New 1,2,3-Triazole-Coumarin-Glycoside Hybrids and Their 1,2,4-Triazolyl Thioglycoside Analogs Targeting Mitochondria Apoptotic Pathway: Synthesis, Anticancer Activity and Docking Simulation
Abstract
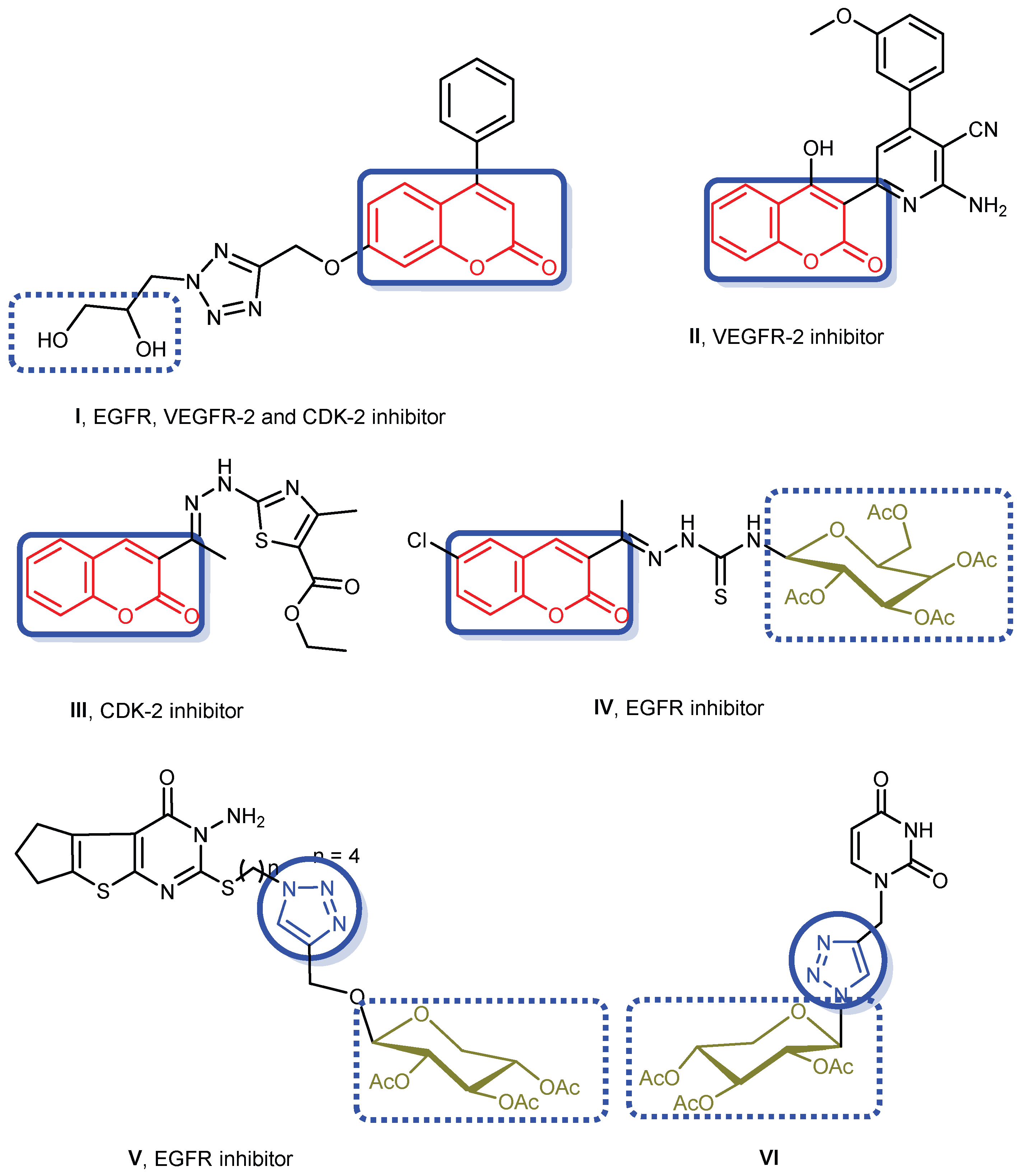
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Biological Evaluation
2.2.1. In Vitro Cytotoxicity Using MTT Assay
Structure Activity Relationship (SAR)
2.2.2. Effect on the Level of Bax, Bcl-2, Cytochrome c and Caspase-7 Protein in MCF-7 Cells
2.2.3. Cell Cycle Arrest
2.2.4. Kinase Inhibitory Assessment against EGFR, VEGFR-2 and CDK-2
2.3. Molecular Docking Study
3. Experimental
3.1. Chemistry
- 4-Phenyl-7-((4-(methyl/ethyl)-5-(1-(O-acetyl-β-D-glycopyranosyl)thio)-4H-1,2,4-triazol-3-yl)methoxy)-2H-chromen-2-one (3-6)
- 4-Phenyl-7-((4-methyl-5-(1-(2,3,4,6-tetra-O-acetyl-β-D-galactopyranosyl)thio)-4H-1,2,4-triazol-3-yl)methoxy)-2H-chromen-2-one (3)
- 4-Phenyl-7-((4-methyl-5-(1-(2,3,4-tri-O-acetyl-β-D-xylopyranosyl)thio)-4H-1,2,4-triazol-3-yl)methoxy)-2H-chromen-2-one (4)
- 4-Phenyl-7-((4-ethyl-5-(1-(2,3,4,6-tetra-O-acetyl-β-D-galactopyranosyl)thio)-4H-1,2,4-triazol-3-yl)methoxy)-2H-chromen-2-one (5)
- 4-Phenyl-7-((4-ethyl-5-(1-(2,3,4-tri-O-acetyl-β-D-xylopyranosyl)thio)-4H-1,2,4-triazol-3-yl)methoxy)-2H-chromen-2-one (6)
- 4-Phenyl-7-((4-methyl-5-(1-(β-D-glycopyranosyl)thio)-4H-1,2,4-triazol-3-yl)methoxy)-2H-chromen-2-one (7-10)
- 4-Phenyl-7-((4-methyl-5-(1-(β-D-galactopyranosyl)thio)-4H-1,2,4-triazol-3-yl)methoxy)-2H-chromen-2-one (7)
- 4-Phenyl-7-((4-methyl-5-(1-(β-D-xylopyranosyl)thio)-4H-1,2,4-triazol-3-yl)meth-oxy)-2H-chromen-2-one (8)
- 4-Phenyl-7-((4-ethyl-5-(1-(β-D-galactopyranosyl)thio)-4H-1,2,4-triazol-3-yl)meth-oxy)-2H-chromen-2-one (9)
- 4-Phenyl-7-((4-ethyl-5-(1-(β-D-xylopyranosyl)thio)-4H-1,2,4-triazol-3-yl)meth-oxy)-2H-chromen-2-one (10)
- Synthesis of disubstituted-1,2,4-triazol compounds 11–14
- 4-Phenyl-7-((5-((2-(2-hydroxyethoxy)ethyl)thio)-4-methyl-4H-1,2,4-triazol-3-yl)methoxy)-2H-chromen-2-one (11)
- 4-Phenyl-7-((4-ethyl-5-((2-(2-hydroxyethoxy)ethyl)thio)-4H-1,2,4-triazol-3-yl)methoxy)-2H-chromen-2-one (12)
- 4-Phenyl-7-((5-((2,3-dihydroxypropyl)thio)-4-methyl-4H-1,2,4-triazol-3-yl)meth-oxy)-2H-chromen-2-one (13)
- 4-Phenyl-7-((5-((2,3-dihydroxypropyl)thio)-4-ethyl-4H-1,2,4-triazol-3-yl)methoxy)-2H-chromen-2-one (14)
- 7-(3-Iodopropoxy&4-iodobutoxy )-4-phenyl-2H-chromen-2-one (16, 17)
- 7-(3-Iodopropoxy)-4-phenyl-2H-chromen-2-one (16)
- 7-(4-Iodobutoxy)-4-phenyl-2H-chromen-2-one (17)
- 7-(3-Azidoalkyloxy)-4-phenyl-2H-chromen-2-one (18, 19)
- 7-(3-Azidopropoxy)-4-phenyl-2H-chromen-2-one (18)
- 7-(4-Azidobutoxy)-4-phenyl-2H-chromen-2-one (19)
- 4-Phenyl-7-(3-(4-(1-(O-acetyl-β-D-glycopyranosyl))-1H-1,2,3-triazol-1-yl)propoxy & butoxy)-2H-chromen-2-one (21-23)
- 4-Phenyl-7-(3-(4-(1-(2,3,4,6-tetra-O-acetyl-β-D-galactopyranosyl))-1H-1,2,3-triazol-1-yl)propoxy)-2H-chromen-2-one (21)
- 4-Phenyl-7-(3-(4-(1-(2,3,4–tria-O-acetyl-β-D-xylopyranosyl))-1H-1,2,3-triazol-1-yl)propoxy)-2H-chromen-2-one (22)
- 4-Phenyl-7-(3-(4-(1-(2,3,4,6-tetra-O-acetyl-β-D-galactopyranosyl))-1H-1,2,3-triazol-1-yl)butoxy)-2H-chromen-2-one (23)
- 4-Phenyl-7-(3-(4-(1-(-β-D-glycopyranosyl))-1H-1,2,3-triazol-1-yl)alkyloxy)-2H-chromen-2-one (24-26)
- 4-Phenyl-7-(3-(4-(1-(β-D-galactopyranosyl))-1H-1,2,3-triazol-1-yl)propoxy)-2H-chromen-2-one (24).
- 4-Phenyl-7-(3-(4-(1-(β-D-xylopyranosyl))-1H-1,2,3-triazol-1-yl)propoxy)-2H-chromen-2-one (25)
- 4-Phenyl-7-(3-(4-(1-(β-D-galactopyranosyl))-1H-1,2,3-triazol-1-yl)butoxy)-2H-chromen-2-one (26)
3.2. Biological Evaluation
3.2.1. Cytotoxicity Assay
Cell Lines
3.2.2. Cell Cycle Analysis and Apoptosis Detection
3.2.3. Human CASP-7 (Caspase-7) Estimation
3.2.4. Human Cytochrome c Estimation
3.2.5. Measurement of Bcl-2 Levels
3.2.6. Measurement of Bax Levels
3.2.7. In Vitro Kinase Inhibitory Assessment against EGFR, VEGFR-2 and CDK-2
3.3. Molecular M7odelling Study upon EGFR, VEGFR-2 and CDK-2
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- An, W.; Lai, H.; Zhang, Y.; Liu, M.; Lin, X.; Cao, S. Apoptotic pathway as the therapeutic target for anticancer traditional Chinese medicines. Front. Pharmacol. 2019, 10, 758. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.L.; Shi, F.; Tan, Z.; Li, Y.; Bode, A.M.; Cao, Y. Mitochondrial network structure homeostasis and cell death. Cancer Sci. 2018, 109, 3686–3694. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Cao, Y.; Lu, L.; Xu, Y.; Chen, H.; Liu, C.; Tao, Z. Plasmodium infection suppresses colon cancer growth by inhibiting proliferation and promoting apoptosis associated with disrupting mitochondrial biogenesis and mitophagy in mice. Parasit. Vectors 2022, 15, 192. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer. 2021, 149, 778–789. [Google Scholar] [CrossRef]
- Gerber, D.E. Targeted therapies: A new generation of cancer treatments. Am. Fam. Physician 2008, 77, 311–319. [Google Scholar]
- Du, Z.; Lovly, C.M. Mechanisms of receptor tyrosine kinase activation in cancer. Mol. Cancer 2018, 17, 58. [Google Scholar] [CrossRef]
- Iqbal, N.; Iqbal, N. Imatinib: A breakthrough of targeted therapy in cancer. Chemother. Res. Pract. 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Abd El-Meguid, E.A.; Moustafa, G.O.; Awad, H.M.; Zaki, E.R.; Nossier, E.S. Novel benzothiazole hybrids targeting EGFR: Design, synthesis, biological evaluation and molecular docking studies. J. Mol. Struct. 2021, 1240, 130595. [Google Scholar] [CrossRef]
- Khattab, R.R.; Hassan, A.A.; Osman, D.A.A.; Abdel-Megeid, F.M.; Awad, H.M.; Nossier, E.S.; El-Sayed, W.A. Synthesis, anticancer activity and molecular docking of new triazolo [4, 5-d] pyrimidines based thienopyrimidine system and their derived N-glycosides and thioglycosides. Nucleosides Nucleotides Nucleic Acids 2021, 40, 1090–1113. [Google Scholar] [CrossRef]
- Ciardiello, F.; Tortora, G. A novel approach in the treatment of cancer: Targeting the epidermal growth factor receptor. Clin. Cancer Res. 2001, 7, 2958–2970. [Google Scholar]
- Khattab, R.R.; Alshamari, A.K.; Hassan, A.A.; Elganzory, H.H.; El-Sayed, W.A.; Awad, H.M.; Nossier, E.S.; Hassan, N.A. Click chemistry based synthesis, cytotoxic activity and molecular docking of novel triazole-thienopyrimidine hybrid glycosides targeting EGFR. J. Enzym. Inhib. Med. Chem. 2021, 36, 504–516. [Google Scholar] [CrossRef]
- Othman, I.M.; Alamshany, Z.M.; Tashkandi, N.Y.; Gad-Elkareem, M.A.; Anwar, M.M.; Nossier, E.S. New pyrimidine and pyrazole-based compounds as potential EGFR inhibitors: Synthesis, anticancer, antimicrobial evaluation and computational studies. Bioorganic Chem. 2021, 114, 105078. [Google Scholar] [CrossRef]
- Carmeliet, P. VEGF as a key mediator of angiogenesis in cancer. Oncology 2005, 69, 4–10. [Google Scholar] [CrossRef]
- Hicklin, D.J.; Ellis, L.M. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J. Clin. Oncol. 2005, 23, 1011–1027. [Google Scholar] [CrossRef]
- Otrock, Z.K.; Hatoum, H.A.; Musallam, K.M.; Awada, A.H.; Shamseddine, A.I. Is VEGF a predictive biomarker to anti-angiogenic therapy? Crit. Rev. Oncol. Hematol. 2011, 79, 103–111. [Google Scholar] [CrossRef]
- Tugues, S.; Koch, S.; Gualandi, L.; Li, X.; Claesson-Welsh, L. Vascular endothelial growth factors and receptors: Anti-angiogenic therapy in the treatment of cancer. Mol. Asp. Med. 2011, 32, 88–111. [Google Scholar] [CrossRef]
- Musgrove, E.A.; Caldon, C.E.; Barraclough, J.; Stone, A. Cyclin D as a therapeutic target in cancer. Nat. Rev. Cancer 2011, 11, 558–572. [Google Scholar] [CrossRef]
- Chohan, T.A.; Qian, H.; Pan, Y.; Chen, J.Z. Cyclin-dependent kinase-2 as a target for cancer therapy: Progress in the development of CDK2 inhibitors as anti-cancer agents. Curr. Med. Chem. 2015, 22, 237–263. [Google Scholar] [CrossRef]
- Peyressatre, M.; Prevel, C.; Pellerano, M.; Morris, M.C. Targeting cyclin-dependent kinases in human cancers: From small molecules to peptide inhibitors. Cancers 2015, 7, 179–237. [Google Scholar] [CrossRef]
- Manidhar, D.M.; Kesharwani, R.K.; Reddy, N.B.; Reddy, C.S.; Misra, K. Designing, synthesis, and characterization of some novel coumarin derivatives as probable anticancer drugs. Med. Chem. Res. 2013, 22, 4146–4157. [Google Scholar] [CrossRef]
- Khan, M.S.; Agrawal, R.; Ubaidullah, M.; Hassan, M.I.; Tarannum, N. Design, synthesis and validation of anti-microbial coumarin derivatives: An efficient green approach. Heliyon 2019, 5, e02615. [Google Scholar] [CrossRef]
- Alshibl, H.M.; Al-Abdullah, E.S.; Haiba, M.E.; Alkahtani, H.M.; Awad, G.E.A.; Mahmoud, A.H.; Ibrahim, B.M.M.; Bari, A.; Villinger, A. Synthesis and evaluation of new coumarin derivatives as antioxidant, antimicrobial, and anti-inflammatory agents. Molecules 2020, 25, 3251. [Google Scholar] [CrossRef]
- Narayan, B.; Anupa, A.K.; Yuba, R.P.; Paras, N.Y. Anticancer potential of coumarin and its derivatives. Mini-Rev. Med. Chem. 2021, 21, 2996–3029. [Google Scholar] [CrossRef]
- Agarwal, R. Synthesis & biological screening of some novel coumarin derivatives. Biochem. Pharmacol. 2000, 6, 1042–1051. [Google Scholar]
- El-Sayed, W.A.; Alminderej, F.M.; Mounier, M.M.; Nossier, E.S.; Saleh, S.M.; Kassem, A.F. Novel 1, 2, 3-Triazole-Coumarin Hybrid Glycosides and Their Tetrazolyl Analogues: Design, Anticancer Evaluation and Molecular Docking Targeting EGFR, VEGFR-2 and CDK-2. Molecules 2022, 27, 2047. [Google Scholar] [CrossRef]
- Vu, N.T.; Nguyen, D.T. Synthesis and antiproliferative activity of hybrid thiosemicarbazone derivatives bearing coumarin and D-galactose moieties with EGFR inhibitory activity and molecular docking study. Med. Chem. Res. 2021, 30, 1868–1885. [Google Scholar]
- Ahmed, E.Y.; Elserwy, W.S.; El-Mansy, M.F.; Serry, A.M.; Salem, A.M.; Abdou, A.M.; Abdelrahman, B.A.; Elsayed, K.H.; Abd Elaziz, M.R. Angiokinase inhibition of VEGFR-2, PDGFR and FGFR and cell growth inhibition in lung cancer: Design, synthesis, biological evaluation and molecular docking of novel azaheterocyclic coumarin derivatives. Bioorganic Med. Chem. Lett. 2021, 48, 128258. [Google Scholar] [CrossRef]
- Abd El-Karim, S.S.; Syam, Y.M.; El Kerdawy, A.M.; Abdelghany, T.M. New thiazol-hydrazono-coumarin hybrids targeting human cervical cancer cells: Synthesis, CDK2 inhibition, QSAR and molecular docking studies. Bioorganic Chem. 2019, 86, 80–96. [Google Scholar] [CrossRef]
- Kassem, A.F.; Abbas, E.M.H.; El-Kady, D.S.; Awad, H.A.; El-Sayed, W.A. Design, Synthesis and Anticancer Activity of new thiazole- Tetrazole or triazole hybrid glycosides targeting CDK-2 via structure-based virtual screening. Mini-Rev. Med. Chem. 2019, 19, 933–948. [Google Scholar] [CrossRef]
- Gurrapu, N.; Kumar, E.P.; Kolluri, P.K.; Putta, S.; Sivan, S.K.; Subhashini, N.J.P. Synthesis, biological evaluation and molecular docking studies of novel 1, 2, 3-triazole tethered chalcone hybrids as potential anticancer agents. J. Mol. Struct. 2020, 1217, 128356. [Google Scholar] [CrossRef]
- Wang, X.; Huang, B.; Liu, X.; Zhan, P. Discovery of bioactive molecules from CuAAC click-chemistry-based combinatorial libraries. Drug Discov. Today 2016, 120, 118–132. [Google Scholar] [CrossRef] [PubMed]
- Fahad, M.A.; Hussein, H.E.; Mohamed, N.B.; Hanem, M.A.; El-Sayed, W.A. Synthesis and Cytotoxic Activity of New 1,3,4-Thiadiazole Thioglycosides and 1,2,3-Triazolyl-1,3,4-Thiadiazole N-glycosides. Molecules 2019, 24, 3738–3752. [Google Scholar]
- Flefel, E.E.; Tantawy, W.A.; El-Sayed, W.A.; Sayed, H.H.; Fathy, N.M. Synthesis and Anticancer Activity of New Substituted Pyrazoles and Their Derived 1,2,4-Triazoles And Their Sugar Derivatives. J. Heterocycl. Chem. 2013, 50, 344–350. [Google Scholar] [CrossRef]
- El-Sayed, W.A.; Hemat, S.K.; Dalia, A.A.O.; Hebat-Allah, S.A.; Mahmoud, M.A. Synthesis and Anticancer Activity of New Pyrazolyl and Oxadiazolyl Glycosides Based on Theinopyrimidine Nucleus and Their Acyclic Analogs. Acta Pol. Pharm. 2017, 74, 1739–1751. [Google Scholar]
- Abbas, H.S.; El-Sayed, W.A.; Fathy, N.M. Synthesis and antitumor activity of new dihydropyridine thioglycosides and their corresponding dehydrogenated forms. Eur. J. Med. Chem. 2010, 45, 973–982. [Google Scholar] [CrossRef]
- Abdel-Rahman, A.A.H.; Nassar, F.N.; Saaban, A.K.F.; El-Kady, D.S.; Awad, H.M.; El-Sayed, W.A. Synthesis, Docking Studies into CDK-2 and Anticancer Activity of New Derivatives Based Pyrimidine Scaffold and Their Derived Glycosides. Mini-Rev. Med. Chem. 2019, 19, 1093–1110. [Google Scholar] [CrossRef]
- El-Sayed, W.A.; Fathi, N.M.; Gad, W.A.; El-Ashry, E.S.H. Synthesis, and Antiviral Evaluation of Some 5-N-Arylaminomethyl-2-glycosylsulphanyl-1,3,4-oxadiazoles and their analogues against Hepatitis A and Herpes simplex viruses. J. Carbohydr. Chem. 2008, 27, 357–372. [Google Scholar] [CrossRef]
- El-Sayed, W.A.; Abdel-Rahman, A.A.H.; Ramiz, M.M.M. Anti-Hepatitis B Virus Activity of New N4-β-D-Glycoside pyrazolo[3,4-d]pyrimidine derivatives. Z. Nat. C 2009, 64, 323–328. [Google Scholar] [CrossRef]
- Kassem, A.F.; Batran, R.Z.; Abbas, E.M.; Elseginy, S.A.; Shaheen, M.N.; Elmahdy, E.M. New 4-phenylcoumarin derivatives as potent 3C protease inhibitors: Design, synthesis, anti-HAV effect and molecular modeling. Eur. J. Med. Chem. 2019, 168, 447–460. [Google Scholar] [CrossRef]
- Mansour, A.K.; Ibrahim, Y.A.; Khalil, N.S.A.M. Selective synthesis and structure of 6-arylvinyl-2- and 4-glucosyl-1,2,4-triazines of expected interesting biological activity. Nucleosides Nucleotides 1999, 18, 2256–2283. [Google Scholar] [CrossRef]
- Missiroli, S.; Perrone, M.; Genovese, I.; Pinton, P.; Giorgi, C. Cancer metabolism and mitochondria: Finding novel mechanisms to fight tumours. eBioMedicine 2020, 59, 102943. [Google Scholar] [CrossRef]
- Xiao, L.; Xu, C.; Lin, P.; Mu, L.; Yang, X. Novel dihydroartemisinin derivative Mito-DHA5 induces apoptosis associated with mitochondrial pathway in bladder cancer cells. BMC Pharmacol. Toxicol. 2022, 23, 10. [Google Scholar] [CrossRef]
- Hashem, H.E.; Amr, A.E.G.E.; Nossier, E.S.; Anwar, M.M.; Azmy, E.M. New Benzimidazole-, 1, 2, 4-Triazole-, and 1, 3, 5-Triazine-Based Derivatives as Potential EGFRWT and EGFRT790M Inhibitors: Microwave-Assisted Synthesis, Anticancer Evaluation, and Molecular Docking Study. ACS Omega 2022, 7, 7155–7171. [Google Scholar] [CrossRef]
- Dawood, D.H.; Nossier, E.S.; Ali, M.M.; Mahmoud, A.E. Synthesis and molecular docking study of new pyrazole derivatives as potent anti-breast cancer agents targeting VEGFR-2 kinase. Bioorganic Chem. 2020, 101, 103916. [Google Scholar] [CrossRef]
- Othman, I.M.; Alamshany, Z.M.; Tashkandi, N.Y.; Gad-Elkareem, M.A.; Abd El-Karim, S.S.; Nossier, E.S. Synthesis and biological evaluation of new derivatives of thieno-thiazole and dihydrothiazolo-thiazole scaffolds integrated with a pyrazoline nucleus as anticancer and multi-targeting kinase inhibitors. RSC Adv. 2022, 12, 561–577. [Google Scholar] [CrossRef]
- El-Sayed, A.A.; Nossier, E.S.; Almehizia, A.A.; Amr, A.E.G.E. Design, synthesis, anticancer evaluation and molecular docking study of novel 2,4-dichlorophenoxymethyl-based derivatives linked to nitrogenous heterocyclic ring systems as potential CDK-2 inhibitors. J. Mol. Struct. 2022, 1247, 131285. [Google Scholar] [CrossRef]
- Hawata, M.A.; El-Sayed, W.A.; Nossier, E.S.; Abdel-Rahman, A.A.H. Synthesis and Cytotoxic Activity of New Pyrimido[1,2-c]quinazolines[1,2,4]triazolo[4,3-c]quinazolines and (quinazolin-4-yl)-1H-pyrazoles Hybrids. Biointerface Res. Appl. Chem. 2022, 12, 5217–5233. [Google Scholar]
- El-serwy, W.S.; Mohamed, H.S.; El-serwy, W.S.; Mohamed, N.A.; Kassem, E.M.; Mahmoud, K.; Nossier, E.S. Thiopyrimidine-5-carbonitrile Derivatives as VEGFR-2 Inhibitors: Synthesis, Anticancer Evaluation, Molecular Docking, ADME Predictions and QSAR Studies. ChemistrySelect 2020, 5, 15243–15253. [Google Scholar] [CrossRef]
- Hashem, H.E.; Amr, A.E.G.E.; Nossier, E.S.; Elsayed, E.A.; Azmy, E.M. Synthesis, antimicrobial activity and molecular docking of novel thiourea derivatives tagged with thiadiazole, imidazole and triazine moieties as potential DNA gyrase and topoisomerase IV inhibitors. Molecules 2020, 25, 2766. [Google Scholar] [CrossRef]
- Abd El-Meguid, E.A.; Mohi El-Deen, E.M.; Moustafa, G.O.; Awad, H.M.; Nossier, E.S. Synthesis, anticancer evaluation and molecular docking of new benzothiazole scaffolds targeting FGFR-1. Bioorganic Chem. 2022, 119, 105504. [Google Scholar] [CrossRef]
- Hassan, A.S.; Moustafa, G.O.; Awad, H.M.; Nossier, E.S.; Mady, M.F. Design, Synthesis, Anticancer Evaluation, Enzymatic Assays, and a Molecular Modeling Study of Novel Pyrazole–Indole Hybrids. ACS Omega 2021, 6, 12361–12374. [Google Scholar] [CrossRef] [PubMed]
- Mounier, M.M.; Shehata, S.H.; Soliman, T.N. Anticancer activity of nanoencapsulated ginger in whey proteins against human tumor cell lines. Egypt. Pharm. J. 2020, 19, 87. [Google Scholar] [CrossRef]
- Mohamed, F.H.; Shalaby, A.M.; Soliman, H.A.; Abdelazem, A.Z.; Mounier, M.M.; Nossier, E.S.; Moustafa, G.O. Design, synthesis and molecular docking studies of novel cyclic pentapeptides based on phthaloyl chloride with expected anticancer activity. Egypt. J. Chem. 2020, 63, 1723–1736. [Google Scholar] [CrossRef]
- Diab, S.; Teo, T.; Kumarasiri, M. Discovery of 5-(2-(phenylamino)pyrimidin-4-yl) thiazol-2(3H)-one derivatives as potent Mnk2 inhibitors: Synthesis. SAR analysis and biological evaluation. ChemMedChem 2014, 9, 962–972. [Google Scholar] [CrossRef]
- Denault, J.-B.; Salvesen, G.S. Human Caspase-7 Activity and Regulation by Its N-terminal Peptide. J. Biol. Chem. 2003, 278, 34042–34050. [Google Scholar] [CrossRef]
- Jiang, W.; Jin, P.; Wei, W.; Jiang, W. Apoptosis in cerebrospinal fluid as outcome predictors in severe traumatic brain injury: An observational study. Medicine 2020, 99, e20922. [Google Scholar] [CrossRef]
- Barbareschi, M.; Caffo, O.; Veronese, S.; Leek, R.D.; Fina, P.; Fox, S.; Bonzanini, M.; Girlando, S.; Morelli, L.; Eccher, C. Bcl-2 and p53 expression in node-negative breast carcinoma: A study with long-term follow-up. Hum. Pathol. 1996, 27, 1149–1155. [Google Scholar] [CrossRef]
- Alamshany, Z.M.; Tashkandi, N.Y.; Othman, I.M.; Anwar, M.M.; Nossier, E.S. New thiophene, thienopyridine and thiazoline-based derivatives: Design, synthesis and biological evaluation as antiproliferative agents and multitargeting kinase inhibitors. Bioorganic Chem. 2022, 127, 105964. [Google Scholar] [CrossRef]















| Cytotoxicity % at 100 µM | |||||
|---|---|---|---|---|---|
| Compound | HOS | MDA | MCF-7 | Caco-2 | HCT-116 |
| 1a | 42.5 ± 1.2 | 24.1 ± 1.4 | 15.6 ± 0.2 | 34.8 ± 1.3 | 16.8 ± 0.1 |
| 1b | 92 ± 2.1 | 69.7 ± 2.4 | 88 ± 1.4 | 77.7 ± 0.8 | 81 ± 1.2 |
| 3 | 18.4 ± 0.7 | 15.4 ± 0.5 | 9.8 ± 0.6 | 23.2 ± 1.1 | 11.1 ± 0.4 |
| 4 | 45.5 ± 0.6 | 36.9 ± 1.2 | 17.1 ± 0.3 | 24.9 ± 0.6 | 15 ± 0.6 |
| 5 | 24.9 ± 0.6 | 18.5 ± 0.6 | 2.8 ± 0.2 | 20.6 ± 0.3 | 7.6 ± 0.2 |
| 6 | 33.1 ± 0.2 | 7.4 ± 0.3 | 14.3 ± 0.1 | 30.1 ± 0.1 | 18.9 ± 0.6 |
| 7 | 7.3 ± 0.3 | 15.5 ± 1.1 | 14.3 ± 0.2 | 35.7 ± 0.3 | 16.6 ± 0.8 |
| 8 | 41.9 ± 0.7 | 37.1 ± 2.3 | 60.1 ± 0.7 | 28.4 ± 0.6 | 23 ± 0.6 |
| 9 | 20.4 ± 0.4 | 27.7 ± 0.4 | 37.8 ± 0.3 | 1.7 ± 0.1 | 22.7 ± 0.9 |
| 10 | 5.3 ± 0.2 | 20.6 ± 0.5 | 66.3 ± 1 | 0.7 ± 0.003 | 27.2 ± 0.7 |
| 11 | 39.7 ± 0.8 | 62.4 ± 0.7 | 86.7 ± 0.7 | 19.1 ± 0.4 | 91.7 ± 1.1 |
| 12 | 97.2 ± 1.3 | 91.2 ± 0.8 | 97.6 ± 0.9 | 92 ± 1.3 | 84.4 ± 0.8 |
| 13 | 2.8 ± 0.2 | 0 ± 0.01 | 51.9 ± 0.2 | 16.6 ± 0.7 | 26.1 ± 0.3 |
| 14 | 90.5 ± 1.1 | 97.8 ± 0.7 | 98.1 ± 0.3 | 91.2 ± 2.1 | 95.2 ± 0.9 |
| 15 | 62.7 ± 1.5 | 34.5 ± 0.4 | 36.8 ± 1.1 | 46.1 ± 0.6 | 89.3 ± 0.7 |
| 16 | 91.2 ± 2.2 | 88.3 ± 1.4 | 35.6 ± 0.8 | 54.3 ± 0.4 | 12.8 ± 1.1 |
| 17 | 50.8 ± 0.7 | 7.7 ± 0.4 | 38.5 ± 1.2 | 34.3 ± 0.3 | 38.2 ± 0.7 |
| 21 | 58.3 ± 1.7 | 26.4 ± 0.3 | 85.4 ± 1.8 | 59.2 ± 1.1 | 64.9 ± 0.5 |
| 22 | 90.6 ± 1.5 | 97.1 ± 1.1 | 91.9 ± 0.8 | 57.5 ± 0.5 | 97.3 ± 0.8 |
| 23 | 0 ± 0.001 | 28.6 ± 0.2 | 35.2 ± 0.3 | 13.6 ± 0.4 | 30.4 ± 0.7 |
| 24 | 97.6 ± 1.3 | 97.7 ± 0.8 | 88.2 ± 1.1 | 86.5 ± 0.9 | 99.1 ± 0.8 |
| 25 | 90.4 ± 0.8 | 94.2 ± 2.2 | 81.9 ± 0.9 | 82.7 ± 1.1 | 97.1 ± 1.1 |
| 26 | 66.9 ± 2.2 | 31.4 ± 0.5 | 91.8 ± 1.2 | 2.6 ± 0.2 | 83.9 ± 0.9 |
| IC50 (µM) | ||||
|---|---|---|---|---|
| Compound | HOS | MDA | MCF-7 | HCT-116 |
| 8 | --- | --- | 52.2 ± 1.3 | --- |
| 10 | --- | --- | 19.6 ± 0.7 | --- |
| 15 | 88.3 ± 2.2 | --- | --- | 46 ± 2.2 |
| 16 | 26.2 ± 1.4 | 39.5 ± 0.9 | --- | --- |
| 21 | --- | --- | 38.9 ± 0.8 | 82.8 ± 2.3 |
| Compound No. | IC50 (Mean ± SEM) (µM) | ||
|---|---|---|---|
| EGFR | VEGFR-2 | CDK-2/Cyclin A2 | |
| Erlotinib | 0.18 ± 0.05 | - | - |
| Sorafenib | - | 1.58 ± 0.11 | - |
| Roscovitine | - | - | 0.46 ± 0.30 |
| 8 | 0.22 ± 0.01 | 0.93 ± 0.42 | 0.24 ± 0.20 |
| 10 | 0.12 ± 0.50 | 0.79 ± 0.14 | 0.15± 0. 60 |
| 21 | 123 ± 0.10 | 172 ± 0.02 | 211± 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Sayed, W.A.; Alminderej, F.M.; Mounier, M.M.; Nossier, E.S.; Saleh, S.M.; F. Kassem, A. New 1,2,3-Triazole-Coumarin-Glycoside Hybrids and Their 1,2,4-Triazolyl Thioglycoside Analogs Targeting Mitochondria Apoptotic Pathway: Synthesis, Anticancer Activity and Docking Simulation. Molecules 2022, 27, 5688. https://doi.org/10.3390/molecules27175688
El-Sayed WA, Alminderej FM, Mounier MM, Nossier ES, Saleh SM, F. Kassem A. New 1,2,3-Triazole-Coumarin-Glycoside Hybrids and Their 1,2,4-Triazolyl Thioglycoside Analogs Targeting Mitochondria Apoptotic Pathway: Synthesis, Anticancer Activity and Docking Simulation. Molecules. 2022; 27(17):5688. https://doi.org/10.3390/molecules27175688
Chicago/Turabian StyleEl-Sayed, Wael A., Fahad M. Alminderej, Marwa M. Mounier, Eman S. Nossier, Sayed M. Saleh, and Asmaa F. Kassem. 2022. "New 1,2,3-Triazole-Coumarin-Glycoside Hybrids and Their 1,2,4-Triazolyl Thioglycoside Analogs Targeting Mitochondria Apoptotic Pathway: Synthesis, Anticancer Activity and Docking Simulation" Molecules 27, no. 17: 5688. https://doi.org/10.3390/molecules27175688
APA StyleEl-Sayed, W. A., Alminderej, F. M., Mounier, M. M., Nossier, E. S., Saleh, S. M., & F. Kassem, A. (2022). New 1,2,3-Triazole-Coumarin-Glycoside Hybrids and Their 1,2,4-Triazolyl Thioglycoside Analogs Targeting Mitochondria Apoptotic Pathway: Synthesis, Anticancer Activity and Docking Simulation. Molecules, 27(17), 5688. https://doi.org/10.3390/molecules27175688








