Abstract
Citri Reticulatae Pericarpium (CRP), also known as “chenpi”, is the most common qi-regulating drug in traditional Chinese medicine. It is often used to treat cough and indigestion, but in recent years, it has been found to have multi-faceted anti-cancer effects. This article reviews the pharmacology of CRP and the mechanism of the action of flavonoids, the key components of CRP, against cancers including breast cancer, lung cancer, prostate cancer, hepatic carcinoma, gastric cancer, colorectal cancer, esophageal cancer, cervical cancer, bladder cancer and other cancers with a high diagnosis rate. Finally, the specific roles of CRP in important phenotypes such as cell proliferation, apoptosis, autophagy and migration–invasion in cancer were analyzed, and the possible prospects and deficiencies of CRP as an anticancer agent were evaluated.
1. Introduction
Citri Reticulatae Pericarpium is a commonly used traditional Chinese medicine derived from the ripe peel of the Rutaceae plant Citrus reticulate Blanco and its cultivars [1], which was first recorded in Shen Nong Ben Cao Jing and has a history of thousands of years in China. As a botanical medicine with the same origin of medicine and food, CRP has various pharmacological effects, which can be used alone or combined with other traditional Chinese medicines to form many well-known classical prescriptions. It is widely used in the clinical treatment of diseases of various systems and has outstanding advantages for diseases of the respiratory and digestive system, especially diseases with cough, expectoration, nausea and vomiting as the main symptoms.
The clinical efficacy of CRP has been affirmed, especially its contribution in cancer treatment. Researchers began to explore its mechanism, trying to find out its countermeasures against cancer in cancer cell lines and animal models, and concluded that the natural compounds flavonoids contained in CRP are the implementers of its anti-cancer effect. According to reports, flavonoids of CRP have outstanding performance in terms of regulating key signaling pathways and related effectors, blocking the cancer cell cycle to resist proliferation, inducing cell apoptosis, enhancing autophagy and inhibiting cell migration and invasion. Thanks to these research results and data, the core code of CRP as an anticancer agent has gradually been revealed.
As a novel anticancer agent, CRP has received extensive attention, and many studies have confirmed that CRP and its active ingredients have inhibitory effects on cancer. This review aims to collect and introduce the mechanism of CRP and its components in inhibiting cancer, focusing on breast cancer, lung cancer, prostate cancer, hepatic carcinoma, gastric cancer, colorectal cancer, esophageal cancer, cervical cancer, bladder cancer and other cancers with a high diagnosis rate.
2. Pharmacological Effects and Chemical Composition of CRP
Pharmacological studies have found that when applied to the digestive system, the efficacy of CRP is to ameliorate gastrointestinal smooth muscle activity, accelerate gastric emptying and intestinal push, alter gut microbiota, protect the esophagus and gastrointestinal mucosa and resist peptic ulcer [2,3,4,5,6,7]. To date, CRP has proven effects in the respiratory system, including but not limited to inhibiting airway inflammation, fighting acute lung injury and pulmonary fibrosis [8,9,10,11,12,13]. In terms of the cardiovascular system, CRP can improve cardiac insufficiency, alleviate cardiac hypertrophy and myocardial fibrosis, prevent hyperlipidemia and keep cardiomyocytes away from the damage of hypercholesterolemia [14,15,16,17,18]. Experiments have found that CRP’s neuroprotective properties confer the ability to prevent neurodegeneration caused by Parkinson’s, Alzheimer’s, Huntington’s disease and multiple sclerosis [19,20,21,22]. Unlike the above systems, CRP has relatively few studies in the related direction of the urinary system and has been reported to be anti-inflammatory and enhance renal function [23,24,25]. It is worth mentioning that CRP exhibits anti-inflammatory activity not only in the urinary system, but also in various systems of the body. Nobiletin is considered to be a marker of anti-inflammatory effect of CRP, while Xue N. et al. suggested that naringenin inhibits reactive oxygen species (ROS) and inflammatory cytokines involved in the process of CRP against inflammatory damage [26,27]. The antioxidant capacity of CRP is also an important part of its pharmacological action, and it is positively correlated with the aging of CRP [28]. Data proves that long-term storage benefits the quality of CRP, in which phenolic acids play a key role [29]. Moreover, the antioxidant activity of CRP is closely related to the fungi represented by Aspergillus niger growing on its surface, which can promote the transformation of flavonoids in CRP to increase its content [30].
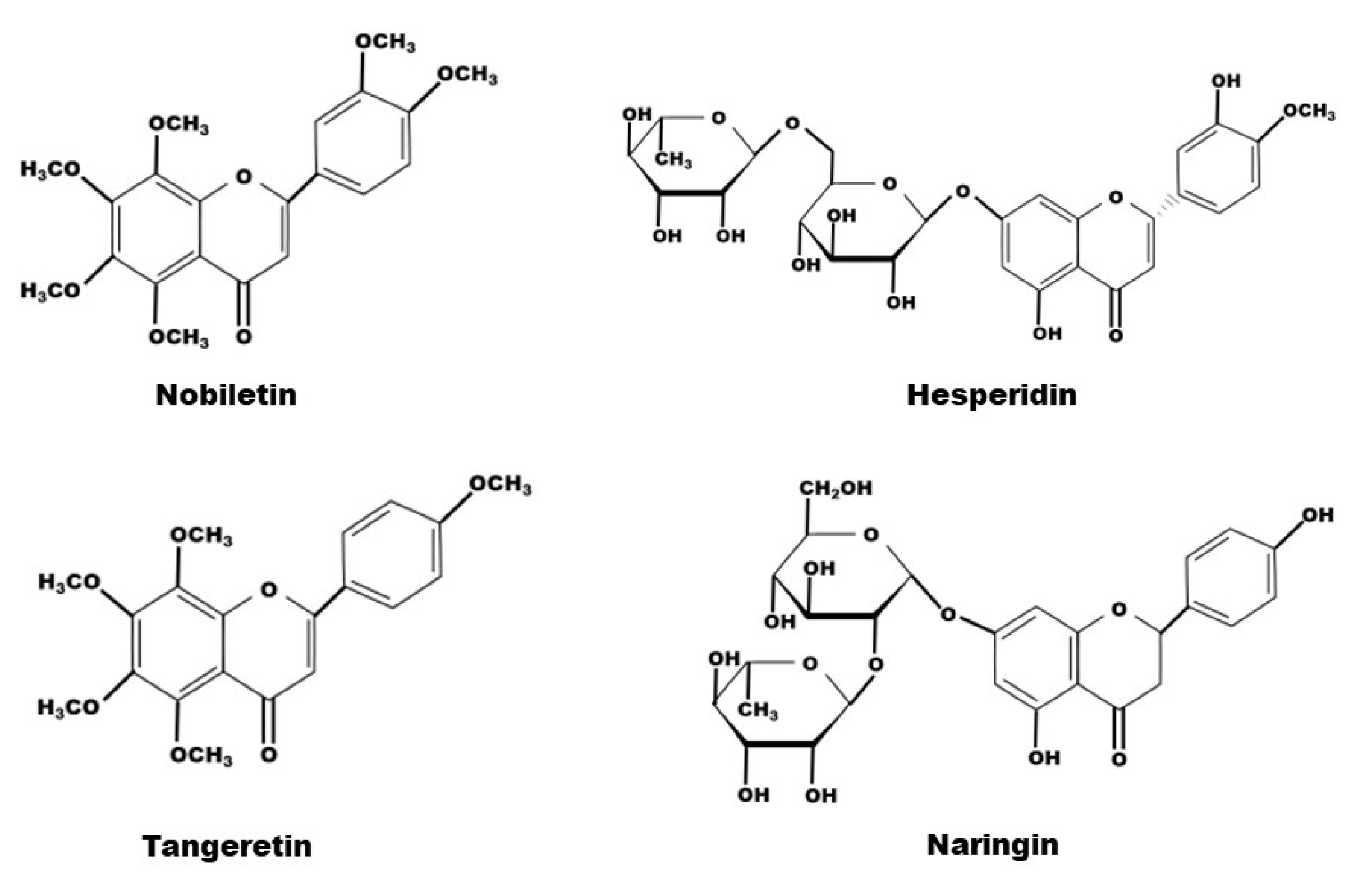
The composition of natural compounds of CRP is complex. Through supercritical CO2 extraction [31,32], Soxhlet extraction [1,33,34], pressurized liquid extraction (Wei L) and high-speed countercurrent separation [35], 161 constituents from CRP have been extracted and identified, including 65 flavonoids, 51 phenolic acids, 27 fatty acids and 18 amino acids [29,36]. Certainly, flavonoids are the most relevant class of all compounds with the pharmacological effects of CRP, and flavanone, flavone, and polymethoxyflavone aglycones, flavanone-and flavone-O-glycosides and flavone-C-glycosides are all the compounds it contains [37,38,39]. Due to its abundant content, hesperidin has been used as a chemical reference for CRP quality control by the Chinese Pharmacopoeia. Among them, in terms of exerting medical effects, the representative flavonoids are tangeretin and nobiletin, belonging to the polymethoxyflavones, and naringin and hesperidin, belonging to the flavanone O-glacosides (Table 1, Figure 1 and Figure 2).

Table 1.
The Flavonoids in CRP.

Figure 1.
(A) Citrus reticulata Blanco tree (B) fresh ripe citrus (C) fresh mature pericarps (D) CRP.
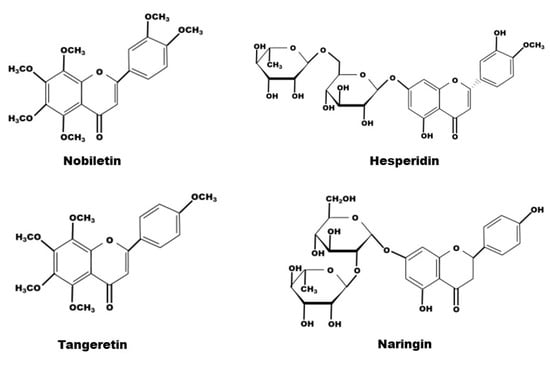
Figure 2.
The chemical structure of nobiletin, hesperidin, tangeretin and naringin contained in CRP.
3. Epidemiological Investigation of Cancer
Cancer develops from the clonal expansion of abnormal cells inside the body [41]. As changes in the prevalence and distribution of the main risk factors, cancer incidence and mortality are rapidly growing worldwide. Cancer was the leading cause of premature death in 57 countries until 2020 [42]. Based on cancer incidence and mortality from the GLOBOCAN 2020 and The World Health Organization database, the most commonly diagnosed cancers worldwide were female breast cancer, lung and prostate cancer; the most common cause of cancer death were lung, liver and stomach cancers [43,44]. Female breast cancer has now surpassed lung cancer as the leading cause of global cancer incidence in 2020 with more than 6 million deaths [45]. The causes of cancer are multifaceted, with multiple external factors combined with internal genetic changes leading to cancer, but it mainly originates from both environment and genetics [46]. Unhealthy lifestyles, such as cigarette smoking, alcohol abuse, excess fat and red meat intake and lack of fiber, can also affect the development of cancer [47,48]. Conventional treatment modalities for cancer include surgery, radiation therapy, chemotherapy, targeted therapy, hormonal therapy and immunotherapy [49]. However, current treatments are often accompanied by various physical and psychological side effects, which severely impact the prognosis and life expectancy of patients. Throughout the past few years, both clinical and laboratory studies of the treatment of cancer through traditional Chinese medicine have gained great attention. The efficacy and safety of herbal medicine make it unique in the treatment of cancer. At the same time, Chinese medicine can also be used as an adjuvant to reduce the side effects of conventional cancer treatment.
4. The Performance of CRP in the Typical Phenotype of Cancer
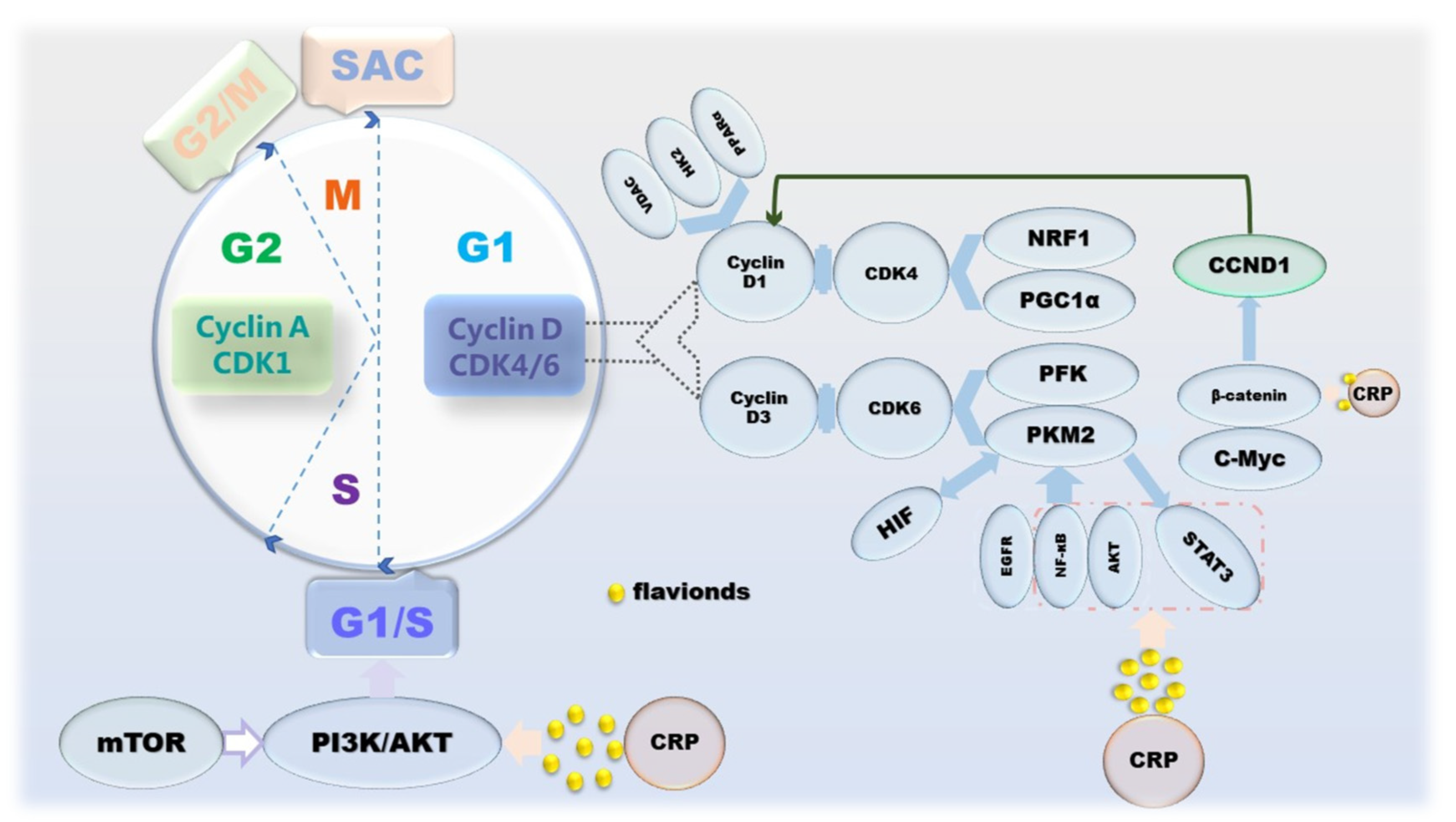
Broadly speaking, cancer is a disease caused by dysregulation of cell proliferation [50]. The manifestation of cell proliferation is cell division, and mitosis is the main means of cell division in eukaryotes, which is periodic. The cell cycle goes through 4 phases: G1, S, G2 and M; and 3 checkpoints: G1/S, G2/M and SAC [51]. Apparently, this program is regulated by proliferation signals, cyclins and corresponding cyclin-dependent kinases (CDKs) [52]. Inactivation or mutation of growth suppressor genes and overexpression of oncogenes lead to uncontrolled proliferation of cancer cells and upregulation of cyclin-CDKs’ expression, which in turn affects cell cycle progression and mitosis [53]. However, mTOR, which coordinates growth metabolism, suppresses the PI3K/AKT pathway to attenuate its anti-proliferative effect [54]. From the perspective of cancer metabolism, one of the biological markers of cancer, the Warburg effect, a form of energy metabolism manifested in aerobic glycolysis, enhances the proliferation and division, invasion and anti-apoptosis of cancer cells [55]. It is worth noting that in the G1 phase, on the one hand, cyclin D1 inactivates the inhibition of a mitochondria through the action of a series of factors, such as NRF-1, PPARγ and PGC-1α [56,57,58]. On the other hand, during G1 phase in cancer cells, PKM2 affected by PFK1, Ras, HIF-1 and PI3K/AKT/mTOR upregulates the gene CCND1 encoding cyclin D1 by increasing c-Myc expression and promoting β-catenin transactivation [59,60,61]. Conversely, PKM2 activated under the action of proliferative signals, such as EGFR, NF-κB and AKT, can also upregulate the expression of HIF-1, STAT3 and c-Myc through the STAT3 signaling pathway to maintain cell cycle progression [62,63,64]. Naringin and tangeretin were determined to affect cyclin D and beta-catenin, resulting in cell cycle arrest, which in turn counteracts the disordered proliferation of cancer cells. In addition, in the anti-proliferation process of CRP, effectors on JAK/STAT, PI3K/AKT/mTOR pathways are also involved (Figure 3).
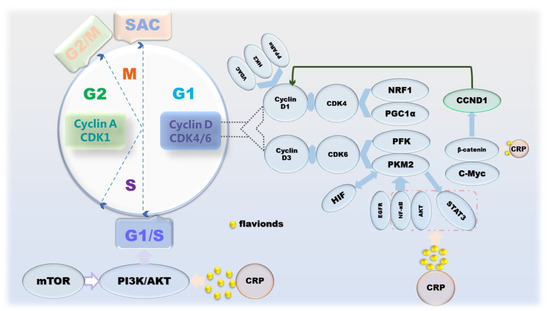
Figure 3.
The mechanism of CRP causing cancer cell cycle arrest.
Interestingly, some proteins are involved in both cell proliferation and apoptosis. For example, p53 not only acts on its key effectors PUMA and p21 to induce cell cycle arrest to inhibit proliferation, but also acts as an upstream activator of pro-apoptotic proteins to initiate apoptosis [65,66]. It is reasonable to think that the cycle arrest caused by the regulation of P53 by hesperidin and tangeretin is the result of this.
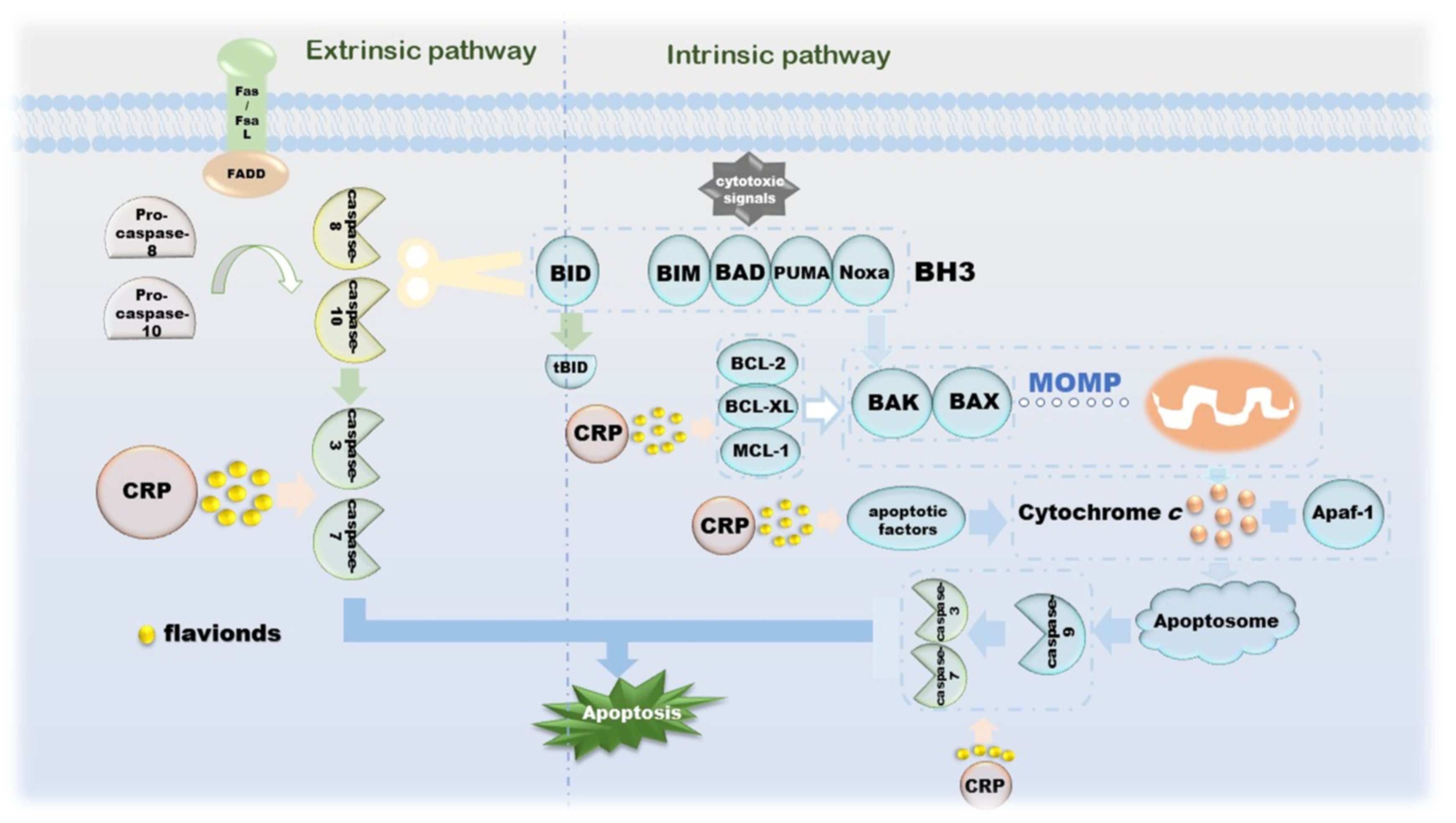
The morphological changes of apoptosis are cytoplasmic shrinkage, chromatin pyknosis, nuclear fragmentation and plasma membrane blebbing, which finally form an apoptotic body [67]. Biochemical changes that can be observed during this process include caspase activation, DNA and protein breakdown, membrane changes and recognition by phagocytes [68]. The intrinsic apoptotic pathway is due to growth factor loss, DNA damage, ER stress, ROS overload, replication stress, microtubule alterations and mitotic defects, resulting in irreversible enhancement of mitochondrial outer membrane permeability and the release of pro-apoptotic factors [67,69]. The BCL-2 family is an indispensable regulatory protein in the internal apoptosis pathway, including the pro-apoptotic BAX, BAK, BAD, BCL-XS, BID, BIK, BIM and Hrk, and the anti-apoptotic BCL-2, BCL -Xl, BCL-W, BFL-1 and MCL-1 [70]. Under the action of cytotoxic signals, BH3-like proteins, such as BIM, BAD, PUMA and Noxa, transmit the signals to the downstream BAX and BAK. Under normal conditions, BAX located in the cytoplasm is enriched in mitochondria and becomes an activated state [71,72,73]. During this process, BAK is also activated. The apoptosis-inhibiting proteins BCL-2, BCL-XL, MCL-1, etc., can combine with BAX and BAK to suppress their activity [74]. Cytochrome c is released under the action of these two diametrically opposed proteins and then binds to Apaf-1 under the induction of apoptotic factors, such as Smac, DIABLO and Omi/HtrA2, to form apoptosome to further activate caspase-3 [75]. The apoptosis inhibitor IAP family binds to and prevents the activation of caspase, forming a negative feedback mechanism [76]. Another apoptosis-inducing factor induces apoptosis in a caspase-independent manner. Excessive DNA damage leads to the onset of the PPAR-1-dependent cell death program; AIF then translocates from the mitochondria to the nucleus, followed by nuclear condensation, phosphatidylserine exposure at the plasma membrane and mitochondrial transmembrane collapse, a process unaffected by caspase inhibitors [77]. The extrinsic apoptosis pathway is completed by death receptors that are members of the TNFR superfamily, and the death receptors that receive cytotoxic signals bind to their ligands. For instance, Fas/FasL, the most representative one, activates pro-caspase-8 into caspase-8 through the combination of FADD and DED and then activates other executioners of the caspase family in turn [78,79,80]. Activated caspase-8 activates BAX and BAK by cleaving BID to release cytochrome c, a link that links the extrinsic and intrinsic apoptotic pathways. Activated caspase-3 and caspase-7 cleave ICAD, which is an inhibitor of caspase-activated DNase, and then release CAD, resulting in DNA fragmentation and ultimately, complete apoptosis [78]. CRP is involved in the release of related proteins on the internal/external apoptotic pathway, as well as upstream and downstream signaling pathways, including but not limited to ERK and PPARγ-dependent or independent (Figure 4).
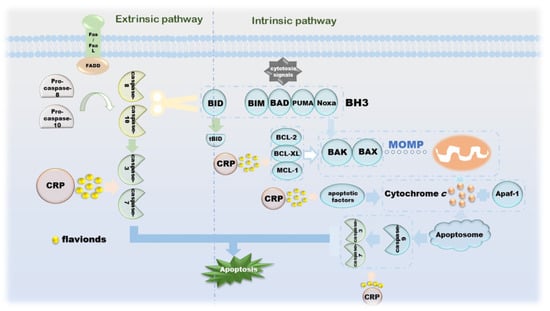
Figure 4.
The mechanism of CRP promoting apoptosis of cancer cells.
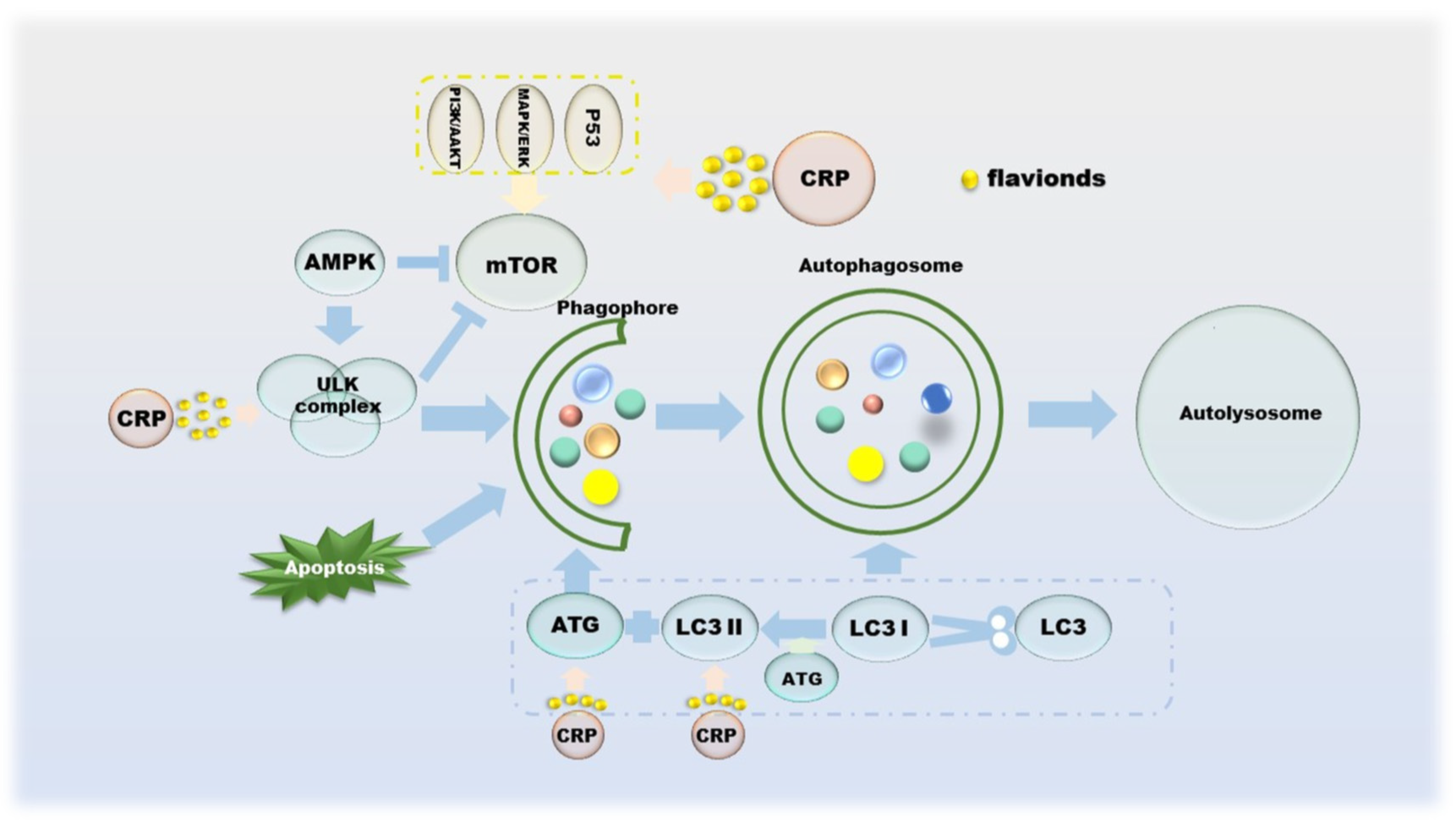
Autophagy suppresses tumors by eliminating oncogenic substrate proteins, toxic unfolded proteins and damaged organelles, preventing chronic tissue damage and cancer development; conversely, autophagy-mediated intracellular recycling promotes tumor growth metabolism in cancer [81]. Autophagy was originally thought to be tumor-suppressive, as the absence of the autophagy-related protein ATG6 has been observed in human breast, ovarian and other cancers [82]. The mTOR and ULK complexes mediated by AMPK, p53, PI3K/AKT and MAPK/ERK cooperate with ATG on the autophagic membrane to form autophagosomes, which in turn control the entire autophagy process [83,84,85,86]. The formation of autophagosomes involves a series of proteins and multimolecular complexes, involving BECLIN 1, LC3, Rab complex, etc. [86]. The damaged organelles are captured by the autophagosome membrane, recognized by the autophagy substrate p62, and then degraded by lysosomes [87]. Autophagy inhibition is beneficial to tumor growth, as its deficiency leads to the accumulation of p62, and the binding of p62 to mTORC1 inhibits autophagy and activates NF-κB and NRF-2, which further promotes tumor cell proliferation [88,89,90]. From a metabolic point of view, the existence of autophagy allows cells to still metabolize in a starved state, which means that the activation of autophagy is sufficient to maintain tumor cell survival [87,91]. CRP has not been researched in the field of autophagy for a long time, but it is currently certain that its effects on autophagy include but are not limited to regulating the PI3K/AKT/GSK-3c/mTOR pathway (Figure 5).
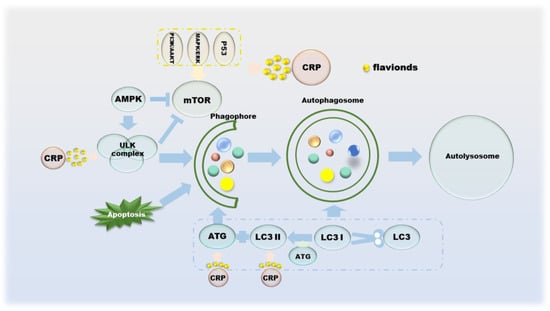
Figure 5.
The role of CRP in the process of autophagy in cancer cells.
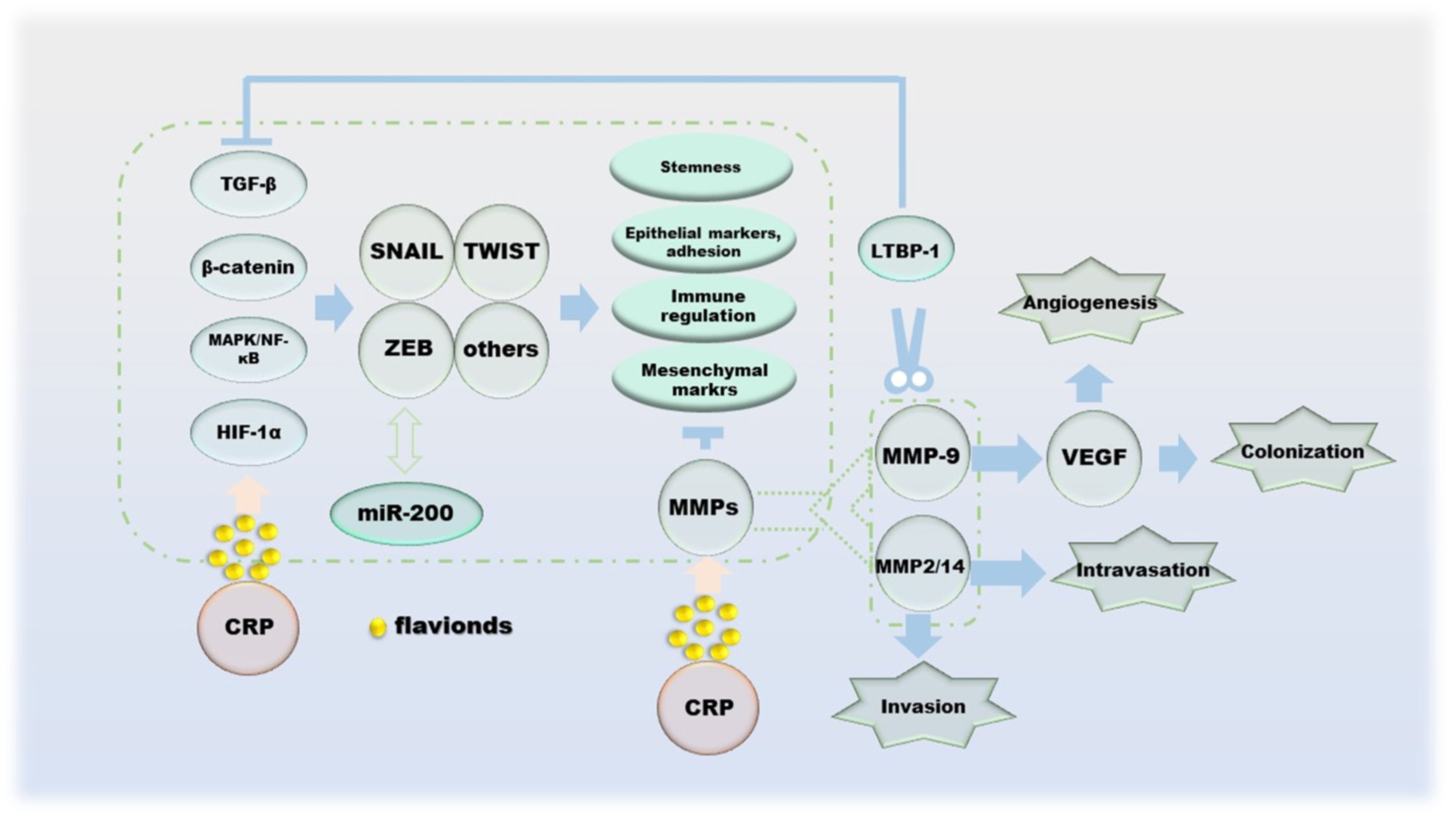
Cancer metastasis is the spread of cancer cells to distant organs, and the invasion–migration of cancer cells during this process is controlled by multiple factors. The process of cell invasion–migration consists of the following aspects: (a) local invasion and cell migration of the basement membrane; (b) intravascular deep vasculature and/or lymphatic system; (c) survival in the circulation; (d) arrest and extravasation at distant organ sites; and (e) colonization at metastatic sites [92]. Protocols for single cell invasion involve protease, actin cytoskeleton, integrin-dependent, integrin-independent and Rho- and Rock/MLCK-dependent modes of migration into mesenchymal migration and glide-like “amoematoid-migration” [93,94]. Epithelial–mesenchymal transitions (EMT) during cell migration and invasion is regulated by transcription factors such as slug, Twist, ZEB1 and ZEB2, which inhibit E-cadherin, the cornerstone of the epithelial state [95,96]. In addition, the double negative feedback loop formed by miR-200 and ZEB1/ZEB2 is another important mechanism for regulating EMT [96]. The invasion–metastasis cascade of cancer cells begins with the destruction of BM, and its damage is the result of MMPs as enzymatically hydrolyzed active proteins [97,98]. Indeed, the effects of MMPs on cancer are multifaceted, including inflammation, cell proliferation, extracellular matrix (ECM) degradation, cell migration, resistance to cell death, replicative immortality and metastatic niches; the most critical role is the degradation and remodeling of the ECM during cancer cell invasion and metastasis [99]. One of the sources of TGF-β, which has the function of inhibiting tumor cell differentiation, is the inactive precursor after proteolysis of MMP-9. At the same time, the regulation of VEGF by MMP-9 can promote tumor angiogenesis, which is beneficial to tumor colonization [100,101,102]. In addition, TGF-β1 can also be activated by MMP-14 and MMP-2 [103]. All three of the above proteins cleave LTBP-1 of the ECM to indirectly regulate the activity of TGF-β [104,105]. NF-κB enhances the production of MMPs, and its ability is restricted by TIMPs, with which it can form complexes that inhibit proteolysis [106,107]. In particular, MMP-7 inhibits apoptosis by cleaving Fas ligands, making it a place in the process of apoptosis [108]. The ability of hesperidin, naringin to downregulate MMPs has been demonstrated, which allows it to successfully reverse EMT (Figure 6).
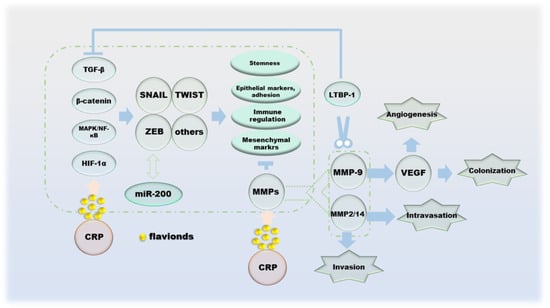
Figure 6.
The effect of CRP during the migration of cancer cells.
5. Inhibitory Effect of CRP and Its Active Components on Cancer
5.1. Breast Cancer
With the highest diagnosis rate in women, breast cancer severely threatens the survival and quality of life of women around the world. The mechanism of CRP in the treatment of breast cancer is still under study. According to the existing results, flavonoids such as nobiletin, tangeretin, hesperidin and naringin play a crucial role. The study found that nobiletin can simultaneously inhibit the ERK1/2 and PI3K/AKT pathways to suppress the growth of TNBC MDA-MB-468 cells and perform anti-tumor effects through anti-proliferation and induction of apoptosis [109]. Tangeretin inhibits breast cancer cell metastasis by targeting TP53, PTGS2, MMP9 and PIK3CA and regulating the PI3K/AKT pathway [110]. Tangeretin inhibits the formation of BCSCs and targets BCSCs by inhibiting the Stat3/Sox2 signaling pathway, thereby treating breast cancer and BCSCs [111]. Tangeretin also attenuates DMBA-induced oxidative stress, reduces kidney DNA damage and has chemo preventive activity against DMBA-induced breast cancer with cellular engraftment [112,113]. Hesperidin exhibits a concentration-dependent cytotoxicity effect on human breast cancer cell line MCF-7, which induces apoptosis and causes DNA damage [114,115]. The combination of hesperidin and chlorogenic acid modulates mitochondrial and ATP production via the estrogen receptor pathway and synergistically inhibits the growth of MCF-7 [116]. Naringin inhibits cell proliferation and promotes apoptosis and G1 cycle arrest through regulating the β-catenin pathway, thereby suppressing the growth potential of TNBC cells [117]. What’s more, hesperidin performed the inhibitory activity of the proliferation of MCF-7-GFP-Tubulin cells, fought against drug-resistant cancer cells and amelioratde the cell migration of MDA-MB 231 cells [118,119,120].
5.2. Lung Cancer
As the second most common cancer worldwide, lung cancer is the leading cause of cancer death in 2020, with incidence and mortality rates approximately twice as high in men as in women [45]. Under the current situation of extremely low survival rate, how to improve the rate and the quality of life of patients diagnosed with lung cancer has become the main subject of medical research. CRP is good at treating respiratory system diseases, and pharmacological research has carried out in-depth exploration on the treatment of lung cancer. Smoking greatly increases the risk of lung cancer. Hesperidin is able to down-regulate the expression of MMPs and enhance antioxidant status to combat nicotine toxicity and suppression of smoking-induced lung cancer [121]. The antioxidant capacity of hesperidin also inhibits tumor cell proliferation in benzo(a)pyrene-induced lung cancer mouse models [122]. Another study found that inhibition of NSCLC cells proliferation and promotion of apoptosis through the miR-132/ZEB2 signaling pathway may be one of the mechanisms by which hesperidin alleviates NSCLC [123]. Hesperidin also induces apoptosis through the mitochondrial pathway, up-regulates the expression of P21 and P53 to triggers G0/G1 phase arrest in A549 cells and down-regulates cyclin D1 for anti-proliferation [124,125]. Another set of experiments by Xia R found that blocking the SDF-1/CXCR-4 pathway to inhibit the migration of A549 cells and the suppression of EMT phenotype transformation are also the approach for hesperidin to prevent tumors and its metastasis [126]. Taking A549 cells as the research object, another important natural compound of CRP, naringin, attenuates the EGF-induced MUC5AC mucin and mRNA overexpression by inhibiting the synergistic activity of MAPKs/AP-1 and IKKs/IκB/NF-κB signaling pathways [127]. Chen M found that naringin exhibited the capacity to inhibit PI3K/AKT/mTOR and NF-κB pathways and activate the expression of miR-126 in H69 cells, thereby preventing cell growth and inducing apoptosis in SCLC cells [128].
5.3. Prostate Cancer
Prostate cancer is the most common malignancy among men worldwide, with 1.4 million cases diagnosed in 2016 and more than 380,000 deaths [129]. A series of experimental studies have demonstrated that flavonoids in CRP have positive effects on weakening cell viability and inducing cytotoxicity in prostate cancer. The expressions of NF-κB and HIF-1α were down-regulated in nobiletin-treated prostate cell lines DU145 and PC-3 cells, accompanied by decreased phosphorylation of AKT, which in turn impairs cell viability [130]. Coincidentally, tangeretin also down-regulated the expression of AKT and AR in C4-2 cells and synergistically antagonized the resistance of CRPC cells to sorafenib or cisplatin [131]. In PC-3 cells, the pathways of tangeretin-induced cytotoxicity include not only caspase-30mediated apoptosis, but also inhibition of PI3K/AKT/mTOR pathway to reverse the EMT process [132].
5.4. Liver Cancer
Primary liver cancer, including hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma, was the sixth most commonly diagnosed cancer and the third leading cause of cancer death globally in 2020, with HCC accounting for 75–85% of these [45,133]. Hence, the exploration of CRP in the treatment of liver cancer mainly focuses on the direction of HCC. Zheng J. et al. verified that the regulation of JNK/Bcl-2/BECLIN1 pathway-mediated autophagy is the mechanism by which tangeretin antagonizes the proliferation and migration of HepG2 cells [134]. Hesperidin’ fight against HCC cells’ invasiveness is achieved by suppressing the activities of NF-κB and AP-1 to down-regulate the expression and secretion of MMP-9 in acetaldehyde- and TPA-induced HCC [135,136]. The pro-apoptotic protein BAX is the key to cell apoptosis, and up-regulation of BAX to induce apoptosis in HepG2 cells is an effective way for hesperidin and naringin to inhibit liver cancer [137,138]. The fact that naringin reduces cell proliferation in DEN-induced hepatocarcinoma rats is manifested by a marked decrease in AgNOR/nuclear and PCNA levels, as well as altered DNA fragmentation in liver tissue [139].
5.5. Gastric Cancer
According to statistics, in 2020, there were more than 1 million new cases of gastric cancer, causing 769,000 deaths, ranking fourth and fifth in the world in morbidity and mortality, respectively [45]. Relying on the long-term clinical experience of CRP in the treatment of digestive system diseases, related experiments have thus explored multiple mechanisms of anti-gastric cancer. Endoplasmic reticulum stress mediates apoptosis and autophagy. Nobiletin down-regulates AKT/mTOR signaling pathway to promote endoplasmic reticulum stress response in SNU-16 cells, which may be an integral part of its anticancer activity [140]. Mitochondrial apoptosis mediated by activated caspase-9 and Fas/Fas L synergistically enables tangeretin to fulfill its mission of inhibiting AGS cells [141]. Mitochondrial-mediated apoptosis is also applicable to the killing effect of hesperidin on AGS cells. The level of reactive oxygen species in AGS cells after hesperidin intervention increases, and the MAPK signaling pathway is regulated to induce cell apoptosis [142]. Radiation therapy is one of the main methods for the treatment of tumors at present, through downregulating the expression of Notch-, Jagged1/2, Hey-1 and Hes-1, downregulating the expression of miR-410, causing attenuated invasion and migration in GC cells; tangeretin greatly enhanced the radiosensitivity of GC cells [143].
5.6. Colorectal Cancer
As the third most common cancer in terms of incidence and the second in mortality, colorectal cancer accounts for one tenth of all diagnosed cancers and deaths [45]. Similar to gastric cancer, the advantages of CRP in digestive diseases are also reflected in colorectal cancer. Nobiletin and its major metabolites M1, M2 and M3 in the colon have established roles in cell cycle arrest and apoptosis, thus effectively inhibiting AOM/DSS induced colitis-associated colon cancer in CD-1 male mice [144]. In rectal cancer, the mechanism of nobiletin is downregulating MMP-7 gene expression to inhibit the invasion and metastasis of cancer cells [145]. Tangeretin and 5-FU synergistically up-regulate P21 in HCT-116 cells, which in turn activates the P53-mediated DNA damage response and triggers apoptosis via the JNK pathway, suggesting that the combination of tangeretin and 5-FU suppresses the autophagy pathway, enabling cancer cells susceptible to oxidative stress-induced programmed cell death [146]. Inhibition of colon cancer by hesperidin involves multiple alterations, including caspase-3-mediated apoptosis, the autophagy program initiated by PI3K/Akt/GSK-3c and mTOR pathway and, down-regulation of NF-κB and its target molecules iNOS and COX-2 to attenuate oxidative stress and enhance antioxidants to fight tumor-induced inflammation [147,148,149,150]. The above phenomenon also occurred in naringin-treated CRC cells, where the suppression of the PI3K/AKT/mTOR pathway resulted in ameliorated abnormal proliferation and apoptosis [151].
5.7. Esophageal Cancer
Esophageal cancer, another intractable disease of the digestive system, is also accompanied by a particularly high fatality rate, with about 1 in every 18 cancer patients dying from it [45]. The way that naringin combats esophageal cancer is to suppress the proliferation and colony formation and the invasion of Eca109 cells by regulating related proteins to block the JAK/STAT signaling pathway, so as to promote cell apoptosis [152]. In nude mice, synephrine has a significant inhibitory effect on ESCC xenografts, and in vitro experiments have observed that it down-regulates Galectin-3 to inactivate the AKT/ERK pathway in ESCC cells [153].
5.8. Cervical Cancer
Although cervical cancer is the fourth leading cause of cancer death in women, the most common cancer and the leading cause of cancer death in many countries worldwide, it is considered a preventable cancer [45]. The inhibition of NEU3 activity in HeLa cells and A549 cells mediated by naringin resulted in the accumulation of GM3 ganglioside, which further led to the weakening of EGFR signaling, and finally resulted in cell growth restriction. At the same time, the down-regulation of phosphorylation of EGFR and ERK was also involved in the process [154]. Lin R’s data demonstrated that naringin abolishes Wnt/β-catenin signaling and ultimately triggers cell cycle arrest at G0/G1 phase in Cervical cancer cells, while ER stress-induced cell killing is also a pathway for naringin to act on [155]. Naringin also induces cell cycle arrest in the G2/M phase, inhibits cell growth and induces apoptosis via the NF-κB/COX-2-caspase-1 pathway, thereby exerting its anticancer activity on SiHa cells and HeLa cells [156,157].
5.9. Bladder Cancer
The incidence of bladder cancer in men is much higher than women, making it the sixth most common cancer and the ninth cause of cancer death [45]. Apoptosis is an important mechanism of CRP against bladder cancer discovered in current research. Nobiletin-induced apoptosis is accomplished through the regulation of endoplasmic reticulum stress via PERK/elF2α/ATF4/CHOP pathway and PI3K/AKT/mTOR pathway, and its inhibitory effect on BFTC cell growth is positively correlated with concentration range [158]. Anti-tumor formation through apoptosis is also suitable for tangeretin by inducing the release of pro-apoptotic factors such as cytochrome c to form an apoptotic complex with activated caspase-9, thereby initiating the apoptotic response and disrupting mitochondrial function, resulting in BFTC-905 cells being cytotoxic [159].
5.10. Other Cancers with High Diagnosis Rate
In addition to the above-mentioned cancers, CRP is quite successful in the treatment of other common cancers. The mechanism of nobiletin against osteosarcoma metastasis is to down-regulate the expression of MMP-2 and MMP-9 via ERK/JNK pathways and inhibit the movement, migration and invasion of U2OS and HOS cells through activation of NF-κB, CREB and SP-1 proteins [160]. Moreover, naringin suppresses the migration and invasion of human chondrosarcoma by up-regulating the expression of miR-126 and downregulating VCAM-1 expression [161]. By inhibiting MAPK and AKT/protein kinase B signaling pathway and downregulating cell cycle-related factors, nobiletin achieves the purpose of combating glioma cell proliferation and migration [162]. Suppression of the cyclin-D/cdc-2 complex formation leads to cell cycle arrest at G2/M arrest, decreased glioblastoma cell growth after tangeretin treatment, increased G2/M phase cells and induces apoptosis [163]. Blocking the MAPKs signaling pathway, downregulating the activity and expression of MMPs, thereby inhibiting the invasion, migration and adhesion of U87 cells, is a non-negligible characteristic of naringin’s anti-metastatic properties [164]. Negative effects on cell proliferation by inhibiting the FAK/cyclin D1 pathway, promoting apoptosis via affecting the FAK/BADs pathway and attenuating cell invasion and metastasis through the FAK/MMPs pathway are another example of naringin in the treatment of glioblastoma cells [165]. Naringin-treated Walker 256 carcinosarcoma rats inhibited tumor growth, down-regulated the expression of IL-6 and TNF-α and significantly prolonged the survival rate without the occurrence of cachexia [166]. Induction of apoptosis by activating caspase-3 and up-regulation of intracellular ROS and blocking cell cycle progression in G2 phase are the main ways that hesperidin reduces the viability of gallbladder cancer cells [167]. Inhibition of MAPK pathway, STAT3 activation and down-regulation of ER signaling pathway in the genome seems to be an important mechanism by which hesperidin induces apoptosis or autophagy in ECC-1 cancer cells [168]. Hesperidin inhibits mesothelioma cell growth by inducing apoptosis by downregulating the mRNA and protein expression levels of Sp1 and its regulatory proteins [169]. Hesperidin triggers apoptosis in lymphocyte lineages in a PPARγ-dependent or PPARγ-independent manner and inactivates NF-κB, which in turn sensitizes Ramos cells to chemotherapeutic agent-induced apoptosis [170]. The mechanism of action of hesperidin in NALM-6 cells is manifested in multiple aspects. It can not only play pro-apoptotic and anti-proliferative effects via PPARγ-dependent and PPARγ-independent pathways, but also affect apoptosis and cytotoxicity through PI3K/AKT/IKK signaling pathway [171,172]. As early as 1998, some scholars have found that hesperidin has an inhibitory effect on 12-O-tetradecanoyl-13-phorbo lactate-induced skin tumor [173]. In vitro, hesperidin affected PD-L1 expression in HN6 cells and HN15 cells by reducing the phosphorylation of STAT1 and STAT3, thereby inhibiting cancer cell survival and avoiding evasion of antitumor immunity. Correspondingly, in vivo experiments found that hesperidin had a negative effect on 4-NQO-induced proliferation of rat oral cancer [174,175,176]. Nobiletin inhibited the proliferation of TCA-8113 cells and CAL-27 cells through cell cycle arrest in G1 phase, accompanied by changes in intracellular levels of acidified PKA and phosphorylated CREB, impaired mitochondrial function, glucose consumption and pyruvate and lactate production [177]. The antagonism of nobiletin on LPS- and INF-γ-induced PGE2, COX-2, NO endows it with the property of preventing inflammation-related tumors [178]. Nobiletin has positive and negative regulatory effects on MMPs and TMP-1, which is a specific manifestation of its interference with PI3K signaling pathway to suppress tumors [179].
6. Conclusions
To sum up, the performance of CRP, especially its flavonoids, in the fight against cancer, is worthy of recognition because, while it does not prevent the process of a certain aspect of cancer alone, it reverses or suppresses the development of cancer through various pathways, which is a characteristic that traditional anti-cancer agents lack compared to traditional Chinese medicine. Because of the multiple functions of CRP, its ability has not been thoroughly studied. In addition, CRP is also commendable as an advantage in that it is a natural product that is homologous to medicine and food, which makes it inexpensive and easy to obtain. However, the most prominent defect of CRP as an anti-cancer agent is the lack of clinical research, which will be an important content of CRP to be explored next.
Author Contributions
Conceptualization, L.S.; methodology, L.S.; resources, L.S., P.X., W.Z. and H.H.; writing—review and editing, L.S.; Supervision, W.H., S.T. and B.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation Of China [Grant No.81860816].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Committee of National Pharmacopoeia. Pharmacopoeia of RP China; Chemical Industry Press: Beijing, China, 2015; p. 1329. [Google Scholar]
- Li, W.; Zheng, T.Z.; Qu, S.Y.; Tian, Z.F.; Qiu, X.Q.; Ding, G.H.; Wei, Y.L. Effects of tangerine peel on gastric emptying and intestinal propulsion in mice. Chin. Mater. Med. Clin. 2002, 18, 22–23. [Google Scholar]
- Chen, B.; Luo, J.K.; Han, Y.H.; Du, H.J.; Liu, J.; Wei, H.; Zhu, J.H.; Xiao, J.; Wang, J.; Cao, Y.; et al. Dietary Tangeretin Alleviated Dextran Sulfate Sodium-Induced Colitis in Mice via Inhibiting Inflammatory Response, Restoring Intestinal Barrier Function, and Modulating Gut Microbiota. J. Agric. Food Chem. 2021, 69, 7663–7674. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, X.; Zhu, J.Y.; Zhao, D.G.; Ma, Y.Y.; Li, L.D.; He, Z.T.; Huang, Q.R. Bidirectional interaction of nobiletin and gut microbiota in mice fed with a high-fat diet. Food Funct. 2021, 12, 3516–3526. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Li, Y.; Gu, Y. Hesperidin Improves Colonic Motility in Loeramide-Induced Constipation Rat Model via 5-Hydroxytryptamine 4R/cAMP Signaling Pathway. Digestion 2020, 101, 692–705. [Google Scholar] [CrossRef]
- Lee, J.A.; Shin, M.R.; Park, H.J.; Roh, S.S. Scutellariae Radix and Citri Reticulatae Pericarpium Mixture Regulate PPARγ/RXR Signaling in Reflux Esophagitis. Evid. Based Complement Altern. Med. 2022, 2022, 6969241. [Google Scholar] [CrossRef]
- Bae, E.A.; Han, M.J.; Kim, D.H. In vitro anti-Helicobacter pylori activity of some flavonoids and their metabolites. Planta Med. 1999, 65, 442–443. [Google Scholar] [CrossRef]
- Hao, Y.; Cheung, C.S.; Yip, W.C.; Ko, W.H. Nobiletin Stimulates Chloride Secretion in Human Bronchial Epithelia via a cAMP/PKA-Dependent Pathway. Cell Physiol. Biochem. 2015, 37, 306–320. [Google Scholar] [CrossRef]
- Wei, D.J.; Ci, X.X.; Chu, X.; Wei, M.M.; Hua, S.C.; Deng, X.M. Hesperidin suppresses ovalbumin-induced airway inflammation in a mouse allergic asthma model. Inflammation 2012, 35, 114–121. [Google Scholar] [CrossRef]
- Xu, J.J.; Liu, Z.; Tang, W.; Wang, G.C.; Hou, Z.X.; Liu, Q.Y.; Ling, Z.; Li, Y.L. Tangeretin from Citrus reticulate Inhibits Respiratory Syncytial Virus Replication and Associated Inflammation in vivo. J. Agric. Food Chem. 2015, 63, 9520–9527. [Google Scholar] [CrossRef]
- Li, M.Q.; Zhao, Y.; Qi, D.; Jing, H.; Wang, D.X. Tangeretin attenuates lipopolysaccharide-induced acute lung injury through Notch signaling pathway via suppressing Th17 cell response in mice. Microb. Pathog. 2020, 138, 103826. [Google Scholar] [CrossRef]
- Ding, Z.; Sun, G.; Zhu, Z. Hesperidin attenuates influenza A virus (H1N1) induced lung injury in rats through its anti-inflammatory effect. Antivir. Ther. 2018, 23, 611–615. [Google Scholar] [CrossRef]
- Zhou, Z.; Kandhare, A.D.; Kandhare, A.A.; Bodhankar, S.L. Hesperidin ameliorates bleomycin-induced experimental pulmonary fibrosis via inhibition of TGF-beta1/Smad3/AMPK and IkappaBalpha/NF-kappaB pathways. EXCLI J. 2019, 18, 723–745. [Google Scholar] [PubMed]
- Cheng, H.L.; Wu, X.D.; Ni, G.H.; Wang, S.Q.; Peng, W.J.; Zhang, H.F.; Gao, J.; Li, X.L. Citri Reticulatae Pericarpium protects against isoproterenol-induced chronic heart failure via activation of PPARγ. Ann. Transl. Med. 2020, 8, 1396. [Google Scholar] [CrossRef]
- Chen, M.; Zhu, H.; Zhu, Q.; Wu, X.; Zhou, Y.; Gao, R.; Shi, M.; Zhang, T.; Yin, T.; Zhang, H. Citri Reticulatae Pericarpium alleviates postmyocardial infarction heart failure by upregulating PPARγ expression. Clin. Exp. Pharmacol. Physiol. 2022, 49, 661–673. [Google Scholar] [CrossRef] [PubMed]
- Green, C.O.; Wheatley, A.O.; McGrowder, D.A.; Dilworth, L.L.; Asemota, H.N. Citrus peel polymethoxylated flavones extract modulates liver and heart function parameters in diet induced hypercholesterolemic rats. Food Chem. Toxicol. 2013, 51, 306–309. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Huang, K.E.; Luo, Y.; Dong, X.L.; Chen, W.; Yu, X.Q.; Ke, X.H. Nontargeted urine metabolomics analysis of the protective and therapeutic effects of Citri Reticulatae Chachiensis Pericarpium on high-fat feed-induced hyperlipidemia in rats. Biomed. Chromatogr. 2020, 34, e4795. [Google Scholar] [CrossRef]
- DU, Y.Z.; Su, J.; Yan, M.Q.; Chen, S.H.; Lyu, G.Y.; Yu, J.J. Improvement effect and mechanism of ethanol extract from Citri Reticulatae Pericarpium on triglyceride in hyperlipidemia model rat. Zhongguo Zhong Yao Za Zhi 2021, 46, 190–195. [Google Scholar]
- Hwang, S.L.; Shih, P.H.; Yen, G.C. Neuroprotective effects of citrus flavonoids. J. Agric. Food Chem. 2012, 60, 877–885. [Google Scholar] [CrossRef]
- Hajialyani, M.; Hosein, F.M.; Echeverría, J.; Nabavi, S.M.; Uriarte, E.; Sobarzo-Sánchez, E. Hesperidin as a Neuroprotective Agent: A Review of Animal and Clinical Evidence. Molecules 2019, 24, 648. [Google Scholar] [CrossRef]
- Cirmi, S.; Ferlazzo, N.; Lombardo, G.E.; Ventura-Spagnolo, E.; Gangemi, S.; Calapai, G.; Navarra, M. Neurodegenerative Diseases: Might Citrus Flavonoids Play a Protective Role? Molecules 2016, 21, 1312. [Google Scholar] [CrossRef]
- Kim, J.; Wie, M.B.; Ahn, M.; Tanaka, A.; Matsuda, H.; Shin, T. Benefits of hesperidin in central nervous system disorders: A review. Anat. Cell Biol. 2019, 52, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Huang, R.; Qin, Z.; Liu, F. Influence of Tangeretin on the Exponential Regression of Inflammation and Oxidative Stress in Streptozotocin-Induced Diabetic Nephropathy. Appl. Biochem. Biotechnol. 2022, 194, 3914–3929. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, Y.M.; Deng, Z.K. Tangeretin ameliorates renal failure via regulating oxidative stress, NF-κB-TNF-α/iNOS signalling and improves memory and cognitive deficits in 5/6 nephrectomized rats. Inflammopharmacology 2018, 26, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.M.; Gabbar, M.A.; Abdel-Twab, S.M.; Fahmy, E.M.; Ebaid, H.; Alhazza, I.M.; Ahmed, O.M. Antidiabetic Potency, Antioxidant Effects, and Mode of Actions of Citrus reticulata Fruit Peel Hydroethanolic Extract, Hesperidin, and Quercetin in Nicotinamide/Streptozotocin-Induced Wistar Diabetic Rats. Oxid. Med. Cell Longev. 2020, 2020, 1730492. [Google Scholar] [CrossRef]
- Chen, X.M.; Tait, A.R.; Kitts, D.D. Flavonoid composition of orange peel and its association with antioxidant and anti-inflammatory activities. Food Chem. 2017, 218, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Xue, N.; Wu, X.; Wu, L.; Li, L.; Wang, F. Antinociceptive and anti-inflammatory effect of Naringenin in different nociceptive and inflammatory mice models. Life Sci. 2019, 217, 148–154. [Google Scholar] [CrossRef]
- Yu, Q.; Tao, Y.; Huang, Y.; Zogona, D.; Wu, T.; Liu, R.; Pan, S.; Xu, X. Aged Pericarpium Citri Reticulatae ‘Chachi’ Attenuates Oxidative Damage Induced by tert-Butyl Hydroperoxide (t-BHP) in HepG2 Cells. Foods 2022, 11, 273. [Google Scholar] [CrossRef]
- Bian, X.; Xie, X.; Cai, J.; Zhao, Y.; Miao, W.; Chen, X.; Xiao, Y.; Li, N.; Wu, J.L. Dynamic changes of phenolic acids and antioxidant activity of Citri Reticulatae Pericarpium during aging processes. Food Chem. 2022, 373, 131399. [Google Scholar] [CrossRef]
- Wang, F.; Chen, L.; Li, F.Q.; Liu, S.J.; Chen, H.P.; Liu, Y.P. The Increase of Flavonoids in Pericarpium Citri Reticulatae (PCR) Induced by Fungi Promotes the Increase of Antioxidant Activity. Evid. Based Complement Altern. Med. 2018, 2018, 2506037. [Google Scholar] [CrossRef]
- Lv, X.J.; XU, Y.; Dong, P.F.; Xu, L.Y.; Li, S.M.; Long, T.; Wang, Y.L. Optimization of Supercritical CO2 Extraction of Polymethoxyflavonoes from Citri Reticulate Pericarpium by Response Surface Methodology. Food Res. Develop. 2019, 40, 16–20. [Google Scholar]
- Long, T.; Lv, X.; Xu, Y.; Yang, G.; Xu, L.Y.; Li, S. Supercritical fluid CO2 extraction of three polymethoxyflavones from Citri reticulatae pericarpium and subsequent preparative separation by continuous high-speed counter-current chromatography. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2019, 1124, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.J.; Yang, Y.T.; Huang, S.G.; Pan, H.J.; Luo, M.X.; Zheng, G.D. Analysis and comparison of flavonoids extracted from tangerine peel by ultrasonic extraction and Soxhlet extraction. Chinese Mater. Med. 2016, 39, 371–374. [Google Scholar]
- Anagnostopoulou, M.A.; Kefalas, P.; Kokkalou, E.; Assimopoulou, A.N.; Papageorgiou, V.P. Analysis of antioxidant compounds in sweet orange peel by HPLC-diode array detection-electrospray ionization mass spectrometry. Biomed. Chromatogr. 2005, 19, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.D.; Zhou, F.; Jiang, L.; Yang, D.P.; Yang, X.; Lin, L.W. Isolation and purification of polymethoxylated flavonoids from Pericarpium Citri Reticulatae by high-speed counter-current chromatography. Chin. Tradit. Herbal Drugs 2010, 41, 52–55. [Google Scholar]
- Yu, X.; Sun, S.; Guo, Y.; Liu, Y.; Yang, D.; Li, G.; Lü, S. Citri Reticulatae Pericarpium (Chenpi): Botany, ethnopharmacology, phytochemistry, and pharmacology of a frequently used traditional Chinese medicine. J. Ethnopharmacol. 2018, 220, 265–282. [Google Scholar] [CrossRef] [PubMed]
- Roowi, S.; Crozier, A. Flavonoids in tropical citrus species. J. Agric. Food Chem. 2011, 59, 12217–12225. [Google Scholar] [CrossRef]
- Gattuso, G.; Barreca, D.; Gargiulli, C.; Leuzzi, U.; Caristi, C. Flavonoid composition of Citrus juices. Molecules 2007, 12, 1641–1673. [Google Scholar] [CrossRef]
- Zheng, G.D.; Zhou, P.; Yang, H.; Li, Y.S.; Li, P.; Liu, E.H. Rapid resolution liquid chromatography-electrospray ionisation tandem mass spectrometry method for identification of chemical constituents in Citri Reticulatae Pericarpium. Food Chem. 2013, 136, 604–611. [Google Scholar] [CrossRef]
- Duan, L.; Guo, L.; Liu, K.; Liu, E.H.; Li, P. Characterization and classification of seven citrus herbs by liquid chromatography-quadrupole time-of-flight mass spectrometry and genetic algorithm optimized support vector machines. J. Chromatogr. A 2014, 1339, 118–127. [Google Scholar] [CrossRef]
- Martincorena, I.; Campbell, P.J. Somatic mutation in cancer and normal cells. Science 2015, 349, 1483–1489. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Weiderpass, E.; Soerjomataram, I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 2021, 127, 3029–3030. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soeriomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Parsa, N. Environmental factors inducing human cancers. Iran. J. Public Health 2012, 41, 1–9. [Google Scholar]
- Mbemi, A.; Khanna, S.; Njiki, S.; Yedjou, C.G.; Tchounwou, P.B. Impact of Gene-Environment Interactions on Cancer Development. Int. J. Environ. Res. Public Health 2020, 17, 8089. [Google Scholar] [CrossRef] [PubMed]
- Bagot, R.C.; Meaney, M.J. Epigenetics and the biological basis of gene x environment interactions. J. Am. Acad. Child. Adolesc. Psychiatry 2010, 49, 752–771. [Google Scholar]
- Miller, K.D.; Nogueira, L.; Mariotto, A.B.; Rowland, J.H.; Yabroff, K.R.; Alfano, C.M.; Jemal, A.; Kramer, J.L.; Siegel, R.L. Cancer treatment and survivorship statistics, 2019. CA Cancer J. Clin. 2019, 69, 363–385. [Google Scholar] [CrossRef]
- Evan, G.I.; Vousden, K.H. Proliferation, cell cycle and apoptosis in cancer. Nature 2001, 411, 342–348. [Google Scholar] [CrossRef]
- Liu, J.; Peng, Y.; Wei, W. Cell cycle on the crossroad of tumorigenesis and cancer therapy. Trends Cell Biol. 2022, 32, 30–44. [Google Scholar] [CrossRef]
- Malumbres, M. Cyclin-dependent kinases. Genome Biol. 2014, 15, 122. [Google Scholar] [CrossRef] [PubMed]
- Icard, P.; Fournel, L.; Wu, Z.; Alifano, M.; Lincet, H. Interconnection between Metabolism and Cell Cycle in Cancer. Trends Biochem. Sci. 2019, 44, 490–501. [Google Scholar] [CrossRef]
- O’Reilly, K.E.; Rojo, F.; She, Q.B.; Solit, D.; Mills, G.B.; Smith, D.; Lane, H.; Hofmann, F.; Hicklin, D.J.; Ludwig, D.L.; et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006, 66, 1500–1508. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Dominy, J.E., Jr.; Lee, Y.; Gerhart-Hines, Z.; Puigserver, P. Nutrient-dependent regulation of PGC-1alpha’s acetylation state and metabolic function through the enzymatic activities of Sirt1/GCN5. Biochim. Biophys. Acta 2010, 1804, 1676–1683. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, Z.; Lu, Y.; Du, R.; Katiyar, S.; Yang, J.; Fu, M.; Leader, J.E.; Quong, A.; Novikoff, P.M.; et al. Cyclin D1 repression of nuclear respiratory factor 1 integrates nuclear DNA synthesis and mitochondrial function. Proc. Natl. Acad. Sci. USA 2006, 103, 11567–11572. [Google Scholar] [CrossRef]
- Lee, Y.; Dominy, J.E.; Choi, Y.J.; Jurczak, M.; Tolliday, N.; Camporez, J.P.; Chim, H.; Lim, J.H.; Ruan, H.B.; Yang, X.; et al. Cyclin D1-Cdk4 controls glucose metabolism independently of cell cycle progression. Nature 2014, 510, 547–551. [Google Scholar] [CrossRef] [Green Version]
- Courtnay, R.; Ngo, D.C.; Malik, N.; Ververis, K.; Tortorella, S.M.; Karagiannis, T.C. Cancer metabolism and the Warburg effect: The role of HIF-1 and PI3K. Mol. Biol. Rep. 2015, 42, 841–851. [Google Scholar] [CrossRef]
- Yang, W.; Xia, Y.; Ji, H.; Zheng, Y.; Liang, J.; Huang, W.; Gao, X.; Aldape, K.; Lu, Z. Nuclear PKM2 regulates β-catenin transactivation upon EGFR activation. Nature 2011, 480, 118–122. [Google Scholar] [CrossRef]
- Icard, P.; Shulman, S.; Farhat, D.; Steyaert, J.M.; Alifano, M.; Lincet, H. How the Warburg effect supports aggressiveness and drug resistance of cancer cells? Drug Resist. Updat. 2018, 38, 1–11. [Google Scholar] [CrossRef]
- Lu, Z.; Hunter, T. Metabolic Kinases Moonlighting as Protein Kinases. Trends Biochem. Sci. 2018, 43, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Prakasam, G.; Iqbal, M.A.; Bamezai, R.N.K.; Mazurek, S. Posttranslational Modifications of Pyruvate Kinase M2: Tweaks that Benefit Cancer. Front. Oncol. 2018, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Cao, R.; Zhang, Y.; Xia, Y.; Zheng, Y.; Li, X.; Wang, L.; Yang, W.; Lu, Z. PKM2 dephosphorylation by Cdc25A promotes the Warburg effect and tumorigenesis. Nat. Commun. 2016, 7, 12431. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.A.; Hwang, P.M.; Hermeking, H.; Kinzler, K.W.; Vogelstein, B. Cooperative effects of genes controlling the G(2)/M checkpoint. Genes Dev. 2000, 14, 1584–1588. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, L. The transcriptional targets of p53 in apoptosis control. Biochem. Biophys. Res. Commun. 2005, 331, 851–858. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef]
- Kumar, V.; Abbas, A.K.; Fausto, N.; Aster, J.C. Robins and Cotran: Pathologic Basis of Disease; Saunders Elsevier: Philadelphia, PA, USA, 2010; pp. 25–32. [Google Scholar]
- Danial, N.N.; Korsmeyer, S.J. Cell death: Critical control points. Cell 2004, 116, 205–219. [Google Scholar] [CrossRef] [Green Version]
- Wong, R.S. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011, 30, 87. [Google Scholar] [CrossRef]
- Czabotar, P.E.; Lessene, G.; Strasser, A.; Adams, J.M. Control of apoptosis by the BCL-2 protein family: Implications for physiology and therapy. Nat. Rev. Mol. Cell Biol. 2014, 15, 49–63. [Google Scholar] [CrossRef]
- Willis, S.N.; Fletcher, J.I.; Kaufmann, T.; Delft, M.F.; Chen, L.; Czabotar, P.E.; Lerino, H.; Lee, E.F.; Fairlie, W.D.; Bouillet, P.; et al. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science 2007, 315, 856–859. [Google Scholar] [CrossRef]
- Kim, H.; Rafiuddin-Shah, M.; Tu, H.C.; Jeffers, J.R.; Zambetti, G.P.; Hsieh, J.J.D.; Cheng, E.H.Y. Hierarchical regulation of mitochondrion-dependent apoptosis by BCL-2 subfamilies. Nat. Cell Biol. 2006, 8, 1348–1358. [Google Scholar] [CrossRef] [PubMed]
- Ku, B.; Liang, C.; Jung, J.U.; Oh, B.H. Evidence that inhibition of BAX activation by BCL-2 involves its tight and preferential interaction with the BH3 domain of BAX. Cell Res. 2011, 21, 627–641. [Google Scholar] [CrossRef]
- Kroemer, G.; Galluzzi, L.; Brenner, C. Mitochondrial membrane permeabilization in cell death. Physiol. Rev. 2007, 87, 99–163. [Google Scholar] [CrossRef] [PubMed]
- Varfolomeev, E.; Vucic, D. Inhibitor of apoptosis proteins: Fascinating biology leads to attractive tumor therapeutic targets. Future Oncol. 2011, 7, 633–648. [Google Scholar] [CrossRef] [PubMed]
- Joza, N.; Pospisilik, J.A.; Hangen, E.; Hanada, T.; Modjtahedi, N.; Penninger, J.M.; Kroemer, G. AIF: Not just an apoptosis-inducing factor. Ann. N. Y. Acad. Sci. 2009, 1171, 2–11. [Google Scholar] [CrossRef]
- Toda, S.; Nishi, C.; Yanagihashi, Y.; Segawa, K.; Nagata, S. Clearance of Apoptotic Cells and Pyrenocytes. Curr. Top Dev. Biol. 2015, 114, 267–295. [Google Scholar]
- Strasser, A.; Jost, P.J.; Nagata, S. The many roles of FAS receptor signaling in the immune system. Immunity 2009, 30, 180–192. [Google Scholar] [CrossRef]
- Schneider, P.; Tschopp, J. Apoptosis induced by death receptors. Pharm. Acta Helv. 2000, 74, 281–286. [Google Scholar] [CrossRef]
- White, E. Deconvoluting the context-dependent role for autophagy in cancer. Nat. Rev. Cancer 2012, 12, 401–410. [Google Scholar] [CrossRef]
- Liang, X.H.; Jackson, S.; Seaman, M.; Brown, K.; Kempkes, B.; Hibshoosh, H.; Levine, B. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature 1999, 402, 672–676. [Google Scholar] [CrossRef]
- White, E.; Mehnert, J.M.; Chan, C.S. Autophagy, Metabolism, and Cancer. Clin. Cancer Res. 2015, 21, 5037–5046. [Google Scholar] [CrossRef] [PubMed]
- Lamb, C.A.; Yoshimori, T.; Tooze, S.A. The autophagosome: Origins unknown, biogenesis complex. Nat. Rev. Mol. Cell Biol. 2013, 14, 759–774. [Google Scholar] [CrossRef] [PubMed]
- Rubinsztein, D.C.; Shpilka, T.; Elazar, Z. Mechanisms of autophagosome biogenesis. Curr. Biol. 2012, 22, R29–R34. [Google Scholar] [CrossRef] [PubMed]
- Tanida, I. Autophagosome formation and molecular mechanism of autophagy. Antioxid. Redox Signal. 2011, 14, 2201–2214. [Google Scholar] [CrossRef]
- Rabinowitz, J.D.; White, E. Autophagy and metabolism. Science 2010, 330, 1344–1348. [Google Scholar] [CrossRef]
- Duran, A.; Amanchy, R.; Linares, J.F.; Joshi, J.; Abu-Baker, S.; Porollo, A.; Hansen, M.; Moscat, J.; Diaz-Meco, M.T. P62 is a key regulator of nutrient sensing in the mTORC1 pathway. Mol. Cell 2011, 44, 134–146. [Google Scholar] [CrossRef]
- Duran, A.; Linares, J.F.; Galvez, A.S.; Wikenheiser, K.; Flores, J.M.; Diaz-Meco, M.T.; Moscat, J. The signaling adaptor p62 is an important NF-kappaB mediator in tumorigenesis. Cancer Cell 2008, 13, 343–354. [Google Scholar] [CrossRef]
- Komatsu, M.; Kurokawa, H.; Waguri, S.; Taguchi, K.; Kobayashi, A.; Ichimura, Y.; Sou, Y.S.; Ueno, I.; Sakamoto, A.; Tong, K.I.; et al. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat. Cell Biol. 2010, 12, 213–223. [Google Scholar] [CrossRef]
- Degenhardt, K.; Mathew, R.; Beaudoin, B.; Bray, K.; Anderson, D.; Chen, G.; Mukherjee, C.; Shi, Y.; Gélinas, C.; Fan, Y.; et al. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell 2006, 10, 51–64. [Google Scholar] [CrossRef]
- Zanotelli, M.R.; Zhang, J.; Reinhart-King, C.A. Mechanoresponsive metabolism in cancer cell migration and metastasis. Cell Metab. 2021, 33, 1307–1321. [Google Scholar] [CrossRef]
- Friedl, P.; Wolf, K. Tumour-cell invasion and migration: Diversity and escape mechanisms. Nat. Rev. Cancer 2003, 3, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Wolf, K.; Friedl, P. Molecular mechanisms of cancer cell invasion and plasticity. Br. J. Dermatol. 2006, 154 (Suppl. S1), 11–15. [Google Scholar] [CrossRef] [PubMed]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.; Nieto, M.A. Epithelial-mesenchymal transitions in development and disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Kang, Y. Epithelial-Mesenchymal Plasticity in Cancer Progression and Metastasis. Dev. Cell 2019, 49, 361–374. [Google Scholar] [CrossRef]
- Valastyan, S.; Weinberg, R.A. Tumor metastasis: Molecular insights and evolving paradigms. Cell 2011, 147, 275–292. [Google Scholar] [CrossRef]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef]
- Siddhartha, R.; Garg, M. Molecular and clinical insights of matrix metalloproteinases into cancer spread and potential therapeutic interventions. Toxicol. Appl. Pharmacol. 2021, 426, 115593. [Google Scholar] [CrossRef]
- Yu, Q.; Stamenkovic, I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev. 2000, 14, 163–176. [Google Scholar] [CrossRef]
- Bergers, G.; Brekken, R.; McMahon, G.; Vu, T.H.; Itoh, T.; Tamaki, K.; Tanzawa, K.; Thorpe, P.; Itohara, S.; Werb, Z.; et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat. Cell Biol. 2000, 2, 737–744. [Google Scholar] [CrossRef]
- Kaplan, R.N.; Riba, R.D.; Zacharoulis, S.; Bramley, A.H.; Vincent, L.; Costa, C.; Macdonald, D.D.; Jin, D.K.; Shido, K.; Kerns, S.A.; et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 2005, 438, 820–827. [Google Scholar] [CrossRef]
- Mu, D.; Cambier, S.; Fjellbirkeland, L.; Baron, J.L.; Munger, J.S.; Kawakatsu, H.; Sheppard, D.; Broaddus, V.C.; Nishimura, S.L. The integrin alpha(v)beta8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-beta1. J. Cell Biol. 2002, 157, 493–507. [Google Scholar] [CrossRef] [PubMed]
- Dallas, S.L.; Rosser, J.L.; Mundy, G.R.; Bonewald, L.F. Proteolysis of latent transforming growth factor-beta (TGF-beta)-binding protein-1 by osteoclasts. A cellular mechanism for release of TGF-beta from bone matrix. J. Biol. Chem. 2002, 277, 21352–21360. [Google Scholar] [CrossRef]
- Tatti, O.; Vehviläinen, P.; Lehti, K.; Keski-Oja, J. MT1-MMP releases latent TGF-beta1 from endothelial cell extracellular matrix via proteolytic processing of LTBP-1. Exp. Cell Res. 2008, 314, 2501–2514. [Google Scholar] [CrossRef] [PubMed]
- Deryugina, E.I.; Quigley, J.P. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006, 25, 9–34. [Google Scholar] [CrossRef] [PubMed]
- Bond, M.; Chase, A.J.; Baker, A.H.; Newby, A.C. Inhibition of transcription factor NF-kappaB reduces matrix metalloproteinase-1, -3 and -9 production by vascular smooth muscle cells. Cardiovasc. Res. 2001, 50, 556–565. [Google Scholar] [CrossRef]
- Wang, W.S.; Chen, P.M.; Wang, H.S.; Liang, W.Y.; Su, Y. Matrix metalloproteinase-7 increases resistance to Fas-mediated apoptosis and is a poor prognostic factor of patients with colorectal carcinoma. Carcinogenesis 2006, 27, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Ono, M.; Takeshima, M.; Nakano, S. Antiproliferative and apoptosis-inducing activity of nobiletin against three subtypes of human breast cancer cell lines. Anticancer Res. 2014, 34, 1785–1792. [Google Scholar] [PubMed]
- Hermawan, A.; Putri, H.; Hanif, N.; Ikawati, M. Integrative Bioinformatics Study of Tangeretin Potential Targets for Preventing Metastatic Breast Cancer. Evid. Based Complement Alternat. Med. 2021, 2021, 2234554. [Google Scholar] [CrossRef]
- Ko, Y.C.; Choi, H.S.; Liu, R.; Kim, J.H.; Kim, S.L.; Yun, B.S.; Lee, D.S. Inhibitory Effects of Tangeretin, A Citrus Peel-Derived Flavonoid, on Breast Cancer Stem Cell Formation through Suppression of Stat3 Signaling. Molecules 2020, 25, 2599. [Google Scholar] [CrossRef]
- Lakshmi, A.; Subramanian, S.P. Tangeretin ameliorates oxidative stress in the renal tissues of rats with experimental breast cancer induced by 7, 12-dimethylbenz[a]anthracene. Toxicol. Lett. 2014, 229, 333–348. [Google Scholar] [CrossRef]
- Gul, H.F.; Ilhan, N.; Ilhan, N.; Ozercan, I.H.; Kuloglu, T. The combined effect of pomegranate extract and tangeretin on the DMBA-induced breast cancer model. J. Nutr. Biochem. 2021, 89, 108566. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, N.; Thamaraiselvan, R.; Lingaiah, H.; Srinivasan, P.; Periyasamy, B.M. Effect of flavonone hesperidin on the apoptosis of human mammary carcinoma cell line MCF-7. Biomed. Prev. Nutr. 2011, 1, 207–215. [Google Scholar] [CrossRef]
- Sulaiman, G.M.; Waheeb, H.M.; Jabir, M.S.; Khazaal, S.H.; Dewir, Y.H.; Naidoo, Y. Hesperidin Loaded on Gold Nanoparticles as a Drug Delivery System for a Successful Biocompatible, Anti-Cancer, Anti-Inflammatory and Phagocytosis Inducer Model. Sci. Rep. 2020, 10, 9362. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.H.; Chen, W.H.; Juan-Lu, C.; Hsieh, S.C.; Lin, S.C.; Mai, R.T.; Chen, S.Y. Hesperidin and Chlorogenic Acid Synergistically Inhibit the Growth of Breast Cancer Cells via Estrogen Receptor/Mitochondrial Pathway. Life 2021, 11, 950. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, B.; Huang, J.; XIang, T.; Yin, X.; Wan, J.; Luo, F.; Zhang, L.; Li, H.; Ren, G. Naringin inhibits growth potential of human triple-negative breast cancer cells by targeting β-catenin signaling pathway. Toxicol. Lett. 2013, 220, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.J.; Wilson, L.; Jordan, M.A.; Nguyen, V.; Tang, J.; Smiyun, G. Hesperidin suppressed proliferations of both human breast cancer and androgen-dependent prostate cancer cells. Phytother. Res. 2010, 24, S15–S19. [Google Scholar] [CrossRef]
- El-Sisi, A.E.; Sokkar, S.S.; Ibrahim, H.A.; Hamed, M.F.; Abu-Risha, S.E. Targeting MDR-1 gene expression, BAX/BCL2, caspase-3, and Ki-67 by nanoencapsulated imatinib and hesperidin to enhance anticancer activity and ameliorate cardiotoxicity. Fundam. Clin. Pharmacol. 2020, 34, 458–475. [Google Scholar] [CrossRef]
- Kongtawelert, P.; Wudtiwai, B.; Shwe, T.H.; Pothacharoen, P.; Phitak, T. Inhibitory Effect of Hesperidin on the Expression of Programmed Death Ligand (PD-L1) in Breast Cancer. Molecules 2020, 25, 252. [Google Scholar] [CrossRef] [Green Version]
- Balakrishnan, A.; Menon, V.P. Effect of hesperidin on matrix metalloproteinases and antioxidant status during nicotine-induced toxicity. Toxicology 2007, 238, 90–98. [Google Scholar] [CrossRef]
- Kamaraj, S.; Ramakrishnan, G.; Anandakumar, P.; Jagan, S.; Devaki, T. Antioxidant and anticancer efficacy of hesperidin in benzo(a)pyrene induced lung carcinogenesis in mice. Investig. New Drugs 2009, 27, 214–222. [Google Scholar] [CrossRef]
- Tan, S.; Dai, L.; Tan, P.; Liu, W.; Mu, Y.; Wang, J.; Huang, X.; Hou, A. Hesperidin administration suppresses the proliferation of lung cancer cells by promoting apoptosis via targeting the miR-132/ZEB2 signalling pathway. Int. J. Mol. Med. 2020, 46, 2069–2077. [Google Scholar] [CrossRef]
- Xia, R.; Sheng, X.; Xu, X.; Yu, C.; Lu, H. Hesperidin induces apoptosis and G0/G1 arrest in human non-small cell lung cancer A549 cells. Int. J. Mol. Med. 2018, 41, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Kamaraj, S.; Anandakumar, P.; Jagan, S.; Ramakrishnan, G.; Periyasamy, P.; Asokkumar, S.; Subramanian, R.; Devaki, T. Hesperidin inhibits cell proliferation and induces mitochondrial-mediated apoptosis in human lung cancer cells through down regulation of β-catenin/c-myc. Biocatal. Agric. Biotechnol. 2019, 18, 101065. [Google Scholar] [CrossRef]
- Xia, R.; Xu, G.; Huang, Y.; Sheng, X.; Xu, X.; Lu, H. Hesperidin suppresses the migration and invasion of non-small cell lung cancer cells by inhibiting the SDF-1/CXCR-4 pathway. Life Sci. 2018, 201, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.C.; Wu, H.; Li, P.B.; Xie, L.M.; Luo, Y.L.; Shen, J.G.; Su, W.W. Naringin attenuates EGF-induced MUC5AC secretion in A549 cells by suppressing the cooperative activities of MAPKs-AP-1 and IKKs-IκB-NF-κB signaling pathways. Eur. J. Pharmacol. 2012, 690, 207–213. [Google Scholar] [CrossRef]
- Chen, M.; Peng, W.; Hu, S.; Deng, J. miR-126/VCAM-1 regulation by naringin suppresses cell growth of human non-small cell lung cancer. Oncol. Lett. 2018, 16, 4754–4760. [Google Scholar] [CrossRef]
- Global Burden of Disease Cancer Collaboration; Fitzmaurice, C.; Akinyemiju, T.F.; Lami, F.H.A.; Alam, T.; Alizadeh-Navaei, R.; Allen, C.; Alsharif, U.; Alvis-Guzman, N.; Amini, E.; et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2016: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2018, 4, 1553–1568. [Google Scholar] [CrossRef]
- Chen, J.; Creed, A.; Chen, A.Y.; Huang, H.; Li, Z.; Rankin, G.O.; Ye, X.; Xu, G.; Chen, Y.C. Nobiletin suppresses cell viability through AKT pathways in PC-3 and DU-145 prostate cancer cells. BMC Pharmacol. Toxicol. 2014, 15, 59. [Google Scholar] [CrossRef] [Green Version]
- Zhang, N.; Wu, W.; Huang, Y.; An, L.; He, Z.; Chang, Z.; He, Z.; Lai, Y. Citrus Flavone Tangeretin Inhibits CRPC Cell Proliferation by Regulating Cx26, AKT, and AR Signaling. Evid. Based Complement. Alternat. Med. 2022, 2022, 6422500. [Google Scholar] [CrossRef]
- Zhu, W.B.; Xiao, N.; Liu, X.J. Dietary flavonoid tangeretin induces reprogramming of epithelial to mesenchymal transition in prostate cancer cells by targeting the PI3K/Akt/mTOR signaling pathway. Oncol. Lett. 2018, 15, 433–440. [Google Scholar] [CrossRef]
- Petrick, J.L.; Florio, A.A.; Znaor, A.; Ruggieri, D.; Laversanne, M.; Alvarez, C.S.; Ferlay, J.; Valery, P.C.; Bray, F.; McGlynn, K.A. International trends in hepatocellular carcinoma incidence, 1978–2012. Int. J. Cancer 2020, 147, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Shao, Y.; Jiang, Y.; Chen, F.; Liu, S.; Yu, N.; Zhang, D.; Liu, X.; Zou, L. Tangeretin inhibits hepatocellular carcinoma proliferation and migration by promoting autophagy-related BECLIN1. Cancer Manag. Res. 2019, 11, 5231–5242. [Google Scholar] [CrossRef] [PubMed]
- Yeh, M.H.; Kao, S.T.; Hung, C.M.; Liu, C.J.; Lee, K.H.; Yeh, C.C. Hesperidin inhibited acetaldehyde-induced matrix metalloproteinase-9 gene expression in human hepatocellular carcinoma cells. Toxicol. Lett. 2009, 184, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Yeh, M.H.; Kao, S.T.; Hung, C.M.; Liu, C.J.; Huang, Y.Y.; Yeh, C.C. The inhibitory effect of hesperidin on tumor cell invasiveness occurs via suppression of activator protein 1 and nuclear factor-kappaB in human hepatocellular carcinoma cells. Toxicol. Lett. 2010, 194, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Naz, H.; Tarique, M.; Ahamad, S.; Alajmi, M.F.; Hussain, A.; Rehman, M.T.; Luqman, S.; Hassan, M.I. Hesperidin-CAMKIV interaction and its impact on cell proliferation and apoptosis in the human hepatic carcinoma and neuroblastoma cells. J. Cell Biochem. 2019, 120, 15119–15130. [Google Scholar] [CrossRef]
- Banjerdpongchai, R.; Wudtiwai, B.; Khawon, P. Induction of Human Hepatocellular Carcinoma HepG2 Cell Apoptosis by Naringin. Asian Pac. J. Cancer Prev. 2016, 17, 3289–3294. [Google Scholar]
- Prabu, T.; Manju, V. Antiproliferative and apoptotic effects of naringin on diethylnitrosamine induced hepatocellular carcinoma in rats. Biomed. Aging Pathol. 2013, 3, 59–64. [Google Scholar]
- Moon, J.Y.; Cho, S.K. Nobiletin Induces Protective Autophagy Accompanied by ER-Stress Mediated Apoptosis in Human Gastric Cancer SNU-16 Cells. Molecules 2016, 21, 914. [Google Scholar] [CrossRef]
- Dong, Y.; Cao, A.; Shi, J.; Yin, P.; Wang, L.; Ji, G.; Xie, J.; Wu, D. Tangeretin, a citrus polymethoxyflavonoid, induces apoptosis of human gastric cancer AGS cells through extrinsic and intrinsic signaling pathways. Oncol. Rep. 2014, 31, 1788–1794. [Google Scholar] [CrossRef]
- Yu, W.; Xie, X.; Yu, Z.; Jin, Q.; Wu, H. Mechanism of hesperidin-induced apoptosis in human gastric cancer AGS cells. Trop. J. Pharm. Res. 2019, 50, 2363–2369. [Google Scholar]
- Zhang, X.; Zheng, L.; Sun, Y.; Wang, T.; Wang, B. Tangeretin enhances radiosensitivity and inhibits the radiation-induced epithelial-mesenchymal transition of gastric cancer cells. Oncol. Rep. 2015, 34, 302–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Song, M.; Wang, M.; Zheng, J.; Gao, Z.; Xu, F.; Zhang, G.; Xiao, H. Chemopreventive effects of nobiletin and its colonic metabolites on colon carcinogenesis. Mol Nutr Food Res. 2015, 59, 2383–2394. [Google Scholar] [CrossRef]
- Kawabata, K.; Murakami, A.; Ohigashi, H. Nobiletin, a citrus flavonoid, down-regulates matrix metalloproteinase-7 (matrilysin) expression in HT-29 human colorectal cancer cells. Biosci. Biotechnol. Biochem. 2005, 69, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Dey, D.K.; Chang, S.N.; Vadlamudi, Y.; Park, J.G.; Kang, S.C. Synergistic therapy with tangeretin and 5-fluorouracil accelerates the ROS/JNK mediated apoptotic pathway in human colorectal cancer cell. Food Chem. Toxicol. 2020, 143, 111529. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Kim, M.J.; Ha, E.; Chung, J.H. Apoptotic effect of hesperidin through caspase3 activation in human colon cancer cells, SNU-C4. Phytomedicine 2008, 15, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Yang, H.; Dong, X.; Pu, M.; Ji, F. Hesperidin loaded Zn2+@ SA/PCT nanocomposites inhibit the proliferation and induces the apoptosis in colon cancer cells (HCT116) through the enhancement of pro-apoptotic protein expressions. J. Photochem. Photobiol. B 2020, 204, 111767. [Google Scholar] [CrossRef] [PubMed]
- Saiprasad, G.; Chitra, P.; Manikandan, R.; Sudhandiran, G. Hesperidin induces apoptosis and triggers autophagic markers through inhibition of Aurora-A mediated phosphoinositide-3-kinase/Akt/mammalian target of rapamycin and glycogen synthase kinase-3 beta signalling cascades in experimental colon carcinogenesis. Eur. J. Cancer 2014, 50, 2489–2507. [Google Scholar] [CrossRef]
- Saiprasad, G.; Chitra, P.; Manikandan, R.; Sudhandiran, G. Hesperidin alleviates oxidative stress and downregulates the expressions of proliferative and inflammatory markers in azoxymethane-induced experimental colon carcinogenesis in mice. Inflamm. Res. 2013, 62, 425–440. [Google Scholar] [CrossRef]
- Cheng, H.; Jiang, X.; Zhang, Q.; Alma, J.; Cheng, R.; Yong, H.; Shi, H.; Zhou, X.; Ge, L.; Gao, G. Naringin inhibits colorectal cancer cell growth by repressing the PI3K/AKT/mTOR signaling pathway. Exp. Ther. Med. 2020, 19, 3798–3804. [Google Scholar] [CrossRef]
- Yang, F.; Jiang, T.; Zhang, L.; Qi, Y.; Yang, Z. Naringin inhibits the proliferation and invasion of Eca109 esophageal cancer cells and promotes its apoptosis by blocking JAK/STAT signal pathway. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2021, 37, 1085–1091. [Google Scholar]
- Xu, W.W.; Zheng, C.C.; Huang, Y.N.; Chen, W.Y.; Yang, Q.S.; Ren, J.Y.; Wang, Y.M.; He, Q.Y.; Liao, H.X.; Li, B. Synephrine Hydrochloride Suppresses Esophageal Cancer Tumor Growth and Metastatic Potential through Inhibition of Galectin-3-AKT/ERK Signaling. J. Agric. Food Chem. 2018, 66, 9248–9258. [Google Scholar] [CrossRef] [PubMed]
- Yoshinaga, A.; Kajiya, N.; Oishi, K.; Kamada, Y.; Ikeda, A.; Chiwechokha, P.K.; Kibe, T.; Kishida, M.; Kishida, S.; Komatsu, M.; et al. NEU3 inhibitory effect of naringin suppresses cancer cell growth by attenuation of EGFR signaling through GM3 ganglioside accumulation. Eur. J. Pharmacol. 2016, 782, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Hu, X.; Chen, S.; Shi, Q.; Chen, H. Naringin induces endoplasmic reticulum stress-mediated apoptosis, inhibits β-catenin pathway and arrests cell cycle in cervical cancer cells. Acta Biochim. Pol. 2020, 67, 181–188. [Google Scholar] [PubMed]
- Ramesh, E.; Alshatwi, A.A. Naringin induces death receptor and mitochondria-mediated apoptosis in human cervical cancer (SiHa) cells. Food Chem. Toxicol. 2013, 51, 97–105. [Google Scholar] [CrossRef]
- Zeng, L.; Zhen, Y.; Chen, Y.; Zou, L.; Zhang, Y.; Hu, F.; Feng, J.; Shen, J.; Wei, B. Naringin inhibits growth and induces apoptosis by a mechanism dependent on reduced activation of NF-κB/COX-2-caspase-1 pathway in HeLa cervical cancer cells. Int. J. Oncol. 2014, 45, 1929–1936. [Google Scholar] [CrossRef]
- Goan, Y.G.; Wu, W.T.; Liu, C.I.; Neoh, C.A.; Wu, Y.J. Involvement of Mitochondrial Dysfunction, Endoplasmic Reticulum Stress, and the PI3K/AKT/mTOR Pathway in Nobiletin-Induced Apoptosis of Human Bladder Cancer Cells. Molecules 2019, 24, 2881. [Google Scholar] [CrossRef]
- Lin, J.J.; Huang, C.C.; Su, Y.L.; Luo, H.L.; Lee, N.L.; Sung, M.T.; Wu, Y.J. Proteomics Analysis of Tangeretin-Induced Apoptosis through Mitochondrial Dysfunction in Bladder Cancer Cells. Int. J. Mol. Sci. 2019, 20, 1017. [Google Scholar] [CrossRef]
- Cheng, H.L.; Hsieh, M.J.; Yang, J.S.; Lin, C.W.; Lue, K.H.; Lu, K.H.; Yang, S.F. Nobiletin inhibits human osteosarcoma cells metastasis by blocking ERK and JNK-mediated MMPs expression. Oncotarget 2016, 7, 35208–35223. [Google Scholar] [CrossRef]
- Tan, T.W.; Chou, Y.E.; Yang, W.H.; Hsu, C.J.; Fong, Y.C.; Tang, C.H. Naringin suppress chondrosarcoma migration through inhibition vascular adhesion molecule-1 expression by modulating miR-126. Int. Immunopharmacol. 2014, 22, 107–114. [Google Scholar] [CrossRef]
- Lien, L.M.; Wang, M.J.; Chen, R.J.; Chiu, H.C.; Wu, J.L.; Shen, M.Y.; Chou, D.S.; Sheu, J.R.; Lin, K.H.; Lu, W.J. Nobiletin, a Polymethoxylated Flavone, Inhibits Glioma Cell Growth and Migration via Arresting Cell Cycle and Suppressing MAPK and Akt Pathways. Phytother. Res. 2016, 30, 214–221. [Google Scholar] [CrossRef]
- Ma, L.L.; Wang, D.W.; Yu, X.D. Tangeretin induces cell cycle arrest and apoptosis through upregulation of PTEN expression in glioma cells. Biomed. Pharmacother. 2016, 81, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Sonia, A.; Bakhta, A.; Yassine, C. Naringin suppresses cell metastasis and the expression of matrix metalloproteinases (MMP-2 and MMP-9) via the inhibition of ERK-P38-JNK signaling pathway in human glioblastoma. Chem.-Biolog. Interact. 2016, 244, 195–203. [Google Scholar]
- Li, J.; Dong, Y.; Hao, G.; Wang, B.; Wang, J.; Liang, Y.; Liu, Y.; Zhen, E.; Feng, D.; Liang, G. Naringin suppresses the development of glioblastoma by inhibiting FAK activity. J. Drug Target. 2017, 25, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Camargo, C.A.; Gomes-Marcondes, M.C.; Wutzki, N.C.; Aoyama, H. Naringin inhibits tumor growth and reduces interleukin-6 and tumor necrosis factor α levels in rats with Walker 256 carcinosarcoma. Anticancer Res. 2012, 32, 129–133. [Google Scholar] [PubMed]
- Pandey, P.; Sayyed, U.; Tiwari, R.K.; Siddiqui, M.H.; Pathak, N.; Bajpai, P. Hesperidin Induces ROS-Mediated Apoptosis along with Cell Cycle Arrest at G2/M Phase in Human Gall Bladder Carcinoma. Nutr. Cancer. 2019, 71, 676–687. [Google Scholar] [CrossRef]
- Cincin, Z.B.; Kiran, B.; Baran, Y.; Cakmakoglu, B. Hesperidin promotes programmed cell death by downregulation of nongenomic estrogen receptor signalling pathway in endometrial cancer cells. Biomed. Pharmacother. 2018, 103, 336–345. [Google Scholar] [CrossRef]
- Lee, K.A.; Lee, S.H.; Lee, Y.J.; Baeg, S.M.; Shim, J.H. Hesperidin induces apoptosis by inhibiting Sp1 and its regulatory protein in MSTO-211H cells. Biomol. Ther. 2012, 20, 273–279. [Google Scholar] [CrossRef]
- Nazari, M.; Ghorbani, A.; Hekmat-Doost, A.; Jeddi-Tehrani, M.; Zand, H. Inactivation of nuclear factor-κB by citrus flavanone hesperidin contributes to apoptosis and chemo-sensitizing effect in Ramos cells. Eur. J. Pharmacol. 2011, 650, 526–533. [Google Scholar] [CrossRef]
- Ghorbani, A.; Nazari, M.; Jeddi-Tehrani, M.; Zand, H. The citrus flavonoid hesperidin induces p53 and inhibits NF-κB activation in order to trigger apoptosis in NALM-6 cells: Involvement of PPARγ-dependent mechanism. Eur. J. Nutr. 2012, 51, 39–46. [Google Scholar] [CrossRef]
- Shahbazi, R.; Cheraghpour, M.; Homayounfar, R.; Nazari, M.; Nasrollahzadeh, J.; Davoodi, S.H. Hesperidin inhibits insulin-induced phosphoinositide 3-kinase/Akt activation in human pre-B cell line NALM-6. J. Cancer Res. Ther. 2018, 14, 503–508. [Google Scholar]
- Berkarda, B.; Koyuncu, H.; Soybir, G.; Baykut, F. Inhibitory effect of Hesperidin on tumor initiation and promotion in mouse skin. Res. Exp. Med. 1998, 198, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Wudtiwai, B.; Makeudom, A.; Krisanaprakornkit, S.; Pothacharoen, P.; Kongtawelert, P. Anticancer Activities of Hesperidin via Suppression of Up-Regulated Programmed Death-Ligand 1 Expression in Oral Cancer Cells. Molecules 2021, 26, 5345. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Makita, H.; Ohnishi, M.; Hirose, Y.; Wang, A.; Mori, H.; Satoh, K.; Hara, A.; Ogawa, H. Chemoprevention of 4-nitroquinoline 1-oxide-induced oral carcinogenesis by dietary curcumin and hesperidin: Comparison with the protective effect of beta-carotene. Cancer Res. 1994, 54, 4653–4659. [Google Scholar] [PubMed]
- Tanaka, T.; Makita, H.; Ohnishi, M.; Mori, H.; Satoh, K.; Hara, A.; Sumida, T.; Fukutani, K.; Tanaka, T.; Ogawa, H. Chemoprevention of 4-nitroquinoline 1-oxide-induced oral carcinogenesis in rats by flavonoids diosmin and hesperidin, each alone and in combination. Cancer Res. 1997, 57, 246–252. [Google Scholar]
- Lin, C.X.; Tu, C.W.; Ma, Y.K.; Ye, P.C.; Shao, X.; Yang, Z.A.; Fang, Y.M. Nobiletin inhibits cell growth through restraining aerobic glycolysis via PKA-CREB pathway in oral squamous cell carcinoma. Food Sci. Nutr. 2020, 8, 3515–3524. [Google Scholar] [CrossRef]
- Murakami, A.; Nakamura, Y.; Torikai, K.; Tanaka, T.; Koshiba, T.; Koshimizu, K.; Kuwahara, S.; Takahashi, Y.; Ogawa, K.; Yano, M.; et al. Inhibitory effect of citrus nobiletin on phorbol ester-induced skin inflammation, oxidative stress, and tumor promotion in mice. Cancer Res. 2000, 60, 5059–5066. [Google Scholar]
- Sato, T.; Koike, L.; Miyata, Y.; Hirata, M.; Mimaki, Y.; Sashida, Y.; Yano, M.; Ito, A. Inhibition of activator protein-1 binding activity and phosphatidylinositol 3-kinase pathway by nobiletin, a polymethoxy flavonoid, results in augmentation of tissue inhibitor of metalloproteinases-1 production and suppression of production of matrix metalloproteinases-1 and -9 in human fibrosarcoma HT-1080 cells. Cancer Res. 2002, 62, 1025–1029. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).