The Chemical and Pharmacological Research Progress on a Kind of Chinese Herbal Medicine, Fructus Malvae
Abstract
:1. Introduction
2. Research Progress on Chemical Constituents
2.1. Acid Compounds
2.2. Flavonoids
2.3. Sterols
2.4. Glycerides
2.5. Volatile Oils
2.6. Polysaccharides
2.7. Amino Acids
2.8. Other Substances
3. Research Progress on Pharmacological Activity
3.1. Diuretic Effect
3.2. Anti-Diabetic Effect
3.3. Antioxidant Effect
3.4. Antitumor Effect
3.5. Treatment of Hair Loss
3.6. Other Pharmacological Effects
4. Summary and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gruber, J.; Prinstein, M.J.; Clark, L.A.; Rottenberg, J.; Abramowitz, J.S.; Albano, A.M.; Aldao, A.; Borelli, J.L.; Chung, T.; Davila, J.; et al. Mental health and clinical psychological science in the time of COVID-19: Challenges, opportunities, and a call to action. Am. Psychol. 2021, 76, 409–426. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.; Lan, W.; Zhang, J.; Zhao, S.; Ou, J.; Wu, X.; Yan, Y.; Wu, J.; Zhang, Q. COVID-19: Antiviral Agents, Antibody Development and Traditional Chinese Medicine. Virol. Sin. 2020, 35, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y. Artemisinin-A Gift from Traditional Chinese Medicine to the World (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 2016, 55, 10210–10226. [Google Scholar] [CrossRef] [PubMed]
- National Pharmacopoeia Commission, Pharmacopoeia of the People’s Republic of China, (2020 Edition Part one); China Pharmaceutical Science and Technology Press: Beijing, China, 2020; p. 120.
- Wu, Z. Identification of Malvae Semen and Abutili Semen. J. Clin. Med. 2018, 5, 184–186. [Google Scholar]
- Gao, T.; Xin, T.; Song, J.; Song, J. Identification of Malvae Semen and Abutili Semen using ITS2 DNA barcode. Chin. Tradit. Herb. Drugs 2017, 48, 2740–2745. [Google Scholar]
- Ma, Q.; Dong, Y.; Na, S.; Li, X.; Li, S. Determination of Total Phenolic Acid in Fructus malvae. Li Shizhen Med. Mater. Med. Res. 2010, 21, 2583–2584. [Google Scholar]
- Zhong, Y. Interpretation of “Shen Nong’s Materia Medica”—Malvae Semen. Yishou Baodian 2018, 34, 40. [Google Scholar]
- Cai, Y.; Li, C.; Zou, J. Examination and discussion on some Chinese herb medicine names recorded in “Chinese Pharmacopoeia”. Chin. Tradit. Herb. Drugs 2005, 36, 1104–1106. [Google Scholar]
- Qi, Z. The evolution of “Kui” and textual research of its original plant. HeBei J. For. Orchard Res. 2010, 25, 255–262. [Google Scholar]
- Ma, Q.; Dong, Y.; Zhu, X.; Li, S. TLC Identification of Mongolia Medicine Fructus Malvae. China Pharm. 2010, 19, 17–18. [Google Scholar]
- Dong, Y.; Ma, Q.; Na, S.; Li, X.; Li, S. Quantitative determination of caffeic acid in Dongkuiguo (Fructus Malvae, Mongolian medicated herb) by HPLC. J. Beijing Univ. Tradit. Chin. Med. 2010, 33, 117–119. [Google Scholar]
- Wang, H.; Wang, S.; Dong, Y.; Li, S. The experimental study on traditional mongolian materia medica of Fructus Malvae. J. Inn. Mong. Med. Coll. 2012, 34, 69–72. [Google Scholar]
- Menghebilige; Wu, X. Research progress of Mongolian medicinal material Fructus Malva. Chin. J. Ethn. Med. 2012, 18, 37–40. [Google Scholar]
- Hu, Y.; Li, X.; Shi, H.; Liu, Y.; Mao, Q.; Yan, L. Effects of Different Extraction Methods on the Content of Caffeic Acid and Ferulic Acid in Malvae Fructus. Asia-Pac. Tradit. Med. 2022, 18, 57–60. [Google Scholar]
- Zhang, A.; Liu, L.; Luo, S.; Qian, Y. Determination of Ferulic Acid in Malvae Fructus by HPLC. Chin. Pharm. Assoc. 2010, 5, 2752–2756. [Google Scholar]
- Wunierqiqige; Liu, B. Disperses the Puncture Vine, the Mallow Fruit quality specification fumble research to Therr Taste Puncture Vines. North. Pharm. 2014, 11, 1–3. [Google Scholar]
- He, X. Study on Material Base and Quality Specification of Fruit Malva verticillate; Chengdu University of Chinese Medicine: Chengdu, China, 2006. [Google Scholar]
- Gao, F. Analysis on volatile oil of Mongolian medicine Malvae Fructus. J. Med. Pharm. Chin. Minoritie. 2021, 27, 41–43. [Google Scholar]
- Li, Z.; Xu, N.; Yang, L.; Zhang, L.; Wang, Q. Analysis on chemical constituents of volatile oil from Mongolian medicine Malvae Fructus. Chin. Tradit. Pat. Med. 2008, 06, 922–924. [Google Scholar]
- Ryu, H.S.; Jeong, J.; Lee, C.M.; Lee, K.S.; Lee, J.N.; Park, S.M.; Lee, Y.M. Activation of Hair Cell Growth Factors by Linoleic Acid in Malva verticillata Seed. Molecules 2021, 26, 2117. [Google Scholar] [CrossRef]
- Lee, E.Y.; Choi, E.J.; Kim, J.A.; Hwang, Y.L.; Kim, C.D.; Lee, M.H.; Roh, S.S.; Kim, Y.H.; Han, I.; Kang, S. Malva verticillata seed extracts upregulate the Wnt pathway in human dermal papilla cells. Int. J. Cosmet. Sci. 2016, 38, 148–154. [Google Scholar] [CrossRef]
- Shim, K.S.; Lee, C.J.; Yim, N.H.; Ha, H.; Ma, J.Y. A water extract of Malva verticillata seeds suppresses osteoclastogenesis and bone resorption stimulated by RANK ligand. BMC Complement. Altern. Med. 2016, 16, 332. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.-T.; Song, C.-H. Antidiabetic activities of extract from Malva verticillata seed via the activation of AMP-activated protein kinase. J. Microbiol. Biotechnol. 2011, 21, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Gonda, R.; Tomoda, M.; Kanari, M.; Shimizu, N.; Yamada, H. Constituents of the seed of Malva verticillata. VI. Characterization and immunological activities of a novel acidic polysaccharide. Chem. Pharm. Bull. (Tokyo) 1990, 38, 2771–2774. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, N.; Tomoda, M. Constituents of the seed of Malva verticillata. I. Structural features of the major neutral polysaccharide. Chem. Pharm. Bull. (Tokyo) 1987, 35, 4981–4984. [Google Scholar] [CrossRef] [PubMed]
- Tomoda, M.; Shimizu, N.; Gonda, R.; Kanari, M.; Yamada, H.; Hikino, H. Anti-complementary and hypoglycemic activities of the glycans from the seeds of Malva verticillata. Planta Med. 1990, 56, 168–170. [Google Scholar] [CrossRef] [PubMed]
- Tomoda, M.; Asahara, H.; Gonda, R.; Takada, K. Constituents of the seed of Malva verticillata. VIII. Smith degradation of MVS-VI, the major acidic polysaccharide, and anti-complementary activity of products. Chem. Pharm. Bull. (Tokyo) 1992, 40, 2219–2221. [Google Scholar] [CrossRef]
- Shimizu, N.; Asahara, H.; Tomoda, M.; Gonda, R.; Ohara, N. Constituents of seed of Malva verticillata. VII. Structural features and reticuloendothelial system-potentiating activity of MVS-I, the major neutral polysaccharide. Chem. Pharm. Bull. (Tokyo) 1991, 39, 2630–2632. [Google Scholar] [CrossRef]
- Gonda, R.; Tomoda, M.; Shimizu, N.; Kanari, M. Characterization of an acidic polysaccharide from the seeds of Malva verticillata stimulating the phagocytic activity of cells of the RES. Planta Med. 1990, 56, 73–76. [Google Scholar] [CrossRef]
- Bao, L.; Bao, X.; Li, P.; Wang, X.; Ao, W. Chemical profiling of Malva verticillata L. by UPLC-Q-TOF-MS and their antioxidant activity in vitro. J. Pharm. Biomed. Anal. 2018, 150, 420–426. [Google Scholar] [CrossRef]
- Ko, J.-H.; Nam, Y.H.; Joo, S.-W.; Kim, H.G.; Lee, Y.G.; Kang, T.H.; Baek, N.I. Flavonoid 8-O-Glucuronides from the aerial parts of Malva verticillata and their recovery effects on alloxan-induced pancreatic islets in zebrafish. Molecules 2018, 23, 833. [Google Scholar] [CrossRef]
- Ko, J.-H.; Castaneda, R.; Joo, S.-W.; Kim, H.G.; Lee, Y.G.; Lee, Y.H.; Kang, T.H.; Baek, N.I. Glycerides isolated from the aerial parts of Malva verticillata cause immunomodulation effects via splenocyte function and NK anti-tumor activity. Food Sci. Biotechnol. 2018, 27, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.-H.; Cho, S.M.; Joo, S.-W.; Kim, H.G.; Lee, Y.G.; Kang, S.C.; Baek, N.I. Glycosyl glycerides from the aerial parts of Malva verticillata and their chemopreventive effects. Bio. Chem. 2018, 78, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.-H.; Rodriguez, I.; Joo, S.-W.; Kim, H.G.; Lee, Y.G.; Kang, T.H.; Baek, N.I. Synergistic effect of two major components of Malva verticillata in the recovery of alloxan-damaged pancreatic islet cells in zebrafish. J. Med. Food 2019, 22, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.; Zhang, G.; Wang, J. Determination of Great and Trace Elements in Mongolia Traditional Herbs Malva verticillata L. Chin. J. Ethn. Med. 2005, 02, 31–45. [Google Scholar]
- Felker, G.M.; Ellison, D.H.; Mullens, W.; Cox, Z.L.; Testani, J.M. Diuretic Therapy for Patients With Heart Failure: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 1178–1195. [Google Scholar] [CrossRef]
- Liu, X. Study on the intensive diuretic effect of Xinshuai No. 1 formula in the treatment of chronic heart failure. Stud. Trace Elem. Health 2018, 35, 8–9. [Google Scholar]
- Liu, Q.; Wei, L.; Chen, Y.; Li, W.; Liang, X. Study on the Effect of Abutilon indicum(L.) Sweet on Cirrhotic Ascites and Diuretic. J. Liaoning Univ. TCM 2021, 23, 29–33. [Google Scholar]
- Uojima, H.; Hidaka, H.; Nakayama, T.; Sung, J.H.; Ichita, C.; Tokoro, S.; Masuda, S.; Sasaki, A.; Koizumi, K.; Egashira, H.; et al. Efficacy of combination therapy with natriuretic and aquaretic drugs in cirrhotic ascites patients: A randomized study. World J. Gastroenterol. 2017, 23, 8062–8072. [Google Scholar] [CrossRef]
- Mariano, L.N.B.; Boeing, T.; Filho, V.C.; Niero, R.; da Silva, L.M.; Souza, P. 1,3,5,6-tetrahydroxyxanthone promotes diuresis, renal protection and antiurolithic properties in normotensive and hypertensive rats. J. Pharm. Pharmacol. 2021, 73, 700–708. [Google Scholar] [CrossRef]
- Jing, S.; Gai, Q.; Zhao, X.; Wang, J.; Gong, Y.; Pang, Y.; Peng, C.; Tian, Y.; Wang, Y.; Wang, Z. Physical therapy in the management of stone fragments: Progress, status, and needs. Urolithiasis 2018, 46, 223–229. [Google Scholar] [CrossRef]
- Avaid, F.; Mehmood, M.H.; Shaukat, B. Hydroethanolic Extract of A. officinarum Hance Ameliorates Hypertension and Causes Diuresis in Obesogenic Feed-Fed Rat Mode l. Front. Pharmacol. 2021, 12, 670433. [Google Scholar]
- Li, M.; Zeng, M.; Zhang, B.; Fan, H.; Wu, G.; La, Z.; Feng, W.; Kuang, H.; Zheng, X. Laboratory Study on Diuretic Effect of Ephedra Decoction and Its Splitting Fractions on Rats. Chin. Arch. Tradit. Chin. Med. 2018, 36, 2203–2206. [Google Scholar]
- Ye, X.; Zhu, X.; Liu, T.; Liu, X.; Hui, L.; Feng, W.; Yang, L.; Li, C.; Wang, Z. Diuretic effect and material basis of Clematidis Armandii Caulis in rats. China J. Chin. Mater. Med. 2019, 44, 1889–1894. [Google Scholar]
- He, J.; Yang, L. Diuretic effect of Lagopsis supina fraction in saline-loaded rats is mediated through inhibition of aquaporin and renin-angiotensin-aldosterone systems and up-regulation of atriopeptin. Biomed. Pharmacother. 2021, 139, 111554. [Google Scholar] [CrossRef] [PubMed]
- Hakim, E.M.; Sivak, K.V.; Kaukhova, I.E. Evaluation of the diuretic effect of crude ethanol and saponin-rich extracts of Herniaria glabra L. in rats. J. Ethnopharmacol. 2021, 273, 113942. [Google Scholar] [CrossRef]
- Meng, X.; Chen, Y.; Lili; Bi, L.; Wu, R.; Bai, M. Diuretic mechanism of alcohol extract of Mongolian medicine Althaea rosa on water load model rats. Chin. J. Ethn. Med. 2022, 28, 50–54. [Google Scholar]
- Tufer, S.; Engidawork, E.; Ayele, A.G.; Bashea, C. Evaluation of the Diuretic Activity of Aqueous and 80% Methanol Extracts of Croton macrostachyus (Euphorbiaceae) Leaves in Saline-Loaded Rats. J. Exp. Pharmacol. 2021, 13, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, X.Y.; Lin, N.; Zhao, W.L.; Huang, X.Q.; Chen, Y.; Huang, M.Q.; Xu, W.; Wu, S.S. Diuretic Activity of Compatible Triterpene Components of Alismatis rhizoma. Molecules 2017, 22, 1459. [Google Scholar] [CrossRef]
- Lu, Q.; Zheng, R.; Zhu, P.; Bian, J.; Liu, Z.; Du, J. Hinokinin alleviates high fat diet/streptozotocin-induced cardiac injury in mice through modulation in oxidative stress, inflammation and apoptosis. Biomed. Pharmacother. 2021, 137, 111361. [Google Scholar] [CrossRef]
- Wang, P.; Liu, Y.; Zhang, T.; Yin, C.; Kang, S.Y.; Kim, S.j.; Park, Y.K.; Jung, H.W. Effects of Root Extract of Morinda officinalis in Mice with High-Fat-Diet/Streptozotocin-Induced Diabetes and C2C12 Myoblast Differentiation. ACS Omega 2021, 6, 26959–26968. [Google Scholar] [CrossRef]
- Kaul, K.; Tarr, J.M.; Ahmad, S.I.; Kohner, E.M.; Chibber, R. Introduction to diabetes mellitus. Adv. Exp. Med. Biol. 2012, 771, 1–11. [Google Scholar] [PubMed]
- Shepard, B.D. Sex differences in diabetes and kidney disease: Mechanisms and consequences. Am. J. Physiol. Renal. Physiol. 2019, 317, F456–F462. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Hu, H.; Guo, R.; Wang, H.; Jiang, H. Mesenchymal Stem Cell Exosomes as a New Strategy for the Treatment of Diabetes Complications. Front. Endocrinol. 2021, 12, 646233. [Google Scholar] [CrossRef] [PubMed]
- Strain, W.D.; Paldánius, P.M. Diabetes, cardiovascular disease and the microcirculation. Cardiovasc. Diabetol. 2018, 17, 57. [Google Scholar] [CrossRef] [PubMed]
- MacPherson, M.; Cranston, K.; Locke, S.; Vis-Dunbar, M.; Jung, M.E. Diet and exercise interventions for individuals at risk for type 2 diabetes: A scoping review protocol. BMJ Open 2020, 10, e039532. [Google Scholar] [CrossRef] [PubMed]
- Padhi, S.; Nayak, A.K.; Behera, A. Type II diabetes mellitus: A review on recent drug based therapeutics. Biomed. Pharmacother. 2020, 131, 110708. [Google Scholar] [CrossRef]
- Zhang, C.; Yuan, C.; Cheng, Y.; She, R.; Xiao, W. Antioxidant Activity of Alien Invasive Species Eupatorium adenophorum Spreng. Chem. Bioeng. 2022, 39, 35–39, 55. [Google Scholar]
- He, W.; Li, X.; Peng, Y.; He, X.; Pan, S. Anti-Oxidant and Anti-Melanogenic Properties of Essential Oil from Peel of Pomelo cv. Guan Xi. Molecules 2019, 24, 242. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, Y.; Li, Y.; Zhang, X.; Yuan, P.; Xia, K.; Tan, Z. Study on in vitro anti-inflflammatory and antioxidant activities of flavonoids from sea buckthorn. China Food Addit. 2021, 32, 67–74. [Google Scholar]
- Aarland, R.C.; Bañuelos-Hernández, A.E.; Fragoso-Serrano, M.; Sierra-Palacios, E.D.C.; León-Sánchez, F.D.D.; Pérez-Flores, L.J.; Rivera-Cabrera, F.; Mendoza-Espinoza, J. A Studies on phytochemical, antioxidant, anti-inflammatory, hypoglycaemic and antiproliferative activities of Echinacea purpurea and Echinacea angustifolia extracts. Pharm. Biol. 2017, 55, 649–656. [Google Scholar] [CrossRef]
- Dżugan, M.; Tomczyk, M.; Sowa, P.; Grabek-Lejko, D. Antioxidant Activity as Biomarker of Honey Variety. Molecules 2018, 23, 2069. [Google Scholar] [CrossRef] [PubMed]
- Kuang, X.; Huang, R.; Han, S.; Zhang, Q.; Yan, C.; Li, W. Comparison of Antioxidant Activity of Several Animal Protein-derived Peptides by ORAC. Guangdong Chem. Ind. 2022, 49, 14–15, 52. [Google Scholar]
- Xie, J.; Su, T.; Wei, Y.; Li, G.; Wu, J.; Huang, L. Research progress of antioxidant drugs in myocardial ischemiareperfusion injury. Acta Pharm. Sin. 2021, 56, 1845–1855. [Google Scholar]
- Choi, S.; Liu, X.; Pan, Z. Zinc deficiency and cellular oxidative stress: Prognostic implications in cardiovascular diseases. Acta Pharm. Sin. 2018, 39, 1120–1132. [Google Scholar] [CrossRef] [PubMed]
- Karami, S.; Poustchi, H.; Sarmadi, N.; Radmard, A.R.; Yari, F.A.; Pakdel, A.; Shabani, P. Association of anti-oxidative capacity of HDL with subclinical atherosclerosis in subjects with and without non-alcoholic fatty liver disease. Diabetol. Metab. Syndr. 2021, 13, 121. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.X.; Hu, Y.; Li, Y.W.; Gao, J. Levels of anti-oxidative molecules and inflammatory factors in patients with vascular dementia and their clinical significance. Pak. J. Med. Sci. 2021, 37, 1509–1513. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.; Kim, J.Y.; Kim, J.W.; Yoon, J.S. Anti-oxidative and anti-adipogenic effects of caffeine in an in vitro model of Graves’ orbitopathy. Endocr. J. 2020, 67, 439–447. [Google Scholar] [CrossRef]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef]
- Srinivas, U.S.; Tan, B.W.Q.; Vellayappan, B.A.; Jeyasekharan, A.D. ROS and the DNA damage response in cancer. Redox Biol. 2019, 25, 101084. [Google Scholar] [CrossRef] [PubMed]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef]
- Yang, S.; Lian, G. ROS and diseases: Role in metabolism and energy supply. Mol. Cell. Biochem. 2020, 467, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; He, D.; Pan, Z.; Luo, G.; Deng, J. Reactive-oxygen-species-scavenging nanomaterials for resolving inflammation. Mater. Today Bio 2021, 11, 100124. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, Y.; Dai, X.; Jiang, W.; Zhao, H. Nanozymes Regulate Redox Homeostasis in ROS-Related Inflammation. Front. Chem. 2021, 9, 740607. [Google Scholar] [CrossRef]
- Herb, M.; Gluschko, A.; Schramm, M. Reactive Oxygen Species: Not Omnipresent but Important in Many Locations. Front. Cell. Dev. Biol. 2021, 9, 716406. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Li, P.; Yang, H.G.; Wang, Y.Z.; Huang, G.X.; Wang, J.Q.; Zheng, N. Investigation and comparison of the anti-tumor activities of lactoferrin, α-lactalbumin, and β-lactoglobulin in A549, HT29, HepG2, and MDA231-LM2 tumor models. J. Dairy Sci. 2019, 102, 9586–9597. [Google Scholar] [CrossRef]
- Xue, X.; Qian, C.; Tao, Q.; Dai, Y.; Lv, M.; Dong, J.; Su, Z.; Qian, Y.; Zhao, J.; Liu, H.; et al. Using bio-orthogonally catalyzed lethality strategy to generate mitochondria-targeting anti-tumor metallodrugs in vitro and in vivo. Natl. Sci. Rev. 2020, 8, nwaa286. [Google Scholar] [CrossRef]
- Ju, A.; Du, H.; Yuan, J.; Wan, M.; Zhang, Y.; Li, Z.; Li, D.; Gao, W. Study on anti-tumor effect of Yiqi Fumai Lyophilized Injection combined with cisplatin on breast cancer mice. Drug Eval. Res. 2021, 44, 2372–2378. [Google Scholar]
- Zhang, Y.; Yue, Q.; Zhang, L.; Zhang, Y.; Li, P.; Zhang, M. The Effect of Ethanol Extract from Saussurea Medusa Maxim on Anti-tumor Immunity of Lewis Lung Cancer Bearing Mice. Chin. J. Immunol. 2022, 1–17. Available online: http://kns.cnki.net/kcms/detail/22.1126.R.20220303.1819.004.html (accessed on 13 July 2022).
- Cao, Y.; Feng, Y.H.; Gao, L.W.; Li, X.Y.; Jin, Q.X.; Wang, Y.Y.; Xu, Y.Y.; Jin, F.; Lu, S.L.; Wei, M.J. Artemisinin enhances the anti-tumor immune response in 4T1 breast cancer cells in vitro and in vivo. Int. Immunol. Pharmacol. 2019, 70, 110–116. [Google Scholar] [CrossRef]
- He, S.; Bao, H.; Wei, Y.; Liu, Y.; Liu, J. Antitumor effect and mechanism of different extracts of cultivated Phellinus vaninii on H22 tumor bearing mice. Chin. J. Biotechnol. 2022, 38, 1025–1038. [Google Scholar]
- Jin, Y.; Zuo, H.X.; Li, M.Y.; Zhang, Z.H.; Xing, Y.; Wang, J.Y.; Ma, J.; Li, G.; Piao, H.; Gu, P.; et al. Anti-Tumor Effects of Carrimycin and Monomeric Isovalerylspiramycin I on Hepatocellular Carcinoma in Vitro and in Vivo. Front. Pharmacol. 2021, 12, 774231. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, Y.; Mehdiabad, M.V.; Zhou, K.; Chen, Y.; Li, L.; Guo, J.; Xu, C. Enhanced anti-tumor effect of liposomal Fasudil on hepatocellular carcinoma in vitro and in vivo. PLoS ONE 2019, 14, e0223232. [Google Scholar] [CrossRef] [PubMed]
- Youness, R.A.; Gad, A.Z.; Sanber, K.; Ahn, Y.J.; Lee, G.J.; Khallaf, E.; Hafez, H.M.; Motaal, A.A.; Ahmed, N.; Gad, M.Z. Targeting hydrogen sulphide signaling in breast cancer. J. Adv. Res. 2020, 27, 177–190. [Google Scholar] [CrossRef]
- Luo, H.; Xue, W.; Lin, H. Discussion on the Statistical Methods of the Therapeutic Modalities of Tumor Cases. Chin. J. Health Inform. Manag. 2018, 15, 550–553. [Google Scholar]
- Vasan, N.; Baselga, J.; Hyman, D.M. A view on drug resistance in cancer. Nature 2019, 575, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Ning, W.; Chen, Y.; Lv, H.; Fu, C. Drug Resistance of Antineoplastic Drugs and Clinical Therapeutic Strategies. Chin. J. Mod. Appl. Pharm. 2019, 36, 1721–1727. [Google Scholar]
- Ruggiero, A.; Ferrara, P.; Attinà, G.; Rizzo, D.; Riccardi, R. Renal toxicity and chemotherapy in children with cancer. Br. J. Clin. Pharmacol. 2017, 83, 2605–2614. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Liu, M.; Fu, F.; Liu, H.; He, B.; Xiao, D.; Yang, J. High Serum Estradiol Reduces Acute Hepatotoxicity Risk Induced by Epirubicin Plus Cyclophosphamide Chemotherapy in Premenopausal Women with Breast Cancer. Front. Pharmacol. 2021, 11, 572444. [Google Scholar] [CrossRef] [PubMed]
- Freites-Martinez, A.; Shapiro, J.; Goldfarb, S.; Nangia, J.; Jimenez, J.J.; Paus, R.; Lacouture, M.E. Hair disorders in patients with cancer. J. Am. Acad. Dermatol. 2019, 80, 1179–1196. [Google Scholar] [CrossRef]
- Zhu, H.; Long, M.-H.; Wu, J.; Wang, M.; Li, X.; Shen, H.; Xu, J.; Zhou, L.; Fang, Z.; Luo, Y.; et al. Ginseng alleviates cyclophosphamide-induced hepatotoxicity via reversing disordered homeostasis of glutathione and bile acid. Sci. Rep. 2015, 5, 17536. [Google Scholar] [CrossRef]
- Zhou, L. The Function and Mechanisms of Bioactive Molecules in Regulating the Growth and Cycle of Hair Follicle; Zhejiang University(China): Zhejiang, China, 2019. [Google Scholar] [CrossRef]
- Zhang, J. Study on the pharmacological effects of diammonium glycyrrhizinate on hair loss. Zhangjiang Univ. People’s Hosp. Yuyao City Zhejiang Prov. 2015, 12, 15. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=SNAD&dbname=SNAD&filename=SNAD000001700741&uniplatform=NZKPT&v=TjRX_0fSS4t-biFKETF6cmWfszyKo1h7jaaTSJLODhI9eFYXao9JJ2G2mu-l6K4m145XZ7KKFaI%3d (accessed on 13 July 2022).
- Gao, Z.; Xu, Y.; Sun, C.; Wang, X.; Guo, Y.; Shi, Q.; Ma, K. A systematic review of asymptomatic infections with COVID-19. J. Microbiol. Immunol. Infect. 2021, 54, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, S.H.; Mansatta, K.; Mallett, G.; Harris, V.; Emary, K.R.W.; Pollard, A.J. What defines an efficacious COVID-19 vaccine? A review of the challenges assessing the clinical efficacy of vaccines against SARS-CoV-2. Lancet Infect. Dis. 2021, 21, e26–e35. [Google Scholar] [CrossRef]
- Luo, H.; Gao, Y.; Zou, J.; Zhang, S.; Chen, H.; Liu, Q.; Tan, D.; Han, Y.; Zhao, Y.; Wang, S. Reflections on treatment of COVID-19 with traditional Chinese medicine. Chin. Med. 2020, 15, 94. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Guan, W.J.; Bi, Y.; Zhang, W.; Li, L.; Zhang, B.; Liu, Q.; Song, Y.; Li, X.; Duan, Z.; et al. Efficacy and safety of Lianhuaqingwen capsules, a repurposed Chinese herb, in patients with coronavirus disease 2019: A multicenter, prospective, randomized controlled trial. Phytomedicine 2021, 85, 153242. [Google Scholar] [CrossRef]
- Ley, S.H.; Hamdy, O.; Mohan, V.; Hu, F.B. Prevention and management of type 2 diabetes: Dietary components and nutritional strategies. Lancet 2014, 383, 1999–2007. [Google Scholar] [CrossRef]
- Du, S.; Cai, Z.; Yang, K.; Liu, Q. Pancreatic autophagy and protein expression of insulin-related genes in type 2 diabetic rats with Periodontitis. Chin. J. Tissue Eng. Res. 2022, 26, 4605–4610. [Google Scholar]
- Tian, M.; Liu, Y.; Xie, L.; Cao, Y.; Lv, H. Protective effffects of tertiary butylhydroquinone on various organs in type 2 diabetic rats. Chin. J. Tissue Eng. Res. 2022, 26, 4616–4623. [Google Scholar]
- Cheng, Y.; Yu, X.; Zhang, J.; Chang, Y.; Xue, M.; Li, X.; Lu, Y.; Li, T.; Meng, Z.; Su, L.; et al. Pancreatic kallikrein protects against diabetic retinopathy in KK Cg-Ay/J and high-fat diet/streptozotocin-induced mouse models of type 2 diabetes. Diabetologia 2019, 62, 1074–1086. [Google Scholar] [CrossRef]
- Wang, Y.; Dilidaxi, D.; Wu, Y.; Sailike, J.; Sun, X.; Nabi, X. Composite probiotics alleviate type 2 diabetes by regulating intestinal microbiota and inducing GLP-1 secretion in db/db mice. Biomed. Pharmacother. 2020, 125, 109914. [Google Scholar] [CrossRef]
- Yu, F.; Han, W.; Zhan, G.; Li, S.; Jiang, X.; Wang, L.; Xiang, S.; Zhu, B.; Yang, L.; Luo, A.; et al. Abnormal gut microbiota composition contributes to the development of type 2 diabetes mellitus in db/db mice. Aging (Albany NY) 2019, 11, 10454–10467. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Guo, W.; Wu, Y.; Yang, C.; Zhong, L.; Deng, G.; Zhu, Y.; Liu, W.; Gu, Y.; Lu, Y.; et al. SHP2 inhibition triggers anti-tumor immunity and synergizes with PD-1 blockade. Acta Pharm. Sin. B 2019, 9, 304–315. [Google Scholar] [CrossRef] [PubMed]

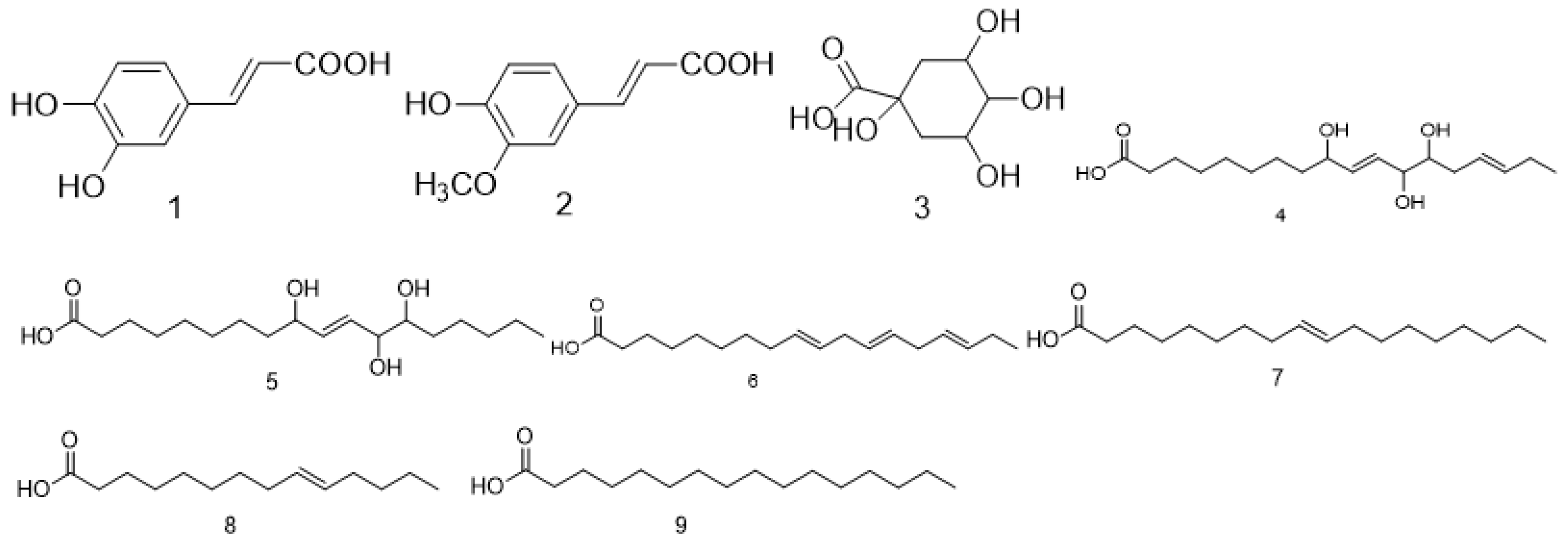
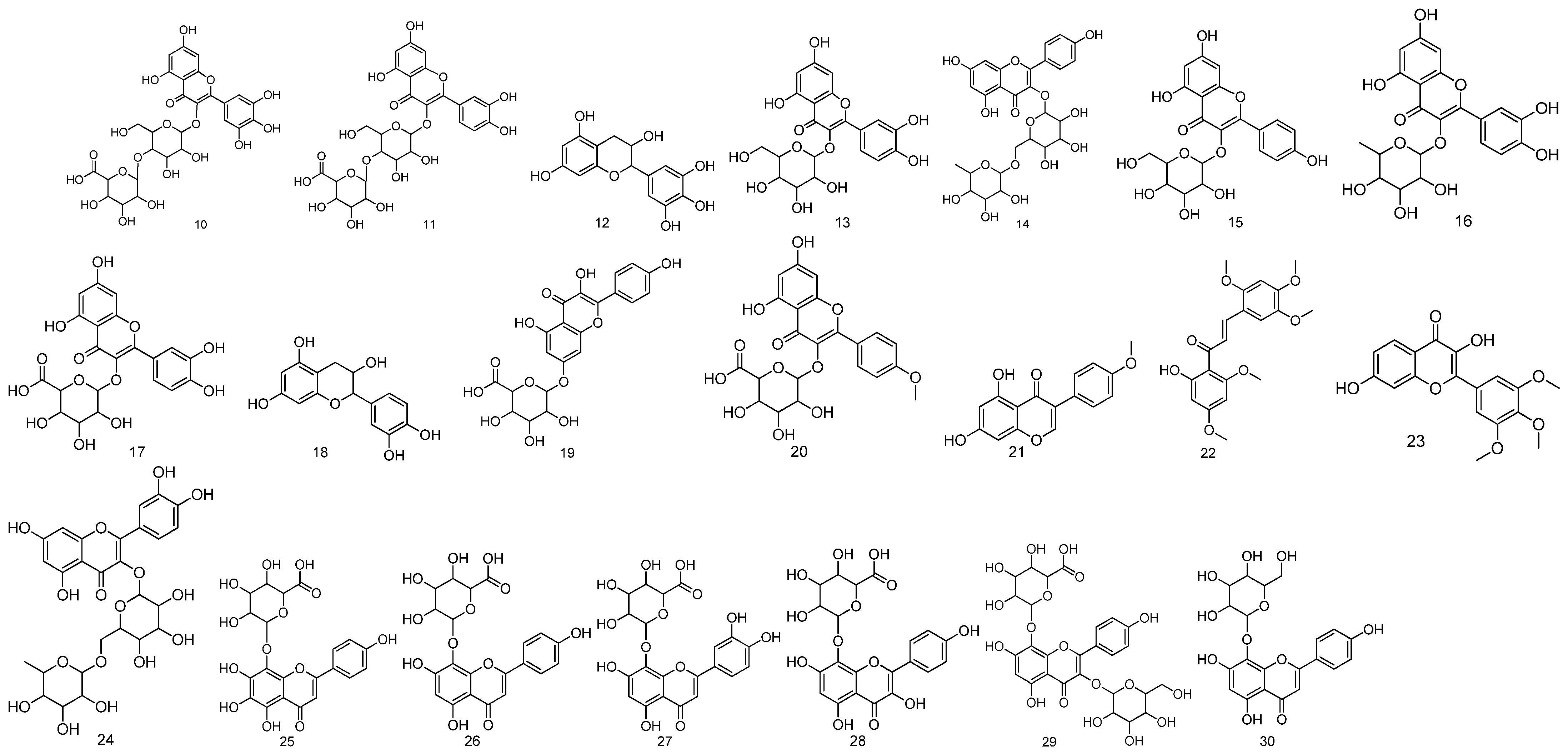
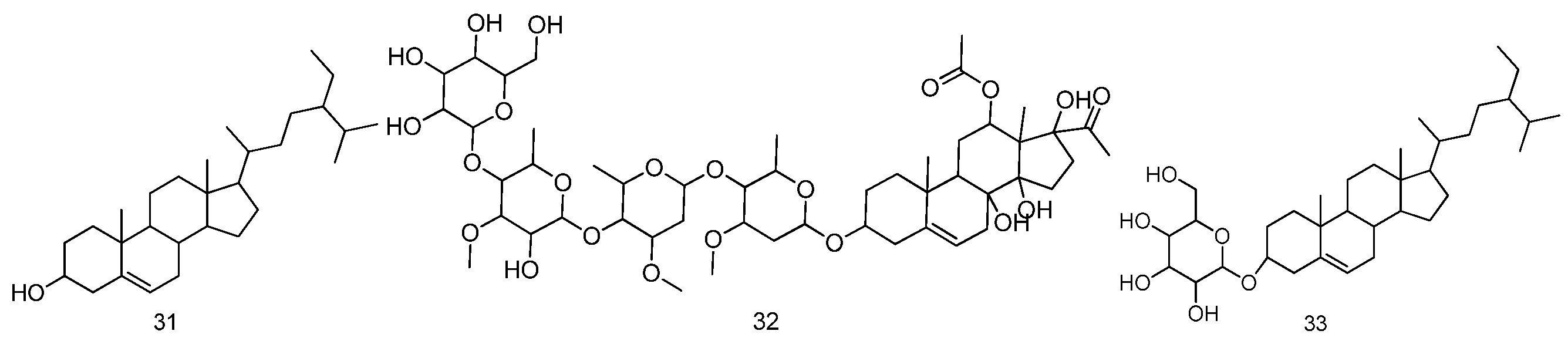
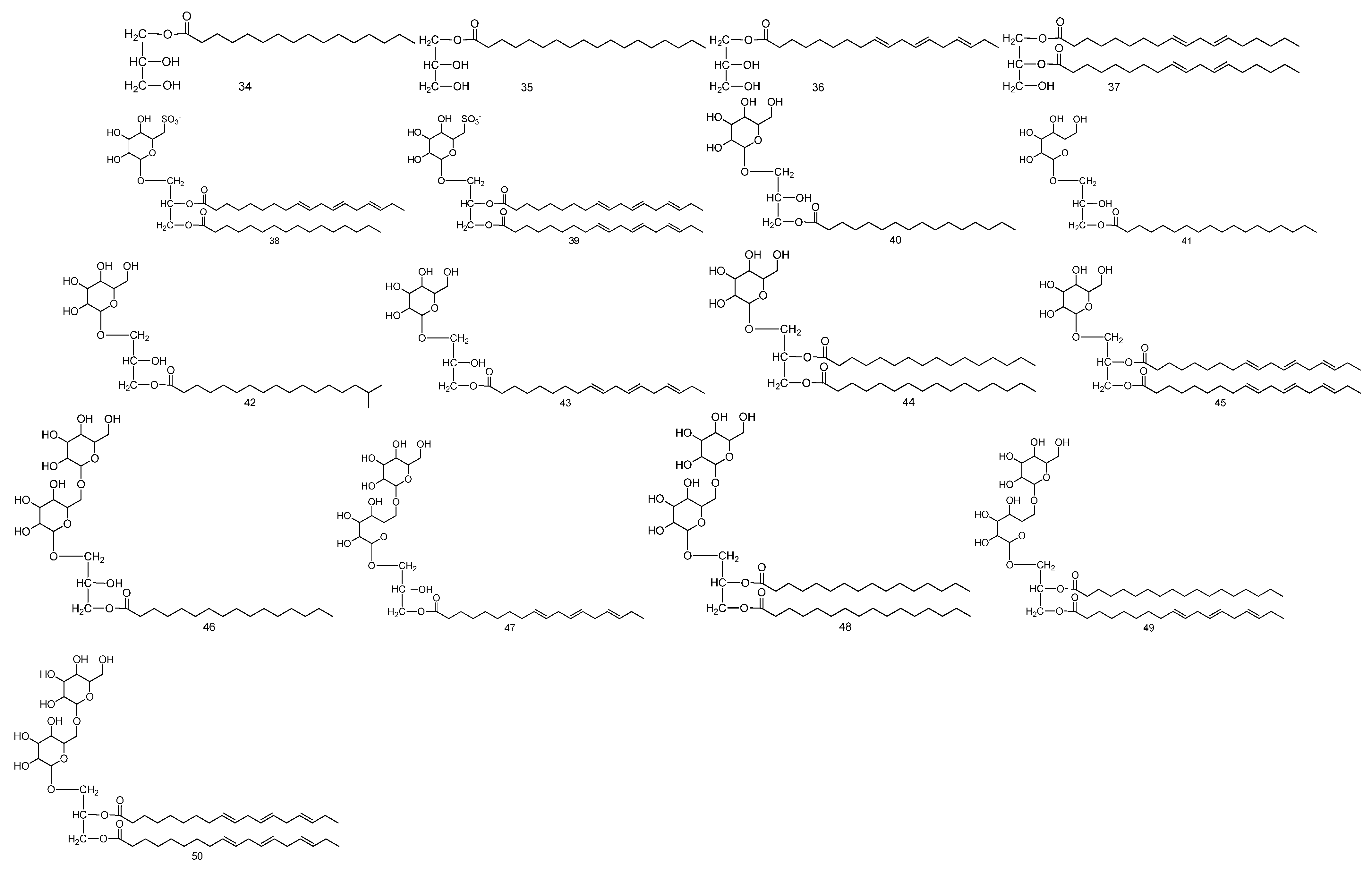




| No. | Compound Name | Molecular Formula | Medicinal Parts | References |
|---|---|---|---|---|
| 1 | Caffeic acid | C9H8O4 | The fruit of Malva verticillata L. | [7,11,12,13,14,15] |
| 2 | Ferulic acid | C10H10O4 | The fruit of Malva verticillata L. | [7,15,16,17] |
| 3 | Quinic acid | C7H12O6 | The stem, leaf and seed mixture of Malva verticillata L. | [31] |
| 4 | 9,12,13-trihydroxy-octadecadienoic acid | C18H32O5 | The stem, leaf and seed mixture of Malva verticillata L. | [31] |
| 5 | 9,12,13-trihydroxy-octadecenoic acid | C18H34O5 | The stem, leaf and seed mixture of Malva verticillata L. | [31] |
| 6 | Linolenic acid | C18H30O2 | The stem, leaf and seed mixture of Malva verticillata L. | [21,31] |
| 7 | Oleic acid | C18H34O2 | The seed of Malva verticillata L. | [21] |
| 8 | Myristoleic acid | C14H26O2 | The seed of Malva verticillata L. | [22] |
| 9 | Palmitic acid | C16H32O2 | The seed of Malva verticillata L. | [23] |
| No. | Chemical Name | Molecular Formula | Medicinal Parts | Reference |
|---|---|---|---|---|
| 10 | Myricetin-3-hexoside-glucuronide | C27H28O19 | The stem, leaf and seed mixture of Malva verticillata L. | [31] |
| 11 | Quercetin-3-O-hexoside-glucuronide | C27H28O18 | The stem, leaf and seed mixture of Malva verticillata L. | [31] |
| 12 | Epigallocatechin | C15H14O7 | The stem, leaf and seed mixture of Malva verticillata L. | [31] |
| 13 | Hyperin | C21H20O12 | The stem, leaf and seed mixture of Malva verticillata L. | [31] |
| 14 | Kaempferol-3-O-rutinoside | C27H30O15 | The stem, leaf and seed mixture of Malva verticillata L. | [31] |
| 15 | Kaempferol-3-O-glucoside | C21H20O11 | The stem, leaf and seed mixture of Malva verticillata L. | [31] |
| 16 | Quercitrin | C21H20O11 | The stem, leaf and seed mixture of Malva verticillata L. | [31] |
| 17 | Quercetin-3-O-glucuronide | C21H18O13 | The stem, leaf and seed mixture of Malva verticillata L. | [31] |
| 18 | Catechin | C15H14O6 | The stem, leaf and seed mixture of Malva verticillata L. | [31] |
| 19 | Kaempferol (or luteolin)-3-O-glucuronid | C21H18O12 | The stem, leaf and seed mixture of Malva verticillata L. | [31] |
| 20 | Kaempferide-3-glucuronide | C22H20O12 | The stem, leaf and seed mixture of Malva verticillata L. | [31] |
| 21 | Biochanin A | C16H12O5 | The stem, leaf and seed mixture of Malva verticillata L. | [31] |
| 22 | Rubone | C20H22O7 | The stem, leaf and seed mixture of Malva verticillata L. | [31] |
| 23 | Robinetin trimethyl ether | C18H16O7 | The stem, leaf and seed mixture of Malva verticillata L. | [31] |
| 24 | Rutin | C27H30O16 | The fruit of Malva verticillata L. | [18] |
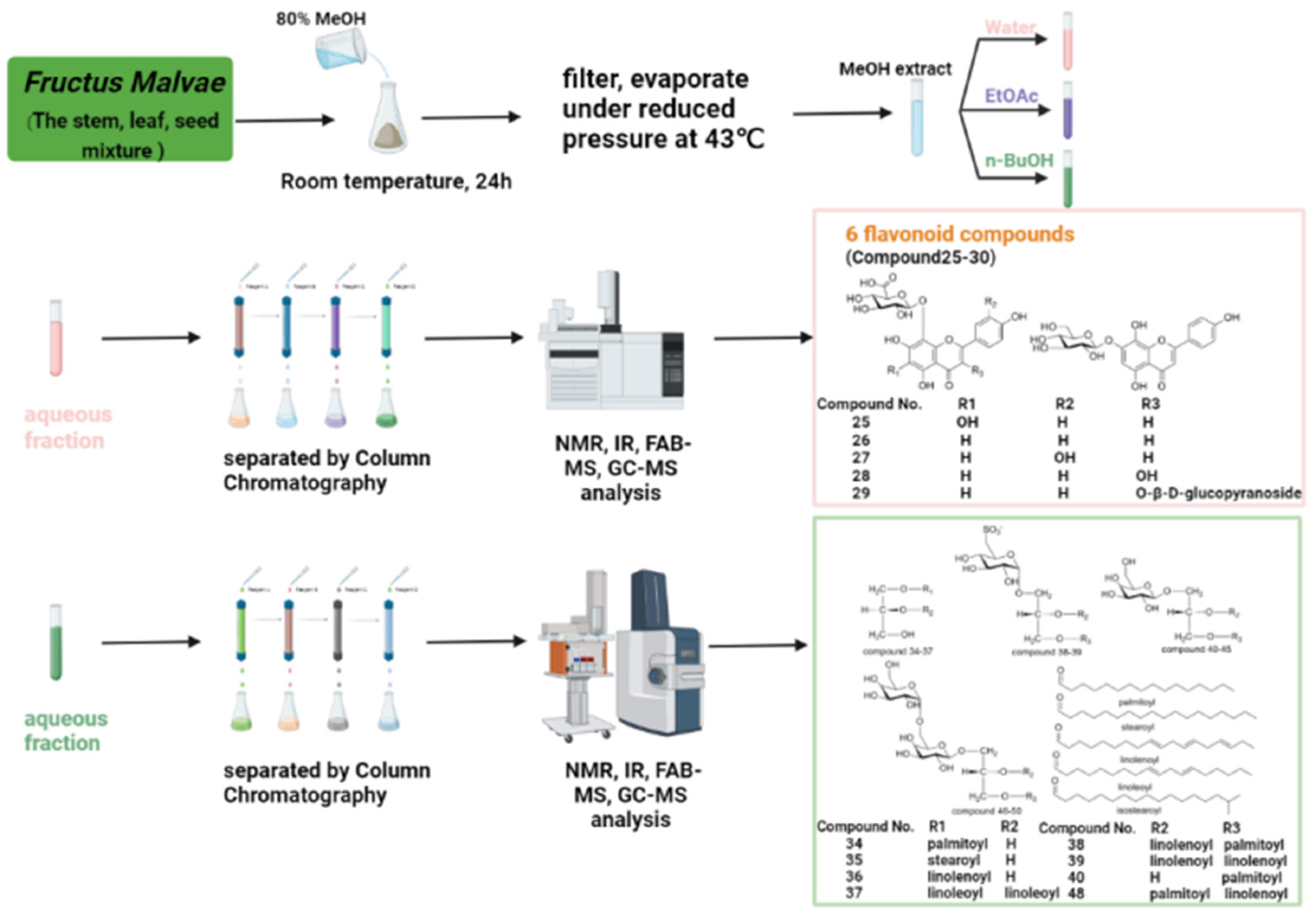
| 25 | Nortangeretin-8-O-β-d-glucuronide | C21H18O13 | The stem, leaf and seed mixture of Malva verticillata L. | [32] |
| 26 | Isoscutellarein 8-O-glucuronopyranoside | C21H18O12 | The stem, leaf and seed mixture of Malva verticillata L. | [32] |
| 27 | Hypolaetin 8-O-glucuronopyranoside | C21H18O13 | The stem, leaf and seed mixture of Malva verticillata L. | [32] |
| 28 | Herbacetin 8-O-glucuronopyranoside | C21H18O13 | The stem, leaf and seed mixture of Malva verticillata L. | [32] |
| 29 | Herbacetin 3-O-glucopyranosyl-8-O-glucuronopyranoside | C27H28O18 | The stem, leaf and seed mixture of Malva verticillata L. | [32] |
| 30 | Isoscutellarein 7-O-glucopyranoside | C21H20O11 | The stem, leaf and seed mixture of Malva verticillata L. | [32] |
| No. | Chemical Name | Molecular Formula | Medicinal Parts | References |
|---|---|---|---|---|
| 31 | β-sitosterol | C29H50O | The seed of Malva verticillata L. | [18,22,23,24] |
| 32 | Verticilloside | C50H80O22 | The seed of Malva verticillata L. | [22] |
| 33 | Daucosterol | C35H60O6 | The seed of Malva verticillata L. | [18,22] |
| No. | Chemical Name | Molecular Formula | Medicinal Parts | Reference |
|---|---|---|---|---|
| 34 | 1-O-palmitoyl glyceride | C19H38O4 | The stem, leaf and seed mixture of Malva verticillata L. | [33] |
| 35 | 1-O-stearoyl glyceride | C21H42O4 | The stem, leaf and seed mixture of Malva verticillata L. | [33] |
| 36 | 1-O-linolenoyl glyceride | C21H36O4 | The stem, leaf and seed mixture of Malva verticillata L. | [33] |
| 37 | 1,2-di-O-linoleoyl glyceride | C39H68O5 | The stem, leaf and seed mixture of Malva verticillata L. | [33] |
| 38 | 1-O-(6-deoxy-6-sulfo)-glucopyranosyl-2-O-linolenoyl-3-O-palmitoyl glyceride | C43H75O12S− | The stem, leaf and seed mixture of Malva verticillata L. | [34] |
| 39 | 1-O-(6-deoxy-6-sulfo)-glucopyranosyl-2,3-di-O-linolenoyl glyceride | C45H73O12S− | The stem, leaf and seed mixture of Malva verticillata L. | [34] |
| 40 | 1-O-galactopyranosyl-3-O-palmitoyl glyceride | C25H48O9 | The stem, leaf and seed mixture of Malva verticillata L. | [34] |
| 41 | 1-O-galactopyranosyl-3-O-stearoyl glyceride | C27H52O9 | The stem, leaf and seed mixture of Malva verticillata L. | [34] |
| 42 | 1-O-galactopyranosyl-3-O-isostearoyl glyceride | C27H52O9 | The stem, leaf and seed mixture of Malva verticillata L. | [34] |
| 43 | 1-O-galactopyranosyl-3-O-linolenoyl glyceride | C27H46O9 | The stem, leaf and seed mixture of Malva verticillata L. | [34] |
| 44 | 1-O-galactopyranosyl-2,3-di-O-palmitoyl glyceride | C41H78O10 | The stem, leaf and seed mixture of Malva verticillata L. | [34] |
| 45 | 1-O-galactopyranosyl-2,3-di-O-linolenoyl glyceride | C45H74O10 | The stem, leaf and seed mixture of Malva verticillata L. | [34] |
| 46 | 1-O-6′-O-(-galactopyranosyl)-galactopyranosyl-3-O-palmitoyl glyceride | C31H58O14 | The stem, leaf and seed mixture of Malva verticillata L. | [34] |
| 47 | O-6′-O-(-galactopyranosyl)-galactopyranosyl-3-O-2-linolenoyl glyceride | C33H56O14 | The stem, leaf and seed mixture of Malva verticillata L. | [34] |
| 48 | 1-O-6′-O-(-galactopyranosyl)-galactopyranosyl-2,3-di-O-palmitoyl glyceride | C47H88O15 | The stem, leaf and seed mixture of Malva verticillata L. | [34] |
| 49 | 1-O-(6-O-galactopyranosyl)-galactopyranosyl-2-O-stearolyl-3-O-linolenoyl glyceride | C51H90O15 | The stem, leaf and seed mixture of Malva verticillata L. | [34] |
| 50 | 1-O-(6-O-galactopyranosyl)-galactopyranosyl-2,3-di-O-linolenoyl glyceride | C51H84O15 | The stem, leaf and seed mixture of Malva verticillata L. | [34] |
| No. | Chemical Name | Molecular Formula | Medicinal Parts | References |
|---|---|---|---|---|
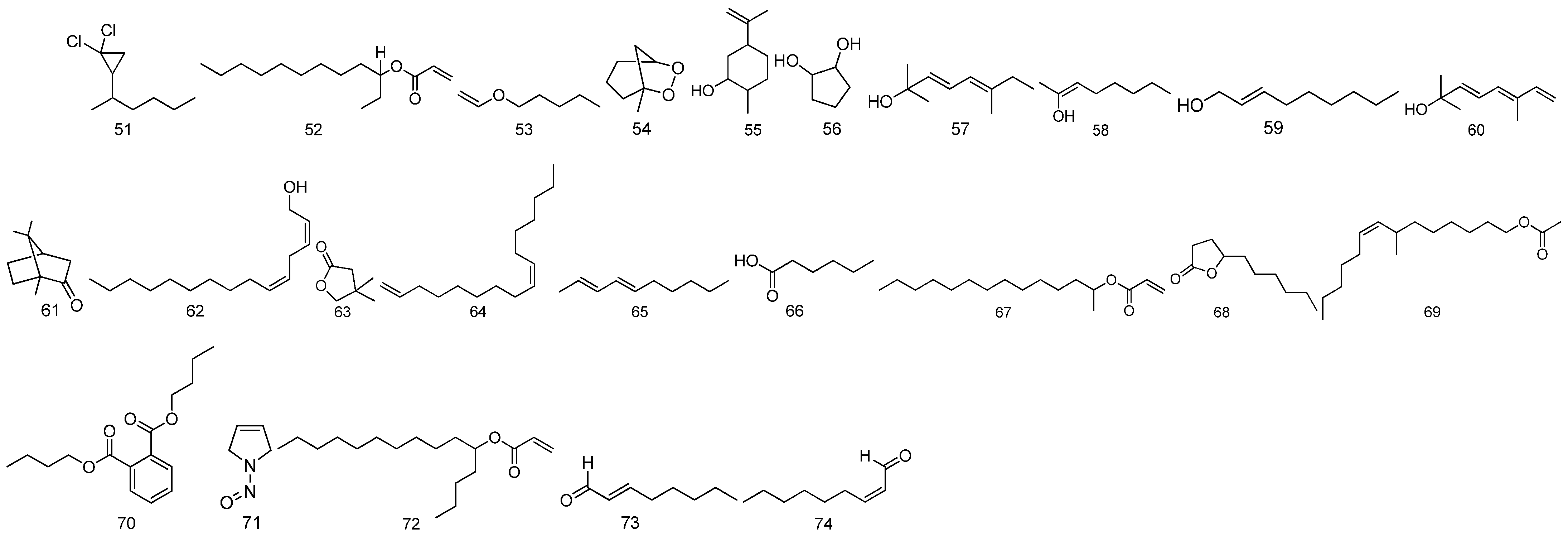
| 51 | 1,1-dichloro-2-hexyl-Cyclopropan | C9H16Cl2 | The fruit of Malva verticillata L. | [19,20] |
| 52 | 3-(Prop-2-enoyloxy)dodecane | C15H28O2 | The fruit of Malva verticillata L. | [19,20] |
| 53 | 1-(ethenyloxy)-pentane | C7H14O | The fruit of Malva verticillata L. | [19,20] |
| 54 | 1-methyl-6,7-Dioxabicyclo[3.2.1]octane | C7H12O2 | The fruit of Malva verticillata L. | [19,20] |
| 55 | 2-methyl-5-(1-methylethenyl)-Cyclohexanol | C10H18O | The fruit of Malva verticillata L. | [19,20] |
| 56 | trans-1,2-Cyclopentanediol | C5H10O2 | The fruit of Malva verticillata L. | [19,20] |
| 57 | 3, 5-Octadien-2-ol | C10H18O | The fruit of Malva verticillata L. | [19,20] |
| 58 | (Z)-2-Octen-2-ol | C8H16O | The fruit of Malva verticillata L. | [19,20] |
| 59 | Nona-2-en-1-ol | C9H18O | The fruit of Malva verticillata L. | [19,20] |
| 60 | (E)-2,6-Dimethyl-3,5,7-octatriene-2-ol | C10H16O | The fruit of Malva verticillata L. | [19,20] |
| 61 | (1S)-1,7,7-trimethyl-Bicyclo[2.2.1] heptan-2-one | C10H16O | The fruit of Malva verticillata L. | [19,20] |
| 62 | Z,Z-2,5-Pentadecadien-1-ol | C15H28O | The fruit of Malva verticillata L. | [19,20] |
| 63 | Dihydro-4,4-dimethyl-2(3H)-Furano | C6H10O2 | The fruit of Malva verticillata L. | [19,20] |
| 64 | Z-1,9-Hexadecadiene | C16H30 | The fruit of Malva verticillata L. | [19,20] |
| 65 | (E, E)-2,4-Decadiene | C10H18 | The fruit of Malva verticillata L. | [19,20] |
| 66 | Hexanoic acid | C16H12O2 | The fruit of Malva verticillata L. | [19,20] |
| 67 | 2-(Prop-2-enoytoxy) tetradecane | C17H32O2 | The fruit of Malva verticillata L. | [19,20] |
| 68 | 5-hexyldihydro-2(3H)-Furanone | C10H18O2 | The fruit of Malva verticillata L. | [19,20] |
| 69 | 7-Methyl-Z-tetradecen-1-ol acetate | C17H32O2 | The fruit of Malva verticillata L. | [19,20] |
| 70 | Dibutylphthalate | C16H22O4 | The fruit of Malva verticillata L. | [19,20] |
| 71 | 2,5-dihydro-1-nitroso-1H-Pyrrole | C4H6N2O | The fruit of Malva verticillata L. | [19,20] |
| 72 | 5-(Prop-2-enoyloxy)pentadecane | C18H34O2 | The fruit of Malva verticillata L. | [19,20] |
| 73 | (E)-2-Octenal | C8H14O | The fruit of Malva verticillata L. | [19,20] |
| 74 | (Z)-2-Nonenal | C9H16O | The fruit of Malva verticillata L. | [19,20] |
| No. | Chemical Name | Molecular Formula | Medicinal Parts | References |
|---|---|---|---|---|
| 75 | MVS-I | -- | The seed of Malva verticillata L. | [25,26,28,29] |
| 76 | MVS-IIA | -- | The seed of Malva verticillata L. | [25,26,29] |
| 77 | MVS-IIG | -- | The seed of Malva verticillata L. | [25,26,29] |
| 78 | MVS-IIIA | -- | The seed of Malva verticillata L. | [25,26,29] |
| 79 | MVS-IVA | -- | The seed of Malva verticillata L. | [25,26,29,30] |
| 80 | MVS-VI | -- | The seed of Malva verticillata L. | [25,26,27,29] |
| 81 | MVS-V | -- | The seed of Malva verticillata L. | [25,26,29] |
| 82 | Sucrose | C12H22O11 | The seed of Malva verticillata L. | [22] |
| 83 | Raffinose | C18H32O16 | The seed of Malva verticillata L. | [22] |
| No. | Chemical Name | Molecular Formula | Medicinal Parts | References |
|---|---|---|---|---|
| 84 | d-alanine | C3H7NO2 | The seed of Malva verticillata L. | [14,18,23] |
| 85 | tryptophan | C11H12N2O2 | The stem, leaf and seed mixture of Malva verticillata L. | [31,35] |
| 86 | Aspartic acid | C4H7NO4 | The fruit of Malva verticillata L. | [14,18] |
| 87 | Threonine | C4H9NO3 | The fruit of Malva verticillata L. | [14,18] |
| 88 | Serine | C3H7NO3 | The fruit of Malva verticillata L. | [14,18] |
| 89 | Glutamic acid | C5H9NO4 | The fruit of Malva verticillata L. | [14,18] |
| 90 | Proline | C5H9NO2 | The fruit of Malva verticillata L. | [14,18] |
| 91 | Glycine | C2H5NO2 | The fruit of Malva verticillata L. | [14,18] |
| 92 | Valine | C5H11NO2 | The fruit of Malva verticillata L. | [14,18] |
| 93 | l-isoleucine | C6H13NO2 | The fruit of Malva verticillata L. | [14,18] |
| 94 | Leucine | C6H13NO2 | The fruit of Malva verticillata L. | [14,18] |
| 95 | Tyrosine | C9H11NO3 | The fruit of Malva verticillata L. | [14,18] |
| 96 | Phenylalanine | C9H11NO2 | The fruit of Malva verticillata L. | [14,18] |
| 97 | Histidine | C6H9N3O2 | The fruit of Malva verticillata L. | [14,18] |
| 98 | Arginine | C6H14N4O2 | The fruit of Malva verticillata L. | [18] |
| No. | Chemical Name | Molecular Formula | Medicinal Parts | Reference |
|---|---|---|---|---|
| 99 | oleamide | C18H35NO | The seed of Malva verticillata L. | [23] |
| 100 | 1,3-dihydroxyacetone dimer | C6H12O6 | The seed of Malva verticillata L. | [23] |
| 101 | 5-hydroxymethyl furfural | C6H6O3 | The seed of Malva verticillata L. | [23] |
| 102 | 2-hydroxy-gamma-butyrolactone | C4H6O3 | The seed of Malva verticillata L. | [23] |
| 103 | 3,5,6,9-tetrahydroxy-7-megastigmene | C13H24O4 | The stem, leaf and seed mixture of Malva verticillata L. | [35] |
| Pharmacological Activity | Compound/Extract | Experimental Level | Experimental Model | Administration Method | Dosage/Concentration | Detection Indicator | Effective Dose | Reference |
|---|---|---|---|---|---|---|---|---|
| Diuretic effect | Petroleum ether extract | Whole animal | Water-loaded rat model | Oral administration | 25 mL/kg | Urine volume, urine sodium content, urine potassium content, urine chlorine content (mg) | Effective dose: 25 mL/kg | [23] |
| Ethyl acetate extract | Whole animal | Water-loaded rat model | Oral administration | 25 mL/kg | Urine volume, urine sodium content, urine potassium content, urine chlorine content (mg) | Effective dose: 25 mL/kg | [23] | |
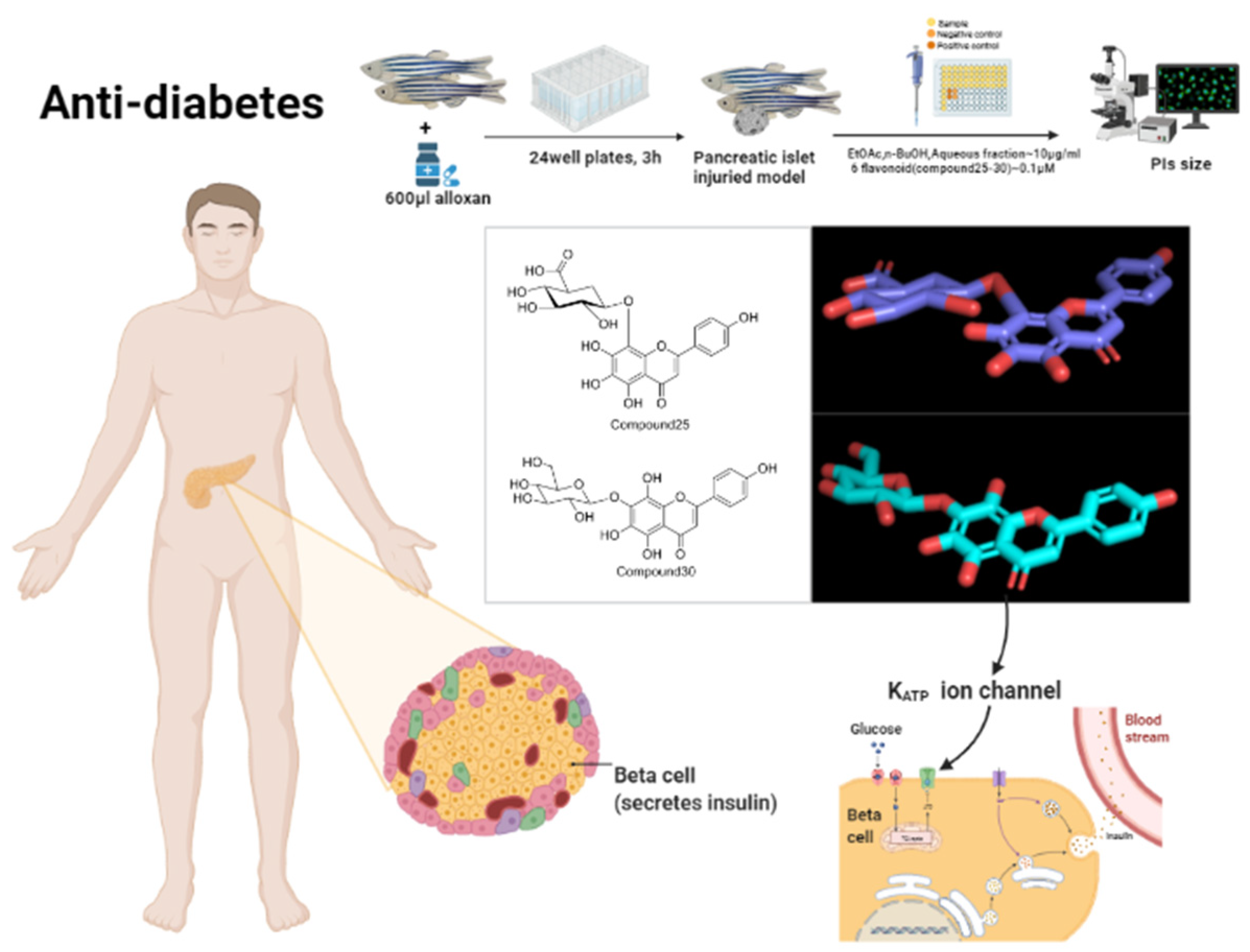
| Anti-diabetic | Ethyl acetate extract | Whole animal | Alloxan-induced islet damage model in zebrafish larvae | Soak absorption | 25~600 μg/mL, 10 μg/mL | 50% lethal concentration LC50; changes in islet area, changes in fluorescence intensity caused by 2-NBDG | LC50:91.5 μg/mL; Effective dose: 10 μg/mL | [18] |
| n-Butanol extract | Whole animal | Alloxan-induced islet damage model in zebrafish larvae | Soak absorption | 25~600 μg/mL, 10 μg/mL | 50% lethal concentration LC50; changes in islet area, changes in fluorescence intensity caused by 2-NBDG | LC50:270.9 μg/mL; Effective dose: 10 μg/mL | [18] | |
| Whole animal | Alloxan-induced islet damage model in zebrafish larvae | Soak absorption | 10 μg/mL | Changes in islet area, changes in fluorescence intensity caused by 2-NBDG | Effective dose: 10 μg/mL | [30] | ||
| Water extract | Whole animal | Alloxan-induced islet damage model in zebrafish larvae | Soak absorption | 25~600 μg/mL, 10 μg/mL | 50% lethal concentration LC50; changes in islet area, changes in fluorescence intensity caused by 2-NBDG | LC50:401.1 μg/mL; Effective dose: 10 μg/mL | [18] | |
| Nortangeretin-8-O-β-d-glucuronide | Whole animal | Alloxan-induced islet damage model in zebrafish larvae | Soak absorption | 0.1 μM | Changes in islet area, changes in fluorescence intensity caused by 2-NBDG | Effective dose: 0.1 μM | [18] | |
| Hypolaetin 8-O-β-d-glucuronopyranoside | Whole animal | Alloxan-induced islet damage model in zebrafish larvae | Soak absorption | 0.1 μM | Changes in islet area, changes in fluorescence intensity caused by 2-NBDG | Effective dose: 0.1 μM | [18] | |
| Herbacetin 8-O-β-d-glucuronopyranoside | Whole animal | Alloxan-induced islet damage model in zebrafish larvae | Soak absorption | 0.1 μM | Changes in islet area, changes in fluorescence intensity caused by 2-NBDG | Effective dose: 0.1 μM | [18] | |
| Isoscutellarein 7-O-β-d-glucopyranoside | Whole animal | Alloxan-induced islet damage model in zebrafish larvae | Soak absorption | 0.1 μM | Changes in islet area, changes in fluorescence intensity caused by 2-NBDG | Effective dose: 0.1 μM | [18] | |
| l-tryptophan | Whole animal | Alloxan-induced islet damage model in zebrafish larvae | Soak absorption | 1 μg/m | Changes in islet area, changes in fluorescence intensity caused by 2-NBDG | Effective dose: 1 μg/mL | [30] | |
| 3,5,6,9-tetrahydroxy-7-megastigmene | Whole animal | Alloxan-induced islet damage model in zebrafish larvae | Soak absorption | 1 μg/m | Changes in islet area, changes in fluorescence intensity caused by 2-NBDG | Effective dose: 1 μg/mL | [30] | |
| n-hexane extract | Whole animal | Type 2 diabetes db/db mice | Oral administration | 10~40 mg/kg weight/d | Fasting blood glucose levels, non-fasting blood glucose levels, triglycerides, total cholesterol, high-density lipoprotein cholesterol, HTR (high-density lipoprotein cholesterol/total cholesterol), phosphorylation levels of AMPK and ACC in soleus muscle and liver | Effective dose: 20 mg/kg | [32] | |
| β-sitosterol | Cellular level | L6 myotube cells | Incubation | 75~300 μM | Phosphorylation levels of AMPK and ACC, glucose uptake | Effective dose: 75 μM | [32] | |
| Neutral polysaccharide MVS-Ⅰ | Whole animal | Male mice | Intraperitoneal injection | 10~100 mg/kg | 0 h, 7 h and 24 h plasma glucose level | Effective dose: 10 mg/kg | [28] | |
| Peptidoglycan MVS-V | Whole animal | Male mice | Intraperitoneal injection | 10~100 mg/kg | 0 h, 7 h and 24 h plasma glucose level | Effective dose: 100 mg/kg | [28] | |
| Peptidoglycan-enriched fraction MVS-V-CH | Whole animal | Male mice | Intraperitoneal injection | 10~100 mg/kg | 0 h, 7 h and 24 h plasma glucose level | Effective dose: 10 mg/kg | [28] | |
| Anti-oxidation | Nortangeretin-8-O-β-d-glucuronide | Physical and chemical reaction | DPPH RS activity, ABTS RS activity, oxygen-radical absorbance capacity (ORAC) assay, superoxide scavenging activity | Incubation | 0.1 mL, 20 μL, | DPPH EC50, ABTS EC50, ORAC, SOD EC50 | DPPH EC50: >50 µM, ABTS EC50: 2.22 ± 0.05 µM, ORAC: 14.38 ± 0.35 µmol TE/µmol SOD EC50: 0.73 ± 0.09 µM | [18] |
| Isoscutellarein 8-O-β-d-glucuronopyranoside | Physical and chemical reaction | DPPH RS activity, ABTS RS activity, oxygen-radical absorbance capacity (ORAC) assay, superoxide scavenging activity | Incubation | DPPH EC50, ABTS EC50, ORAC, SOD EC50 | DPPH EC50: >50 µM, ABTS EC50: 3.38 ± 0.15 µM, ORAC: 8.06 ± 0.36 µmol TE/µmol, SOD EC50:1.51 ± 0.15 µM | [18] | ||
| hypolaetin 8-O-β-d-glucuronopyranoside | Physical and chemical reaction | DPPH RS activity, ABTS RS activity, oxygen-radical absorbance capacity (ORAC) assay, superoxide scavenging activity | Incubation | DPPH EC50, ABTS EC50, ORAC, SOD EC50 | DPPH EC50: 5.98 ± 0.24 µM, ABTS EC50: 1.52 ± 0.04 µM, ORAC: 12.48 ± 1.27 µmol TE/µmol, SOD EC50: 0.98 ± 0.13 µM | [18] | ||
| herbacetin 8-O-β-d-glucuronopyranoside | Physical and chemical reaction | DPPH RS activity, ABTS RS activity, oxygen-radical absorbance capacity (ORAC) assay, superoxide scavenging activity | Incubation | DPPH EC50, ABTS EC50, ORAC, SOD EC50 | DPPH EC50: 31.79 ± 2.22 µM, ABTS EC50: 4.51 ± 0.13 µM, ORAC: 6.56 ± 0.32 µmol TE/µmol, SOD EC50: 1.04 ± 0.21 µM | [18] | ||
| herbacetin 3-O-β-d-glucopyranosyl-8-O-β-d-glucuronopyranoside | Physical and chemical reaction | DPPH RS activity, ABTS RS activity, Oxygen-radical absorbance capacity (ORAC) assay, superoxide scavenging activity | Incubation | DPPH EC50, ABTS EC50, ORAC, SOD EC50 | DPPH EC50: 33.80 ± 1.89 µM, ABTS EC50: 4.05 ± 0.14 µM, ORAC: 6.42 ± 0.18 µmol TE/µmol, SOD EC50: 0.70 ± 0.18 µM | [18] | ||
| isoscutellarein 7-O-d-glucopyranoside | Physical and chemical reaction | DPPH RS activity, ABTS RS activity, oxygen-radical absorbance capacity (ORAC) assay, superoxide scavenging activity | Incubation | DPPH EC50, ABTS EC50, ORAC, SOD EC50 | DPPH EC50: >50 µM, ABTS EC50: 21.62 ± 1.26 µM, ORAC: 3.83 ± 0.30 µmol TE/µmol, SOD EC50: 1.31 ± 0.20 µM | [18] | ||
| 90% ethanol extract | Physical and chemical reaction | DPPH radical scavenging activity assay | Incubation | 100 μL (1–1000 μg/mL) | DPPH anion scavenging activity, ABTS cation scavenging activity, FRAP | [17] | ||
| Antitumor | Ethyl acetate extract | Cellular level | Splenocytes, natural killer (NK) cells | Incubation | 10 μg/mL | splenocyte proliferation ability, natural killer (NK) cell activity | Effective dose: 10 μg/mL | [24] |
| Cellular level | HepG2, AGS, HCT-15, A549 | Incubation | 50% inhibitory concentration IC50 | IC50 ± SD: 83.7 ± 3.98 μg/mL, 79.0 ± 1.47 μg/mL, 80.9 ± 1.56 μg/mL, 87.0 ± 0.98 μg/mL, | [33] | |||
| n-butanol extract | Cellular level | Splenocytes, natural killer (NK) cells | Incubation | 10 μg/mL | splenocyte proliferation ability, natural killer (NK) cell activity | Effective dose: 10 μg/mL | [24] | |
| Cellular level | HepG2, AGS, HCT-15, A549 | Incubation | 10~40 μg/mL | 50% inhibitory concentration IC50, AGS cell apoptosis percentage, Expression of apoptosis proteins PARP, Cleaved APRP, Caspase-3, Cleaved Caspase-3, Bcl-2, Bax, β-actin | IC50 ± SD: 11.3 ± 0.30 μg/mL, 8.2 ± 0.14 μg/mL, 7.4 ± 0.26 μg/mL, 52.2 ± 4.32 μg/mL, | [33] | ||
| Water extract | Cellular level | Splenocytes, Natural Killer (NK) cells | Incubation | 10 μg/mL | splenocyte proliferation ability, natural killer (NK) cell activity | Effective dose: 10 μg/mL | [24] | |
| Cellular level | HepG2, AGS, HCT-15, A549 | Incubation | 50% inhibitory concentration IC50 | IC50 ± SD: 86.0 ± 1.66 μg/mL, 90.0 ± 0.14 μg/mL, 91.5 ± 2.76 μg/mL, 96.3 ± 2.24 μg/mL | [33] | |||
| (2S)-1-O-palmitoyl glyceride | Cellular level | Splenocytes, natural killer (NK) cells | Incubation | 10 μM | splenocyte proliferation ability, natural killer (NK) cell activity | Effective dose: 10 μM | [24] | |
| (2S)-1-O-stearoyl glyceride | Cellular level | Splenocytes, natural killer (NK) cells | Incubation | 10 μM | splenocyte proliferation ability, natural killer (NK) cell activity | Effective dose: 10 μM | [24] | |
| (2S)-1-O-linolenoyl glyceride | Cellular level | Splenocytes, natural killer (NK) cells | Incubation | 10 μM | splenocyte proliferation ability, natural killer (NK) cell activity | Effective dose: 10 μM | [24] | |
| (2S)-1,2-di-O-linoleoyl glyceride | Cellular level | Splenocytes, natural killer (NK) cells | Incubation | 10 μM | splenocyte proliferation ability, natural killer (NK) cell activity | Effective dose: 10 μM | [24] | |
| (2S)-1-O-(6-deoxy-6-sulfo)-α-D glucopyranosyl-2-O-linolenoyl-3-O-palmitoyl glyceride | Cellular level | HepG2, AGS, HCT-15, A549 | Incubation | 25~100 μM | 50% inhibitory concentration IC50, AGS cell apoptosis percentage, Expression of apoptosis proteins PARP, Cleaved APRP, Caspase-3, Cleaved Caspase-3, Bcl-2, Bax, β-actin | IC50 ± SD: 63.7 ± 2.43 μM, 33.7 ± 0.64 μM, 49.6 ± 0.24 μM, 81.8 ± 2.19 μM | [33] | |
| (2S)-1-O-(6-deoxy-6-sulfo)-α-d-glucopyranosyl-2,3-di-O-linolenoyl glyceride | Cellular level | HepG2, AGS, HCT-15, A549 | Incubation | 20~80 μM | 50% inhibitory concentration IC50, AGS cell apoptosis percentage, Expression of apoptosis proteins PARP, Cleaved APRP, Caspase-3, Cleaved Caspase-3, Bcl-2, Bax, β-actin | IC50 ± SD: 34.7 ± 2.26 μM, 11.1 ± 0.07 μM, 49.2 ± 5.16 μM, 76.0 ± 2.62 μM | [33] | |
| (2S)-1-O-β-d- galactopyranosyl-3-O-palmitoyl glyceride | Cellular level | HepG2, AGS, HCT-15, A549 | Incubation | 50% inhibitory concentration IC50 | IC50 ± SD: 83.4 ± 0.55 μM, 86.7 ± 2.02 μM, >100 μM, 96.1 ± 2.23 μM | [33] | ||
| (2S)-1-O-β-d-galactopyranosyl-3-O stearoyl glyceride | Cellular level | HepG2, AGS, HCT-15, A549 | Incubation | 50% inhibitory concentration IC50 | IC50 ± SD: 71.1 ± 2.04 μM, 77.7 ± 6.22 μM, >100 μM, 87.5 ± 3.98 μM | [33] | ||
| (2S)-1-O-β-d-galactopyranosyl-3-O-isostearoyl glyceride | Cellular level | HepG2, AGS, HCT-15, A549 | Incubation | 50% inhibitory concentration IC50 | IC50 ± SD: 77.3 ± 1.76 μM, 89.5 ± 0.88 μM, >100 μM, 91.5 ± 1.76 μM | [33] | ||
| (2S)-1-O-β-d-galactopyranosyl-3-O-linolenoyl glyceride | Cellular level | HepG2, AGS, HCT-15, A549 | Incubation | 50% inhibitory concentration IC50 | IC50 ± SD: 74.9 ± 1.89 μM, 89.3 ± 1.21 μM, 91.8 ± 2.43 μM, 89.9 ± 1.61 μM | [33] | ||
| (2S)-1-O-β-d-galactopyranosyl-2,3-di-O-palmitoyl glyceride | Cellular level | HepG2, AGS, HCT-15, A549 | Incubation | 50% inhibitory concentration IC50 | IC50 ± SD: 83.1 ± 0.48 μM, 90.6 ± 1.00 μM, 90.6 ± 1.00 μM, 87.9 ± 2.69 μM | [33] | ||
| (2S)-1-O-β-d-galactopyranosyl-2,3-di-O-linolenoyl glyceride | Cellular level | HepG2, AGS, HCT-15, A549 | Incubation | 50% inhibitory concentration IC50 | IC50 ± SD: 76.3 ± 1.23 μM, 64.8 ± 2.24 μM, 77.5 ± 4.64 μM, >100 μM | [33] | ||
| (2S)-1-O-6′-O-(α-d-galactopyranosyl)-β-d-galactopyra-nosyl-3-O-palmitoyl glyceride | Cellular level | HepG2, AGS, HCT-15, A549 | Incubation | 50% inhibitory concentration IC50 | IC50 ± SD: 74.4 ± 0.78 μM, 70.6 ± 1.00 μM, 85.9 ± 3.33 μM, 87.8 ± 4.53 μM | [33] | ||
| (2S)-1-O-6′-O-(α-d-galactopyranosyl)-β-d-galactopyran-osyl-3-O-linolenoyl glyceride | Cellular level | HepG2, AGS, HCT-15, A549 | Incubation | 50% inhibitory concentration IC50 | IC50 ± SD: 79.3 ± 1.46 μM, 85.4 ± 1.74 μM, 91.3 ± 3.28 μM, 98.3 ± 0.67 μM | [33] | ||
| (2S)-1-O-6′-O-(α-d-galactopyranosyl)-β-d-galactopyrano-syl-2,3-di-O-palmitoyl glyceride | Cellular level | HepG2, AGS, HCT-15, A549 | Incubation | 10~40 μM | 50% inhibitory concentration IC50, AGS cell apoptosis percentage, Expression of apoptosis proteins PARP, Cleaved APRP, Caspase-3, Cleaved Caspase-3, Bcl-2, Bax, β-actin | IC50 ± SD: 10.0 ± 0.45 μM, 10.6 ± 0.10 μM, 15.3 ± 1.12 μM, 7.1 ± 0.12 μM | [33] | |
| (2S)-1-O-(6-O-α-d-galactopyranosyl)-β-d-galactopyran-osyl-2-O-stearolyl-3-O-linolenoyl glyceride | Cellular level | HepG2, AGS, HCT-15, A549 | Incubation | 50% inhibitory concentration IC50 | IC50 ± SD: 72.8 ± 2.41 μM, 88.2 ± 1.59 μM, 97.1 ± 5.18 μM, >100 μM | [33] | ||
| (2S)-1-O-(6-O-α-d-galactopyranosyl)-β-d-Galactopyrano syl-2-O-stearolyl-3-O-linolenoyl glyceride | Cellular level | HepG2, AGS, HCT-15, A549 | Incubation | 50% inhibitory concentration IC50 | IC50 ± SD: 71.3 ± 0.46 μM, 66.3 ± 1.96 μM, 74.6 ± 1.93 μM, 83.1 ± 3.66 μM | [33] | ||
| Hair-loss treatment | 95% ethanol extract | Cellular level | HFDPC cells | Incubation | 3~100 μg/mL | Cell proliferation rate | Effective dose: 100 μg/mL | [31] |
| n-hexane extract | Cellular level | HFDPC cells | Incubation | 3~100 μg/mL | Cell proliferation rate | Effective dose: 30 μg/mL | [31] | |
| Linoleic acid | Cellular level | HFDPC cells | Incubation | 3~30 μg/mL | Cell proliferation rate, Wnt/β-catenin signaling pathway proteins GSK-3β, β-catenin; Cyclin D1, CDK2, GAPDH; cell growth factor VEGF, IGF-1, HGF, KGF, GAPDH | Effective dose: 10 μg/mL | [31] | |
| Ethanol extract | Cellular level | Human dermal papilla cells (DPCs) | Incubation | 0~50 μg/mL | Wnt reporter activity, expression of intracellular proteins β-catenin and GAPDH | Effective dose: 10 μg/mL | [21] | |
| Dichloromethane extract | Cellular level | Human dermal papilla cells DPCs) | Incubation | 10~100 μg/mL | Wnt reporter activity, expression of intracellular proteins β-catenin and GAPDH | Effective dose: 10 μg/mL | [21] | |
| Myristoleic acid | Cellular level | Human dermal papilla cells (DPCs) | Incubation | 0~100 μg/mL | Wnt reporter activity, cell number, expression of cytokines IGF-1, KGF, VEGF, HGF, GAPDH, Phosphorylation levels of cell-signaling molecules p-38, ERK, CREB, Akt | Effective dose: 10 μg/mL | [21] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Wang, X.; Zhao, M.; Zhang, H.; Liu, C. The Chemical and Pharmacological Research Progress on a Kind of Chinese Herbal Medicine, Fructus Malvae. Molecules 2022, 27, 5678. https://doi.org/10.3390/molecules27175678
Li X, Wang X, Zhao M, Zhang H, Liu C. The Chemical and Pharmacological Research Progress on a Kind of Chinese Herbal Medicine, Fructus Malvae. Molecules. 2022; 27(17):5678. https://doi.org/10.3390/molecules27175678
Chicago/Turabian StyleLi, Xiaoyu, Xianglei Wang, Menglu Zhao, He Zhang, and Chao Liu. 2022. "The Chemical and Pharmacological Research Progress on a Kind of Chinese Herbal Medicine, Fructus Malvae" Molecules 27, no. 17: 5678. https://doi.org/10.3390/molecules27175678
APA StyleLi, X., Wang, X., Zhao, M., Zhang, H., & Liu, C. (2022). The Chemical and Pharmacological Research Progress on a Kind of Chinese Herbal Medicine, Fructus Malvae. Molecules, 27(17), 5678. https://doi.org/10.3390/molecules27175678






