Does Nitrogen Fertilization Affect the Secondary Structures of Gliadin Proteins in Hypoallergenic Wheat?
Abstract
1. Introduction
2. Results
2.1. Analysis of Variance of Protein Content, Gluten Content and Gluten Index in Wheat Kernels
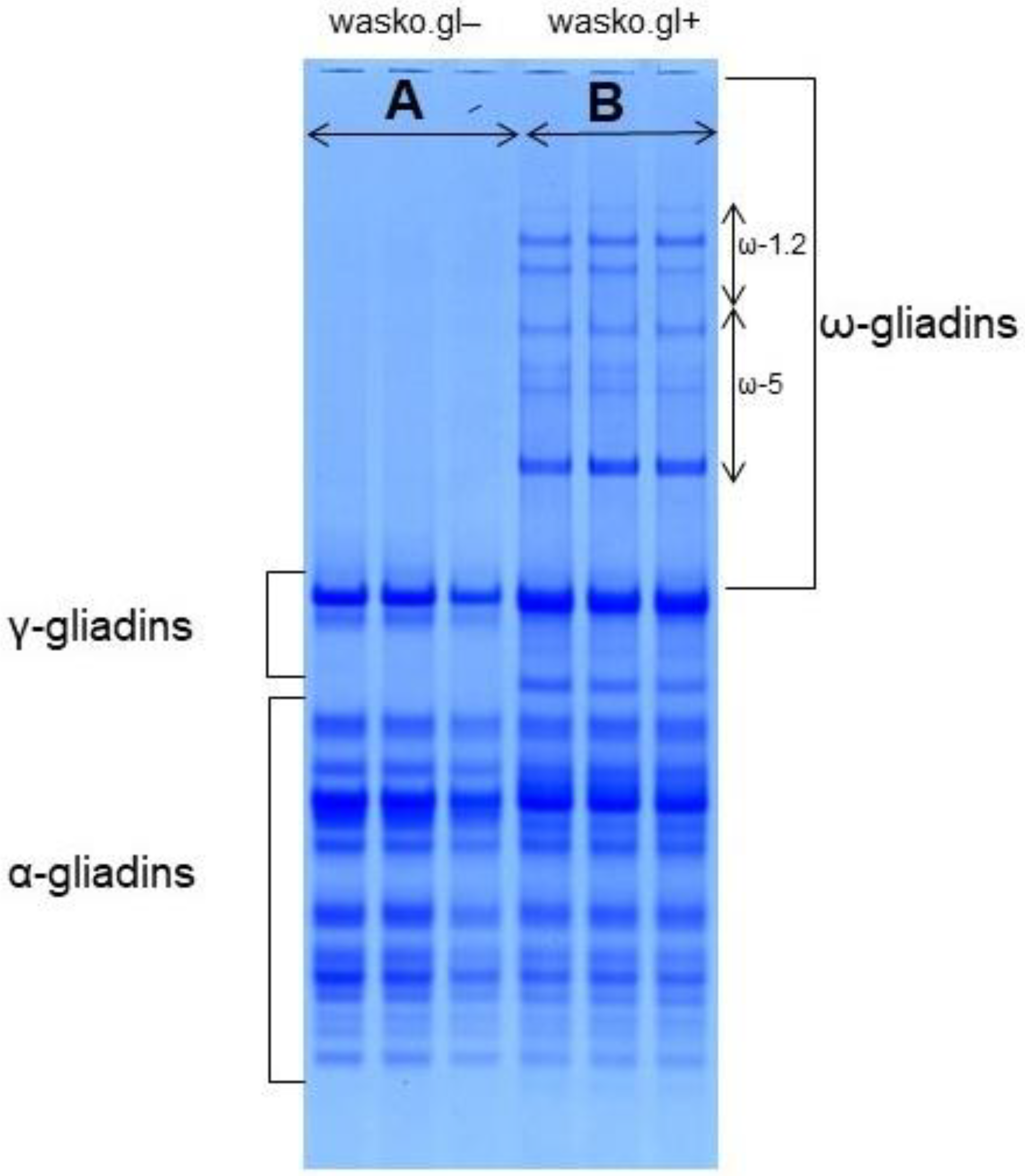
2.2. Electrophoretic Measurements
2.3. FT-Raman Measurements and Curve Fitting Analysis
2.4. HPLC Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Kernels Quality Characteristics
4.3. Wheat Sample Preparation for HPLC and FT-Raman Measurements
4.4. Gliadins and Total Protein Extraction
4.5. Gel Electrophoresis
4.6. Reverse-Phase High-Performance Liquid Chromatography (RP-HPLC) of Gliadin Fraction
4.7. Raman Spectroscopy Measurements and Curve Fitting Analysi
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Aalberse, R.C.; Crameri, R. IgE-binding epitopes: A reappraisal. Allergy 2011, 66, 1261–1274. [Google Scholar] [CrossRef] [PubMed]
- Obtułowicz, K.; Waga, J.; Dyga, W. Gluten—Mechanisms of intolerance, symptoms and treatment possibilities of IgE-related allergy for gluten in the light of actual clinical and immunological studies. Przegląd Lek. 2015, 72, 747–753. (In Polish) [Google Scholar]
- Mowat, A.M. Coeliac disease—A meeting point for genetics, immunology, and protein chemistry. Lancet 2003, 361, 1290–1292. [Google Scholar] [CrossRef]
- Shan, L.; Molberg, O.; Parrot, I.; Hausch, F.; Filiz, F.; Gray, G.M.; Sollid, L.M.; Khosla, C. Structural basis for gluten intolerance in Celiac sprue. Science 2002, 297, 2275–2279. [Google Scholar] [CrossRef] [PubMed]
- Shewry, P.R.; Jenkins, J.A.; Beaudoin, F.; Mills, E.N.C. The Classification, Functions and Evolutionary Relationships of Plant Proteins in Relation to Food Allergies. In Plant Food Allergens; Mills, E.N.C., Shewry, P.R., Eds.; Blackwell Publishing: Oxford, UK, 2003; pp. 24–41. [Google Scholar]
- Palosuo, K.; Varjonen, E.; Kekki, O.-M.; Klemola, T.; Kalkkinen, N.; Alenius, H.; Reunala, T. Wheat ω-5 gliadin is a major allergen in children with immediate allergy to ingested wheat. J. Allergy Clin. Immunol. 2001, 108, 634–638. [Google Scholar] [CrossRef] [PubMed]
- Anderson, O.D.; Gu, Y.Q.; Kong, X.; Lazo, G.R.; Wu, J. The wheat ω-gliadin genes: Structure and EST analysis. Funct. Integr. Genom. 2009, 9, 397–410. [Google Scholar] [CrossRef]
- Shewry, P.R.; Halford, N.G.; Belton, P.S.; Tatham, A.S. Gluten, the elastomeric protein of wheat seeds. In Elastomeric Proteins, Structural, Biomechanical Properties and Biological Roles; Shewry, P.R., Tatham, A.S., Bailey, A.J., Eds.; Cambridge University Press: Cambridge UK, 2003; pp. 279–301. [Google Scholar]
- Matsuo, H.; Kohno, K.; Morita, E. Molecular cloning, recombinant expression and IgE-binding epitope of omega-5 gliadin, a major allergen in wheat-dependent exercise-induced anaphylaxis. Febs J. 2005, 272, 4431–4438. [Google Scholar] [CrossRef]
- Morita, E.; Matsuo, H.; Mihara, S.; Morimoto, K.; Savage, A.W.J.; Tatham, A.S. Fast ω-gliadin is a major allergen in wheat-dependent exercise-induced anaphylaxis. J. Dermatol. Sci. 2003, 33, 99–104. [Google Scholar] [CrossRef]
- Saint Pierre, C.; Peterson, C.J.; Ross, A.S.; Ohm, J.B.; Verhoeven, M.C.; Larson, M.; Hoefer, B. Winter wheat genotypes under different levels of nitrogen and water stress: Changes in grain protein composition. J. Cereal Sci. 2008, 47, 407–416. [Google Scholar] [CrossRef]
- Zhen, S.M.; Deng, X.; Xu, X.X.; Liu, N.N.; Zhu, D.; Wang, Z.M.; Yan, Y.M. Effect of high-nitrogen fertilizer on gliadin and glutenin subproteomes during kernel development in wheat (Triticum aestivum L.). Crop J. 2020, 8, 38–52. [Google Scholar] [CrossRef]
- Jiang, H.; Yu, S.; Yu, Z.; Zhao, Q.; Qiu, H.; Ding, X. Response of Wheat Glutenin Polymerization to Increased Nitrogen Fertilization. J. Triticeae Crops 2003, 23, 43–46. [Google Scholar]
- Zhang, D.; Zhang, Y.; Yang, W.; Yan, C.; JI, H. Effect of nitrogen application on high molecular weight glutenin subunit expression and grain quality of winter wheat. Plant. Nutr. Fertil. Sci. 2008, 14, 235–241. [Google Scholar]
- Rozbicki, J.; Ceglinska, A.; Gozdowski, D.; Jakubczak, M.; Cacak-Pietrzak, G.; Madry, W.; Golba, J.; Piechocinski, M.; Sobczynski, G.; Studnicki, M.; et al. Influence of the cultivar, environment and management on the grain yield and bread-making quality in winter wheat. J. Cereal Sci. 2015, 61, 126–132. [Google Scholar] [CrossRef]
- Yu, X.R.; Chen, X.Y.; Wang, L.L.; Yang, Y.; Zhu, X.W.; Shao, S.S.; Cui, W.X.; Xiong, F. Novel insights into the effect of nitrogen on storage protein biosynthesis and protein body development in wheat caryopsis. J. Exp. Bot. 2017, 68, 2259–2274. [Google Scholar] [CrossRef]
- Matsuo, H.; Morita, E.; Tatham, A.S.; Morimoto, K.; Horikawa, T.; Osuna, H.; Ikezawa, Z.; Kaneko, S.; Kohno, K.; Dekio, S. Identification of the IgE-binding epitope in omega-5 gliadin, a major allergen in wheat-dependent exercise-induced anaphylaxis. J. Biol. Chem. 2004, 279, 12135–12140. [Google Scholar] [CrossRef]
- Maruyama, N.; Ichise, K.; Katsube, T.; Kishimoto, T.; Kawase, S.i.; Matsumura, Y.; Takeuchi, Y.; Sawada, T.; Utsumi, S. Identification of major wheat allergens by means of the Escherichia coli expression system. Eur. J. Biochem. 1998, 255, 739–745. [Google Scholar] [CrossRef]
- Marsh, M.N. Gluten, major histocompatibility complex, and the small-intestine—A molecular and immunobiological approach to the spectrum of gluten sensitivity (celiac sprue). Gastroenterology 1992, 102, 330–354. [Google Scholar] [CrossRef]
- Waga, J.; Skoczowski, A. Development and characteristics of ω-gliadin-free wheat genotypes. Euphytica 2014, 195, 105–116. [Google Scholar] [CrossRef][Green Version]
- Plessis, A.; Ravel, C.; Bordes, J.; Balfourier, F.; Martre, P. Association study of wheat grain protein composition reveals that gliadin and glutenin composition are trans-regulated by different chromosome regions. J. Exp. Bot. 2013, 64, 3627–3644. [Google Scholar] [CrossRef]
- Skoczowski, A.; Obtulowicz, K.; Czarnobilska, E.; Dyga, W.; Mazur, M.; Stawoska, I.; Waga, J. Antibody reactivity in patients with IgE-mediated wheat allergy to various subunits and fractions of gluten and non-gluten proteins from omega-gliadin-free wheat genotypes. Ann. Agric. Environ. Med. 2017, 24, 229–236. [Google Scholar] [CrossRef]
- Stawoska, I.; Wesełucha-Birczyńska, A.; Skoczowski, A.; Dziurka, M.; Waga, J. FT-Raman Spectroscopy as a Tool to Study the Secondary Structures of Wheat Gliadin Proteins. Molecules 2021, 26, 5388. [Google Scholar] [CrossRef] [PubMed]
- Tatham, A.S. The structures of wheat proteins. In Wheat Structure: Biochemistry and Functionality; Schofield, J.D., Ed.; The Royal Society of Chemistry: Cambridge, UK, 1996; pp. 53–62. [Google Scholar]
- Kizil, R.; Irudayaraj, J.; Seetharaman, K. Characterization of Irradiated Starches by Using FT-Raman and FTIR Spectroscopy. J. Agric. Food Chem. 2002, 50, 3912–3918. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.R.; Alves, R.S.; Nascimbem, L.; Stephani, R.; Poppi, R.J.; de Oliveira, L.F.C. Determination of amylose content in starch using Raman spectroscopy and multivariate calibration analysis. Anal. Bioanal. Chem. 2010, 397, 2693–2701. [Google Scholar] [CrossRef]
- Asthir, B.; Jain, D.; Kaur, B.; Bains, N.S. Effect of nitrogen on starch and protein content in grain influence of nitrogen doses on grain starch and protein accumulation in diversified wheat genotypes. J. Environ. Biol. 2017, 38, 427–433. [Google Scholar] [CrossRef]
- Li, W.H.; Shan, Y.L.; Xiao, X.L.; Zheng, J.M.; Luo, Q.G.; Ouyang, S.H.; Zhang, G.Q. Effect of Nitrogen and Sulfur Fertilization on Accumulation Characteristics and Physicochemical Properties of A- and B-Wheat Starch. J. Agric. Food Chem. 2013, 61, 2418–2425. [Google Scholar] [CrossRef]
- Tong, J.; Wang, S.; He, Z.; Zhang, Y. Effects of Reduced Nitrogen Fertilization and Irrigation on Structure and Physicochemical Properties of Starch in Two Bread Wheat Cultivars. Agriculture 2021, 11, 26. [Google Scholar] [CrossRef]
- Shewry, P.R.; Miflin, B.J.; Lew, E.J.L.; Kasarda, D.D. The preparation and characterization of an aggregated gliadin fraction from wheat. J. Exp. Bot. 1983, 34, 1403–1410. [Google Scholar] [CrossRef]
- Payne, P.I.; Corfield, K.G. Subunit composition of wheat glutenin proteins, isolated by gel-filtration in a dissociating medium. Planta 1979, 145, 83–88. [Google Scholar] [CrossRef]
- Masci, S.; Egorov, T.A.; Ronchi, C.; Kuzmicky, D.D.; Kasarda, D.D.; Lafiandra, D. Evidence for the presence of only one cysteine residue in the D-type low molecular weight subunits of wheat subunits of wheat glutenin. J. Cereal Sci. 1999, 29, 17–25. [Google Scholar] [CrossRef]
- Tatham, A.S.; Shewry, P.R. The S-poor prolamins of wheat, barley and rye: Revisited. J. Cereal Sci. 2012, 55, 79–99. [Google Scholar] [CrossRef]
- Shewry, P.R.; Tatham, A.S.; Forde, J.; Kreis, M.; Miflin, B.J. The classification and nomenclature of wheat gluten proteins: A reassessment. J. Cereal Sci. 1986, 4, 97–106. [Google Scholar] [CrossRef]
- Waga, J. Structure and allergenicity of wheat gluten proteins—A review. Pol. J. Food Nutr. Sci. 2004, 54, 327–338. [Google Scholar]
- LiChan, E.C.Y. The applications of Raman spectroscopy in food science. Trends Food Sci. Technol. 1996, 7, 361–370. [Google Scholar] [CrossRef]
- Siamwiza, M.N.; Lord, R.C.; Chen, M.C.; Takamatsu, T.; Harada, I.; Matsuura, H.; Shimanouchi, T. Interpretation of doublet at 850 and 830 cm-1 in Raman spectra of tyrosyl residues in proteins and certain model compounds. Biochemistry 1975, 14, 4870–4876. [Google Scholar] [CrossRef]
- Tu, A.T. Raman Spectroscopy in Biology: Principles and Applications; Wiley: New York, NY, USA, 1982. [Google Scholar]
- Nawrocka, A.; Szymanska-Chargot, M.; Mis, A.; Ptaszynska, A.A.; Kowalski, R.; Wasko, P.; Gruszecki, W.I. Influence of dietary fibre on gluten proteins structure—A study on model flour with application of FT-Raman spectroscopy. J. Raman Spectrosc. 2015, 46, 309–316. [Google Scholar] [CrossRef]
- Ferrer, E.G.; Gomez, A.V.; Anon, M.C.; Puppo, M.C. Structural changes in gluten protein structure after addition of emulsifier. A Raman spectroscopy study. Spectrochim. Acta Part A Mol. Spectrosc. 2011, 79, 278–281. [Google Scholar] [CrossRef]
- De Gelder, J.; De Gussem, K.; Vandenabeele, P.; Moens, L. Reference database of Raman spectra of biological molecules. J. Raman Spectrosc. 2007, 38, 1133–1147. [Google Scholar] [CrossRef]
- Lefevre, T.; Rousseau, M.E.; Pezolet, M. Protein secondary structure and orientation in silk as revealed by Raman spectromicroscopy. Biophys. J. 2007, 92, 2885–2895. [Google Scholar] [CrossRef]
- Brown, B.; Westcott, M.; Christensen, N.; Pan, B.; Stark, J. Nitrogen management for hard wheat protein enhancement. Pac. Northwest. Ext. Publ. PNW 2005, 578, 1–14. [Google Scholar]
- Malik, A.H.; Kuktaite, R.; Johansson, E. Combined effect of genetic and environmental factors on the accumulation of proteins in the wheat grain and their relationship to bread-making quality. J. Cereal Sci. 2013, 57, 170–174. [Google Scholar] [CrossRef]
- Triboi, E.; Triboi-Blondel, A.M. Productivity and grain or seed composition: A new approach to an old problem—Invited paper. Eur. J. Agron. 2002, 16, 163–186. [Google Scholar] [CrossRef]
- Howarth, J.R.; Parmar, S.; Jones, J.; Shepherd, C.E.; Corol, D.I.; Galster, A.M.; Hawkins, N.D.; Miller, S.J.; Baker, J.M.; Verrier, P.J.; et al. Co-ordinated expression of amino acid metabolism in response to N and S deficiency during wheat grain filling. J. Exp. Bot. 2008, 59, 3675–3689. [Google Scholar] [CrossRef]
- Barneix, A.J. Physiology and biochemistry of source-regulated protein accumulation in the wheat grain. J. Plant. Physiol. 2007, 164, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Horvat, D.; Simic, G.; Dvojkovic, K.; Ivic, M.; Plavsin, I.; Novoselovic, D. Gluten Protein Compositional Changes in Response to Nitrogen Application Rate. Agronomy 2021, 11, 325. [Google Scholar] [CrossRef]
- Altenbach, S.B. New insights into the effects of high temperature, drought and post-anthesis fertilizer on wheat grain development. J. Cereal Sci. 2012, 56, 39–50. [Google Scholar] [CrossRef]
- Triboï, E.; Martre, P.; Triboï-Blondel, A.M. Environmentally-induced changes in protein composition in developing grains of wheat are related to changes in total protein content. J. Exp. Bot. 2003, 54, 1731–1742. [Google Scholar] [CrossRef]
- Horvat, D.; Drezner, G.; Sudar, R.; Simic, G.; Dvojkovic, K.; Spanic, V.; Magdic, D. Distribution of wheat protein components under different genetic backgrounds and environments. Turk. J. Field Crops 2015, 20, 150–154. [Google Scholar] [CrossRef][Green Version]
- Yu, Z.T.; Islam, S.; She, M.Y.; Diepeveen, D.; Zhang, Y.J.; Tang, G.X.; Zhang, J.J.; Juhasz, A.; Yang, R.C.; Ma, W.J. Wheat grain protein accumulation and polymerization mechanisms driven by nitrogen fertilization. Plant. J. 2018, 96, 1160–1177. [Google Scholar] [CrossRef]
- Fuertes-Mendizabal, T.; Aizpurua, A.; Gonzalez-Moro, M.B.; Estavillo, J.M. Improving wheat breadmaking quality by splitting the N fertilizer rate. Eur. J. Agron. 2010, 33, 52–61. [Google Scholar] [CrossRef]
- Muller, S.; Wieser, H. The location of disulphide bonds in monomeric gamma-type gliadins. J. Cereal Sci. 1997, 26, 169–176. [Google Scholar] [CrossRef]
- Klosok, K.; Welc, R.; Fornal, E.; Nawrocka, A. Effects of Physical and Chemical Factors on the Structure of Gluten, Gliadins and Glutenins as Studied with Spectroscopic Methods. Molecules 2021, 26, 508. [Google Scholar] [CrossRef] [PubMed]
- Nawrocka, A.; Mis, A.; Szymanska-Chargot, M. Characteristics of relationships between structure of gluten proteins and dough rheology—Influence of dietary fibres studied by FT-raman spectroscopy. Food Biophys. 2016, 11, 81–90. [Google Scholar] [CrossRef]
- Lu, R.; Li, W.W.; Katzir, A.; Raichlin, Y.; Yu, H.Q.; Mizaikoff, B. Probing the secondary structure of bovine serum albumin during heat-induced denaturation using mid-infrared fiberoptic sensors. Analyst 2015, 140, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Nawrocka, A.; Szymanska-Chargot, M.; Mis, A.; Kowalski, R.; Gruszecki, W.I. Raman studies of gluten proteins aggregation induced by dietary fibres. Food Chem. 2016, 194, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.W.; Kang, C.S.; Kang, T.G.; Cho, K.M.; Park, C.S. Influence of different nitrogen application on flour properties, gluten properties by HPLC and end-use quality of Korean wheat. J. Integr. Agric. 2018, 17, 982–993. [Google Scholar] [CrossRef]
- Stawoska, I.; Staszak, A.M.; Ciereszko, I.; Oliwa, J.; Skoczowski, A. Using isothermal calorimetry and FT-Raman spectroscopy for step-by-step monitoring of maize seed germination: Case study. J. Therm. Anal. Calorim. 2020, 142, 755–763. [Google Scholar] [CrossRef]
- Stawoska, I.; Weselucha-Birczynska, A.; Regonesi, M.E.; Riva, M.; Tortora, P.; Stochel, G. Interaction of selected divalent metal ions with human ataxin-3 Q36. J. Biol. Inorg. Chem. 2009, 14, 1175–1185. [Google Scholar] [CrossRef]
- Lancelot, E.; Fontaine, J.; Grua-Priol, J.; Assaf, A.; Thouand, G.; Le-Bail, A. Study of structural changes of gluten proteins during bread dough mixing by Raman spectroscopy. Food Chem. 2021, 358, 7. [Google Scholar] [CrossRef]
- Sadat, A.; Joye, I.J. Peak Fitting Applied to Fourier Transform Infrared and Raman Spectroscopic Analysis of Proteins. Appl. Sci. 2020, 10, 5918. [Google Scholar] [CrossRef]
- Chi, Z.H.; Chen, X.G.; Holtz, J.S.W.; Asher, S.A. UV resonance Raman-selective amide vibrational enhancement: Quantitative methodology for determining protein secondary structure. Biochemistry 1998, 37, 2854–2864. [Google Scholar] [CrossRef]
- Rygula, A.; Majzner, K.; Marzec, K.M.; Kaczor, A.; Pilarczyk, M.; Baranska, M. Raman spectroscopy of proteins: A review. J. Raman Spectrosc. 2013, 44, 1061–1076. [Google Scholar] [CrossRef]
- Ridgley, D.M.; Claunch, E.C.; Barone, J.R. Characterization of Large Amyloid Fibers and Tapes with Fourier Transform Infrared (FT-IR) and Raman Spectroscopy. Appl. Spectrosc. 2013, 67, 1417–1426. [Google Scholar] [CrossRef] [PubMed]
- Tuma, R. Raman spectroscopy of proteins: From peptides to large assemblies. J. Raman Spectrosc. 2005, 36, 307–319. [Google Scholar] [CrossRef]
- Metsalu, T.; Vilo, J. ClustVis: A web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015, 43, W566–W570. [Google Scholar] [CrossRef]
- Fan, D.M.; Ma, W.R.; Wang, L.Y.; Huang, J.L.; Zhao, J.X.; Zhang, H.; Chen, W. Determination of structural changes in microwaved rice starch using Fourier transform infrared and Raman spectroscopy. Starch-Starke 2012, 64, 598–606. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Xu, Y.; Yan, Y.Z.; Hu, D.D.; Yang, L.Z.; Shen, R.L. Application of Raman spectroscopy in structure analysis and crystallinity calculation of corn starch. Starch-Starke 2015, 67, 612–619. [Google Scholar] [CrossRef]
- Corbett, E.C.; Zichy, V.; Goral, J.; Passingham, C. Fourier transform Raman studies of materials and compounds of biological importance—II. The effect of moisture on the molecular structure of the alpha and beta anomers of d-glucose. Spectrochim. Acta Part A Mol. Spectrosc. 1991, 47, 1399–1411. [Google Scholar] [CrossRef]
- Wiercigroch, E.; Szafraniec, E.; Czamara, K.; Pacia, M.Z.; Majzner, K.; Kochan, K.; Kaczor, A.; Baranska, M.; Malek, K. Raman and infrared spectroscopy of carbohydrates: A review. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 185, 317–335. [Google Scholar] [CrossRef]
- Meng, G.T.; Ma, C.Y.; Phillips, D.L. Raman spectroscopic study of globulin from Phaseolus angularis (red bean). Food Chem. 2003, 81, 411–420. [Google Scholar] [CrossRef]
- Linlaud, N.; Ferrer, E.; Puppo, M.C.; Ferrero, C. Hydrocolloid Interaction with water, protein, and starch in wheat dough. J. Agric. Food Chem. 2011, 59, 713–719. [Google Scholar] [CrossRef]
- Petrou, M.; Edwards, H.G.M.; Janaway, R.C.; Thompson, G.B.; Wilson, A.S. Fourier-Transform Raman spectroscopic study of a Neolithic waterlogged wood assemblage. Anal. Bioanal. Chem. 2009, 395, 2131–2138. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Durmaz, S.; Ozgenc, O.; Boyaci, I.H.; Yildiz, U.C.; Erisir, E. Examination of the chemical changes in spruce wood degraded by brown-rot fungi using FT-IR and FT-Raman spectroscopy. Vib. Spectrosc. 2016, 85, 202–207. [Google Scholar] [CrossRef]
- Vitek, P.; Novotna, K.; Hodanova, P.; Rapantova, B.; Klem, K. Detection of herbicide effects on pigment composition and PSII photochemistry in Helianthus annuus by Raman spectroscopy and chlorophyll a fluorescence. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 170, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Heredia-Guerrero, J.A.; Benitez, J.J.; Dominguez, E.; Bayer, I.S.; Cingolani, R.; Athanassiou, A.; Heredia, A. Infrared and Raman spectroscopic features of plant cuticles: A review. Front. Plant Sci. 2014, 5, 14. [Google Scholar] [CrossRef] [PubMed]







| Trials | Protein Content (%) | Gluten Content (%) | Gluten Index | |||
|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |
| Line (C) | 1.89 | 0.2063 | 39.72 | 0.0002 | 428.48 | 0.0000 |
| Nitrogen dose (N) | 17.19 | 0.0032 | 71.66 | 0.0000 | 22.48 | 0.0000 |
| Interaction (CxN) | 0.07 | 0.8004 | 2.36 | 0.1630 | 0.25 | 0.6298 |
| Characteristics | Winter Wheat Line (The Average Value for Both Nitrogen Fertilization Doses) | Nitrogen Fertilization (kg N·ha−1) (The Average Value for Both Winter Wheat Lines) | ||
|---|---|---|---|---|
| wasko.gl− | wasko.gl+ | N0 | N120 | |
| Protein content (%) | 14.3 ± 0.6 a | 13.7 ± 0.5 b | 12.9 ± 0.4 b | 14.9 ± 0.9 a |
| Gluten content (%) | 32.3 ± 2.5 b | 39.1 ± 1.7 a | 31.2 ± 0.7 b | 40.2 ± 0.7 a |
| Gluten index | 93.0 ± 3.0 a | 48.3 ± 2.2 b | 75.7 ± 0.3 a | 65.4 ± 0.9 b |
| Wheat Lines | SSg-g-g [%] | SSt-g-g [%] | SSt-g-t [%] |
| wasko.gl−, N0 | 3 (513) | 54 (519, 523) | 43 (529, 534) |
| wasko.gl−, N120 | 16 (517) | 38 (523) | 46 (529, 537) |
| wasko.gl+, N0 | 5 (514) | 47 (520, 524) | 48 (529, 536) |
| wasko.gl+, N120 | - | 35 (522) | 65 (529, 539) |
| wasko.gl− (N0) | wasko.gl− (N120) | wasko.gl+ (N0) | wasko.gl+ (N120) | |
|---|---|---|---|---|
| β–sheet (1627–33 cm−1) | 19 | 20 | 23 | 20 |
| α-helix (1648–50 cm−1) | 29 | 30 | 31 | 25 |
| RC (1663–1666 cm−1) | 30 | 26 | 24 | 28 |
| β-turn (1680–83 cm−1) | 18 | 20 | 16 | 25 |
| aβ-sheet (1691–98 cm−1) | 4 | 4 | 6 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stawoska, I.; Waga, J.; Wesełucha-Birczyńska, A.; Dziurka, M.; Podolska, G.; Aleksandrowicz, E.; Skoczowski, A. Does Nitrogen Fertilization Affect the Secondary Structures of Gliadin Proteins in Hypoallergenic Wheat? Molecules 2022, 27, 5684. https://doi.org/10.3390/molecules27175684
Stawoska I, Waga J, Wesełucha-Birczyńska A, Dziurka M, Podolska G, Aleksandrowicz E, Skoczowski A. Does Nitrogen Fertilization Affect the Secondary Structures of Gliadin Proteins in Hypoallergenic Wheat? Molecules. 2022; 27(17):5684. https://doi.org/10.3390/molecules27175684
Chicago/Turabian StyleStawoska, Iwona, Jacek Waga, Aleksandra Wesełucha-Birczyńska, Michał Dziurka, Grażyna Podolska, Edyta Aleksandrowicz, and Andrzej Skoczowski. 2022. "Does Nitrogen Fertilization Affect the Secondary Structures of Gliadin Proteins in Hypoallergenic Wheat?" Molecules 27, no. 17: 5684. https://doi.org/10.3390/molecules27175684
APA StyleStawoska, I., Waga, J., Wesełucha-Birczyńska, A., Dziurka, M., Podolska, G., Aleksandrowicz, E., & Skoczowski, A. (2022). Does Nitrogen Fertilization Affect the Secondary Structures of Gliadin Proteins in Hypoallergenic Wheat? Molecules, 27(17), 5684. https://doi.org/10.3390/molecules27175684









