Recent Advances in Design and Synthesis of Diselenafulvenes, Tetraselenafulvalenes, and Their Tellurium Analogs and Application for Materials Sciences
Abstract
:1. Introduction
2. The 1,4-Diselenafulvene and 1,4,5,8-Tetraselenafulvalene Derivatives
2.1. Non-Condensed 1,4-Diselenafulvenes
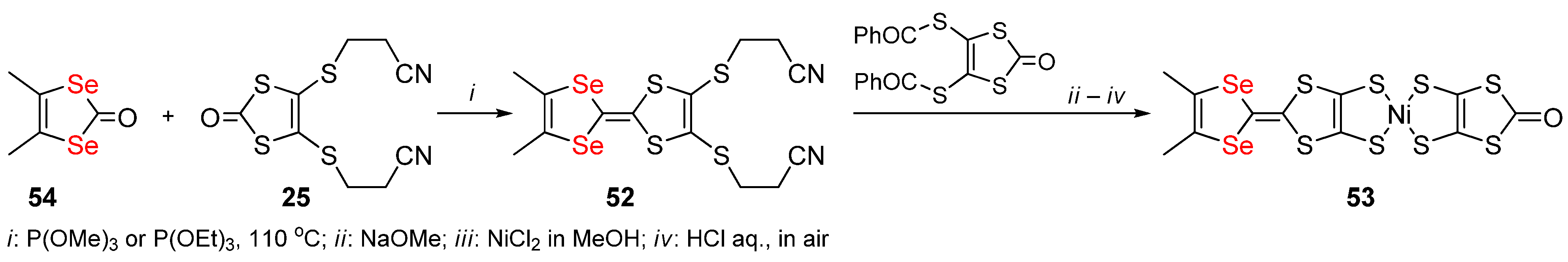
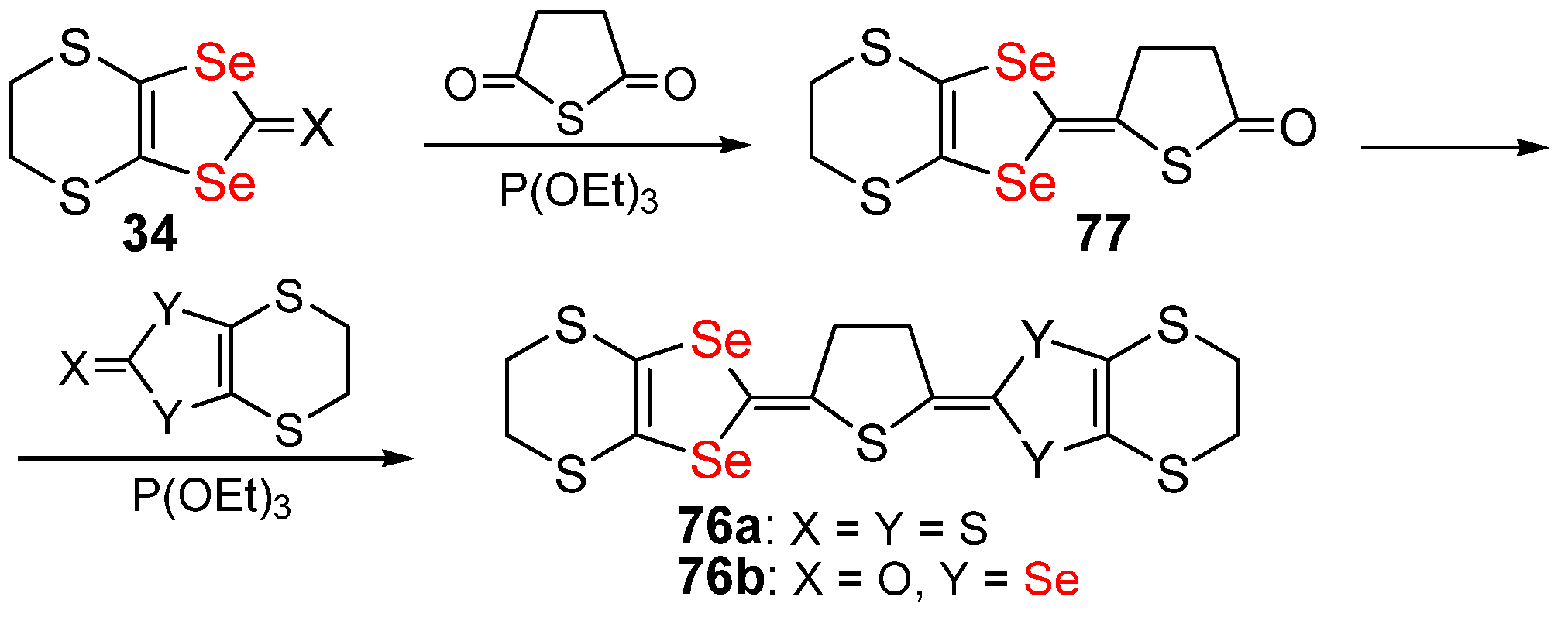
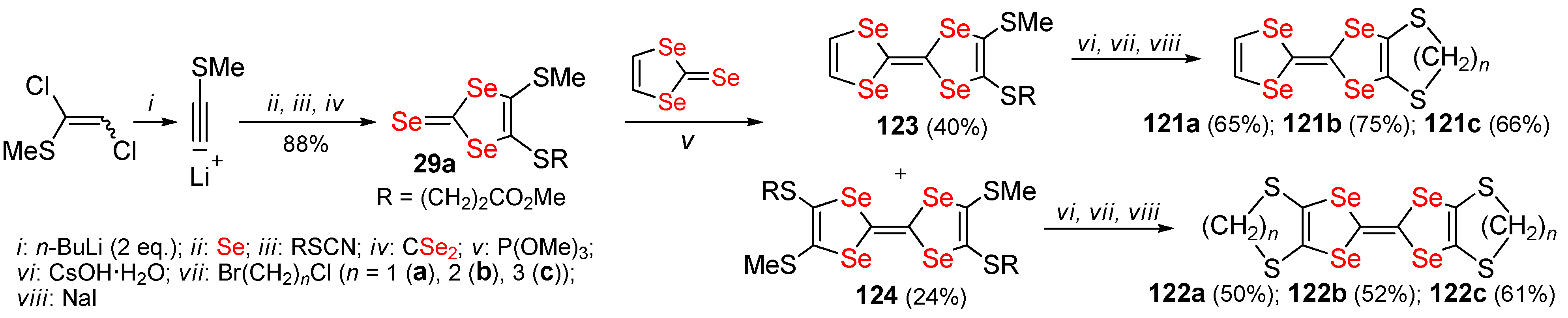
2.2. Condensed 1,4-Diselenafulvenes and 1,4,5,8-Dithiadiselenafulvalenes
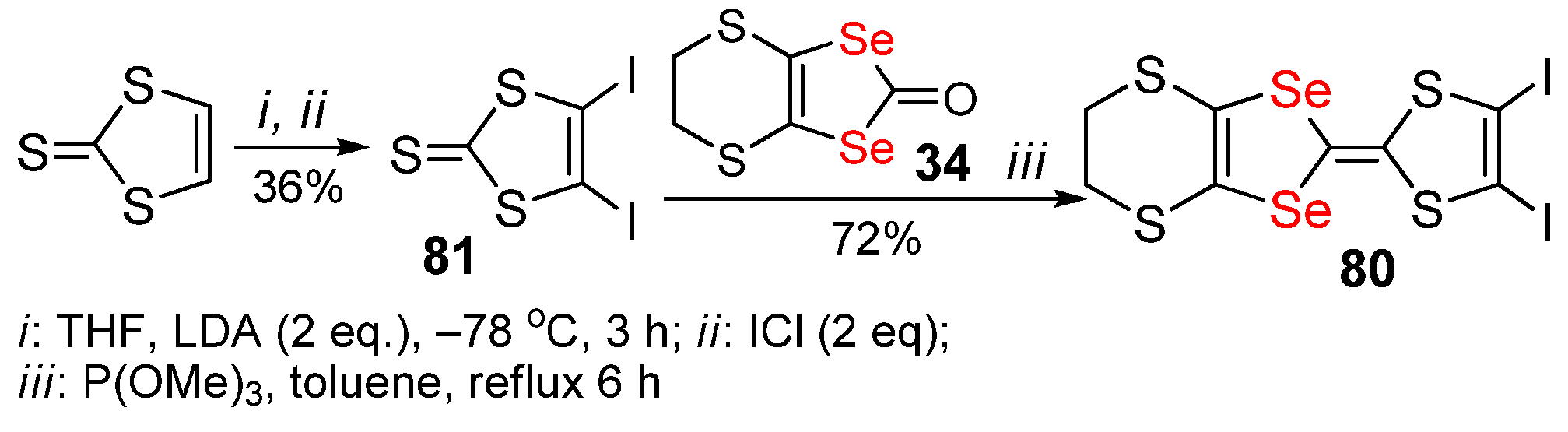
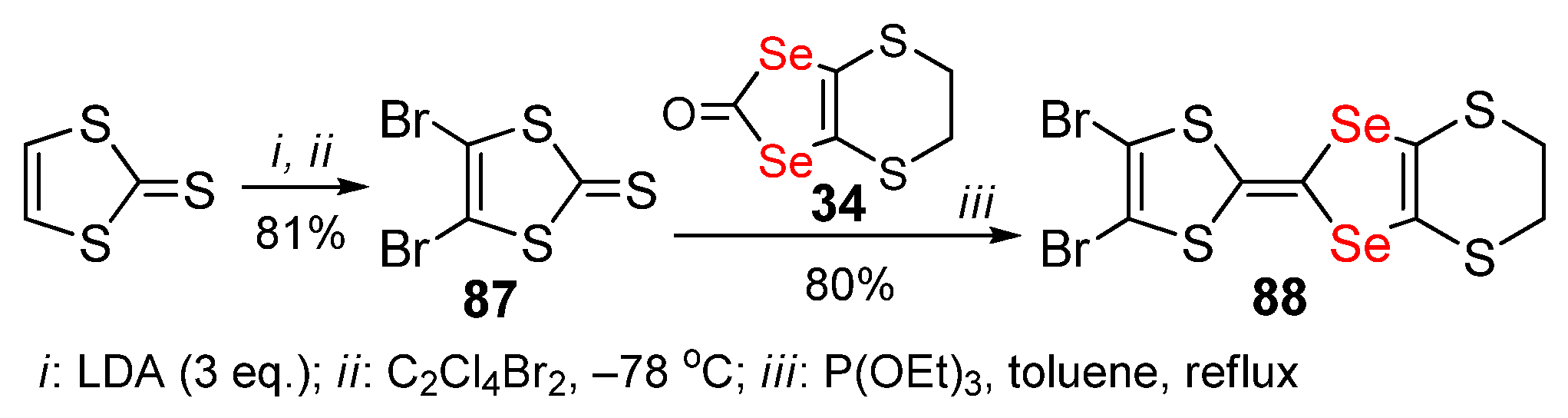
2.3. Halogenated 1,4-Diselenafulvenes and 1,4,5,8-Dithiadiselenafulvalenes
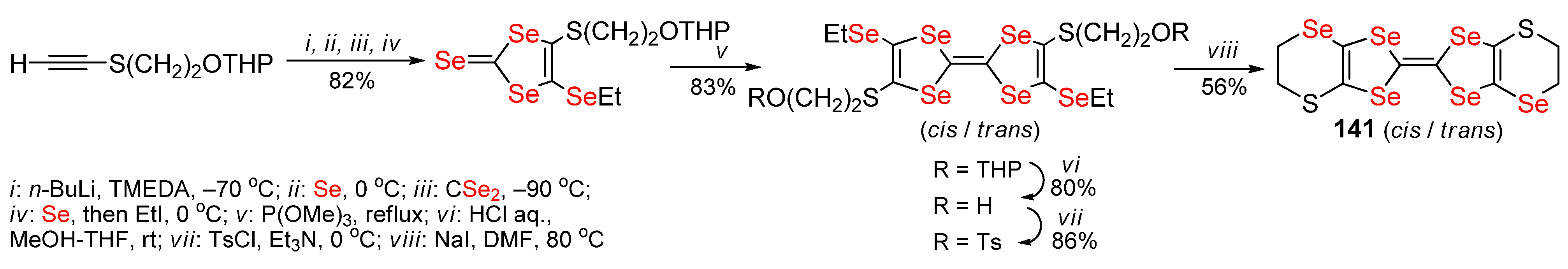
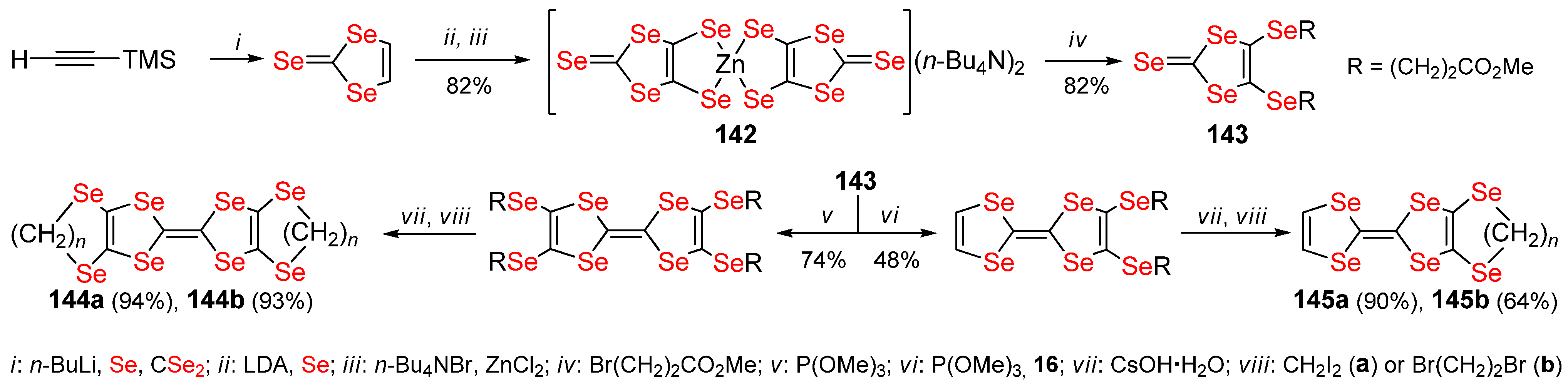
2.4. Non-Condensed 1,4,5,8-Tetraselenafulvalenes
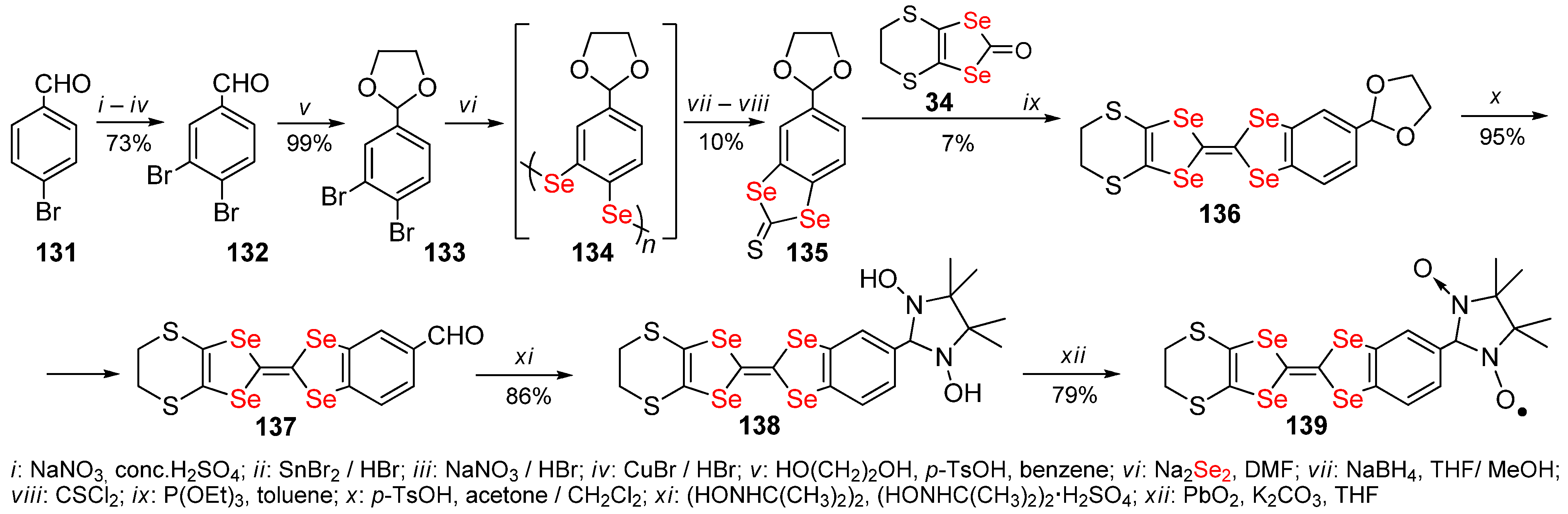
2.5. Condensed 1,4,5,8-Tetraselenafulvalenes
2.6. Other 1,4-Selenafulvene and 1,4,5,8-Selenafulvalene Derivatives
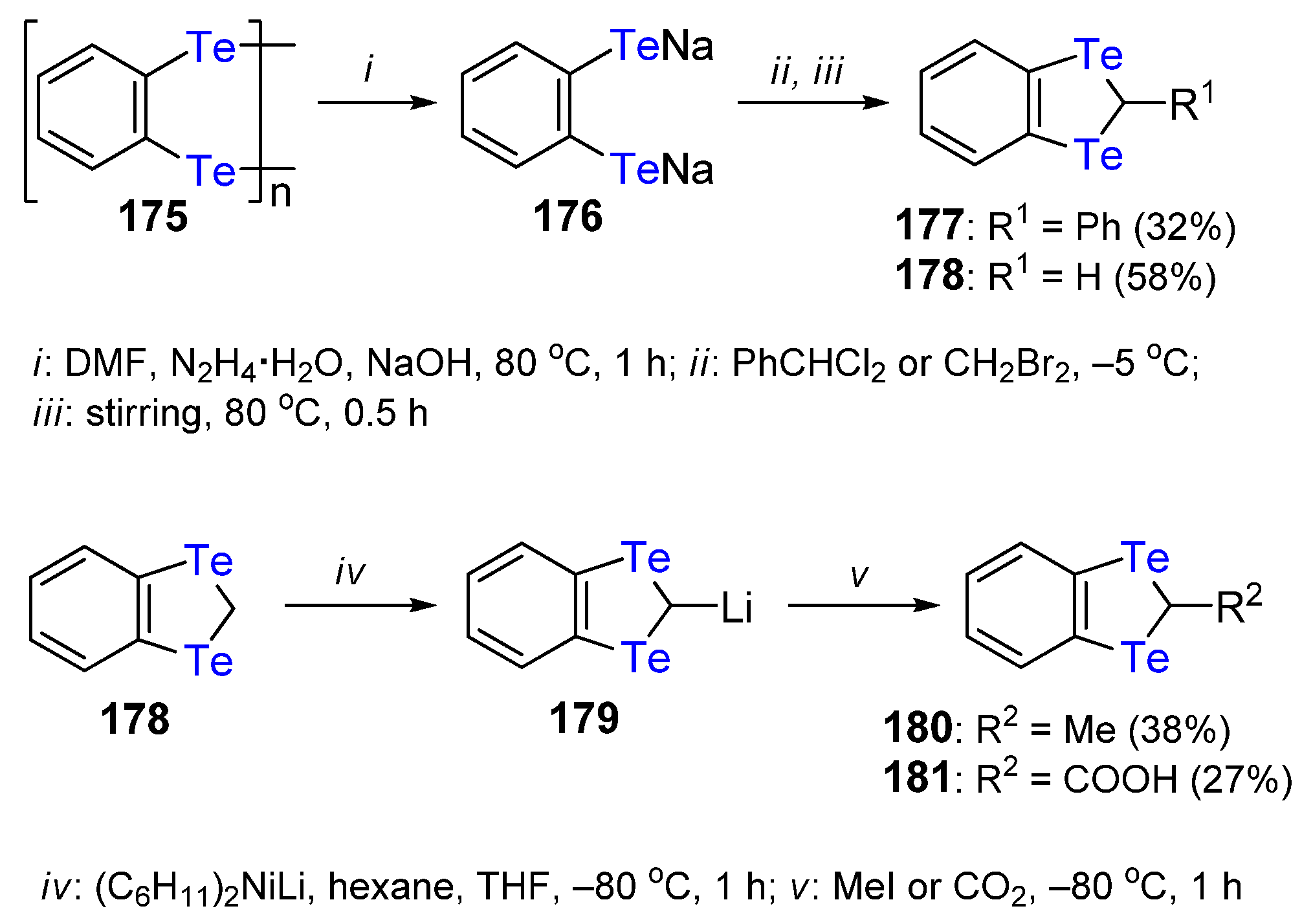
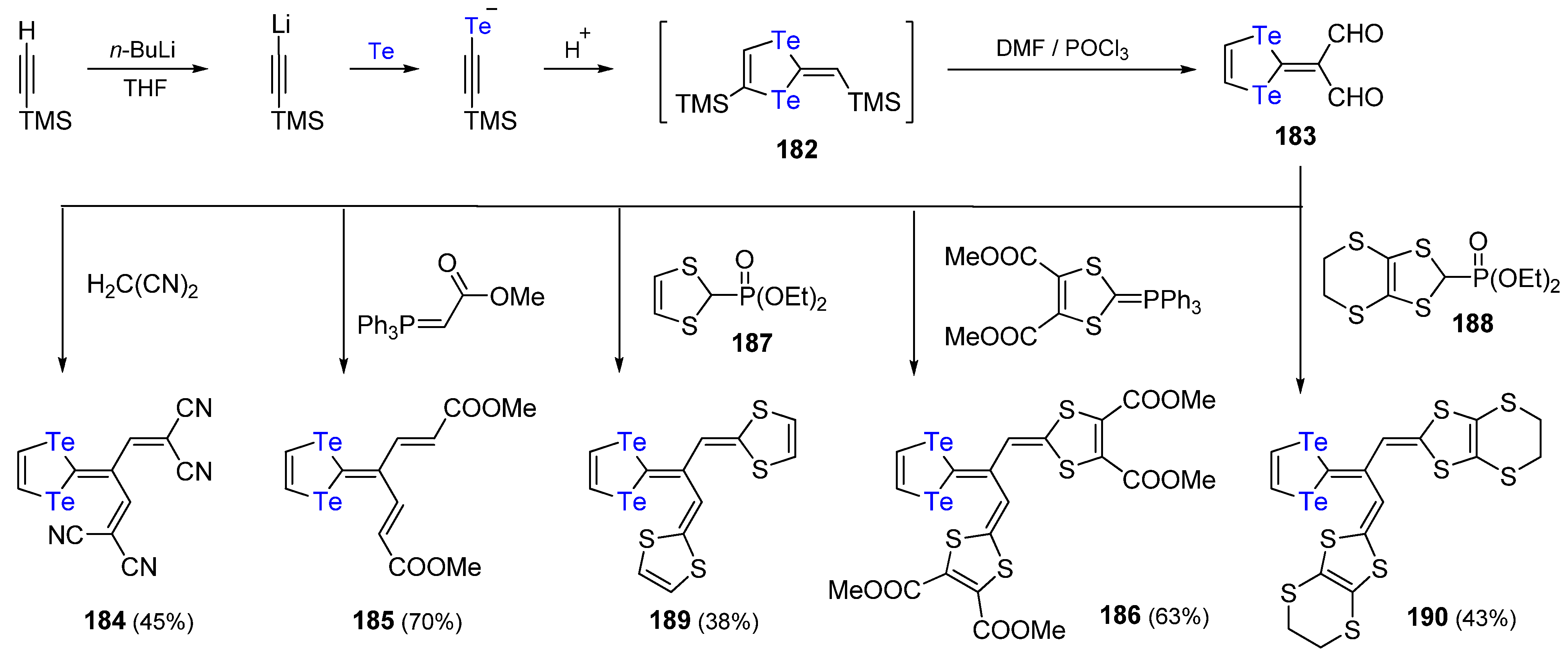
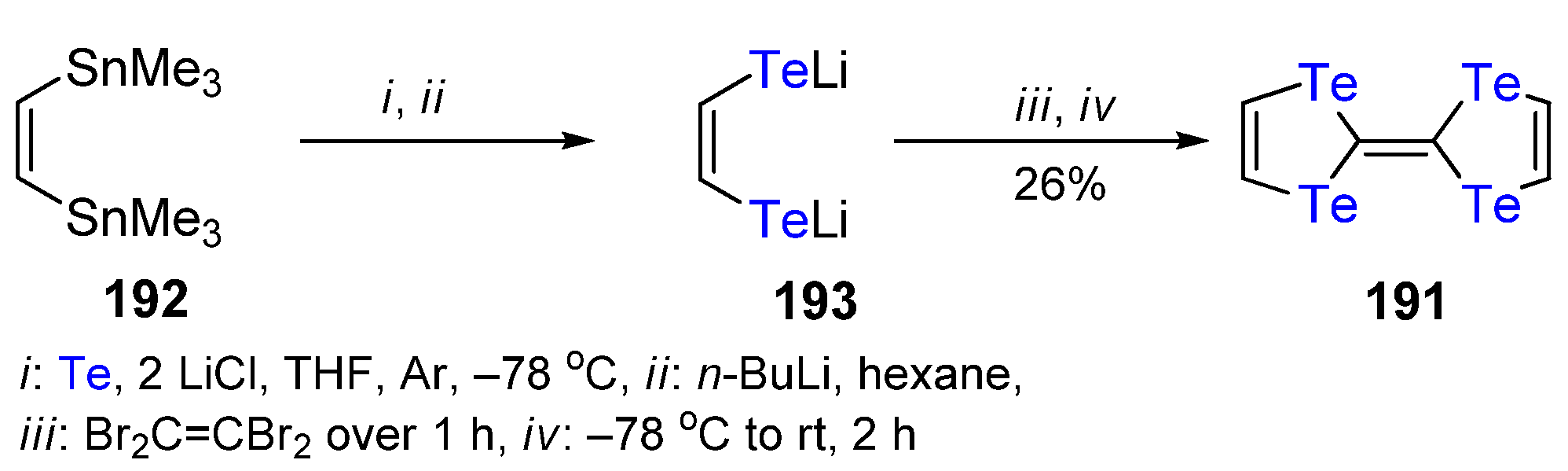
3. The Synthesis of 1,4-Ditellurafulvene and 1,4,5,8-Tetratellurafulvalene Derivatives
4. Application of 1,4,5,8-Tetraselenafulvalene Derivatives and Their Tellurium Analogs for Materials Sciences
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wudl, F.; Wobschall, D.; Hufnagel, E.J. Electrical conductivity by the bis(1,3-dithiole)-bis(1,3-dithiolium) system. J. Am. Chem. Soc. 1972, 94, 670–672. [Google Scholar] [CrossRef]
- Ferraris, J.; Cowan, D.O.; Walatka, V.V., Jr.; Perlstein, J.H. Electron transfer in a new highly conducting donor-acceptor complex. J. Am. Chem. Soc. 1973, 95, 948–949. [Google Scholar] [CrossRef]
- Prokhorova, T.G.; Yagubskii, E.B. Organic conductors and superconductors based on bis(ethylenedithio)tetrathiafulvalene radical cation salts with supramolecular tris(oxalato)metallate anions. Russ. Chem. Rev. 2017, 86, 164–180. [Google Scholar] [CrossRef]
- Williams, J.M.; Ferraro, J.R.; Thorn, R.J.; Carlson, K.D.; Geiser, U.; Wang, H.H.; Kini, A.M.; Whangbo, M.-H. Organic Superconductors (Including Fullerenes): Synthesis, Structure, Properties, and Theory; Prentice Hall: Englewood Cliffs, NJ, USA, 1992; p. 400. [Google Scholar]
- Ishiguro, T.; Yamaji, K.; Saito, G. Organic Superconductors, 2nd ed.; Springer: Berlin, Germany, 1998. [Google Scholar]
- Jérome, D.; Mazaud, A.; Ribault, M.; Bechgaard, K. Superconductivity in a synthetic organic conductor (TMTSF)2PF6. J. Phys. Lett. 1980, 41, 95–98. [Google Scholar] [CrossRef]
- Matsumoto, T.; Kamada, Y.; Sugimoto, T.; Tada, T.; Noguchi, S.; Nakazumi, H.; Shiro, M.; Yoshino, H.; Murata, K. Electrical conducting and magnetic properties of (ethylenedithiotetrathiafulvalenothioquinone-1,3-diselenolemethide)2∙FeBr4 (GaBr4) crystals with two different interlayer arrangements of donor molecules. Inorg. Chem. 2003, 42, 5192–5201. [Google Scholar] [CrossRef]
- Shirahata, T.; Kibune, M.; Maesato, M.; Kawashima, T.; Saito, G.; Imakubo, T. New organic conductors based on dibromo- and diiodo-TSeFs with magnetic and non-magnetic MX4 counter anions (M = Fe, Ga; X = Cl, Br). J. Mater. Chem. 2006, 16, 3381–3390. [Google Scholar] [CrossRef]
- Ichikawa, S.; Mori, H. High conductivity of the new supramoleclar copper complex with oxidized pyrazinoselenathiafulvalene (pyra-STF) as the ligand, [CuICl1.5 (pyra-STF)0.5+. Inorg. Chem. 2009, 48, 4643–4645. [Google Scholar] [CrossRef]
- Akutsu, H.; Koyama, Y.; Turner, S.S.; Furuta, K.; Nakazawa, Y. Structures and properties of new organic conductors: BEDT-TTF, BEST and BETS salts of the HOC2H4SO3− anion. Crystals 2020, 10, 775. [Google Scholar] [CrossRef]
- Morinari, T.; Suzumura, Y. On the possible zero-gap state in organic conductor α-(BEDT-TSF)2I3 under pressure. J. Phys. Soc. Jpn. 2014, 83, 094701. [Google Scholar] [CrossRef]
- Kosaka, Y.; Yamamoto, H.M.; Nakao, A.; Kato, R. Multicomponent molecular conductors with supramolecular assemblies prepared from neutral iodine-bearing pBIB (p-bis(iodoethynyl)benzene) and derivatives. Bull. Chem. Soc. Jpn. 2006, 79, 1148–1154. [Google Scholar] [CrossRef]
- Umemiya, M.; Goto, M.; Kobayashi, N.; Takaishi, S.; Kajiwara, T.; Yamashita, M.; Miyasaka, H.; Sugiura, K.-i.; Watanabe, H.; Suzuki, D.; et al. New charge-transfer salts based on unsymmetrical donor DMET and metal complex anions: (DMET)3[Cr(isoq)2(NCS)4] and (DMET)3[Cr(phen)(NCS)4]∙CH3CN. Chem. Lett. 2006, 35, 368–369. [Google Scholar] [CrossRef]
- Hayashi, T.; Xiao, X.; Fujiwara, H.; Sugimoto, T.; Nakazumi, H.; Noguchi, S.; Katori, H.A. Weak ferromagnetism in a semiconducting (ethylenedithiodiselenadithiafulvalenoquinone-1,3-diselenolemethide)2∙FeBr4 salt. Inorg. Chem. 2007, 46, 8478–8480. [Google Scholar] [CrossRef]
- Takimiya, K.; Takamori, A.; Aso, Y.; Otsubo, T.; Kawamoto, T.; Mori, T. Organic superconductors based on a new electron donor, methylenedithio-diselenadithiafulvalene (MDT-ST). Chem. Mater. 2003, 15, 1225–1227. [Google Scholar] [CrossRef]
- Takimiya, K.; Kodani, M.; Niihara, N.; Aso, Y.; Otsubo, T.; Bando, Y.; Kawamoto, T.; Mori, T. Pressure-induced superconductivity in (MDT-TS)(AuI2)0.441 [MDT-TS) = 5H-2-(1,3-diselenol-2-ylidene)-1,3,4,6-tetrathiapentalene]: A new organic superconductor possessing an incommensurate anion lattice. Chem. Mater. 2004, 16, 5120–5123. [Google Scholar] [CrossRef]
- Imakubo, T.; Kibune, M.; Yoshino, H.; Shirahata, T.; Yoza, K. Hexagonal supramolecular organic conductors based on diiodo(pyrazino)tetraselenafulvalene: High yield recovery of the neutral π-donor by a simple chemical reaction. J. Mater. Chem. 2006, 16, 4110–4116. [Google Scholar] [CrossRef]
- Ashizawa, M.; Yamamoto, H.M.; Nakao, A.; Kato, R. The first methyl antimony linked dimeric tetrathiafulvalene and tetraselenafulvalenes. Tetrahedron Lett. 2006, 47, 8937–8941. [Google Scholar] [CrossRef]
- Imakubo, T.; Shirahata, T.; Kibune, M.; Yoshino, H. Hybrid organic/inorganic supramolecular conductors D2[Au(CN)4] [D = Diiodo(ethylenedichalcogeno)tetrachalcogenofulvalene], including a new ambient pressure superconductor. Eur. J. Inorg. Chem. 2007, 2007, 4727–4735. [Google Scholar] [CrossRef]
- Kobayashi, H.; Cui, H.; Kobayashi, A. Organic metals and superconductors based on BETS (BETS = Bis(ethylenedithio)tetraselenafulvalene). Chem. Rev. 2004, 104, 5265–5288. [Google Scholar] [CrossRef]
- Kobayashi, T.; Taniguchi, H.; Ohnuma, A.; Kawamoto, A. Unconventional superconductivity in λ-(BETS)2GaCl4 probed by 13C NMR. Phys. Rev. B 2020, 102, 121106. [Google Scholar] [CrossRef]
- Kobayashi, H.; Kobayashi, A.; Tajima, H. Studies on molecular conductors: From organic semiconductors to molecular metals and superconductors. Chem. Asian J. 2011, 6, 1688–1704. [Google Scholar] [CrossRef]
- Fujiwara, E.; Yamamoto, K.; Shimamura, M.; Zhou, B.; Kobayashi, A.; Takahashi, K.; Okano, Y.; Cui, H.; Kobayashi, H. (nBu4N)[Ni(dmstfdt)2]: A planar nickel coordination complex with an extended-TTF ligand exhibiting metallic conduction, metal-insulator transition, and weak ferromagnetism. Chem. Mater. 2007, 19, 553–558. [Google Scholar] [CrossRef]
- Wang, M.; Yu, T.; Tan, L.-S.; Urbas, A.M.; Chiang, L.Y. Enhancement of photoswitchable dielectric property by conducting electron donors on plasmonic core-shell gold-fluorenyl C60 nanoparticles. J. Phys. Chem. C 2018, 122, 12512–12523. [Google Scholar] [CrossRef]
- Misaki, Y.; Tani, Y.; Taniguchi, M.; Maitani, T.; Tanaka, K.; Bechgaard, K. Preparation and properties of gold complexes with TTF dithiolato ligands. Mol. Cryst. Liq. Cryst. 2000, 343, 59–64. [Google Scholar] [CrossRef]
- Ashizawa, M.; Akutsu, A.; Noda, B.; Nii, H.; Kawamoto, T.; Mori, T.; Nakayashiki, T.; Misaki, Y.; Tanaka, K.; Takimiya, K.; et al. Synthesis and structures of highly conducting charge-transfer salts of selenium containing TTM-TTP derivatives. Bull. Chem. Soc. Jpn. 2004, 77, 1449–1458. [Google Scholar] [CrossRef]
- Ashizawa, M.; Nakao, A.; Yamamoto, H.M.; Kato, R. Development of the first methyl antimony bridged tetrachalcogenafulvalene systems. J. Low Temp. Phys. 2006, 142, 449–452. [Google Scholar] [CrossRef]
- Mori, T. Organic conductors with unusual band fillings. Chem. Rev. 2004, 104, 4947–4969. [Google Scholar] [CrossRef]
- Yamada, J.-i.; Akutsu, H. New trends in the synthesis of π-electron donors for molecular conductors and superconductors. Chem. Rev. 2004, 104, 5057–5083. [Google Scholar] [CrossRef]
- Fabre, J.M. Synthesis strategies and chemistry of nonsymmetrically substituted tetrachalcogenafulvalenes. Chem. Rev. 2004, 104, 5133–5150. [Google Scholar] [CrossRef]
- Ohki, D.; Yoshimi, K.; Kobayashi, A. Interaction-induced quantum spin Hall insulator in the organic Dirac electron system α-(BEDT-TSeF)2I3. Phys. Rev. B 2022, 105, 205123. [Google Scholar] [CrossRef]
- Naito, T.; Doi, R. Band structure and physical properties of α-STF2I3: Dirac electrons in disordered conduction sheets. Crystals 2020, 10, 270. [Google Scholar] [CrossRef] [Green Version]
- Schukat, G.; Fanghänel, E. Synthesis, reactions, and selected physico-chemical properties of 1,3- and 1,2-tetrachalcogenafulvalenes. Sulfur Rep. 2003, 24, 1–190. [Google Scholar] [CrossRef]
- Potapov, V.A.; Amosova, S.V.; Doron’kina, I.V. Reaction of selenium with acetylene: Synthesis of 1,4-diselenafulvene. Russ. Chem. Bull. 2008, 57, 684–685. [Google Scholar] [CrossRef]
- Bortolatto, C.F.; Wilhelm, E.A.; Roman, S.S.; Nogueira, C.W. (E)-2-Benzylidene-4-phenyl-1,3-diselenole ameliorates signals of renal injury induced by cisplatin in rats. J. Appl. Toxicol. 2014, 34, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, E.A.; Jesse, C.R.; Bortolatto, C.F.; Nogueira, C.W. (E)-2-benzylidene-4-phenyl-1,3-diselenole has antioxidant and hepatoprotective properties against oxidative damage induced by 2-nitropropane in rats. Fundam. Clin. Pharmacol. 2011, 25, 80–90. [Google Scholar] [CrossRef]
- Wilhelm, E.A.; Jesse, C.R.; Roman, S.S.; Bortolatto, C.F.; Nogueira, C.W. Anticonvulsant effect of (E)-2-benzylidene-4-phenyl-1,3-diselenole in a pilocarpine model in mice. Life Sci. 2010, 87, 620–627. [Google Scholar] [CrossRef]
- Pietschnig, R.; Merz, K.; Schäfer, S. Synthesis, charge distribution, and dimerization behavior of lithium alkynylselenolates. Heteroat. Chem. 2005, 16, 169–174. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, C.; Ma, Y.; Zhang, J.; Sun, D.; Chen, L.; Huang, L.; Wu, G. Copper-catalyzed decarboxylative alkylselenation of propiolic acids with Se powder and epoxides. Adv. Synth. Catal. 2021, 363, 1930–1934. [Google Scholar] [CrossRef]
- Potapov, V.A.; Amosova, S.V.; Kashik, A.S. Reactions of selenium and tellurium metals with phenylacetylene in three-phase catalytical systems. Tetrahedron Lett. 1989, 30, 613–616. [Google Scholar] [CrossRef]
- Amosova, S.V.; Elokhina, V.N.; Nakhmanovich, A.S.; Larina, L.I.; Martynov, A.V.; Steele, B.R.; Potapov, V.A. Reactions of selenourea with benzoyl- and 2-thienoylbromoacetylenes: Synthesis of 1,3-diselenetanes and 1,4-diselenafulvenes. Tetrahedron Lett. 2008, 49, 974–976. [Google Scholar] [CrossRef]
- Okuma, K.; Kubota, T. Novel formation of 1,2,4-triselenolanes by the reaction of tert-butylarylmethylenetriphenylphosphoranes with elemental selenium. Tetrahedron Lett. 2001, 42, 3881–3883. [Google Scholar] [CrossRef]
- Rajagopal, D.; Lakshmikantham, M.V.; Cava, M.P.; Broker, G.A.; Rogers, R.D. Synthesis and transformations of some new 2,4-bismethylene-1,3-ditelluretanes. Tetrahedron Lett. 2003, 44, 2397–2400. [Google Scholar] [CrossRef]
- Carland, M.W.; Schiesser, C.H.; White, J.M. Ethyl 2-(2-ethoxy-2-oxoethylidene)-1,3-diselenole-4-carboxylate—an example of unconventional bonding in organic selenides. Aust. J. Chem. 2004, 57, 97–100. [Google Scholar] [CrossRef]
- Nakayama, J.; Kitahara, T.; Sugihara, Y.; Sakamoto, A.; Ishii, A. Isolable, stable diselenocarboxylate and selenothiocarboxylate salts: Syntheses, structures, and reactivities of 2-(1,3-dimethylimidazolidinio)diselenocarboxylate and 2-(1,3-dimethylimidazolidinio)selenothiocarboxylate. J. Am. Chem. Soc. 2000, 122, 9120–9126. [Google Scholar] [CrossRef]
- Lyapunova, A.G.; Petrov, M.L.; Androsov, D.A. A novel synthesis of benzo[b]selenophenes via regioselective intramolecular transformation of 4-(3-nitroaryl)-1,2,3-selenadiazoles. Org. Lett. 2013, 15, 1744–1747. [Google Scholar] [CrossRef]
- Yekhlef, M.; Petrov, M.L.; Pevzner, L.M.; Ponyaev, A.I. Synthesis of 2-naphthylacetylene selenides from 4-(2-naphthyl)-1,2,3-selenadiazole. Russ. J. Gen. Chem. 2018, 88, 151–153. [Google Scholar] [CrossRef]
- Yekhlef, M.; Petrov, M.L.; Pevzner, L.M.; Ponyaev, A.I. Synthesis of 2-naphthylselenoacetic acid dialkylamides from 4-(2-naphthyl)-1,2,3-selenadiazole. Russ. J. Gen. Chem. 2020, 90, 2197–2199. [Google Scholar] [CrossRef]
- Shirahata, T.; Imakubo, T. Synthesis of novel selenium-containing donors as selenium analogues of diiodo(ethylenedithio)diselenadithiafulvalene (DIETS). Org. Biomol. Chem. 2004, 2, 1685–1687. [Google Scholar] [CrossRef]
- Iyoda, M.; Watanabe, R.; Miyake, Y. Anomalous ring cleavage of 1,3-dithiole- and 1,3-diselenole-2-thiones under the cross-coupling conditions using triethyl phosphite. Chem. Lett. 2004, 33, 570–571. [Google Scholar] [CrossRef]
- Wang, G.; Bian, X.; Shen, L.; Liu, X.; Xiao, X. Synthesis, structure and redox property of a new bent tetrathiafulvalene-based donor molecule. Chin. J. Org. Chem. 2011, 31, 2156–2160. [Google Scholar]
- Takimiya, K.; Kataoka, Y.; Nakamura, Y.; Aso, Y.; Otsubo, T. Molecular modifications of methylenedithio-tetraselenafulvalene (MDT-TSF) and methylenedithio-diselenadithiafulvalene (MDT-ST) for superior electron donors. Synthesis 2004, 2004, 1315–1320. [Google Scholar] [CrossRef]
- Naito, T.; Kobayashi, H.; Kobayashi, A. The electrical behavior of charge-transfer salts based on an unsymmetrical donor bis(ethylenedithio)diselenadithiafulvalene (STF): Disorder effect on the transport properties. Bull. Chem. Soc. Jpn. 1997, 70, 107–114. [Google Scholar] [CrossRef]
- Naito, T.; Doi, R.; Suzumura, Y. Exotic Dirac cones on the band structure of α-STF2I3 at ambient temperature and pressure. J. Phys. Soc. Jpn. 2020, 89, 023701. [Google Scholar] [CrossRef]
- Kobara, R.; Igarashi, S.; Kawasugi, Y.; Doi, R.; Naito, T.; Tamura, M.; Kato, R.; Nishio, Y.; Kajita, K.; Tajima, N. Universal behavior of magnetoresistance in organic Dirac electron systems. J. Phys. Soc. Jpn. 2020, 89, 113703. [Google Scholar] [CrossRef]
- Hiraki, K.-I.; Harada, S.; Arai, K.; Takano, Y.; Takahashi, T.; Tajima, N.; Kato, R.; Naito, T. Local spin susceptibility of α-D2I3 (D = bis(ethylendithio)tetraselenafulvalene (BETS) and bis(ethylendithio)dithiadiselenafulvalene (BEDT-STF)) studied by 77Se NMR. J. Phys. Soc. Jpn. 2011, 80, 014715. [Google Scholar] [CrossRef]
- Naito, T.; Suzumura, Y. Theoretical model for a novel electronic state in a Dirac electron system close to merging: An imaginary element between sulphur and selenium. Crystals 2022, 12, 346. [Google Scholar] [CrossRef]
- Nakajima, H.; Katsuhara, M.; Ashizawa, M.; Kawamoto, T.; Mori, T. Ferromagnetic anomaly associated with the antiferromagnetic transitions in (donor)[Ni(mnt)2]-type charge-transfer salts. Inorg. Chem. 2004, 43, 6075–6082. [Google Scholar] [CrossRef]
- Takimiya, K.; Nakamura, Y.; Aso, Y.; Otsubo, T. Two isomeric methylenediseleno-diselenadithiafulvalenes (MDSE-TS and MDSE-ST): Synthesis and properties of new selenium-containing fulvalene-type electron donors. In Multifunctional Conducting Molecular Materials. Special Publication; Saito, G., Wudl, F., Haddon, R.C., Tanigaki, K., Enoki, T., Katz, H.E., Maesato, M., Eds.; Royal Society of Chemistry: London, UK, 2007; Volume 306, pp. 23–30. [Google Scholar]
- Fujiwara, E.; Kobayashi, A.; Okano, Y.; Kobayashi, H.; Fujishiro, Y.; Nishibori, E.; Takata, M.; Sakata, M. New single-component molecular conductors with diselenadithiafulvalene framework. In Multifunctional Conducting Molecular Materials. Special Publication; Saito, G., Wudl, F., Haddon, R.C., Tanigaki, K., Enoki, T., Katz, H.E., Maesato, M., Eds.; Royal Society of Chemistry: London, UK, 2007; Volume 306, pp. 45–48. [Google Scholar]
- Fujiwara, E.; Zhou, B.; Kobayashi, A.; Kobayashi, H.; Fujishiro, Y.; Nishibori, E.; Sakata, M.; Ishibashi, S.; Terakura, K. Structures and physical properties of highly conducting single-component molecular conductors containing Se atoms. Eur. J. Inorg. Chem. 2009, 2009, 1585–1591. [Google Scholar] [CrossRef]
- Anyfantis, G.C.; Papavassiliou, G.C.; Aloukos, P.; Couris, S.; Weng, Y.F.; Yoshino, H.; Murata, K. Unsymmetrical single-component nickel 1,2-dithiolene complexes with extended tetrachalcogenafulvalenedithiolato ligands. Z. Naturforsch. 2007, 62b, 200–204. [Google Scholar] [CrossRef]
- Naito, T.; Karasudani, T.; Mori, S.; Ohara, K.; Konishi, K.; Takano, T.; Takahashi, Y.; Inabe, T.; Nishihara, S.; Inoue, K. Molecular photoconductor with simultaneously photocontrollable localized spins. J. Am. Chem. Soc. 2012, 134, 18656–18666. [Google Scholar] [CrossRef]
- Naito, T.; Karasudani, T.; Ohara, K.; Takano, T.; Takahashi, Y.; Inabe, T.; Furukawa, K.; Nakamura, T. Simultaneous control of carriers and localized spins with light in organic materials. Adv. Mater. 2012, 24, 6153–6157. [Google Scholar] [CrossRef]
- Kimura, T. Preparation and electrochemical property of octaethylphthalocyanine fused with four diselenadithiafulvalene units. Heterocycles 2014, 88, 207–212. [Google Scholar] [CrossRef]
- Imakubo, T.; Iijima, T.; Kobayashi, K.; Kato, R. A new synthetic route to TTF-vinylogues. Synth. Met. 2001, 120, 899–900. [Google Scholar] [CrossRef]
- Ichikawa, S.; Takahashi, K.; Mori, H.; Yamaura, J.-i. Syntheses, crystal structures, and physical properties of copper complexes with dimethylthio-pyrazino-selenathiafulvalene (=Dmt-Pyra-STF) as ligand: Trans-[CuCl2(Dmt-Pyra-STF)2] and [Cu2Br2.5(Dmt-Pyra-STF)]. Solid State Sci. 2008, 10, 1724–1728. [Google Scholar] [CrossRef]
- Yasuda, M.; Fujiwara, E.; Aonuma, S.; Fujiwara, H.; Sugimoto, T.; Nakayashiki, T.; Tanaka, K.; Takahashi, K.; Kobayashi, H.; Misaki, Y. Structures and electrical properties of (BTM-TS-TTP)4PF6. Bull. Chem. Soc. Jpn. 2011, 84, 79–81. [Google Scholar] [CrossRef]
- Kamo, H.; Ueda, A.; Isono, T.; Takahashi, K.; Mori, H. Synthesis and properties of catechol-fused tetrathiafulvalene derivatives and their hydrogen-bonded conductive charge-transfer salts. Tetrahedron Lett. 2012, 53, 4385–4388. [Google Scholar] [CrossRef]
- Ashizawa, M.; Nii, H.; Kawamoto, T.; Mori, T.; Misaki, Y.; Tanaka, K.; Takimiya, K.; Otsubo, T. Synthesis and properties of selenium containing TTM-TTP conductors. Synth. Met. 2003, 135–136, 627–628. [Google Scholar] [CrossRef]
- Ashizawa, M.; Nii, H.; Mori, T.; Misaki, Y.; Tanaka, K.; Takimiya, K.; Otsubo, T. Synthesis and structures of neutral crystals and charge-transfer salts of selenium containing TMET-TTP derivatives. Bull. Chem. Soc. Jpn. 2003, 76, 2091–2097. [Google Scholar] [CrossRef]
- Bando, Y.; Ashizawa, M.; Kawamoto, T.; Mori, T. Flat resistivity in θ-phase charge-transfer salts of selenium-containing TMET-TTP derivatives. Bull. Chem. Soc. Jpn. 2008, 81, 947–955. [Google Scholar] [CrossRef]
- Shirahata, T.; Mori, T.; Kato, R.; Takahashi, K. Synthesis, crystal structures and electrical properties of cation radical salts of selenium-containing π-extended donors, BEDT-HBDST and BEDT-HSTT. Synth. Met. 2003, 133–134, 321–324. [Google Scholar] [CrossRef]
- Imakubo, T.; Shirahata, T. CSe2-free synthesis of [1,3]diselenole-2-thione and its application to syntheses of iodinated tetraselenafulvalenes (TSeFs). Chem. Commun. 2003, 2003, 1940–1941. [Google Scholar] [CrossRef]
- Ueda, K.; Sugimoto, T.; Faulmann, C.; Cassoux, P. Synthesis, crystal structure, and electrical properties of novel radical cation salts of iodine-substituted TTF-derived donors with the nitroprusside anion exhibiting strong -I···NC- interactions. Eur. J. Inorg. Chem. 2003, 2003, 2333–2338. [Google Scholar] [CrossRef]
- Suizu, R.; Imakubo, T. Synthesis and properties of hetero-halogenated TTFs. Org. Biomol. Chem. 2003, 1, 3629–3631. [Google Scholar] [CrossRef] [PubMed]
- Alberola, A.; Collis, R.J.; García, F.; Howard, R.E. Efficient synthesis of brominated tetrathiafulvalene (TTF) derivatives: Solid-state structure and electrochemical behaviour. Tetrahedron 2006, 62, 8152–8157. [Google Scholar] [CrossRef]
- Takimiya, K.; Jeon, H.J.; Otsubo, T. A practical two-step synthesis of tetraselenafulvalene (TSF). Synthesis 2005, 2005, 2810–2813. [Google Scholar] [CrossRef]
- Zorina, L.V.; Simonov, S.V.; Khasanov, S.S.; Shibaeva, R.P.; Suslikova, I.Y.; Kushch, L.A.; Yagubskii, E.B. Crystal structure of the new organic conductor (TSeF)7[FeNO(CN)5]2 with unusual molecular packing of conducting layer. Crystallogr. Rep. 2011, 56, 1042–1046. [Google Scholar] [CrossRef]
- Christensen, J.B.; Bechgaard, K.; Paquignon, G. Synthesis of 13C-labelled tetramethyltetraselenafulvalene. J. Label. Comp. Radiopharm. 2001, 44, 1035–1041. [Google Scholar] [CrossRef]
- Kimura, Y.; Misawa, M.; Kawamoto, A. Correlation between non-Fermi-liquid behavior and antiferromagnetic fluctuations in (TMTSF)2PF6 observed using 13C-NMR spectroscopy. Phys. Rev. B 2011, 84, 045123. [Google Scholar] [CrossRef]
- Saito, G.; Yoshida, Y.; Murofushi, H.; Iwasawa, N.; Hiramatsu, T.; Otsuka, A.; Yamochi, H.; Isa, K.; Mineo-Ota, E.; Konno, M.; et al. Preparation, structures, and physical properties of tetrakis(alkylthio)tetraselenafulvalene (TTCn-TSeF, n = 1 15). Bull. Chem. Soc. Jpn. 2010, 83, 335–344. [Google Scholar] [CrossRef]
- Rajagopal, D.; Lakshmikantham, M.V.; Cava, M. 4-Phenyl-2-methylene-1,3-diselenole: Synthesis and reactions. Sulfur Lett. 2002, 25, 129–133. [Google Scholar] [CrossRef]
- Fabian, J.; Krebs, A.; Schönemann, D.; Schaefer, W. 1,3-Heterocumulene-to-alkyne [3 + 2] cycloaddition reactions: A theoretical and experimental study. J. Org. Chem. 2000, 65, 8940–8947. [Google Scholar] [CrossRef]
- Imakubo, T.; Shirahata, T.; Kibune, M. Synthesis, crystal structure and electrochemical properties of bis(ethylenedioxy)tetraselenafulvalene (BEDO-TSeF). Chem. Commun. 2004, 2004, 1590–1591. [Google Scholar] [CrossRef] [PubMed]
- Takimiya, K.; Kodani, M.; Murakami, S.; Otsubo, T.; Aso, Y. Synthesis and properties of ethylenethiotetraselenafulvalene (ET-TSF) and its conductive radical cation salts. Heterocycles 2006, 67, 655–663. [Google Scholar] [CrossRef]
- Kodani, M.; Murakami, S.; Jigami, T.; Takimiya, K.; Aso, Y.; Otsubo, T. Bis(ethylenethio)tetraselenafulvalene and related hybrid diselenadithiafulvalenes as novel electron donors forming highly conductive complexes with 7,7,8,8-tetracyanoquinodimethane. Heterocycles 2001, 54, 225–235. [Google Scholar] [CrossRef]
- Takimiya, K.; Kataoka, Y.; Niihara, N.; Aso, Y.; Otsubo, T. A general method for the synthesis of alkylenedithio- and bis(alkylenedithio)tetraselenafulvalenes. J. Org. Chem. 2003, 68, 5217–5224. [Google Scholar] [CrossRef] [PubMed]
- Ueda, A.; Kamo, H.; Mori, H. Unexpected formation of ortho-benzoquinone-fused tetraselenafulvalene (TSF): Synthesis, structures, and properties of a novel TSF-based donor acceptor dyad. Chem. Lett. 2015, 44, 1538–1540. [Google Scholar] [CrossRef]
- Komatsu, H.; Mogi, R.; Matsushita, M.M.; Miyagi, T.; Kawada, Y.; Sugawara, T. Synthesis and properties of TSF-based spin-polarized donor. Polyhedron 2009, 28, 1996–2000. [Google Scholar] [CrossRef]
- Jigami, T.; Kodani, M.; Murakami, S.; Takimiya, K.; Aso, Y.; Otsubo, T. Synthesis and properties of novel heterocycle-fused TTF-type electron donors: Bis(propylenethio)tetrathiafulvalene (BPT-TTF), bis(propyleneseleno)tetrathiafulvalene (BPS-TTF), and their tetraselenafulvalene analogues (BPT-TSF and BPS-TSF). J. Mater. Chem. 2001, 11, 1026–1033. [Google Scholar] [CrossRef]
- Elsherbini, M.; Hamama, W.S.; Zoorob, H.H. Recent advances in the chemistry of selenium-containing heterocycles: Five-membered ring systems. Coord. Chem. Rev. 2016, 312, 149–177. [Google Scholar] [CrossRef]
- Takimiya, K.; Jigami, T.; Kawashima, M.; Kodani, M.; Aso, Y.; Otsubo, T. Synthetic procedure for various selenium-containing electron donors of the bis(ethylenedithio)tetrathiafulvalene (BEDT-TTF) type. J. Org. Chem. 2002, 67, 4218–4227. [Google Scholar] [CrossRef]
- Kodani, M.; Takimiya, K.; Aso, Y.; Otsubo, T.; Nakayashiki, T.; Misaki, Y. Effective synthesis of 1,3-diselenole-2-selone-4,5-diselenolate (dsis) and its utilization for the synthesis of selenocycle-fused tetraselenafulvalene (TSF) derivatives. Synthesis 2001, 2001, 1614–1618. [Google Scholar] [CrossRef]
- Ashizawa, M.; Ishidzu, K.; Watanabe, M.; Tanahashi, T.; Shirahata, T.; Kawamoto, T.; Mori, T.; Misaki, Y. Novel bis-fused π-electron donor composed of tetrathiafulvalene and tetraselenafulvalene. Chem. Lett. 2010, 39, 1093–1095. [Google Scholar] [CrossRef]
- Ishizu, K.; Watanabe, M.; Tanahashi, T.; Misaki, Y.; Ashizawa, M.; Mori, T. Synthesis and properties of new TTP donors composed of TTF and TSF moieties. J. Phys. Conf. Ser. 2008, 132, 012021. [Google Scholar] [CrossRef]
- Amaresh, R.R.; Liu, D.; Konovalova, T.; Lakshmikantham, M.V.; Cava, M.P.; Kispert, L.D. Dendralene-type TTF vinylogs containing a 1,3-diselenole ring. J. Org. Chem. 2001, 66, 7757–7764. [Google Scholar] [CrossRef] [PubMed]
- Naka, K.; Uemura, T.; Chujo, Y. π-Conjugated poly(dithiafulvene)s and poly(diselenafulvene)s: Effects of side alkyl chains on optical, electrochemical, and conducting properties. Macromolecules 2002, 35, 3539–3543. [Google Scholar] [CrossRef]
- Takimiya, K.; Oharuda, A.; Morikami, A.; Aso, Y.; Otsubo, T. Improved synthesis of double-bridged tetraselenafulvalenophanes and formation of their conductive radical cation salts. Eur. J. Org. Chem. 2000, 2000, 3013–3039. [Google Scholar] [CrossRef]
- Walwyn, R.J.; Chan, B.; Usov, P.M.; Solomon, M.B.; Duyker, S.G.; Koo, J.Y.; Kawano, M.; Turner, P.; Kepert, C.J.; D’Alessandro, D.M. Spectroscopic, electronic and computational properties of a mixed tetrachalcogenafulvalene and its charge transfer complex. J. Mater. Chem. C 2018, 6, 1092–1104. [Google Scholar] [CrossRef]
- Morikami, A.; Takimiya, K.; Aso, Y.; Otsubo, T. Novel tellurium containing fulvalene-type electron donors, triselenatellurafulvalene (TSTeF) and diselenaditellurafulvalene (DSDTeF); synthesis, conductivities and crystal structures of their TCNQ complexes. J. Mater. Chem. 2001, 11, 2431–2436. [Google Scholar] [CrossRef]
- Torubaev, Y.; Mathur, P.; Jha, B.; Mobin, S.M.; Skabitsky, I.V. Cyclodimerization of phenyliodoacetylene with elemental tellurium: New pathway to 1.3-ditellurofulvenes. J. Organomet. Chem. 2011, 696, 496–503. [Google Scholar] [CrossRef]
- Gadzhieva, P.I.; Sadekov, I.D. Synthesis of 2-substituted benzo-1,3-ditelluroles. Russ. Chem. Bull. 2000, 49, 1127–1129. [Google Scholar] [CrossRef]
- Rajagopal, D.; Lakshmikantham, M.V.; Cava, M.P. Synthesis of the first 1,3-ditellurole-containing radialene-type TTF derivatives. Org. Lett. 2002, 4, 2581–2583. [Google Scholar] [CrossRef]
- Herr, D.E.; Mays, M.D.; McCullough, R.D.; Bailey, A.B.; Cowan, D.O. Optimizing the synthesis of tetratellurafulvalene. J. Org. Chem. 1996, 61, 7006–7011. [Google Scholar] [CrossRef] [PubMed]
- Kazheva, O.N.; Chekhlov, A.N.; Alexandrov, G.G.; Buravov, L.I.; Kravchenko, A.V.; Starodub, V.A.; Sivaev, I.B.; Bregadze, V.I.; Dyachenko, O.A. Synthesis, structure and electrical conductivity of fulvalenium salts of cobalt bis(dicarbollide) anion. J. Organomet. Chem. 2006, 691, 4225–4233. [Google Scholar] [CrossRef]
- Kazheva, O.N.; Alexandrov, G.G.; Kravchenko, A.V.; Starodub, V.A.; Sivaev, I.B.; Lobanova, I.A.; Bregadze, V.I.; Buravov, L.I.; Dyachenko, O.A. New fulvalenium salts of bis(dicarbollide) cobalt and iron: Synthesis, crystal structure and electrical conductivity. J. Organomet. Chem. 2007, 692, 5033–5043. [Google Scholar] [CrossRef]
- Reinheimer, E.W.; Galán-Mascarós, J.R.; Gómez-García, C.J.; Zhao, H.; Fourmigué, M.; Dunbar, K.R. Radical salts of TTF derivatives with the metal—Metal bonded [Re2Cl8]2− anion. J. Mol. Struct. 2008, 890, 81–89. [Google Scholar] [CrossRef]
- Lee, I.J.; Naughton, M.J.; Chaikin, P.M. Exceeding the Pauli limit in (TMTSF)2PF6. Phys. B 2001, 294–295, 413–417. [Google Scholar] [CrossRef]
- Lee, I.J. Mixed phase of superconductor and spin density wave insulator in a quasi-one-dimensional system (TMTSF)2PF6: Revisited. J. Korean Phys. Soc. 2005, 47, 380–384. [Google Scholar]
- Haddad, S.; Charfi-Kaddour, S.; Héritier, M.; Bennaceur, R. Superconductivity, spin density wave and field induced spin density wave versus anion ordering in the (TMTSF)2ClO4 salt. J. Low Temp. Phys. 2006, 142, 461–464. [Google Scholar] [CrossRef]
- Nakano, M.; Alves, H.; Molinari, A.S.; Ono, S.; Minder, N.; Morpurgo, A.F. Small gap semiconducting organic charge-transfer interfaces. Appl. Phys. Lett. 2010, 96, 232102. [Google Scholar] [CrossRef]
- Oza, A.T.; Solanki, G.K.; Ray, A.; Amin, A. Mechanism of conduction in TTF and TMTSF organic conductors from a study of their vibrational spectra. Indian J. Phys. 2010, 84, 1527–1540. [Google Scholar] [CrossRef]
- Saito, G.; Ikegami, H.; Yoshida, Y.; Drozdova, O.O.; Nishimura, K.; Horiuchi, S.; Yamochi, H.; Otsuka, A.; Hiramatsu, T.; Maesato, M.; et al. Ionicity phase diagram of trifluoromethyl-TCNQ (CF3TCNQ) charge-transfer solids. Bull. Chem. Soc. Jpn. 2010, 83, 1462–1480. [Google Scholar] [CrossRef]
- Brown, J.T.; Zeller, M.; Rosokha, S.V. Effects of structural variations on π-dimer formation: Long-distance multicenter bonding of cation-radicals of tetrathiafulvalene analogues. Phys. Chem. Chem. Phys. 2020, 22, 25054. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, J.O.; Kostesha, N.; Johnsen, C.; Nielsen, K.A.; Boisen, A.; Jakobsen, M.H. Multisensor Array for Detection of Analytes or Mixtures Thereof in Gas or Liquid. Phase. Patent WO 2011121077 A1, 6 October 2011. [Google Scholar]
- Malfanta, I.; Courcet, T.; Faulmann, C.; Cassoux, P.; Gornitzka, H.; Granier, F.; Doublet, M.-L.; Guionneau, P.; Howard, J.A.K.; Kushch, N.D.; et al. Structure and properties of BETS salts: κ-(BETS)8(Cu2Cl6)(CuCl4), θ-(BETS)2(CuCl2) and (BETS)2(CuCl4). Comptes Rendus Académie Sci.-Ser. IIC-Chem. 2001, 4, 149–160. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.; Wang, Z.; Gao, S.; Guo, Y.; Liue, F.; Zhua, D. BETS3[Cu2(C2O4)3](CH3OH)2: An organic–inorganic hybrid antiferromagnetic metal (BETS = bisethylene(tetraselenfulvalene)). Cryst. Eng. Comm. 2013, 15, 3529–3535. [Google Scholar] [CrossRef]
- Zhilyaeva, E.I.; Torunova, S.A.; Shilov, G.V.; Flakina, A.M.; Yudanova, E.I.; Lyubovskaya, R.N. Tetrathiafulvalene-based organic conductors with Pb-containing anions. Russ. Chem. Bull. 2017, 66, 986–990. [Google Scholar] [CrossRef]
- Sanchez, M.-E.; Doublet, M.-L.; Faulmann, C.; Malfant, I.; Cassoux, P.; Kushch, L.A.; Yagubskii, E.B. Anion conformation and physical properties in BETS salts with the nitroprusside anion and its related ruthenium halide (X = Cl, Br) mononitrosyl complexes: θ-(BETS)4[Fe(CN)5NO], (BETS)2[RuBr5NO], and (BETS)2[RuCl5NO]. Eur. J. Inorg. Chem. 2001, 2001, 2797–2804. [Google Scholar] [CrossRef]
- Coronado, E.; Curreli, S.; Giménez-Saiz, C.; Gómez-García, C.J.; Alberola, A.; Canadell, E. Molecular conductors based on the mixed-valence polyoxometalates [SMo12O40]n− (n = 3 and 4) and the organic donors bis(ethylenedithio)tetrathiafulvalene and bis(ethylenedithio)tetraselenafulvalene. Inorg. Chem. 2009, 48, 11314–11324. [Google Scholar] [CrossRef]
- Alberola, A.; Coronado, E.; Galán-Mascarós, J.R.; Giménez-Saiz, C.; Gómez-García, C.J. A molecular metal ferromagnet from the organic donor bis(ethylenedithio)tetraselenafulvalene and bimetallic oxalate complexes. J. Am. Chem. Soc. 2003, 125, 10774–10775. [Google Scholar] [CrossRef]
- Zhilyaeva, E.I.; Bogdanova, O.A.; Shilov, G.V.; Lyubovskii, R.B.; Pesotskii, S.I.; Aldoshin, S.M.; Kobayashi, A.; Kobayashi, H.; Lyubovskaya, R.N. Two-dimensional organic metals θ-(BETS)4MBr4(PhBr), M = Cd, Hg with differently oriented conducting layers. Synth. Met. 2009, 159, 1072–1076. [Google Scholar] [CrossRef]
- Gritsenko, V.; Tanaka, H.; Kobayashi, H.; Kobayashi, A. A new molecular superconductor, κ-(BETS)2TlCl4[BETS = bis(ethylenedithio)tetraselenafulvalene]. J. Mater. Chem. 2001, 11, 2410–2411. [Google Scholar] [CrossRef]
- Vlasova, R.M.; Drichko, N.V.; Petrov, B.V.; Semkin, V.N.; Zhilyaeva, E.I.; Lyubovskaya, R.N.; Olejniczak, I.; Kobayashi, A.; Kobayashi, H. Optical properties of new organic conductors based on the BEDT-TSeF molecule (the κ-(BETS)4Hg2.84Br8 superconductor and κ-(BETS)4Hg3Cl8 metal) in the range 300–15 K. Phys. Solid State 2004, 46, 1985–1993. [Google Scholar] [CrossRef]
- Uji, S.; Terashima, T.; Terai, Y.; Yasuzuka, S.; Tokumoto, M.; Tanaka, H.; Kobayashi, A.; Kobayashi, H. Superconductivity and vortex phases in the two-dimensional organic conductor λ-(BETS)2FexGa1−x Cl4 (x = 0.45). Phys. Rev. B 2005, 71, 104525. [Google Scholar] [CrossRef]
- Uji, S.; Yasuzuka, S.; Tokumoto, M.; Tanaka, H.; Kobayashi, A.; Zhang, B.; Kobayashi, H.; Choi, E.S.; Graf, D.; Brooks, J.S. Magnetic-field-induced superconductivity and phase diagrams of λ-(BETS)2FeCl4−xBrx. Phys. Rev. B 2005, 72, 184505. [Google Scholar] [CrossRef]
- Dubrovskii, A.D.; Spitsina, N.G.; Buravov, L.I.; Chekhlov, A.N.; Dyachenko, O.A.; Okano, Y.; Kobayashi, A.; Pesotskii, S.I.; Lyubovskii, R.B. New BETS salt with the square planar [Ni(CN)4]2− anion. Synth. Met. 2005, 148, 213–218. [Google Scholar] [CrossRef]
- Vyaselev, O.M.; Kushch, N.D.; Yagubskii, E.B. Proton NMR study of the organic metal κ-(BETS)2Mn[N(CN)2]3. J. Exp. Theor. Phys. 2011, 113, 835–841. [Google Scholar] [CrossRef]
- Shirahata, T.; Kibune, M.; Yoshino, H.; Imakubo, T. Ambient-pressure organic superconductors κ-(DMEDO-TSeF)2[Au(CN)4](solv.): Tc Tuning by modification of the solvent of crystallization. Chem. Eur. J. 2007, 13, 7619–7630. [Google Scholar] [CrossRef]
- Kawamoto, T.; Mori, T.; Shirahata, T.; Imakubo, T. Two-dimensional superconducting properties of the organic superconductor κL-(DMEDO-TSeF)2[Au(CN)4](THF) with domain structures. J. Phys. Soc. Japan 2011, 80, 054706. [Google Scholar] [CrossRef]
- Shirahata, T.; Kibune, M.; Imakubo, T. New unsymmetrical donor dimethyl(ethylenedioxy)tetraselenafulvalene (DMEDO-TSeF): Structures and properties of its cation radical salts. J. Mater. Chem. 2005, 15, 4399–4402. [Google Scholar] [CrossRef]
- Fujii, K.; Shimizu, A. Graphene Thin Films with High Electric Conductivity and Method for Their Manufacture by Low-Temperature Coating. Process. Patent JP 2011063492 A, 31 March 2011. [Google Scholar]
- Kamesaki, H.; Horiuchi, T.; Torii, M. Functional Devices Using Charge-Transfer Complexes, and Their. Manufacture. Patent JP 2014007053 A, 16 January 2014. [Google Scholar]
- Otsu, S.; Suzurizato, Y.; Kita, H. Organic Electroluminescent Devices for Display Devices and Lighting. Devices. Patent JP 2006041020 A, 9 February 2006. [Google Scholar]
- Pac, S.-S.; Saito, G. Peculiarity of hexamethylenetetratellurafulvalene (HMTTeF) charge transfer complexes of donor-acceptor (D-A) Type. J. Solid State Chem. 2002, 168, 486–496. [Google Scholar] [CrossRef]
- Saito, G.; Pac, S.-S.; Drozdova, O.O. First metallic DA alternating charge transfer complex: (HMTTeF)(Et2TCNQ)(THF)x. Synth. Met. 2001, 120, 667–679. [Google Scholar] [CrossRef]





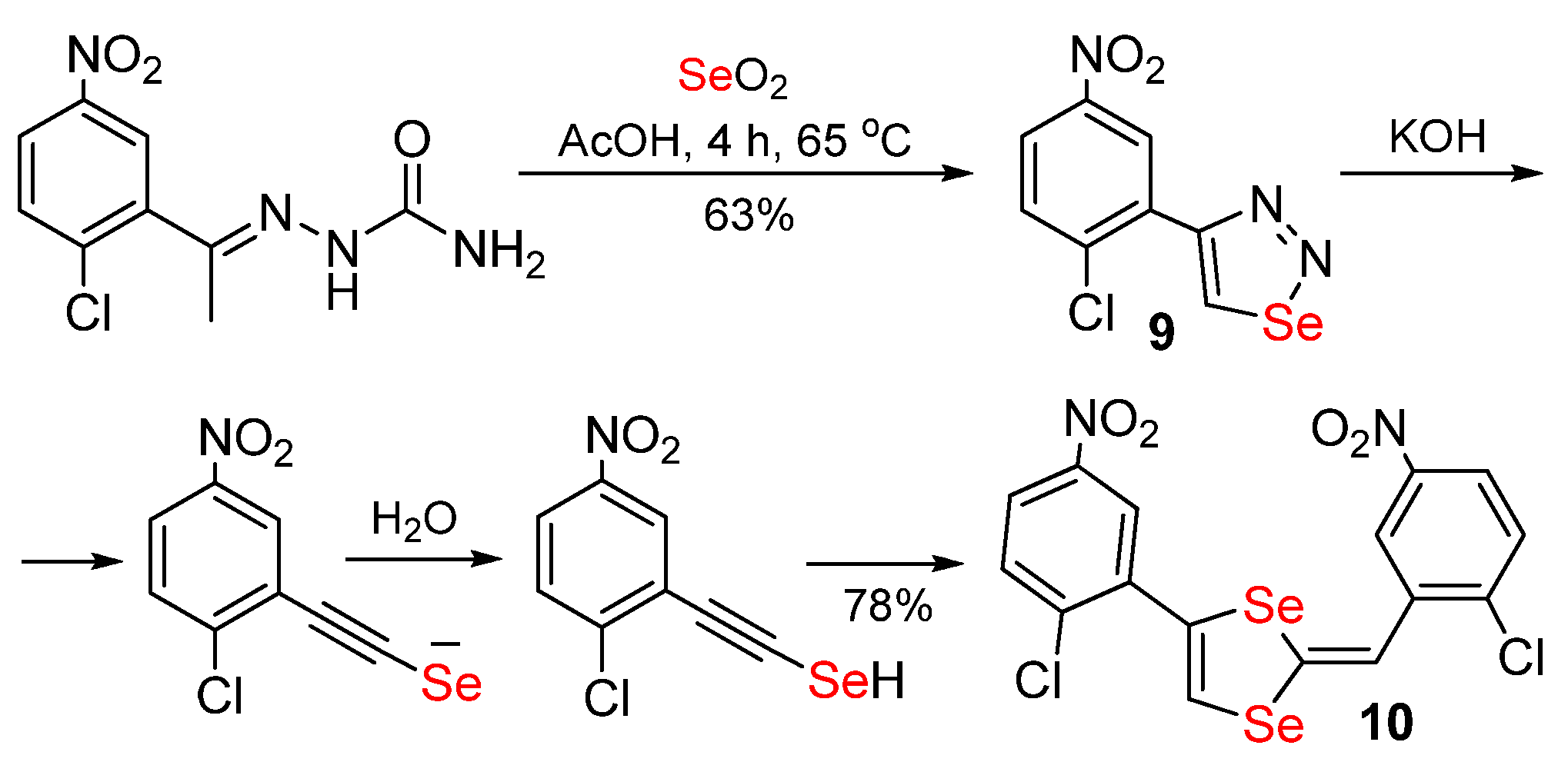
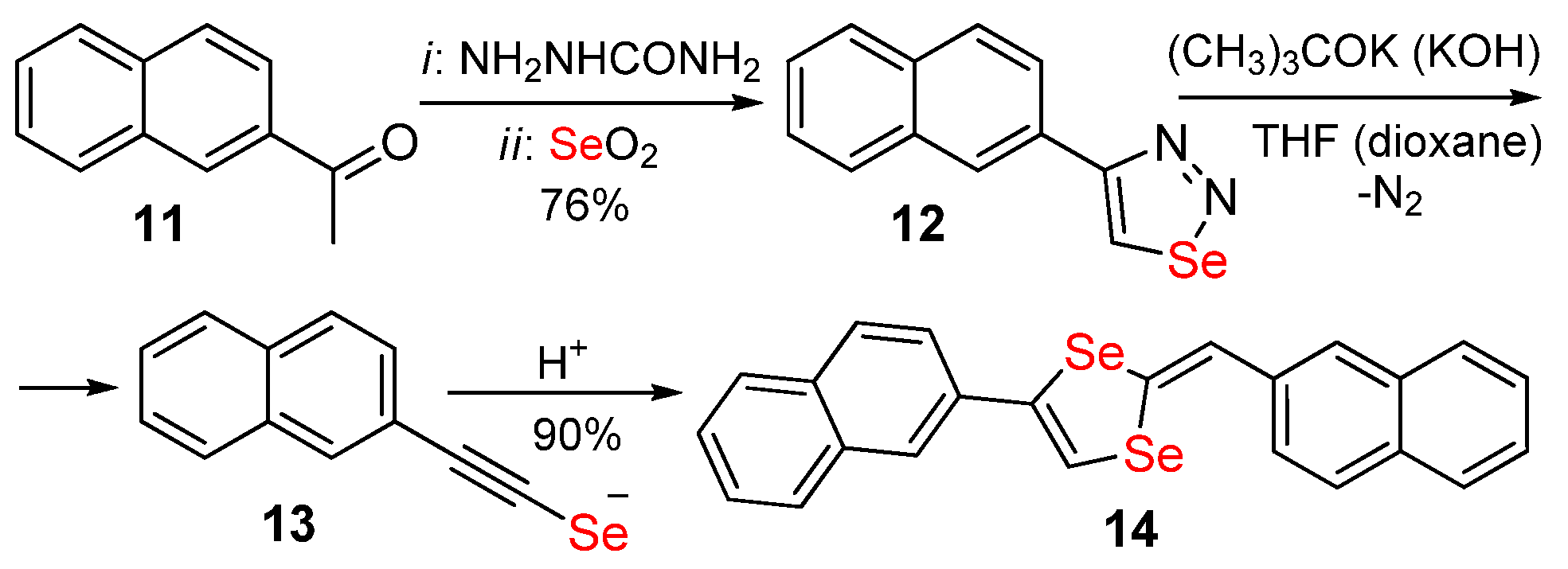
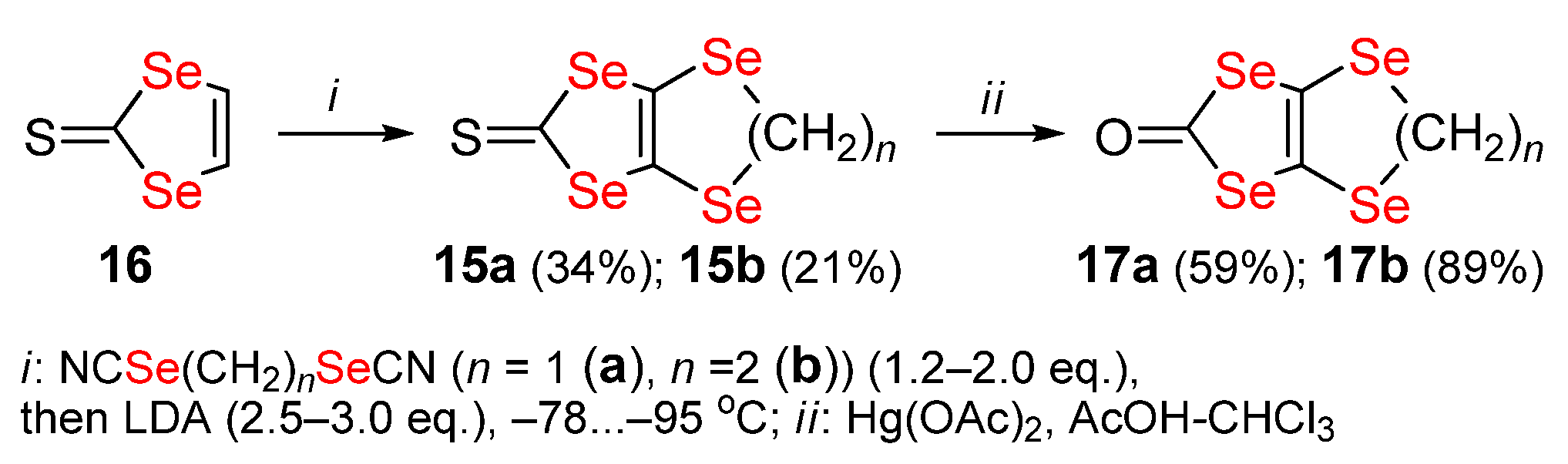
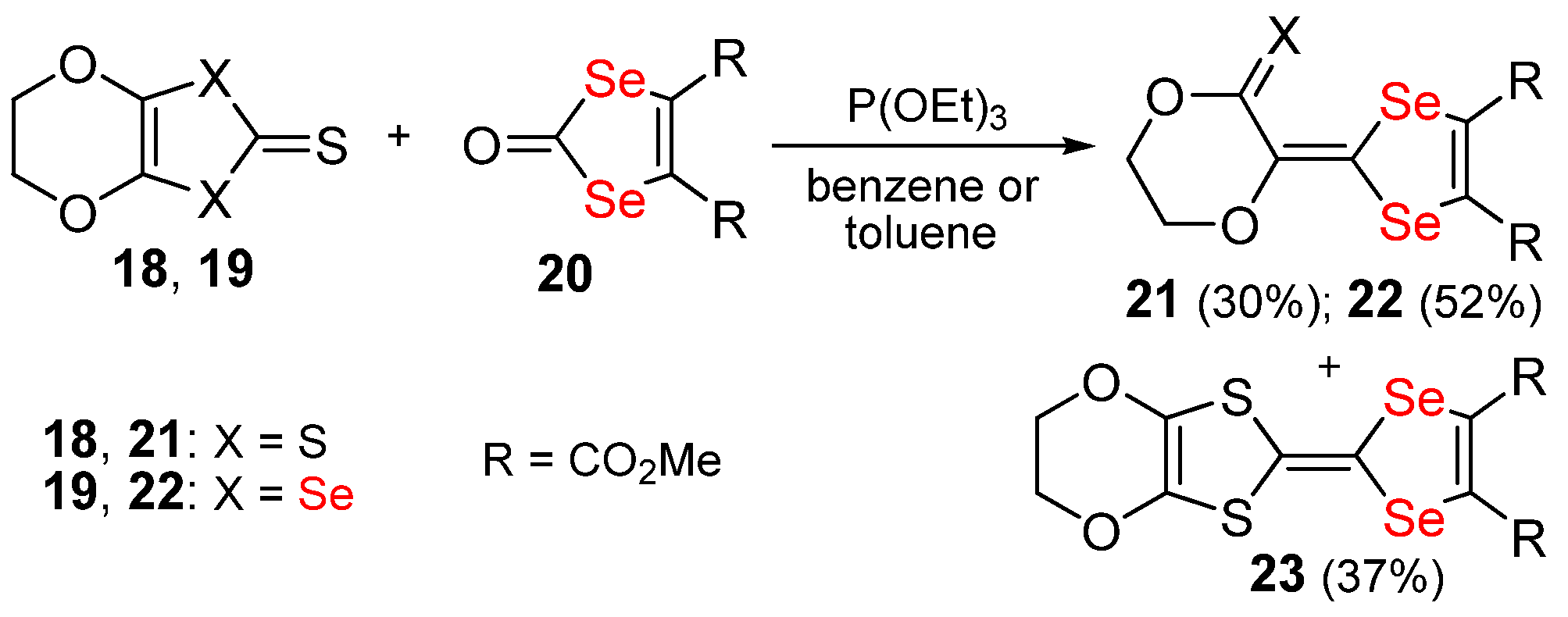

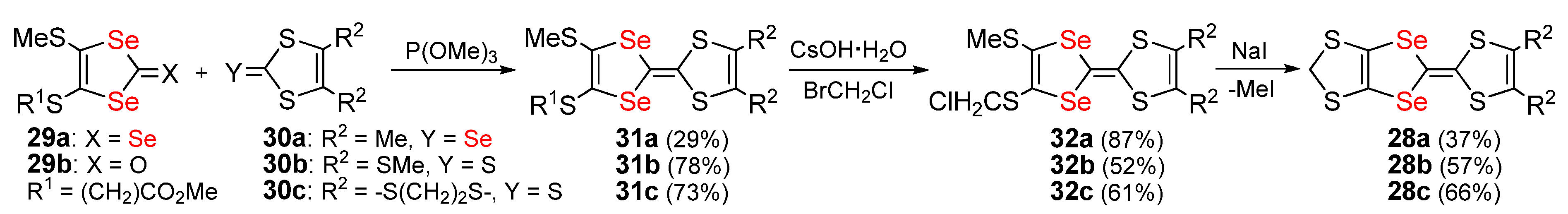
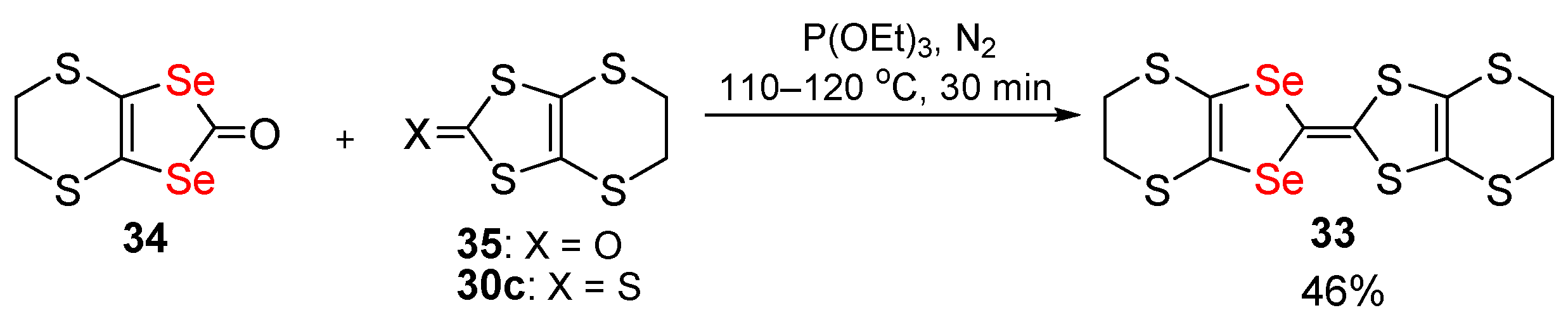
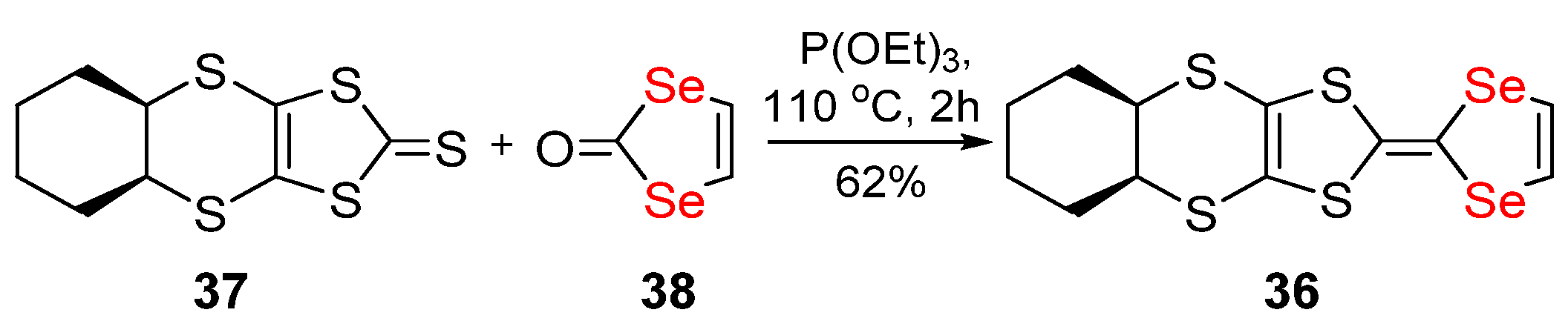
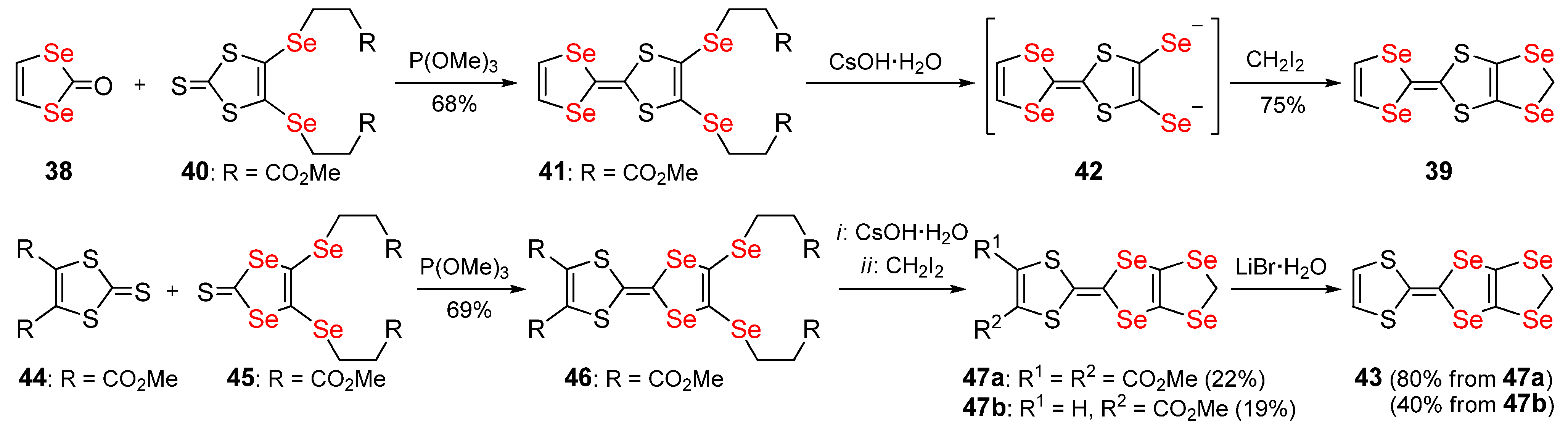









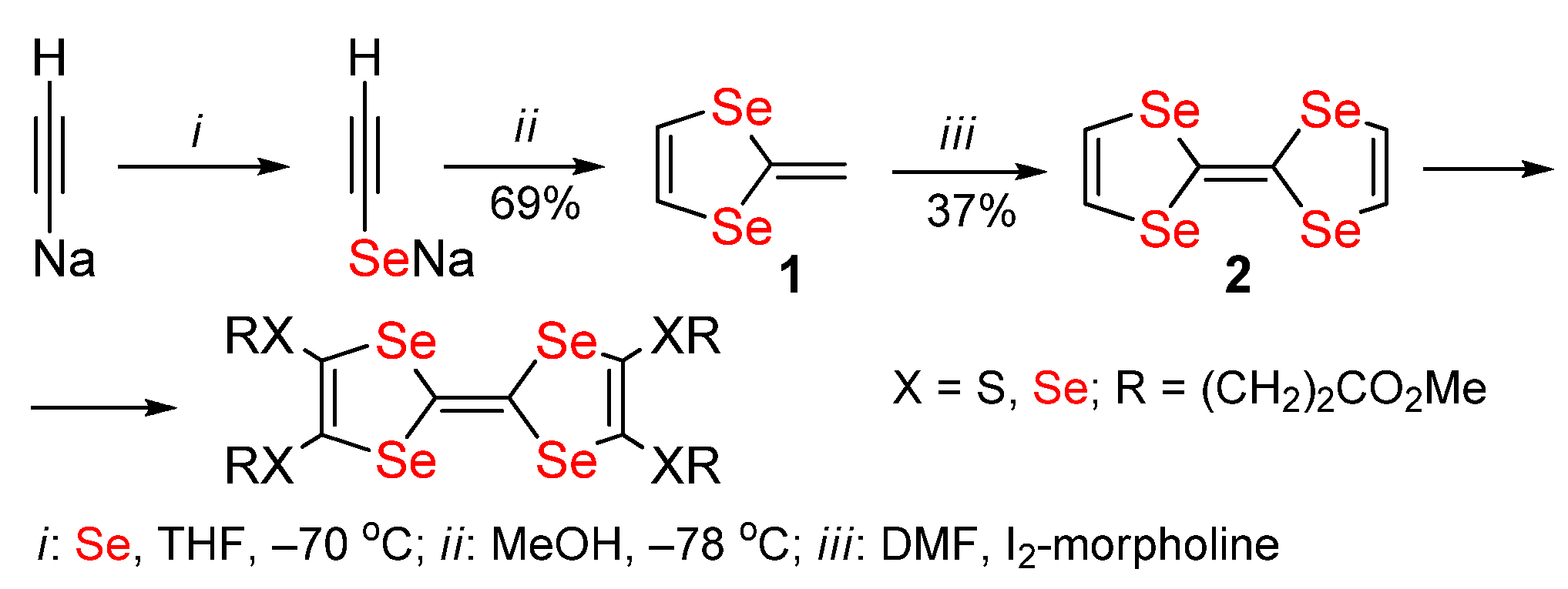
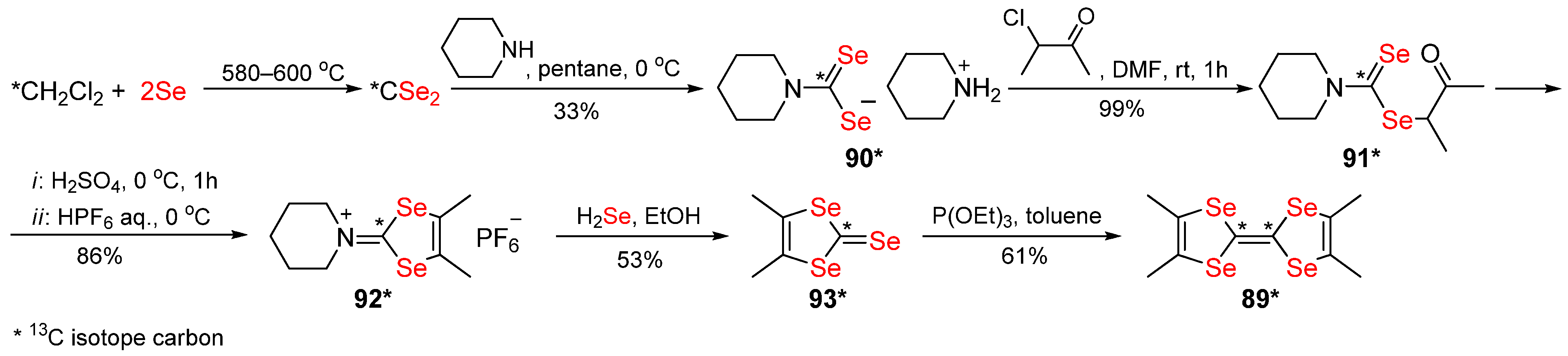

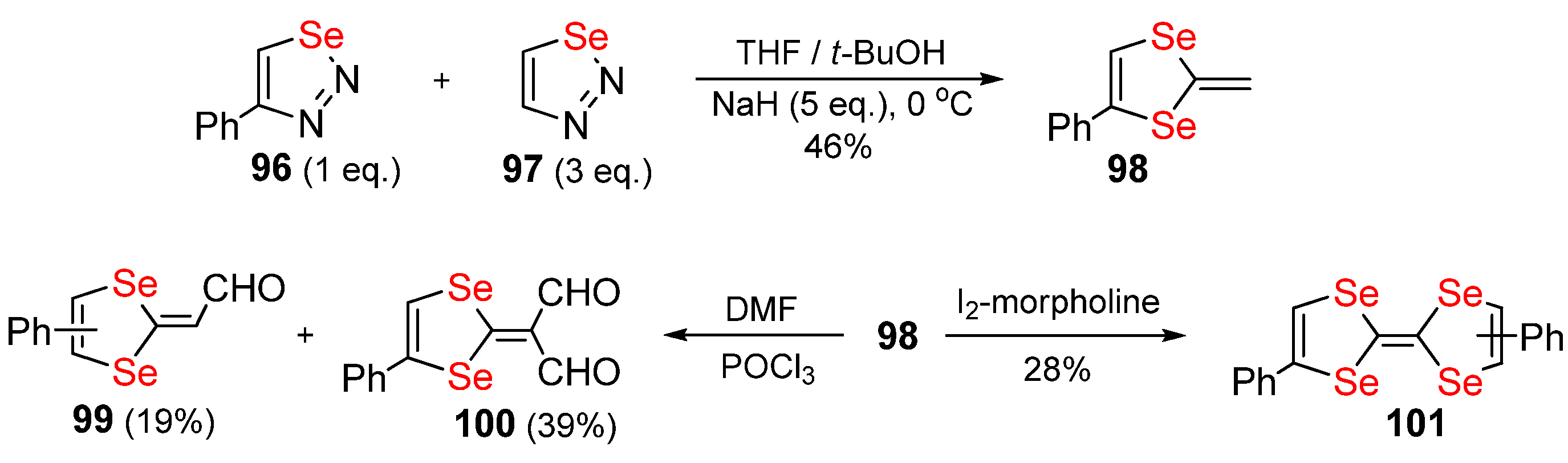
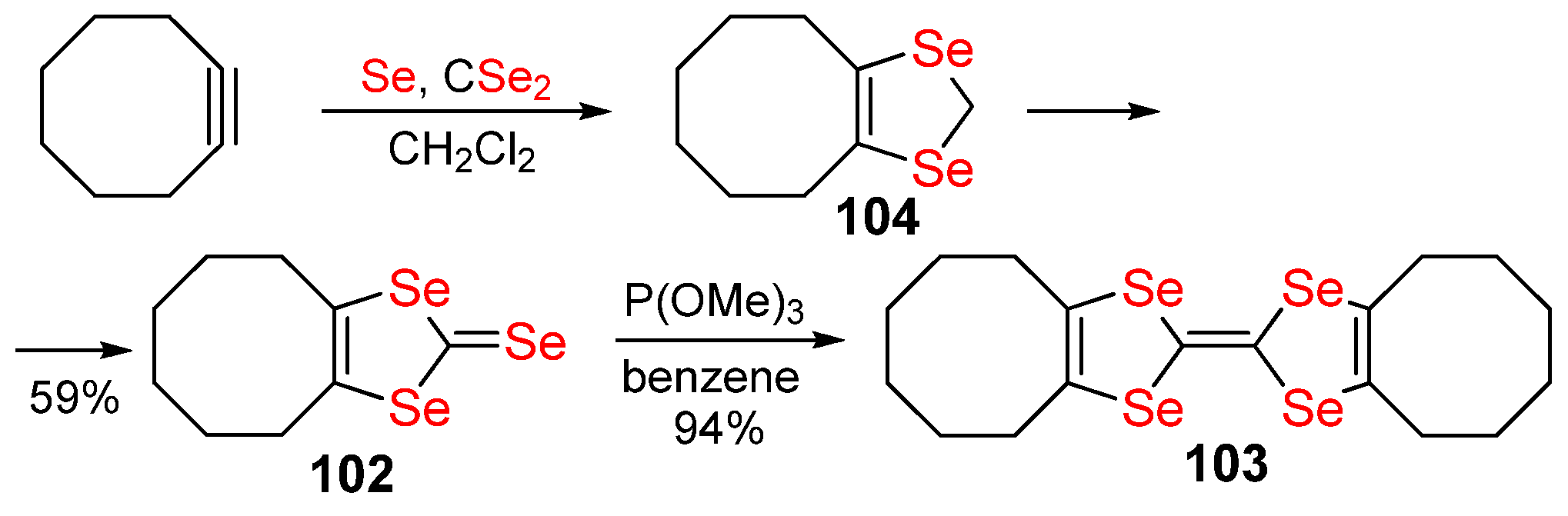











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makhaeva, N.A.; Amosova, S.V.; Potapov, V.A. Recent Advances in Design and Synthesis of Diselenafulvenes, Tetraselenafulvalenes, and Their Tellurium Analogs and Application for Materials Sciences. Molecules 2022, 27, 5613. https://doi.org/10.3390/molecules27175613
Makhaeva NA, Amosova SV, Potapov VA. Recent Advances in Design and Synthesis of Diselenafulvenes, Tetraselenafulvalenes, and Their Tellurium Analogs and Application for Materials Sciences. Molecules. 2022; 27(17):5613. https://doi.org/10.3390/molecules27175613
Chicago/Turabian StyleMakhaeva, Nataliya A., Svetlana V. Amosova, and Vladimir A. Potapov. 2022. "Recent Advances in Design and Synthesis of Diselenafulvenes, Tetraselenafulvalenes, and Their Tellurium Analogs and Application for Materials Sciences" Molecules 27, no. 17: 5613. https://doi.org/10.3390/molecules27175613
APA StyleMakhaeva, N. A., Amosova, S. V., & Potapov, V. A. (2022). Recent Advances in Design and Synthesis of Diselenafulvenes, Tetraselenafulvalenes, and Their Tellurium Analogs and Application for Materials Sciences. Molecules, 27(17), 5613. https://doi.org/10.3390/molecules27175613








