang, Y.Q.; Zhang, A.; Miao, Z.H. New Tanshinone I derivatives S222 and S439 similarly inhibit topoisomerase I/II but reveal different p53-dependency in inducing G2/M arrest and apoptosis. Biochem. Pharmacol. 2018, 154, 255–264. [Google Scholar] [CrossRef]
- Assaraf, Y.G.; Brozovic, A.; Gonçalves, A.C.; Jurkovicova, D.; Linē, A.; Machuqueiro, M.; Saponara, S.; Sarmento-Ribeiro, A.B.; Xavier, C.P.R.; Vasconcelos, M.H. The multi-factorial nature of clinical multidrug resistance in cancer. Drug Resist. Updates 2019, 46, 100645. [Google Scholar] [CrossRef]
- Li, H.; Wang, H.; Ma, Y.; Zhao, J. Clarithromycin combined with tanshinone for rhinosinusal and laryngeal radiation injury in patients with nasopharyngeal carcinoma after radiotherapy. Nan Fang Yi Ke Da Xue Xue Bao 2012, 32, 1168–1170. (In Chinese) [Google Scholar]
- Li, K.; Liu, W.; Zhao, Q.; Wu, C.; Fan, C.; Lai, H.; Li, S. Combination of tanshinone IIA and doxorubicin possesses synergism and attenuation effects on doxorubicin in the treatment of breast cancer. Phytotherapy Res. 2019, 33, 1658–1669. [Google Scholar] [CrossRef]
- Ketola, K.; Viitala, M.; Kohonen, P.; Fey, V.; Culig, Z.; Kallioniemi, O.; Iljin, K. High-throughput cell-based compound screen iden-tifies pinosylvin methyl ether and Tanshinone IIA as inhibitors of castration-resistant prostate cancer. J. Mol. Biochem. 2016, 5, 12–22. [Google Scholar] [PubMed]
- Lin, L.-L.; Hsia, C.-R.; Hsu, C.-L.; Huang, H.-C.; Juan, H.-F. Integrating transcriptomics and proteomics to show that tanshinone IIA suppresses cell growth by blocking glucose metabolism in gastric cancer cells. BMC Genom. 2015, 16, 41. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cao, Y.; Chen, L.; Liu, F.; Qi, Z.; Cheng, X.; Wang, Z. Cryptotanshinone suppresses cell proliferation and glucose me-tabolism via STAT3/SIRT3 signaling pathway in ovarian cancer cells. Cancer Med. 2018, 7, 4610–4618. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhu, X.; Wu, Y. Effects of Glucose Metabolism, Lipid Metabolism, and Glutamine Metabolism on Tumor Microenvi-ronment and Clinical Implications. Biomolecules 2022, 12, 580. [Google Scholar] [CrossRef] [PubMed]
- Pardo, J.C.; de Porras, V.R.; Gil, J.; Font, A.; Puig-Domingo, M.; Jordà, M. Lipid Metabolism and Epigenetics Crosstalk in Prostate Cancer. Nutrients 2022, 14, 851. [Google Scholar] [CrossRef]
- Galbraith, L.; Leung, H.Y.; Ahmad, I. Lipid pathway deregulation in advanced prostate cancer. Pharmacol. Res. 2018, 131, 177–184. [Google Scholar] [CrossRef] [Green Version]
- Stoykova, G.E.; Schlaepfer, I.R. Lipid Metabolism and Endocrine Resistance in Prostate Cancer, and New Opportunities for Therapy. Int. J. Mol. Sci. 2019, 20, 2626. [Google Scholar] [CrossRef]
- Abd Wahab, N.A.; Lajis, N.H.; Abas, F.; Othman, I.; Naidu, R. Mechanism of Anti-Cancer Activity of Curcumin on Andro-gen-Dependent and Androgen-Independent Prostate Cancer. Nutrients 2020, 12, 679. [Google Scholar] [CrossRef]
- Oeckinghaus, A.; Ghosh, S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb. Perspect Biol. 2009, 1, a000034. [Google Scholar] [CrossRef]
- Chi, N.; Tan, Z.; Ma, K.; Bao, L.; Yun, Z. Increased circulating myeloid-derived suppressor cells correlate with cancer stages, interleukin-8 and -6 in prostate cancer. Int. J. Clin. Exp. Med. 2014, 7, 3181–3192. [Google Scholar]
- Maubach, G.; Feige, M.H.; Lim, M.C.; Naumann, M. NF-kappaB-inducing kinase in cancer. Biochim. et Biophys. Acta 2019, 1871, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Nadiminty, N.; Tummala, R.; Liu, C.; Lou, W.; Evans, C.P.; Gao, A.C. NF-κB2/p52:c-Myc:hnRNPA1 Pathway Regulates Expression of AR Splice Variants and Enzalutamide Sensitivity in Prostate Cancer. Mol. Cancer Ther. 2015, 14, 1884–1895. [Google Scholar] [CrossRef]
- Jain, G.; Voogdt, C.; Tobias, A.; Spindler, K.D.; Möller, P.; Cronauer, M.V.; Marienfeld, R.B. IκB kinases modulate the activity of the AR in prostate carcinoma cell lines. Neoplasia 2012, 14, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Ma, J.; Wang, K.S.; Mi, C.; Lee, J.J.; Jin, X. Blockade of TNF-α-induced NF-κB signaling pathway and anti-cancer therapeutic response of Dihydrotanshinone I. Int. Immunopharmacol. 2015, 28, 764–772. [Google Scholar] [CrossRef]
- Ke, F.; Wang, Z.; Song, X.; Ma, Q.; Hu, Y.; Jiang, L.; Zhang, Y.; Liu, Y.; Zhang, Y.; Gong, W. Cryptotanshinone induces cell cycle arrest and apoptosis through the JAK2/STAT3 and PI3K/Akt/NFκB pathways in cholangiocarcinoma cells. Drug Des. Devel. Ther. 2017, 11, 1753–1766. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Han, S.; Li, X.X.; Wang, Q.Q.; Cui, Y.; Chen, Y.; Gao, H.; Huang, L.; Yang, S. Diethyl Blechnic Exhibits Anti-Inflammatory and Antioxidative Activity via the TLR4/MyD88 Signaling Pathway in LPS-Stimulated RAW264.7 Cells. Molecules 2019, 24, 4502. [Google Scholar]
- Wang, X.; Guo, D.; Li, W.; Zhang, Q.; Jiang, Y.; Wang, Q.; Li, C.; Qiu, Q.; Wang, Y. Danshen (Salvia miltiorrhiza) restricts MD2/TLR4-MyD88 complex formation and signalling in acute myocardial infarction-induced heart failure. J. Cell Mol. Med. 2020, 24, 10677–10692. [Google Scholar] [CrossRef]
- Gao, H.; Liu, X.; Sun, W.; Kang, N.; Liu, Y.; Yang, S.; Xu, Q.-M.; Wang, C.; Chen, X. Total tanshinones exhibits anti-inflammatory effects through blocking TLR4 dimerization via the MyD88 pathway. Cell Death Dis. 2017, 8, e3004. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.-H.; Hu, Q.; Sui, H.; Ci, S.-J.; Wang, Y.; Liu, X.; Liu, N.-N.; Yin, P.-H.; Qin, J.-M.; Li, Q. Tanshinone II-A Inhibits Angiogenesis through Down Regulation of COX-2 in Human Colorectal Cancer. Asian Pac. J. Cancer Prev. 2012, 13, 4453–4458. [Google Scholar] [CrossRef]
- Su, C.C. Tanshinone IIA decreases the migratory ability of AGS cells by decreasing the protein expression of matrix metal-loproteinases, nuclear factor κB-p65 and cyclooxygenase-2. Mol. Med. Rep. 2016, 13, 1263–1268. [Google Scholar] [CrossRef]
- Ching, M.M.; Reader, J.; Fulton, A.M. Eicosanoids in Cancer: Prostaglandin E2 Receptor 4 in Cancer Therapeutics and Immu-notherapy. Front Pharmacol. 2020, 11, 819. [Google Scholar] [CrossRef]
- Motolani, A.; Martin, M.; Sun, M.; Lu, T. Phosphorylation of the Regulators, a Complex Facet of NF-κB Signaling in Cancer. Biomolecules 2020, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- Shafi, A.A.; Yen, A.E.; Weigel, N.L. ARs in hormone-dependent and castration-resistant prostate cancer. Pharmacol. Ther. 2013, 140, 223–238. [Google Scholar] [CrossRef]
- Aurilio, G.; Cimadamore, A.; Mazzucchelli, R.; Lopez-Beltran, A.; Verri, E.; Scarpelli, M.; Massari, F.; Cheng, L.; Santoni, M.; Montironi, R. AR Signaling Pathway in Prostate Cancer: From Genetics to Clinical Applications. Cells 2020, 9, 2653. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Heemers, H.; Sharifi, N. Androgen Signaling in Prostate Cancer. Cold Spring Harb. Perspect Med. 2017, 7, a030452. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, K.E. Hormone Whodunit: Clues for Solving the Case of Intratumor Androgen Production. Clin. Cancer Res. 2014, 20, 5343–5345. [Google Scholar] [CrossRef]
- Stanbrough, M.; Bubley, G.J.; Ross, K.; Golub, T.R.; Rubin, M.A.; Penning, T.M.; Febbo, P.G.; Balk, S.P. Increased Expression of Genes Converting Adrenal Androgens to Testosterone in Androgen-Independent Prostate Cancer. Cancer Res. 2006, 66, 2815–2825. [Google Scholar] [CrossRef]
- Yu, J.; Zhai, D.; Hao, L.; Zhang, D.; Bai, L.; Cai, Z.; Yu, C. Cryptotanshinone Reverses Reproductive and Metabolic Disturbances in PCOS Model Rats via Regulating the Expression of CYP17 and AR. Evidence-Based Complement. Altern. Med. 2014, 2014, 670743. [Google Scholar]
- Ye, D.; Li, M.; Zhang, Y.; Wang, X.; Liu, H.; Wu, W.; Ma, W.; Quan, K.; Ng, E.H.Y.; Wu, X.; et al. Cryptotanshinone Regulates Androgen Synthesis through the ERK/c-Fos/CYP17 Pathway in Porcine Granulosa Cells. Evidence-Based Complement. Altern. Med. 2017, 2017, 5985703. [Google Scholar]
- Sharifi, N. The 5α-androstanedione pathway to dihydrotestosterone in castration-resistant prostate cancer. J. Investig. Med. 2012, 60, 504–507. [Google Scholar] [CrossRef]
- Pisolato, R.; Lombardi, A.; Vicente, C.; Lucas, T.; Lazari, M.; Porto, C. Expression and regulation of the estrogen receptors in PC-3 human prostate cancer cells. Steroids 2016, 107, 74–86. [Google Scholar] [CrossRef]
- Wang, C.; Du, X.; Yang, R.; Liu, J.; Xu, D.; Shi, J.; Chen, L.; Shao, R.; Fan, G.; Gao, X.; et al. The prevention and treatment effects of tanshinone IIA on oestrogen/androgen-induced benign prostatic hyperplasia in rats. J. Steroid Biochem. Mol. Biol. 2015, 145, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Bonkhoff, H. Estrogen receptor signaling in prostate cancer: Implications for carcinogenesis and tumor progression. Prostate 2017, 78, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, K.; Piastowska-Ciesielska, A.W. Oestrogens and oestrogen receptors in prostate cancer. SpringerPlus 2016, 5, 522. [Google Scholar] [CrossRef] [PubMed]
- Hua, H.; Kong, Q.; Zhang, H.; Wang, J.; Luo, T.; Jiang, Y. Targeting mTOR for cancer therapy. J. Hematol. Oncol. 2019, 12, 71. [Google Scholar] [CrossRef] [PubMed]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976, Erratum in Cell 2017, 169, 361–371. [Google Scholar] [CrossRef]
- Nagaraj, N.S.; Singh, O.V.; Merchant, N.B. Proteomics: A strategy to understand the novel targets in protein misfolding and cancer therapy. Expert Rev. Proteom. 2010, 7, 613–623. [Google Scholar] [CrossRef]
- Lv, C.; Zeng, H.-W.; Wang, J.-X.; Yuan, X.; Zhang, C.; Fang, T.; Yang, P.-M.; Wu, T.; Zhou, Y.-D.; Nagle, D.G.; et al. The antitumor natural product tanshinone IIA inhibits protein kinase C and acts synergistically with 17-AAG. Cell Death Dis. 2018, 9, 165. [Google Scholar] [CrossRef]
- Deng, L.; Chen, L.; Zhao, L.; Xu, Y.; Peng, X.; Wang, X.; Ding, L.; Jin, J.; Teng, H.; Wang, Y.; et al. Ubiquitination of Rheb governs growth factor-induced mTORC1 activation. Cell Res. 2019, 29, 136–150. [Google Scholar] [CrossRef]
- Tee, A.R.; Manning, B.D.; Roux, P.P.; Cantley, L.C.; Blenis, J. Tuberous sclerosis complex gene products, Tuberin and Hamartin, control mTOR signaling by acting as a GTPase-activating protein complex toward Rheb. Curr. Biol. 2003, 13, 1259–1268. [Google Scholar] [CrossRef] [Green Version]
- Su, C.-C.; Chiu, T.-L. Tanshinone IIA decreases the protein expression of EGFR, and IGFR blocking the PI3K/Akt/mTOR pathway in gastric carcinoma AGS cells both in vitro and in vivo. Oncol. Rep. 2016, 36, 1173–1179. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yu, Z.; Ren, S.; Song, J.; Wang, J.; Du, G. Metabolic reprogramming in colon cancer reversed by DHTS through regulating PTEN/AKT/HIF1α mediated signal pathway. Biochim. et Biophys. Acta (BBA)-Gen. Subj. 2018, 1862, 2281–2292. [Google Scholar] [CrossRef] [PubMed]
- Gwinn, D.M.; Shackelford, D.B.; Egan, D.F.; Mihaylova, M.M.; Mery, A.; Vasquez, D.S.; Turk, B.E.; Shaw, R.J. AMPK Phosphorylation of Raptor Mediates a Metabolic Checkpoint. Mol. Cell 2008, 30, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Inoki, K.; Ouyang, H.; Zhu, T.; Lindvall, C.; Wang, Y.; Zhang, X.; Yang, Q.; Bennett, C.; Harada, Y.; Stankunas, K.; et al. TSC2 Integrates Wnt and Energy Signals via a Coordinated Phosphorylation by AMPK and GSK3 to Regulate Cell Growth. Cell 2006, 126, 955–968. [Google Scholar] [CrossRef]
- Chen, W.; Pan, Y.; Wang, S.; Liu, Y.; Chen, G.; Zhou, L.; Zhang, C.; Ni, W.; Wang, A.; Lu, Y.; et al. Correction to: Cryptotanshinone activates AMPK-TSC2 axis leading to inhibition of mTORC1 signaling in cancer cells. BMC Cancer 2019, 19, 257. [Google Scholar] [CrossRef]
- Parmigiani, A.; Nourbakhsh, A.; Ding, B.; Wang, W.; Kim, Y.C.; Akopiants, K.; Guan, K.-L.; Karin, M.; Budanov, A.V. Sestrins Inhibit mTORC1 Kinase Activation through the GATOR Complex. Cell Rep. 2014, 9, 1281–1291. [Google Scholar] [CrossRef]
- Shorning, B.Y.; Dass, M.S.; Smalley, M.J.; Pearson, H.B. The PI3K-AKT-mTOR Pathway and Prostate Cancer: At the Crossroads of AR, MAPK, and WNT Signaling. Int. J. Mol. Sci. 2020, 21, 4507. [Google Scholar] [CrossRef]
- Yen, J.H.; Huang, S.T.; Huang, H.S.; Fong, Y.C.; Wu, Y.Y.; Chiang, J.H.; Su, Y.C. HGK-sestrin 2 signaling-mediated autophagy contrib-utes to antitumor efficacy of Tanshinone IIA in human osteosarcoma cells. Cell Death Dis. 2018, 9, 1003. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, X.; Wang, Y.; Peng, H.; Wang, Y.; Jing, Y.; Zhang, H. PDK4 protein promotes tumorigenesis through activation of cAMP-response element-binding protein (CREB)-Ras homolog enriched in brain (RHEB)-mTORC1 signaling cascade. J. Biol. Chem. 2014, 289, 29739–29749. [Google Scholar] [CrossRef]
- Tambe, Y.; Terado, T.; Kim, C.J.; Mukaisho, K.; Yoshida, S.; Sugihara, H.; Tanaka, H.; Chida, J.; Kido, H.; Yamaji, K.; et al. Antitumor activity of potent pyruvate dehydrogenase kinase 4 inhibitors from plants in pancreatic cancer. Mol. Carcinog. 2019, 58, 1726–1737. [Google Scholar] [CrossRef]
- Carriere, A.; Romeo, Y.; Acosta-Jaquez, H.A.; Moreau, J.; Bonneil, E.; Thibault, P.; Fingar, D.C.; Roux, P.P. ERK1/2 phosphorylate Raptor to promote Ras-dependent activation of mTOR complex 1 (mTORC1). J. Biol. Chem. 2011, 286, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Carrière, A.; Cargnello, M.; Julien, L.-A.; Gao, H.; Bonneil, E.; Thibault, P.; Roux, P.P. Oncogenic MAPK Signaling Stimulates mTORC1 Activity by Promoting RSK-Mediated Raptor Phosphorylation. Curr. Biol. 2008, 18, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, L.M.; Yuzugullu, H.; Zhao, J.J. PI3K in cancer: Divergent roles of isoforms, modes of activation and therapeutic targeting. Nat. Rev. Cancer 2015, 15, 7–24. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, M.C.; Er, E.E.; Blenis, J. The Ras-ERK and PI3K-mTOR pathways: Cross-talk and compensation. Trends Biochem. Sci. 2011, 36, 320–328. [Google Scholar] [CrossRef]
- Chen, W.; Liu, L.; Luo, Y.; Odaka, Y.; Awate, S.; Zhou, H.; Shen, T.; Zheng, S.; Lu, Y.; Huang, S. Cryptotanshinone activates p38/JNK and inhibits Erk1/2 leading to caspase-independent cell death in tumor cells. Cancer Prev. Res. 2012, 5, 778–787. [Google Scholar] [CrossRef]
- Su, C. Tanshinone IIA can inhibit MiaPaCa-2 human pancreatic cancer cells by dual blockade of the Ras/Raf/MEK/ERK and PI3K/AKT/mTOR pathways. Oncol. Rep. 2018, 40, 3102–3111. [Google Scholar] [CrossRef]
- Liu, P.; Cheng, H.; Roberts, T.M.; Zhao, J.J. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat. Rev. Drug Discov. 2009, 8, 627–644. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, W.; Chen, X.; Wei, G.; Zhu, H.; Xing, S. Tanshinone IIA reverses EGF- and TGF-β1-mediated epitheli-al-mesenchymal transition in HepG2 cells via the PI3K/Akt/ERK signaling pathway. Oncol Lett. 2019, 18, 6554–6562. [Google Scholar]
- Luo, Y.; Song, L.; Wang, X.; Huang, Y.; Liu, Y.; Wang, Q.; Hong, M.; Yuan, Z. Uncovering the Mechanisms of Cryptotanshinone as a Therapeutic Agent Against Hepatocellular Carcinoma. Front. Pharmacol. 2020, 11, 1264. [Google Scholar] [CrossRef]
- Shi, D.; Zhao, P.; Cui, L.; Li, H.; Sun, L.; Niu, J.; Chen, M. Inhibition of PI3K/AKT molecular pathway mediated by membrane es-trogen receptor GPER accounts for Cryptotanshinone induced antiproliferative effect on breast cancer SKBR-3 cells. BMC Pharmacol. Toxicol. 2020, 21, 32. [Google Scholar] [CrossRef]
- Liu, J.J.; Liu, W.D.; Yang, H.Z.; Zhang, Y.; Fang, Z.G.; Liu, P.Q.; Lin, D.J.; Xiao, R.Z.; Hu, Y.; Wang, C.Z.; et al. Inactivation of PI3k/Akt signaling pathway and activation of caspase-3 are involved in Tanshinone I-induced apoptosis in myeloid leu-kemia cells in vitro. Ann. Hematol. 2010, 89, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, X.; Jiang, G.; Zhang, C.; Liu, L.; Kang, J.; Wang, J.; Owusu, L.; Zhou, L.; Zhang, L.; et al. Dihydrotanshinone I inhibits ovarian cancer cell proliferation and migration by transcriptional repression of PIK3CA gene. J. Cell. Mol. Med. 2020, 24, 11177–11187. [Google Scholar] [CrossRef] [PubMed]
- Don, M.-J.; Liao, J.-F.; Lin, L.-Y.; Chiou, W.-F. Cryptotanshinone inhibits chemotactic migration in macrophages through negative regulation of the PI3K signaling pathway. J. Cereb. Blood Flow Metab. 2007, 151, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lin, F.; Chen, Y.; Wang, R.; Liu, J.; Jin, Y.; An, R. Cryptotanshinone Inhibites Bladder Cancer Cell Proliferation and Promotes Apoptosis via the PTEN/PI3K/AKT Pathway. J. Cancer 2020, 11, 488–499. [Google Scholar] [CrossRef]
- Ye, Y.-T.; Zhong, W.; Sun, P.; Wang, D.; Wang, C.; Hu, L.-M.; Qian, J.-Q. Apoptosis induced by the methanol extract of Salvia miltiorrhiza Bunge in non-small cell lung cancer through PTEN-mediated inhibition of PI3K/Akt pathway. J. Ethnopharmacol. 2017, 200, 107–116. [Google Scholar] [CrossRef]
- Papa, A.; Pandolfi, P.P. The PTEN⁻PI3K Axis in Cancer. Biomolecules 2019, 9, 153. [Google Scholar] [CrossRef]
- Geybels, M.S.; Fang, M.; Wright, J.L.; Qu, X.; Bibikova, M.; Klotzle, B.; Fan, J.-B.; Feng, Z.; Ostrander, E.A.; Nelson, P.S.; et al. PTEN loss is associated with prostate cancer recurrence and alterations in tumor DNA methylation profiles. Oncotarget 2017, 8, 84338–84348. [Google Scholar] [CrossRef]
- Wise, H.M.; Hermida, M.A.; Leslie, N.R. Prostate cancer, PI3K, PTEN and prognosis. Clin. Sci. 2017, 131, 197–210. [Google Scholar] [CrossRef]
- Hong, J.-Y.; Park, S.H.; Park, H.J.; Lee, S.K. Anti-proliferative Effect of 15,16-Dihydrotanshinone I Through Cell Cycle Arrest and the Regulation of AMP-activated Protein Kinase/Akt/mTOR and Mitogen-activated Protein Kinase Signaling Pathway in Human Hepatocellular Carcinoma Cells. J. Cancer Prev. 2018, 23, 63–69. [Google Scholar] [CrossRef]
- Holz, M.K.; Ballif, B.A.; Gygi, S.P.; Blenis, J. mTOR and S6K1 mediate assembly of the translation preinitiation complex through dynamic protein interchange and ordered phosphorylation events. Cell 2005, 123, 569–580. [Google Scholar] [CrossRef]
- Jeoung, N.H.; Jeong, J.Y.; Kang, B.S. Cryptotanshinone Prevents the Binding of S6K1 to mTOR/Raptor Leading to the Sup-pression of mTORC1-S6K1 Signaling Activity and Neoplastic Cell Transformation. J. Cancer Prev. 2021, 26, 145–152. [Google Scholar] [CrossRef]
- Li, G.; Shan, C.; Liu, L.; Zhou, T.; Zhou, J.; Hu, X.; Chen, Y.; Cui, H.; Gao, N. Tanshinone IIA inhibits HIF-1α and VEGF expression in breast cancer cells via mTOR/p70S6K/RPS6/4E-BP1 signaling pathway. PLoS ONE 2015, 10, e0117440. [Google Scholar] [CrossRef]
- Barrera-Vázquez, O.S.; Gómez-Verjan, J.C.; Magos-Guerrero, G.A. Chemoinformatic Screening for the Selection of Potential Senolytic Compounds from Natural Products. Biomolecules 2021, 11, 467. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Tao, T.; Li, H.; Zhu, X. mTOR signaling pathway and mTOR inhibitors in cancer: Progress and challenges. Cell Biosci. 2020, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.Q.; Gao, W.; Zhou, Y.J. Application of synthetic biology to sustainable utilization of Chinese materia medica re-sources. Yao Xue Xue Bao 2014, 49, 37–43. (In Chinese) [Google Scholar]
- Chen, M.; Cai, Y.; Zhang, W.; Chen, Z.; Shi, Z.; He, C. Recent insights into the biological activities and drug delivery systems of tanshinones. Int. J. Nanomed. 2016, 11, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Hu, T.-Y.; Guo, J.; Lv, D.-M.; Dai, Z.-B.; Zhou, Y.-J.; Huang, L.-Q. Research progress of synthetic biology for tanshinones. China J. Chin. Mater. Medica 2015, 40, 2486–2491. [Google Scholar]
- Zhou, Y.J.; Gao, W.; Rong, Q.; Jin, G.; Chu, H.; Liu, W.; Yang, W.; Zhu, Z.; Li, G.; Zhu, G.; et al. Modular pathway engi-neering of diterpenoid synthases and the mevalonic acid pathway for miltiradiene production. J. Am. Chem. Soc. 2012, 134, 3234–3241. [Google Scholar] [CrossRef]
- Ma, X.H.; Ma, Y.; Tang, J.F.; He, Y.L.; Liu, Y.C.; Ma, X.J.; Shen, Y.; Cui, G.H.; Lin, H.X.; Rong, Q.X.; et al. The Biosynthetic Pathways of Tanshinones and Phenolic Acids in Salvia miltiorrhiza. Molecules 2015, 20, 16235–16254. [Google Scholar] [CrossRef]
- Song, J.-J.; Fang, X.; Li, C.-Y.; Jiang, Y.; Li, J.-X.; Wu, S.; Guo, J.; Liu, Y.; Fan, H.; Huang, Y.-B.; et al. A 2-oxoglutarate-dependent dioxygenase converts dihydrofuran to furan in Salvia diterpenoids. Plant Physiol. 2022, 188, 1496–1506. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, P.; Ye, M.; Kim, S.-H.; Jiang, C.; Lü, J. Tanshinones: Sources, Pharmacokinetics and Anti-Cancer Activities. Int. J. Mol. Sci. 2012, 13, 13621–13666. [Google Scholar] [CrossRef] [PubMed]
- Estolano-Cobián, A.; Alonso, M.M.; Díaz-Rubio, L.; Ponce, C.N.; Córdova-Guerrero, I.; Marrero, J.G. Tanshinones and their Deriva-tives: Heterocyclic Ring-Fused Diterpenes of Biological Interest. Mini Rev. Med. Chem. 2021, 21, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Deng, H.; Shen, Q.K.; Quan, Z.S. Tanshinone IIA: Pharmacology, Total Synthesis, and Progress in Struc-ture-modifications. Curr. Med. Chem. 2022, 29, 1959–1989. [Google Scholar] [CrossRef]
- Wang, M.; Liu, J.; Zhou, B.; Xu, R.; Tao, L.; Ji, M.; Zhu, L.; Jiang, J.; Shen, J.; Gui, X.; et al. Acute and sub-chronic toxicity studies of Danshen injection in Sprague-Dawley rats. J. Ethnopharmacol. 2012, 141, 96–103. [Google Scholar] [CrossRef]
- Lan, Y.; Wang, B.; Wang, X.; Wang, T.; Wang, C.; Zhang, H.; Chen, J.; Mei, W. Evaluation of tanshinone IIA developmental toxicity in zebrafish embryos. Molecules 2017, 22, 660. [Google Scholar]
- Wang, C.; Wang, T.; Lian, B.-W.; Lai, S.; Li, S.; Li, Y.-M.; Tan, W.-J.; Wang, B.; Mei, W. Developmental toxicity of cryptotanshinone on the early-life stage of zebrafish development. Hum. Exp. Toxicol. 2021, 40, S278–S289. [Google Scholar] [CrossRef]
- Yang, L.-J.; Jeng, C.-J.; Kung, H.-N.; Chang, C.-C.; Wang, A.-G.; Chau, G.-Y.; Don, M.-J.; Chau, Y.-P. Tanshinone IIA isolated from Salvia miltiorrhiza elicits the cell death of human endothelial cells. J. Biomed. Sci. 2005, 12, 347–361. [Google Scholar] [CrossRef]




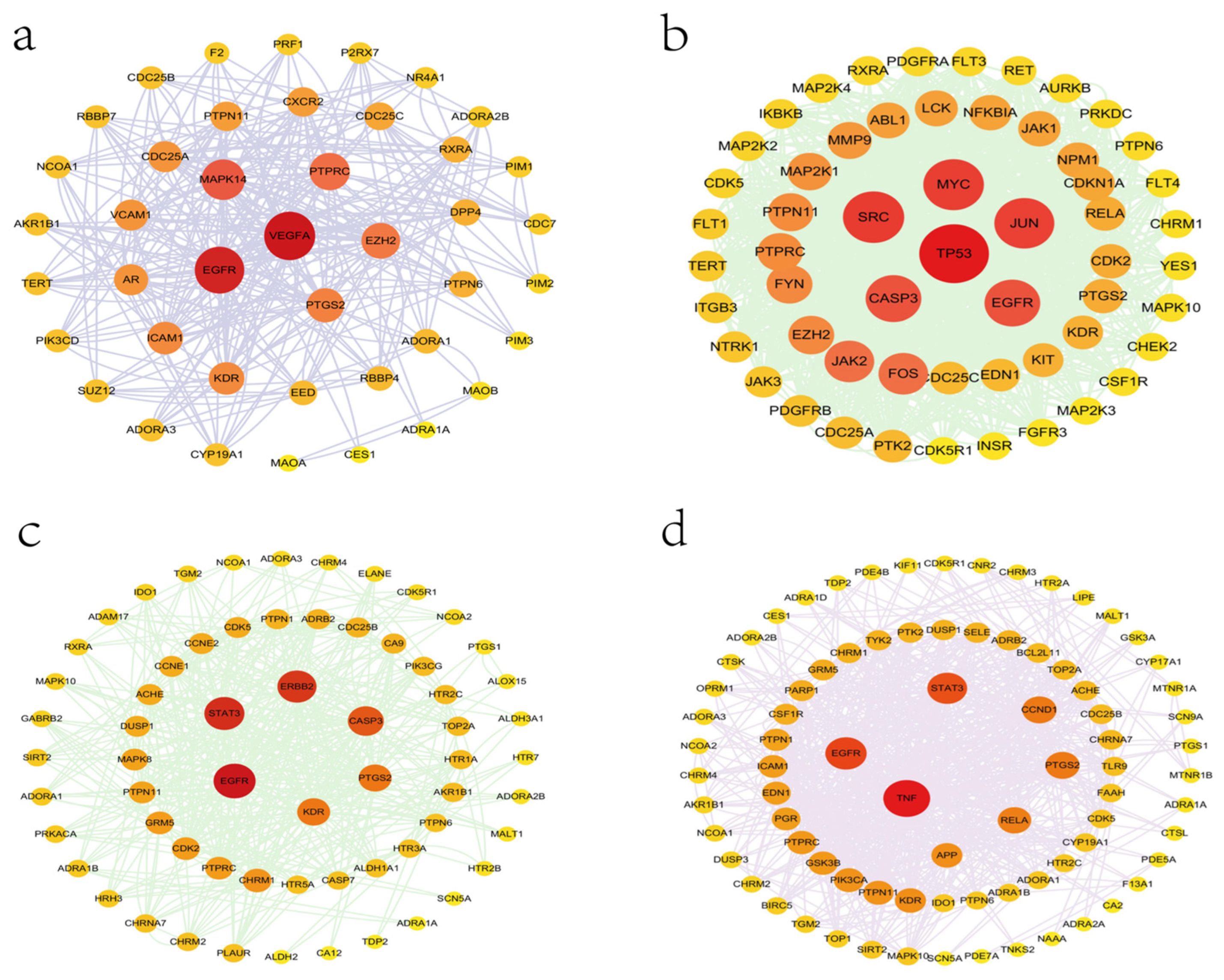
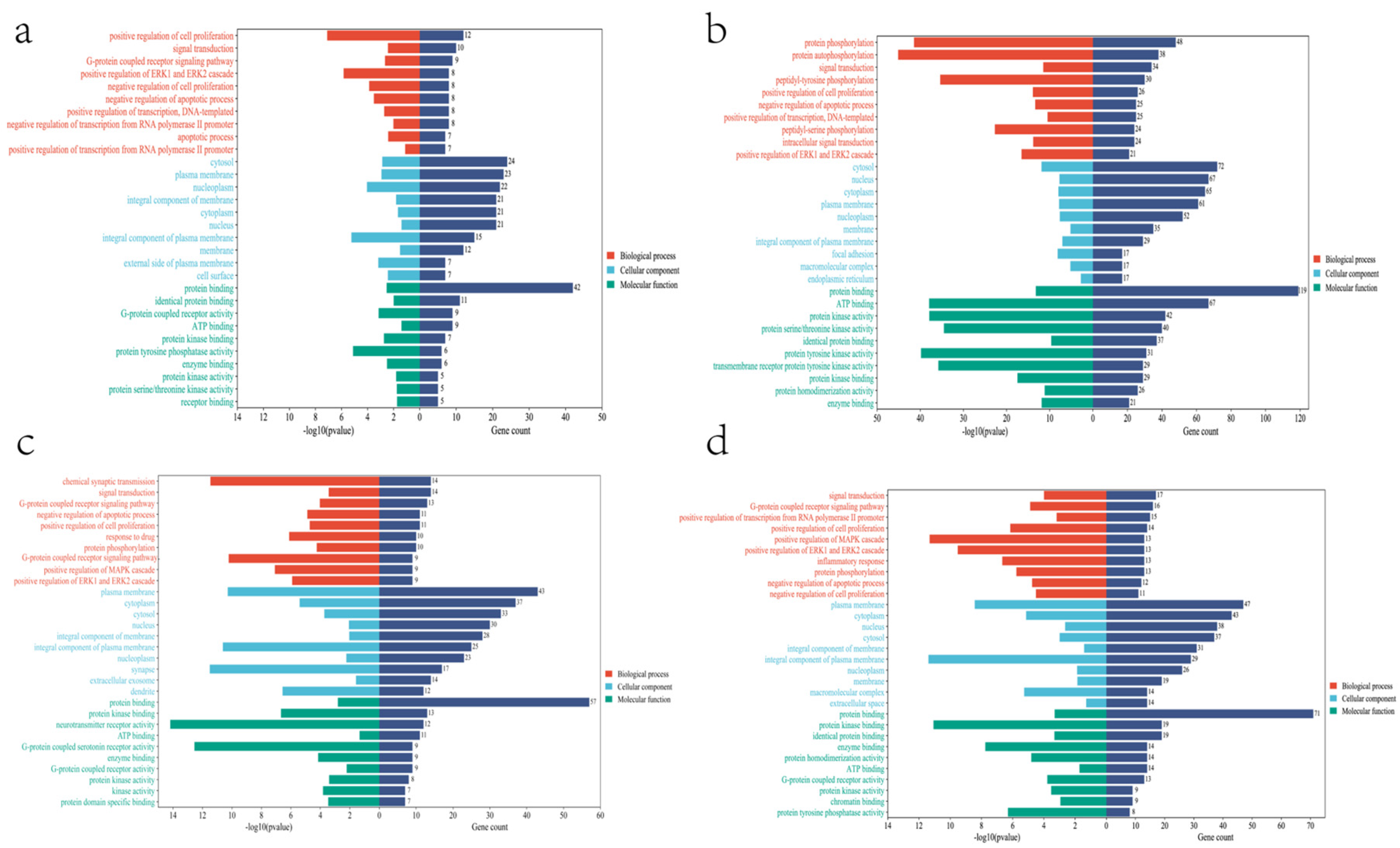
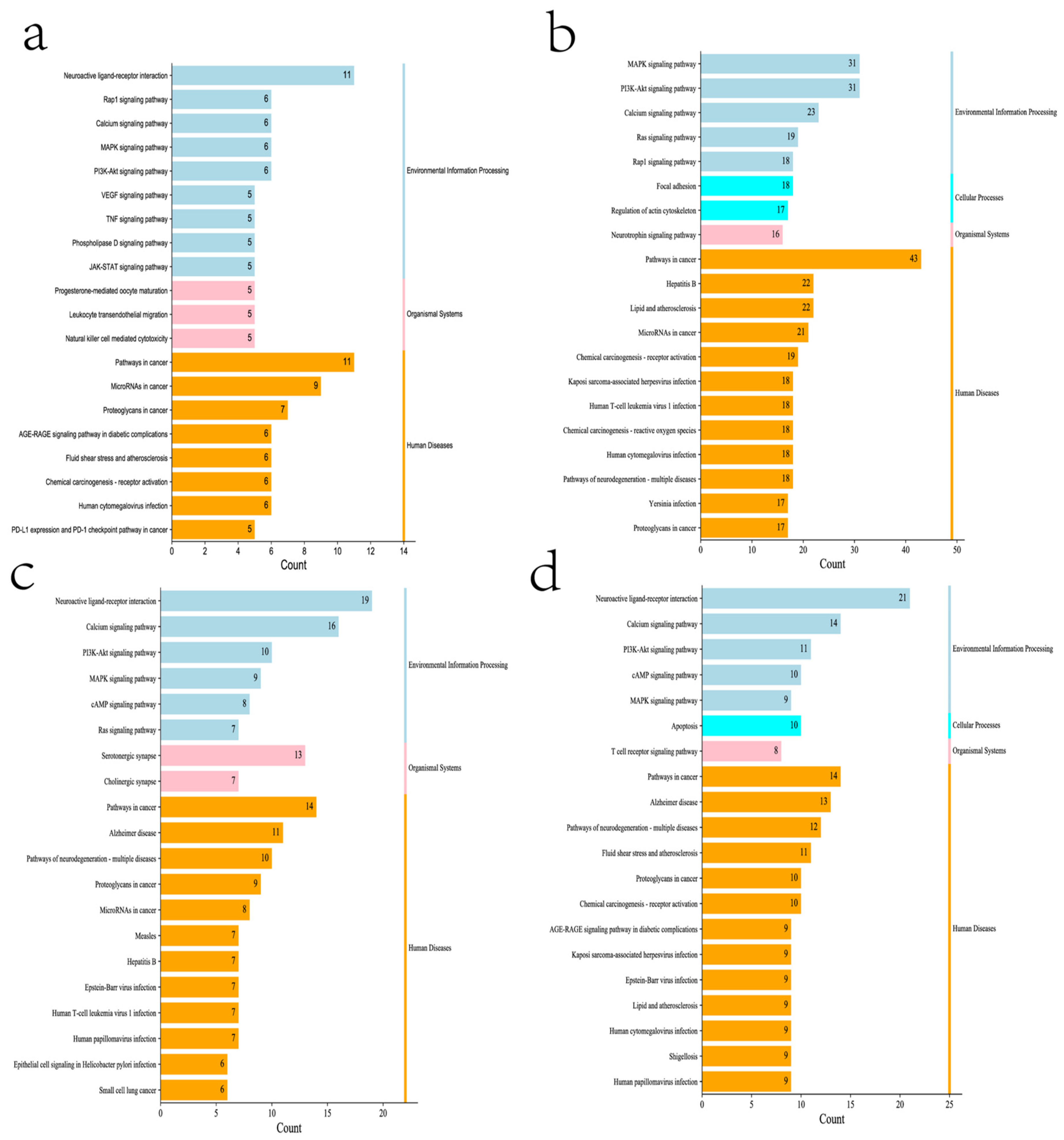
| Compound | Dose | Cell | Mechanism | Reference |
|---|---|---|---|---|
| TsIIA | 0, 1.25, 2.5, 5, 10 uM | LNCaP | Cell cycle arrest and apoptosis are induced by the activation of P53 (dose-dependent). | [34] |
| TsIIA | 5 μM | PC-3 | Induced autophagy and apoptosis | [35] |
| TsIIA | 20 umol/L | LNCaP, PC3 | enhancing the effect of the anti-tumor activity of cisplatin. | [36] |
| TsIIA | 0, 40, 80 µM | PC-3 | Inducing autophagy by up-regulated expression of microtubule-associated protein light chain 3 (LC3) II | [37] |
| TsIIA | 10, 25, 50 uM | LNCaP, PC-3 | inducing mitochondrial-dependent cell apoptosis by inhibiting PIK3/AKT | [38] |
| TsIIA | 2.5, 5 μg/ml | LNCaP | Induced apoptosis and induced cell cycle arrest by endoplasmic reticulum stress | [39] |
| TsIIA | — | LNCaP | Cell proliferation was inhibited by inhibiting the AR signal. | [40] |
| TsIIA | — | — | Maspin expression was induced, AR expression was inhibited, and apoptosis was induced. | [41] |
| TsIIAD | 2.5 μM | PC3 | Binding NQO1 protein causes cell cycle arrest and apoptosis. | [42] |
| CYT | 10 umol/L | DU145 | Apoptosis was induced and the expression of isomucin was inhibited by inhibiting the PI3K/AKT signaling pathway. | [43] |
| CYT | 10 μM | LNCaP, 22Rv1, and PC3 | The activity and expression of AR were inhibited by inhibiting LSD1-mediated H3K9 demethylation. | [44] |
| CYT | 1.0 ug/ml | DU145 | To activate Fas-mediated apoptosis | [45] |
| CYT | 0.5 µM | LNCaP, 22Rv1 | Cell proliferation was inhibited by inhibiting AR expression and activity. | [46] |
| CYT | 1.5 µM | LNCaP | Tumor-initiating cells are influenced by down-regulating dry gene expression. | [47] |
| CYT | 5, 10 μM | DU145, LNCaP, and PC-3 | Inhibiting HIF-1 and AEG-1 inhibits angiogenesis and induces cell cycle arrest and apoptosis. | [31] |
| CYT | 10 μM | PC3 | Cell proliferation is inhibited by decreasing the stability and expression of DNA topoisomerase 2. | [48] |
| CYT | 5 uM | 22Rv1 and PC-3 | AR expression and activity were reduced, and MMP9 secretion was also reduced. | [49] |
| CYT | 0–40 µM | DU145 | Apoptosis was induced by inhibiting phosphorylation of mTOR and Rb. | [50] |
| CYT | 7 μmol/L | DU145 | Inhibition of STAT3Tyr705 and its upstream tyrosine kinase induces cell cycle arrest and apoptosis. | [51] |
| TsI | 20, 40, 80 μM | PC-3, DU145 | Apoptosis is induced by upregulation of microRNA135A-3p and death receptor 5. | [52] |
| TsI | 3–6 μM | PC-3, LNCaP, and DU-145 | inhibiting angiogenesis and inducing apoptosis by down-regulating AuroraA expression. | [30] |
| DHT | 5–10 μM | PC-3, DU145, and 22Rv1 | inhibiting EMT by inhibition of the CCL2/STAT3 axis | [34] |
| DHT | 0.1 ug/mL and 1.5 ug/mL | DU145 | Inducing cell cycle arrest by activating the ER pathway | [53] |
| TsD | 3, 6, 12 μM | PC3, LNCAP | Inducing cell cycle arrest and apoptosis | [54] |
| TsD | 2 µM | LNCaP, C4-2 | AR expression and activity were reduced, and cell proliferation was slowed. | [55] |
| SME | 3.125, 12.5, 25 and 50 μg/mL | DU-145 | Cell cycle arrest and apoptosis are mediated by P53 | [56] |
| SME | 20 µg/ml | PC-3, LNCaP, and DU-145 | Inducing cell cycle arrest and apoptosis | [57] |
| TsIIAN | — | PC-3 and DU145 | Induction of apoptosis | [58] |
| SMEN | — | LNCap | Inducing apoptosis and up-regulating ROS in cells | [59] |
| NCDT | — | LNCaP | Enhancing toxicity of doxorubicin | [60] |
| Animal Models | Dose | Delivery Way | Result | Reference |
|---|---|---|---|---|
| 22Rv1 allograft mouse model | CYT (5 mg/Kg) and CYT (25 mg/Kg) | Intraperitoneal injections were given every two days for four weeks. | Tumor growth was inhibited in both the low-dose and high-dose groups. | [46] |
| PC-3 allograft mouse model | TsI (150 mg/kg) | Tube feeding, once a day, for 2 weeks | Tumor weight (67%) and intratumor blood vessels (80%) were reduced. | [30] |
| PC-3 allograft mouse model | SME (100 mg/kg) | Oral and tube feeding, once a day, for 6 weeks | The incidence and weight of tumors were reduced. | [57] |
| LNCaP allograft mouse model | TsIIA (25 mg/kg) | Orally, once daily for 6 weeks | Tumor growth and the expression of AR were inhibited. | [40] |
| PC-3 allograft mouse model | CYT (10 mg/kg) | Intraperitoneal injection, once a day | Tumor weight (46.4%) and intratumor blood vessels were reduced. | [31] |
| DU-145 allograft mouse model | SME (500 mg/kg) | Orally, once daily for 2 weeks | Tumor growth was inhibited | [56] |
| PC-3 allograft mouse model | TsD (60 mg/kg) | Subcutaneous injections were given every two days for 18 days | Tumor growth was inhibited | [54] |
| LNCaP allograft mouse model | TsIIA (60 or 90 mg/kg) | Subcutaneous injections were given every two days for 13 days | Tumor weight (86.4%) was reduced. | [39] |
| CWR22Rv1 allograft mouse model | CYT (25 mg/kg) | Intraperitoneal injections were given 3 times per week for 4 weeks | Tumor metastasis is inhibited. | [49] |
| LNCaP allograft mouse model | NCDT (5 mg/Kg) | It was injected once every two days for 18 days. | To enhance the toxicity of Doxorubicin | [60] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Huang, T.; Xu, S.; Che, B.; Yu, Y.; Zhang, W.; Tang, K. Molecular Mechanism of Tanshinone against Prostate Cancer. Molecules 2022, 27, 5594. https://doi.org/10.3390/molecules27175594
Li W, Huang T, Xu S, Che B, Yu Y, Zhang W, Tang K. Molecular Mechanism of Tanshinone against Prostate Cancer. Molecules. 2022; 27(17):5594. https://doi.org/10.3390/molecules27175594
Chicago/Turabian StyleLi, Wei, Tao Huang, Shenghan Xu, Bangwei Che, Ying Yu, Wenjun Zhang, and Kaifa Tang. 2022. "Molecular Mechanism of Tanshinone against Prostate Cancer" Molecules 27, no. 17: 5594. https://doi.org/10.3390/molecules27175594
APA StyleLi, W., Huang, T., Xu, S., Che, B., Yu, Y., Zhang, W., & Tang, K. (2022). Molecular Mechanism of Tanshinone against Prostate Cancer. Molecules, 27(17), 5594. https://doi.org/10.3390/molecules27175594





