Comparison of the Characteristics of Recycled Carbon Fibers/Polymer Composites by Different Recycling Techniques
Abstract
:1. Introduction
2. Results and Discussion
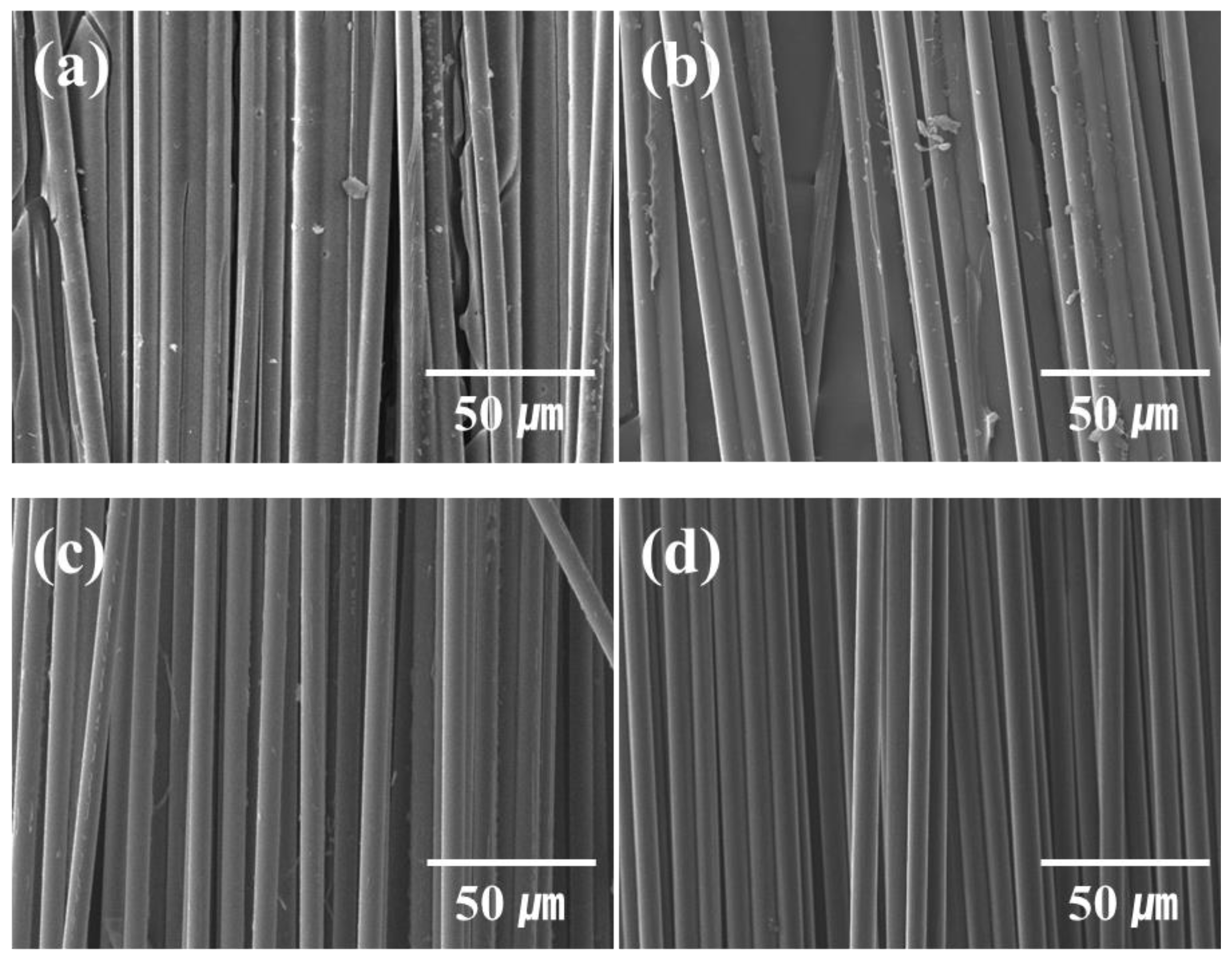
2.1. Characteristics of the CFRPs Acquired through the Studied Recycling Techniques
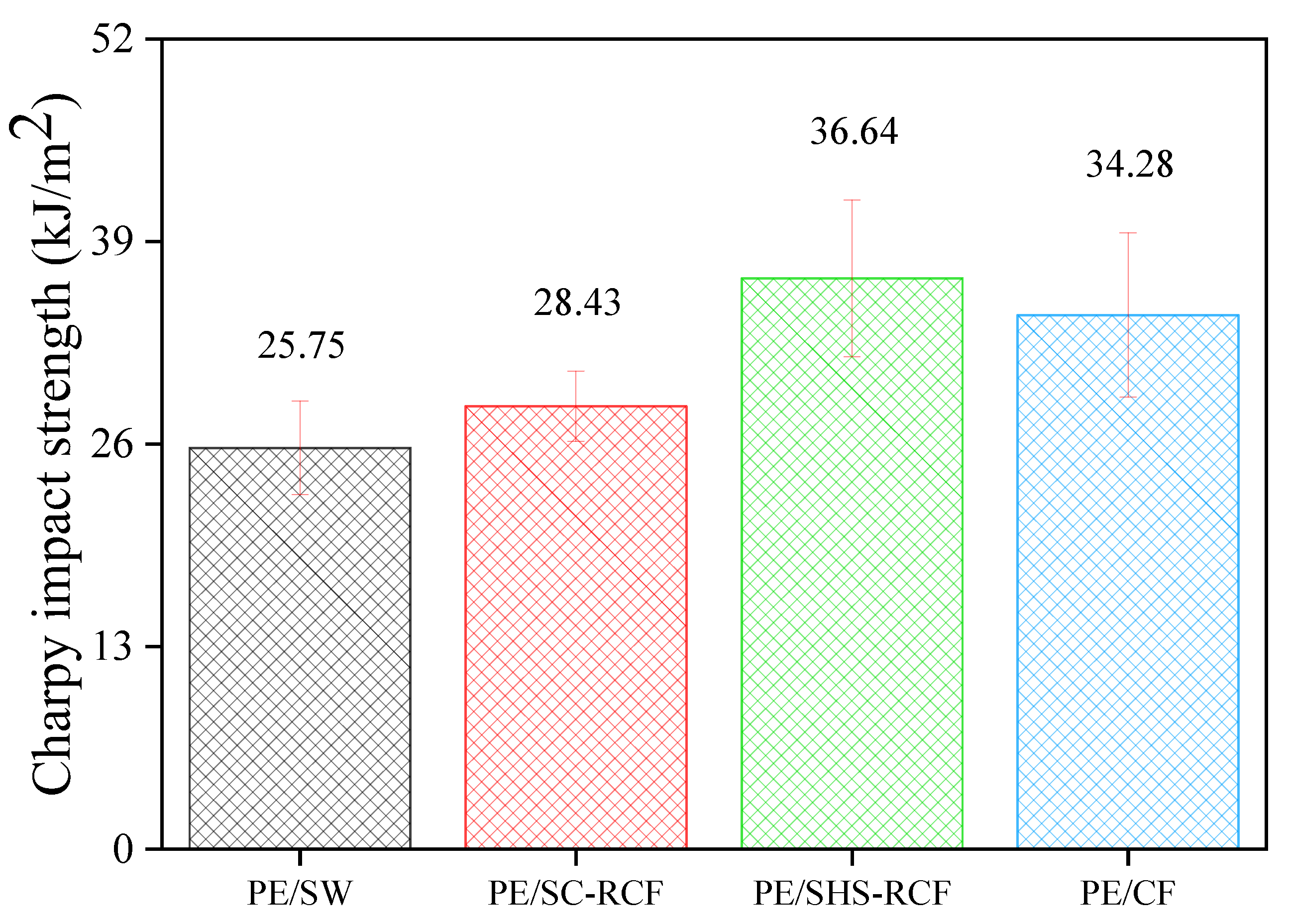
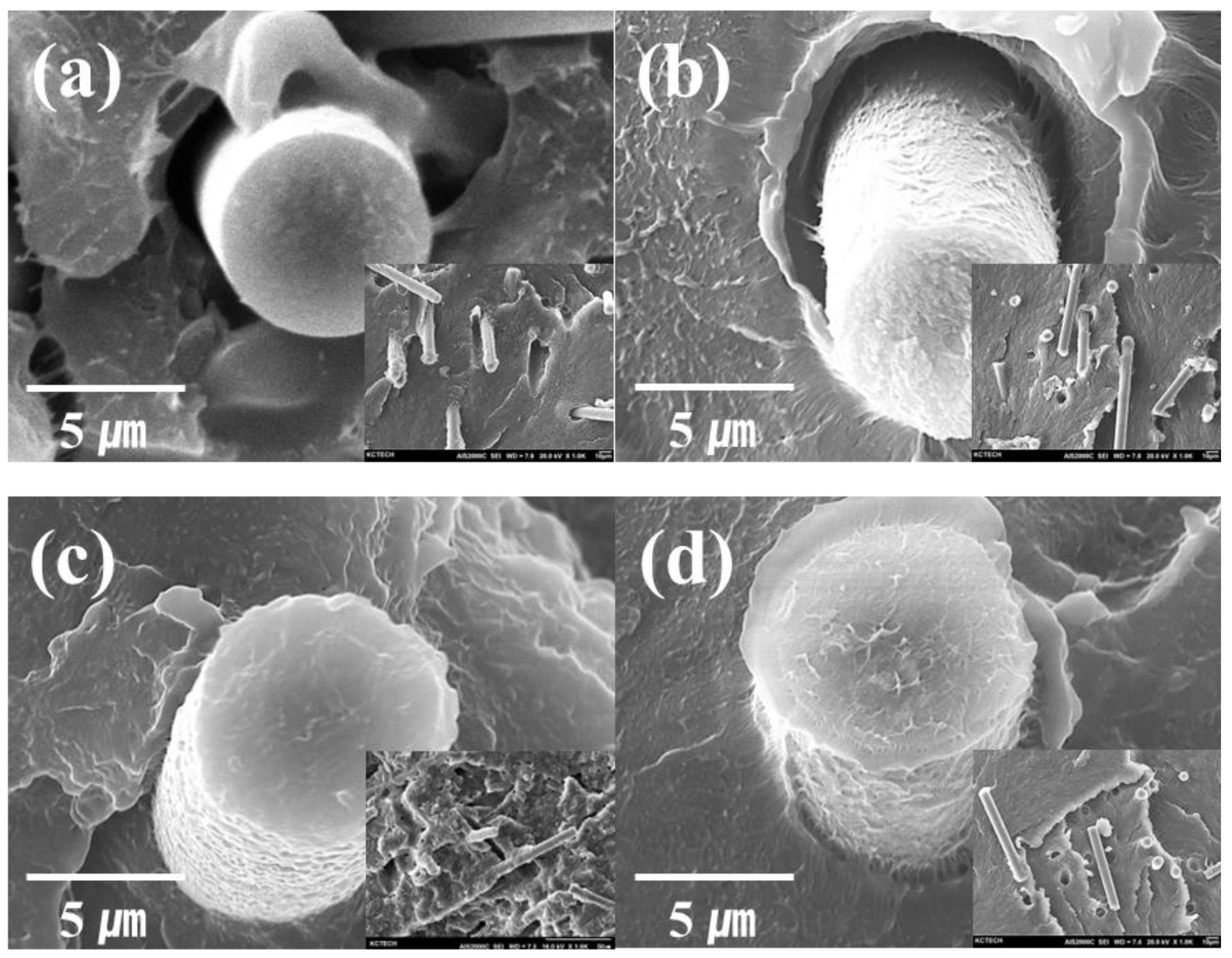
2.2. Physical Characteristics of the Various RCF-Reinforced Composites
2.3. Thermal and Electrical Properties of RCF-Reinforced Composites
3. Materials and Methods
3.1. Materials
3.2. Sample Preparation
3.3. Characterization of Samples
3.4. Measurement of Physical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Yang, J.; Liu, J.; Liu, W.; Wang, J.; Tang, T. Recycling of carbon fibre reinforced epoxy resin composites under various oxygen concentrations in nitrogen–oxygen atmosphere. J. Anal. Appl. Pyrolysis 2015, 112, 253–261. [Google Scholar] [CrossRef]
- Xu, Y.; Dai, S.; Bi, L.; Jiang, J.; Zhang, H.; Chen, Y. Catalyst-free self-healing bio-based vitrimer for a recyclable, reprocessable, and self-adhered carbon fiber reinforced composite. Chem. Eng. J. 2022, 429, 132518. [Google Scholar] [CrossRef]
- Pei, C.; Chen, P.Y.; Kong, S.C.; Wu, J.; Zhu, J.H.; Xing, F. Recyclable separation and recovery of carbon fibers from CFRP composites: Optimization and mechanism. Sep. Purif. Technol. 2021, 278, 119591. [Google Scholar] [CrossRef]
- Jeong, J.S.; Kim, K.W.; Chung, D.C.; An, K.H.; Kim, B.J. A study on the correlation between microstructure change and the mechanical strength of a single CF modified by electron beam. Carbon Lett. 2022, 32, 567–579. [Google Scholar] [CrossRef]
- Lee, D.K.; Kim, Y.E.; Kwon, O.H.; Park, W.H.; Cho, D.H. Carbon fiber coating with MWCNT in the presence of polyethyleneimine of different molecular weights and the effect on the interfacial shear strength of thermoplastic and thermosetting carbon fiber composites. Carbon Lett. 2021, 31, 407–417. [Google Scholar] [CrossRef]
- Wang, X.; Qian, X.; Zhang, Y.; Wang, X.; Song, S.; Zhang, C. Surface oxidation of PAN-based ultrahigh modulus carbon fibers (UHMCFs) and its effect on the properties of UHMCF/EP composites. Carbon Lett. 2021, 31, 449–461. [Google Scholar] [CrossRef]
- Xing, M.; Li, Z.; Zheng, G.; Du, Y.; Chen, C.; Wang, Y. Recycling of carbon fiber-reinforced epoxy resin composite via a novel acetic acid swelling technology. Compos. Part B Eng. 2021, 224, 109230. [Google Scholar] [CrossRef]
- Palmay, P.; Haro, C.; Huacho, I.; Barzallo, D.; Bruno, J.C. Production and Analysis of the Physicochemical Properties of the Pyrolytic Oil Obtained from Pyrolysis of Different Thermoplastics and Plastic Mixtures. Molecules 2022, 27, 3287. [Google Scholar] [CrossRef]
- Park, J.M.; Kwon, D.J.; Wang, Z.J.; Gu, G.Y.; DeVries, K.L. Effect of thermal treatment temperatures on the reinforcing and interfacial properties of recycled carbon fiber–phenolic composites. Compos. Part A Appl. Sci. Manuf. 2013, 47, 156–164. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Jiang, Z.; Tang, T. Chemical recycling of carbon fibre reinforced epoxy resin composites in subcritical water: Synergistic effect of phenol and KOH on the decomposition efficiency. Polym. Degrad. Stab. 2012, 97, 214–220. [Google Scholar] [CrossRef]
- Feraboli, P.; Kawakami, H.; Wade, B.; Gasco, F.; DeOto, L.; Masini, A. Recyclability and reutilization of carbon fiber fabric/epoxy composites. J. Compos. Mater. 2011, 46, 1459–1473. [Google Scholar] [CrossRef]
- Palmer, J.; Ghita, O.R.; Savage, L.; Evans, K.E. Successful closed-loop recycling of thermoset composites. Compos. Part A Appl. Sci. Manuf. 2009, 40, 490–498. [Google Scholar] [CrossRef]
- Kim, D.H.; Lee, M.; Goh, M. Enhanced and Eco-Friendly Recycling of Carbon-Fiber-Reinforced Plastics Using Water at Ambient Pressure. ACS Sustain. Chem. Eng. 2019, 8, 2433–2440. [Google Scholar] [CrossRef]
- Lee, M.; Kim, D.H.; Park, J.J.; You, N.H.; Goh, M. Fast chemical recycling of carbon fiber reinforced plastic at ambient pressure using an aqueous solvent accelerated by a surfactant. Waste Manag. 2021, in press. [Google Scholar] [CrossRef]
- Mustata, F.; Tudorachi, N.; Bicu, I. The kinetic study and thermal characterization of epoxy resins crosslinked with amino carboxylic acids. J. Anal. Appl. Pyrolysis 2015, 112, 180–191. [Google Scholar] [CrossRef]
- Mazzocchetti, L.; Benelli, T.; D’Angelo, E.; Leonardi, C.; Zattini, G.; Giorgini, L. Validation of carbon fibers recycling by pyro-gasification: The influence of oxidation conditions to obtain clean fibers and promote fiber/matrix adhesion in epoxy composites. Compos. Part A Appl. Sci. Manuf. 2018, 112, 504–514. [Google Scholar] [CrossRef]
- Nahil, M.A.; Williams, P.T. Recycling of carbon fibre reinforced polymeric waste for the production of activated carbon fibres. J. Anal. Appl. Pyrolysis 2011, 91, 67–75. [Google Scholar] [CrossRef]
- Obunai, K.; Fukuta, T.; Ozaki, K. Carbon fiber extraction from waste CFRP by microwave irradiation. Compos. Part A Appl. Sci. Manuf. 2015, 78, 160–165. [Google Scholar] [CrossRef]
- Braun, D.; Von Gentzkow, W.; Rudolf, A.P. Hydrogenolytic degradation of thermosets. Polym. Degrad. Stab. 2011, 74, 25–32. [Google Scholar] [CrossRef]
- Dang, W.; Kubouchi, M.; Sembokuya, H.; Tsuda, K. Chemical recycling of glass fiber reinforced epoxy resin cured with amine using nitric acid. Polymer 2005, 46, 1905–1912. [Google Scholar] [CrossRef]
- Gong, X.; Kang, H.; Liu, Y.; Wu, S. Decomposition mechanisms and kinetics of amine/anhydride-cured DGEBA epoxy resin in near-critical water. RSC Adv. 2015, 5, 40269–40282. [Google Scholar] [CrossRef]
- Prinçaud, M.; Aymonier, C.; Loppinet-Serani, A.; Perry, N.; Sonnemann, G. Environmental Feasibility of the Recycling of Carbon Fibers from CFRPs by Solvolysis Using Supercritical Water. ACS Sustain. Chem. Eng. 2014, 2, 1498–1502. [Google Scholar] [CrossRef]
- Kim, K.W.; Lee, H.M.; An, J.H.; Chung, D.C.; An, K.H.; Kim, B.J. Recycling and characterization of carbon fibers from carbon fiber reinforced epoxy matrix composites by a novel super-heated-steam method. J. Environ. Manag. 2017, 203, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.W.; Jeong, J.S.; An, K.H.; Kim, B.J. A Low Energy Recycling Technique of Carbon Fibers-Reinforced Epoxy Matrix Composites. Ind. Eng. Chem. Res. 2019, 58, 618–624. [Google Scholar] [CrossRef]
- Jeong, J.S.; Kim, K.W.; An, K.H.; Kim, B.J. Fast recovery process of carbon fibers from waste carbon fibers-reinforced thermoset plastics. J. Environ. Manag. 2019, 247, 816–821. [Google Scholar] [CrossRef]
- Niakantan, G.; Nutt, S. Reuse and upcycling of thermoset prepreg scrap: Case study with out-of-autoclave carbon fiber/epoxy prepreg. J. Compos. Mater. 2017, 52, 341–360. [Google Scholar] [CrossRef]
- Stanescu, M.D. State of the art of post-consumer textile waste upcycling to reach the zero waste milestone. Environ. Sci. Pollut. Res. 2021, 28, 14253–14270. [Google Scholar] [CrossRef]
- Kim, K.W.; Jeong, J.S.; An, K.H.; Kim, B.J. A study on the microstructural changes and mechanical behaviors of carbon fibers induced by optimized electrochemical etching. Compos. Part B Eng. 2019, 165, 764–771. [Google Scholar] [CrossRef]
- Kim, K.W.; Kim, D.K.; Kim, B.S.; An, K.H.; Park, S.J.; Rhee, K.Y.; Kim, B.J. Cure behaviors and mechanical properties of carbon fiber-reinforced nylon6/epoxy blended matrix composites. Compos. Part B Eng. 2017, 112, 15–21. [Google Scholar] [CrossRef]
- ASTM Standard D6110; Standard Test Method for Determining the Charpy Impact Resistance of Notched Specimens of Plastic. ASTM International: West Conshohocken, PA, USA, 2017. [CrossRef]

| Property | Units | Sample Name | ||
|---|---|---|---|---|
| SC-RCF | * SHS-RCF | * CF(T700) | ||
| Tensile strength | GPa | 3.42 | 3.88 | 4.28 |
| Interfacial shear strength | MPa | 33.62 | 47.06 | 39.19 |
| Oxygen content | % | 13.37 | 14.29 | 8.79 |
| Polarity value | mN/m | - | 12.52 | 5.66 |
| Surface free energy | mN/m | - | 35.35 | 27.77 |
| Property | Units | Sample Name | |
|---|---|---|---|
| PE/SHS-RCF | PE/CF | ||
| Heat conductivity | W/mK | 1.87 | 1.76 |
| Resistivity | Ω·cm | 3.2 × 10−3 | 1.5 × 10−2 |
| Sample Name | Filler Type | LDPE (g) | Filler (g) | Temperature (°C) | Mix (rpm) | Time (min) |
|---|---|---|---|---|---|---|
| PE/SW | CFRP scrap wastes | 150 | 30 | 130 | 70 | 30 |
| PE/SC-RCF | Recycled CFs(supercritical) | |||||
| PE/SHS-RCF | Recycled CFs (superheated steam) | |||||
| PE/CF | T-700 CFs |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.-W.; Kim, D.-K.; Han, W.; Kim, B.-J. Comparison of the Characteristics of Recycled Carbon Fibers/Polymer Composites by Different Recycling Techniques. Molecules 2022, 27, 5663. https://doi.org/10.3390/molecules27175663
Kim K-W, Kim D-K, Han W, Kim B-J. Comparison of the Characteristics of Recycled Carbon Fibers/Polymer Composites by Different Recycling Techniques. Molecules. 2022; 27(17):5663. https://doi.org/10.3390/molecules27175663
Chicago/Turabian StyleKim, Kwan-Woo, Dong-Kyu Kim, Woong Han, and Byung-Joo Kim. 2022. "Comparison of the Characteristics of Recycled Carbon Fibers/Polymer Composites by Different Recycling Techniques" Molecules 27, no. 17: 5663. https://doi.org/10.3390/molecules27175663
APA StyleKim, K.-W., Kim, D.-K., Han, W., & Kim, B.-J. (2022). Comparison of the Characteristics of Recycled Carbon Fibers/Polymer Composites by Different Recycling Techniques. Molecules, 27(17), 5663. https://doi.org/10.3390/molecules27175663







