Whole-Genome Analysis of Acinetobacter baumannii Strain AB43 Containing a Type I-Fb CRISPR-Cas System: Insights into the Relationship with Drug Resistance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strain
2.2. Genome Sequencing and Assembling
2.3. Gene Prediction and Annotation
2.4. Multilocus Sequence Typing
2.5. Sequence Alignment and Phylogenetic Analysis
3. Results
3.1. Genome Sequence and General Features
3.2. CRISPR Array Analysis
3.3. Sequence Alignment and Phylogenetic Tree Analysis
3.4. Genes Related to Antibiotic Resistance
3.5. Insertion Sequences
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xie, R.; Zhang, X.D.; Zhao, Q.; Peng, B.; Zheng, J. Analysis of global prevalence of antibiotic resistance in Acinetobacter baumannii infections disclosed a faster increase in OECD countries. Emerg. Microbes Infect. 2018, 7, 31. [Google Scholar] [CrossRef] [PubMed]
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S.; Edwards, J.E.; Gilbert, D.; Rice, L.B.; Scheld, M.; Spellberg, B.; Bartlett, J. Bad bugs, no drugs: No ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2009, 48, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Makarova, K.S.; Koonin, E.V. Annotation and Classification of CRISPR-Cas Systems. Methods Mol. Biol. 2015, 1311, 47–75. [Google Scholar] [PubMed]
- Makarova, K.S.; Haft, D.H.; Barrangou, R.; Brouns, S.J.; Charpentier, E.; Horvath, P.; Moineau, S.; Mojica, F.J.; Wolf, Y.I.; Yakunin, A.F.; et al. Evolution and classification of the CRISPR-Cas systems. Nat. Rev. Microbiol. 2011, 9, 467–477. [Google Scholar] [CrossRef]
- Goren, M.; Yosef, I.; Qimron, U. Sensitizing pathogens to antibiotics using the CRISPR-Cas system. Drug Resist. Updates 2017, 30, 1–6. [Google Scholar] [CrossRef]
- Bikard, D.; Hatoum-Aslan, A.; Mucida, D.; Marraffini, L.A. CRISPR interference can prevent natural transformation and virulence acquisition during in vivo bacterial infection. Cell Host Microbe 2012, 12, 177–186. [Google Scholar] [CrossRef]
- Karah, N.; Samuelsen, Ø.; Zarrilli, R.; Sahl, J.W.; Wai, S.N.; Uhlin, B.E. CRISPR-cas subtype I-Fb in Acinetobacter baumannii: Evolution and utilization for strain subtyping. PLoS ONE 2015, 10, e0118205. [Google Scholar] [CrossRef]
- Liang, C.; Zhang, X.; Zhou, L.; Meng, G.; Zhong, L.; Peng, P. Trends and correlation between antibacterial consumption and carbapenem resistance in gram-negative bacteria in a tertiary hospital in China from 2012 to 2019. BMC Infect. Dis. 2021, 21, 444. [Google Scholar] [CrossRef]
- Guo, T.; Sun, X.; Li, M.; Wang, Y.; Jiao, H.; Li, G. The Involvement of the csy1 Gene in the Antimicrobial Resistance of Acinetobacter baumannii. Front. Med. 2022, 9, 797104. [Google Scholar] [CrossRef]
- Wiedenheft, B.; van Duijn, E.; Bultema, J.B.; Waghmare, S.P.; Zhou, K.; Barendregt, A.; Westphal, W.; Heck, A.J.; Boekema, E.J.; Dickman, M.J.; et al. RNA-guided complex from a bacterial immune system enhances target recognition through seed sequence interactions. Proc. Natl. Acad. Sci. USA 2011, 108, 10092–10097. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Yang, J.; Sun, X.; Li, M.; Zhang, P.; Zhu, Z.; Jiao, H.; Guo, T.; Li, G. CRISPR-Cas in Acinetobacter baumannii Contributes to Antibiotic Susceptibility by Targeting Endogenous AbaI. Microbiol. Spectr. 2022, 10, e00829-22. [Google Scholar] [CrossRef]
- Delcher, A.L.; Bratke, K.A.; Powers, E.C.; Salzberg, S.L. Identifying bacterial genes and endosymbiont DNA with Glimmer. Bioinformatics 2007, 23, 673–679. [Google Scholar] [CrossRef]
- Lowe, T.M.; Eddy, S.R. tRNAscan-SE: A program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 1997, 25, 955–964. [Google Scholar] [CrossRef]
- Lagesen, K.; Hallin, P.; Rødland, E.A.; Staerfeldt, H.H.; Rognes, T.; Ussery, D.W. RNAmmer: Consistent and rapid annotation of ribosomal RNA genes. Nucleic Acids Res. 2007, 35, 3100–3108. [Google Scholar] [CrossRef]
- Gardner, P.P.; Daub, J.; Tate, J.G.; Nawrocki, E.P.; Kolbe, D.L.; Lindgreen, S.; Wilkinson, A.C.; Finn, R.D.; Griffiths-Jones, S.; Eddy, S.R.; et al. Rfam: Updates to the RNA families database. Nucleic Acids Res. 2009, 37, D136–D140. [Google Scholar] [CrossRef]
- Zhou, Y.; Liang, Y.; Lynch, K.H.; Dennis, J.J.; Wishart, D.S. PHAST: A fast phage search tool. Nucleic Acids Res. 2011, 39, W347–W352. [Google Scholar] [CrossRef]
- Zhang, Z.; Schwartz, S.; Wagner, L.; Miller, W. A greedy algorithm for aligning DNA sequences. J. Comput. Biol. 2000, 7, 203–214. [Google Scholar] [CrossRef]
- Kanehisa, M.; Sato, Y.; Kawashima, M.; Furumichi, M.; Tanabe, M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 2016, 44, D457–D462. [Google Scholar] [CrossRef]
- Galperin, M.Y.; Makarova, K.S.; Wolf, Y.I.; Koonin, E.V. Expanded microbial genome coverage and improved protein family annotation in the COG database. Nucleic Acids Res. 2015, 43, D261–D269. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef] [Green Version]
- Gene Ontology Consortium. The Gene Ontology resource: Enriching a GOld mine. Nucleic Acids Res. 2021, 49, D325–D334. [Google Scholar] [CrossRef]
- Bairoch, A.; Apweiler, R. The SWISS-PROT protein sequence data bank and its supplement TrEMBL in 1999. Nucleic Acids Res. 1999, 27, 49–54. [Google Scholar] [CrossRef]
- Huerta-Cepas, J.; Szklarczyk, D.; Heller, D.; Hernández-Plaza, A.; Forslund, S.K.; Cook, H.; Mende, D.R.; Letunic, I.; Rattei, T.; Jensen, L.J.; et al. eggNOG 5.0: A hierarchical, functionally and phylogenetically annotated orthology resource based on 5090 organisms and 2502 viruses. Nucleic Acids Res. 2019, 47, D309–D314. [Google Scholar] [CrossRef]
- Alcock, B.P.; Raphenya, A.R.; Lau, T.T.Y.; Tsang, K.K.; Bouchard, M.; Edalatmand, A.; Huynh, W.; Nguyen, A.V.; Cheng, A.A.; Liu, S.; et al. CARD 2020: Antibiotic resistome surveillance with the comprehensive antibiotic resistance database. Nucleic Acids Res. 2020, 48, D517–D525. [Google Scholar] [CrossRef] [PubMed]
- Couvin, D.; Bernheim, A.; Toffano-Nioche, C.; Touchon, M.; Michalik, J.; Néron, B.; Rocha, E.P.C.; Vergnaud, G.; Gautheret, D.; Pourcel, C. CRISPRCasFinder, an update of CRISRFinder, includes a portable version, enhanced performance and integrates search for Cas proteins. Nucleic Acids Res. 2018, 46, W246–W251. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.; Gagnon, J.N.; Brouns, S.J.; Fineran, P.C.; Brown, C.M. CRISPRTarget: Bioinformatic prediction and analysis of crRNA targets. RNA Biol. 2013, 10, 817–827. [Google Scholar] [CrossRef] [PubMed]
- Jolley, K.A.; Bray, J.E.; Maiden, M.C.J. Open-access bacterial population genomics: BIGSdb software, the PubMLST.org website and their applications. Wellcome Open Res. 2018, 3, 124. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Palmer, K.L.; Gilmore, M.S. Multidrug-resistant enterococci lack CRISPR-cas. mBio 2010, 1, e00227-10. [Google Scholar] [CrossRef]
- Kyriakidis, I.; Vasileiou, E.; Pana, Z.D.; Tragiannidis, A. Acinetobacter baumannii Antibiotic Resistance Mechanisms. Pathogens 2021, 10, 373. [Google Scholar] [CrossRef]
- Cannon, R.D.; Lamping, E.; Holmes, A.R.; Niimi, K.; Baret, P.V.; Keniya, M.V.; Tanabe, K.; Niimi, M.; Goffeau, A.; Monk, B.C. Efflux-mediated antifungal drug resistance. Clin. Microbiol. Rev. 2009, 22, 291–321. [Google Scholar] [CrossRef]
- Gholizadeh, P.; Köse, Ş.; Dao, S.; Ganbarov, K.; Tanomand, A.; Dal, T.; Aghazadeh, M.; Ghotaslou, R.; Ahangarzadeh Rezaee, M.; Yousefi, B.; et al. How CRISPR-Cas System Could Be Used to Combat Antimicrobial Resistance. Infect. Drug Resist. 2020, 13, 1111–1121. [Google Scholar] [CrossRef]
- Friedland, A.E.; Tzur, Y.B.; Esvelt, K.M.; Colaiácovo, M.P.; Church, G.M.; Calarco, J.A. Heritable genome editing in C. elegans via a CRISPR-Cas9 system. Nat. Methods 2013, 10, 741–743. [Google Scholar] [CrossRef]
- Makarova, K.S.; Wolf, Y.I.; Iranzo, J.; Shmakov, S.A.; Alkhnbashi, O.S.; Brouns, S.J.J.; Charpentier, E.; Cheng, D.; Haft, D.H.; Horvath, P.; et al. Evolutionary classification of CRISPR-Cas systems: A burst of class 2 and derived variants. Nat. Rev. Microbiol. 2020, 18, 67–83. [Google Scholar] [CrossRef]
- Mortensen, K.; Lam, T.J.; Ye, Y. Comparison of CRISPR-Cas Immune Systems in Healthcare-Related Pathogens. Front. Microbiol. 2021, 12, 758782. [Google Scholar] [CrossRef]
- Makarova, K.S.; Wolf, Y.I.; Alkhnbashi, O.S.; Costa, F.; Shah, S.A.; Saunders, S.J.; Barrangou, R.; Brouns, S.J.; Charpentier, E.; Haft, D.H.; et al. An updated evolutionary classification of CRISPR-Cas systems. Nat. Rev. Microbiol. 2015, 13, 722–736. [Google Scholar] [CrossRef]
- Horvath, P.; Barrangou, R. CRISPR/Cas, the immune system of bacteria and archaea. Science 2010, 327, 167–170. [Google Scholar] [CrossRef]
- Richter, C.; Fineran, P.C. The subtype I-F CRISPR-Cas system influences pathogenicity island retention in Pectobacterium atrosepticum via crRNA generation and Csy complex formation. Biochem. Soc. Trans. 2013, 41, 1468–1474. [Google Scholar] [CrossRef]
- Arzanlou, M.; Chai, W.C.; Venter, H. Intrinsic, adaptive and acquired antimicrobial resistance in Gram-negative bacteria. Essays Biochem. 2017, 61, 49–59. [Google Scholar]
- Croteau, F.R.; Rousseau, G.M.; Moineau, S. The CRISPR-Cas system: Beyond genome editing. Med. Sci. M/S 2018, 34, 813–819. [Google Scholar]
- Burley, K.M.; Sedgley, C.M. CRISPR-Cas, a prokaryotic adaptive immune system, in endodontic, oral, and multidrug-resistant hospital-acquired Enterococcus faecalis. J. Endod. 2012, 38, 1511–1515. [Google Scholar] [CrossRef]
- Touchon, M.; Charpentier, S.; Pognard, D.; Picard, B.; Arlet, G.; Rocha, E.P.; Denamur, E.; Branger, C. Antibiotic resistance plasmids spread among natural isolates of Escherichia coli in spite of CRISPR elements. Microbiology 2012, 158, 2997–3004. [Google Scholar] [CrossRef]
- Li, H.Y.; Kao, C.Y.; Lin, W.H.; Zheng, P.X.; Yan, J.J.; Wang, M.C.; Teng, C.H.; Tseng, C.C.; Wu, J.J. Characterization of CRISPR-Cas Systems in Clinical Klebsiella pneumoniae Isolates Uncovers Its Potential Association With Antibiotic Susceptibility. Front. Microbiol. 2018, 9, 1595. [Google Scholar] [CrossRef]
- Tyumentseva, M.; Mikhaylova, Y.; Prelovskaya, A.; Tyumentsev, A.; Petrova, L.; Fomina, V.; Zamyatin, M.; Shelenkov, A.; Akimkin, V. Genomic and Phenotypic Analysis of Multidrug-Resistant Acinetobacter baumannii Clinical Isolates Carrying Different Types of CRISPR/Cas Systems. Pathogens 2021, 10, 205. [Google Scholar] [CrossRef]
- Moran, R.A.; Anantham, S.; Holt, K.E.; Hall, R.M. Prediction of antibiotic resistance from antibiotic resistance genes detected in antibiotic-resistant commensal Escherichia coli using PCR or WGS. J. Antimicrob. Chemother. 2017, 72, 700–704. [Google Scholar]
- D’Souza, R.; Pinto, N.A.; Phuong, N.L.; Higgins, P.G.; Vu, T.N.; Byun, J.H.; Cho, Y.L.; Choi, J.R.; Yong, D. Phenotypic and Genotypic Characterization of Acinetobacter spp. Panel Strains: A Cornerstone to Facilitate Antimicrobial Development. Front. Microbiol. 2019, 10, 559. [Google Scholar] [CrossRef]
- Louwen, R.; Staals, R.H.; Endtz, H.P.; van Baarlen, P.; van der Oost, J. The role of CRISPR-Cas systems in virulence of pathogenic bacteria. Microbiol. Mol. Biol. Rev. MMBR 2014, 78, 74–88. [Google Scholar] [CrossRef]
- Sorokina, J.; Sokolova, I.; Rybolovlev, I.; Shevlyagina, N.; Troitskiy, V.; Zhukhovitsky, V.; Belyi, Y. VirB4- and VirD4-Like ATPases, Components of a Putative Type 4C Secretion System in Clostridioides difficile. J. Bacteriol. 2021, 203, e0035921. [Google Scholar] [CrossRef]

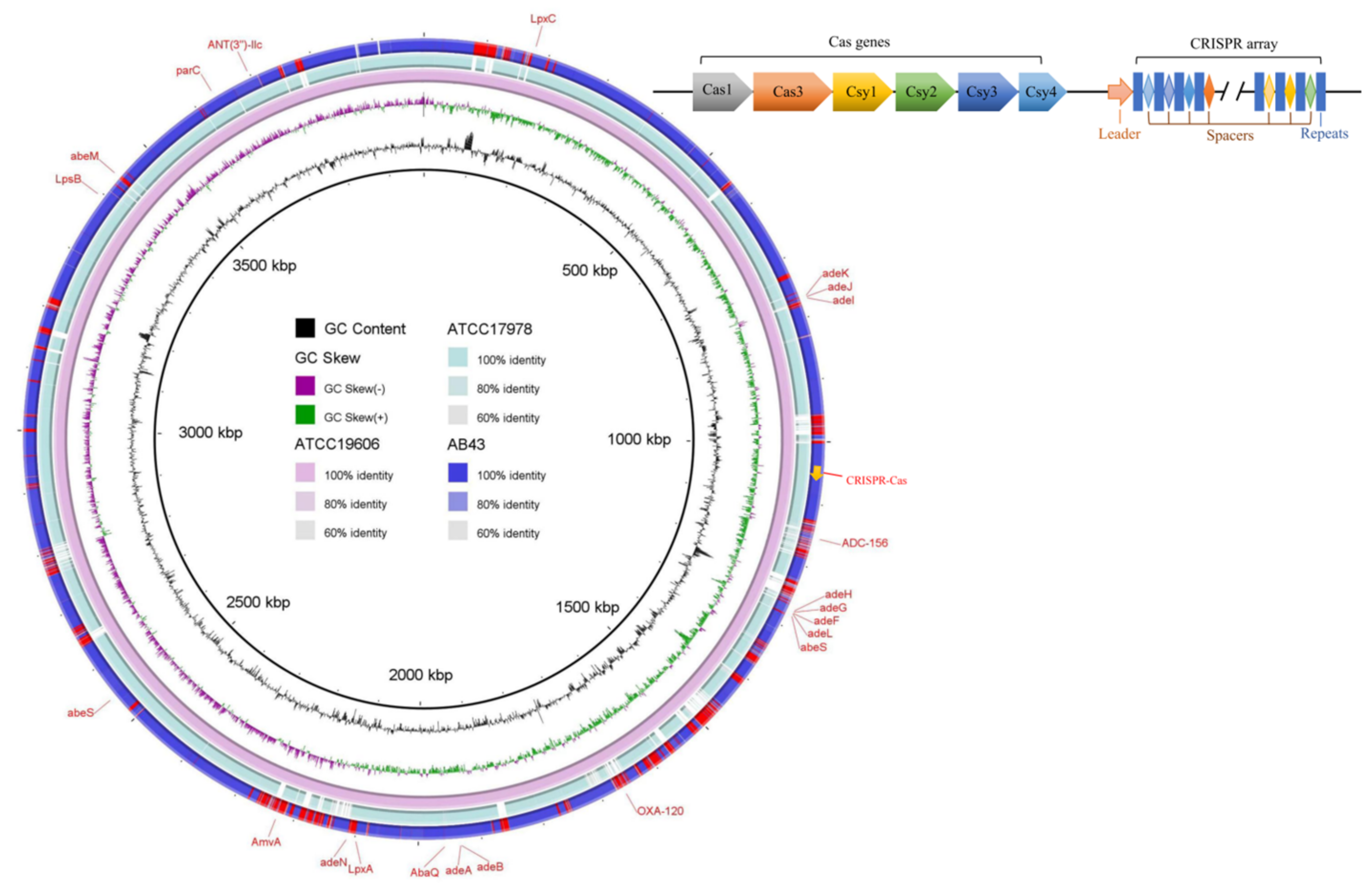

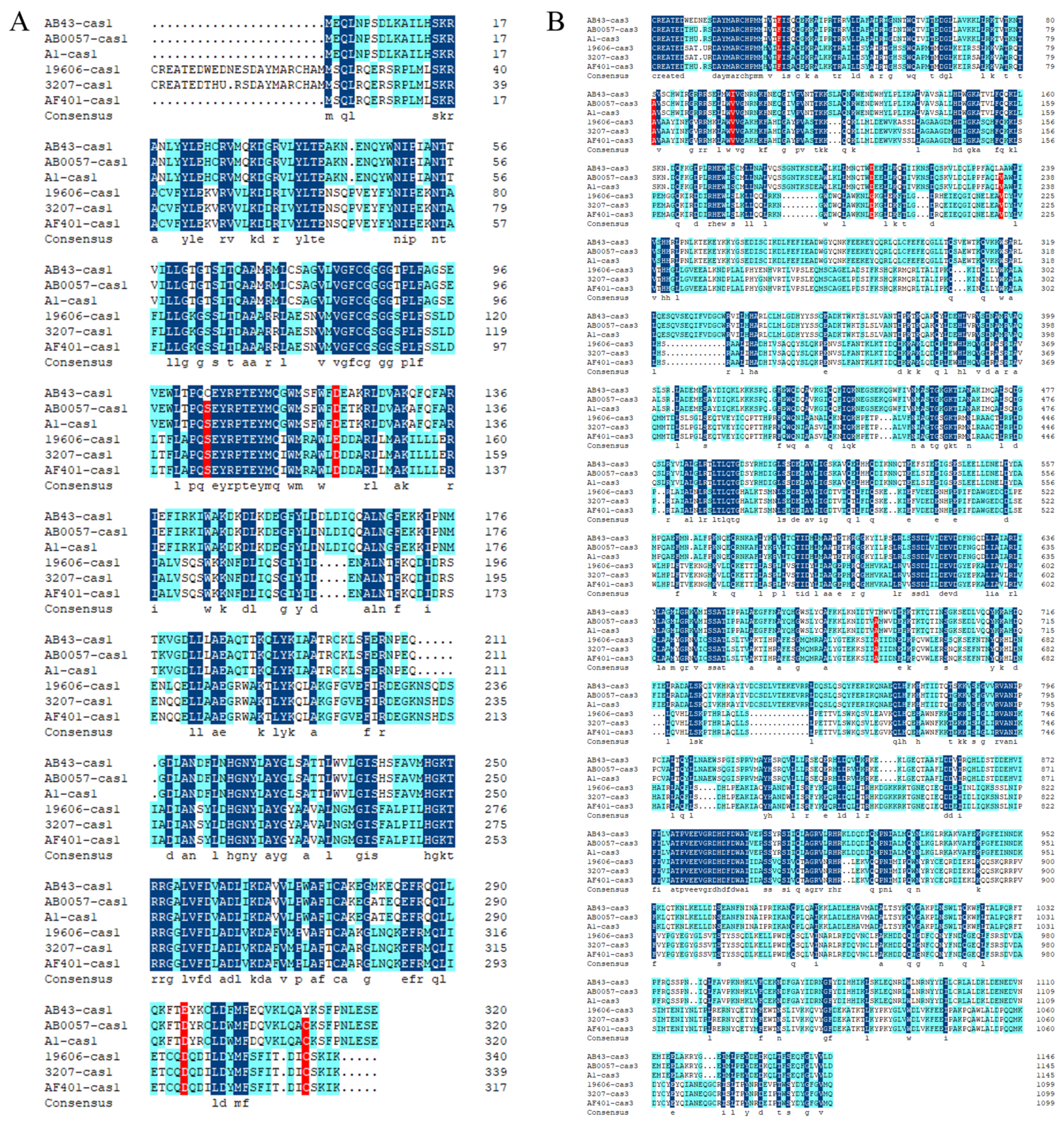

| Feature | Chromosome | Plasmid pAB43-1 |
|---|---|---|
| Size (base pairs) | 3,854,806 | 104,309 |
| Topology | Circular | Circular |
| GC content (%) | 39.10 | 39.99 |
| No. of genes | 3751 | 112 |
| No. of protein-coding sequences | 3487 | 88 |
| No. of rRNA operons | 18 | - |
| No. of tRNA genes | 74 | - |
| No. of small ncRNA genes | 4 | - |
| No. of CRISPR | 1 | 0 |
| No. of prophage | 6 | 3 |
| AMR Gene Family | Coding Genes | Antibiotic Class | Locus Tags |
|---|---|---|---|
| Resistance-nodulation-cell division (RND) antibiotic efflux pump | adeG | Fluoroquinolone, tetracycline | AB43GL001197 |
| adeB | Glycylcycline, tetracycline | AB43GL001861 | |
| adeA | Glycylcycline, tetracycline | AB43GL001862 | |
| adeN | Macrolide antibiotic, fluoroquinolone antibiotic, lincosamide antibiotic, carbapenem, cephalosporin, tetracycline antibiotic, rifamycin antibiotic, diaminopyrimidine antibiotic, phenicol antibiotic, penem | AB43GL002039 | |
| adeK | Macrolide, fluoroquinolone, lincosamide, carbapenem, cephalosporin, tetracycline, rifamycin, diaminopyrimidine, phenicol, penem | AB43GL000719 | |
| adeI | Macrolide, fluoroquinolone, lincosamide, carbapenem, cephalosporin, tetracycline, rifamycin, diaminopyrimidine, phenicol, penem | AB43GL000721 | |
| adeJ | Macrolide, fluoroquinolone, lincosamide, carbapenem, cephalosporin, tetracycline, rifamycin, diaminopyrimidine, phenicol, penem | AB43GL000720 | |
| adeH | Fluoroquinolone, tetracycline | AB43GL001196 | |
| adeL | Fluoroquinolone, tetracycline | AB43GL001199 | |
| adeF | Fluoroquinolone, tetracycline | AB43GL001198 | |
| Major facilitator superfamily (MFS) antibiotic efflux pump | AbaQ | Fluoroquinolone antibiotic | AB43GL001891 |
| AmvA | Macrolide antibiotic, acridine dye, disinfecting agents and intercalating dyes | AB43GL002170 | |
| Small multidrug resistance (SMR) antibiotic efflux pump | AbeS | Fluoroquinolone | AB43GL001204 |
| Multidrug and toxic compound extrusion (MATE) transporter | AbeM | Macrolide, acridine dye | AB43GL003308 |
| Intrinsic peptide antibiotic-resistant Lps | LpsB | Peptide antibiotic | AB43GL003272 |
| LpxA | Peptide antibiotic | AB43GL002024 | |
| LpxC | Peptide antibiotic | AB43GL000163 | |
| ANT(3″) | ANT(3″)-Ⅱc Aminoglycoside | Aminoglycoside | AB43GL003549 |
| Fluoroquinolone-resistant parC | parC | Fluoroquinolone antibiotic | AB43GL003487 |
| ADC beta-lactamases pending classification for carbapenemase activity | ADC-156 | Cephalosporin | AB43GL001071 |
| OXA beta-lactamase | OXA-120 | Carbapenem, cephalosporin, penam | AB43GL001588 |
| Sequences Producing Significant Alignments | IS Family | Group | Origin | Location (Start–End) |
|---|---|---|---|---|
| ISAba2 | IS3 | IS51 | Acinetobacter baumannii | 825271–826579, 889584–890892, |
| 1670545–1671853, 2234086–2235394, | ||||
| 2260215–2261523, 2287668–2288976, | ||||
| 3186375–3187683, 3188421–3189729 | ||||
| ISAba22 | IS3 | IS3 | Acinetobacter baumannii | 1767604–1768877, 1804912–1806185, |
| 1941275–1942548, 2083961–2085234, | ||||
| 2253669–2254929, 2283894–2285167, | ||||
| 2455194–2456467, 3276754–3278027 | ||||
| ISAcsp12 | IS3 | IS3 | Acinetobacter sp. | 90476–90852, 92164–92236, |
| 92308–92376, 3230992–3231229 | ||||
| ISAba5 | IS5 | IS903 | Acinetobacter haemolyticus | 828759–829798, 876433–877472; |
| 942334–943373, 1372703–1373742, | ||||
| 1548861–1549880, 2276631–2277670 | ||||
| ISAba13 | IS5 | IS903 | Acinetobacter baumannii | 141776–142656, 271285–272323, |
| 1882841–1883879,1889761–1890799, | ||||
| 1939734–1940660, 254414–255452 | ||||
| ISAba59 | IS5 | IS903 | Acinetobacter baumannii | 140737–141775, 1118270–1119308, |
| 1646101–1647139, 2091081–2092119, | ||||
| 2289970–2291008, 2431933–2432971, | ||||
| ISAba62 | IS5 | IS427 | Acinetobacter baumannii | 863210–863355, 863912–864022 |
| ISAba64 | IS256 | Acinetobacter baumannii | 3674955–3675184, 3765469–3765698 | |
| ISAba44 | IS481 | Vibrio cholerae | 260755–260818 | |
| ISAcsp2 | IS630 | Acinetobacter baumannii | 420808–421689, 696057–696938, | |
| 1280457–1281338, 1528383–1529264, | ||||
| 1846480–1847361, 2467893–2468774, | ||||
| 2546510–2547391, 3009474–3010355 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, T.; Yang, J.; Sun, X.; Wang, Y.; Yang, L.; Kong, G.; Jiao, H.; Bao, G.; Li, G. Whole-Genome Analysis of Acinetobacter baumannii Strain AB43 Containing a Type I-Fb CRISPR-Cas System: Insights into the Relationship with Drug Resistance. Molecules 2022, 27, 5665. https://doi.org/10.3390/molecules27175665
Guo T, Yang J, Sun X, Wang Y, Yang L, Kong G, Jiao H, Bao G, Li G. Whole-Genome Analysis of Acinetobacter baumannii Strain AB43 Containing a Type I-Fb CRISPR-Cas System: Insights into the Relationship with Drug Resistance. Molecules. 2022; 27(17):5665. https://doi.org/10.3390/molecules27175665
Chicago/Turabian StyleGuo, Tingting, Jie Yang, Xiaoli Sun, Yuhang Wang, Liying Yang, Guimei Kong, Hongmei Jiao, Guangyu Bao, and Guocai Li. 2022. "Whole-Genome Analysis of Acinetobacter baumannii Strain AB43 Containing a Type I-Fb CRISPR-Cas System: Insights into the Relationship with Drug Resistance" Molecules 27, no. 17: 5665. https://doi.org/10.3390/molecules27175665
APA StyleGuo, T., Yang, J., Sun, X., Wang, Y., Yang, L., Kong, G., Jiao, H., Bao, G., & Li, G. (2022). Whole-Genome Analysis of Acinetobacter baumannii Strain AB43 Containing a Type I-Fb CRISPR-Cas System: Insights into the Relationship with Drug Resistance. Molecules, 27(17), 5665. https://doi.org/10.3390/molecules27175665






