Compound Capecitabine Colon-Targeted Microparticle Prepared by Coaxial Electrospray for Treatment of Colon Tumors
Abstract
:1. Introduction
2. Results
2.1. Formula Ratio
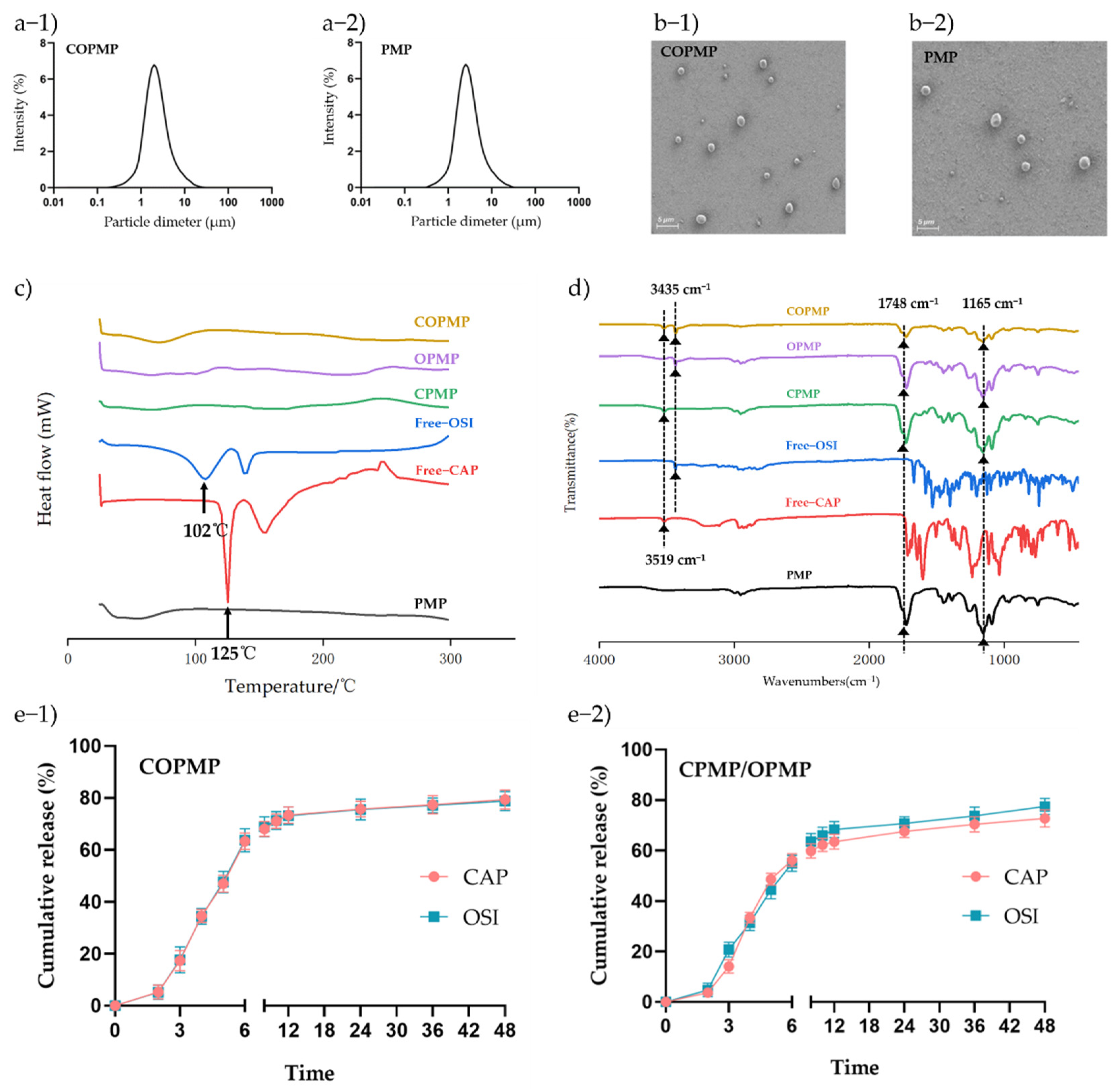
2.2. Preparation and Characterization of Microparticles
2.3. In Vitro Drug Release
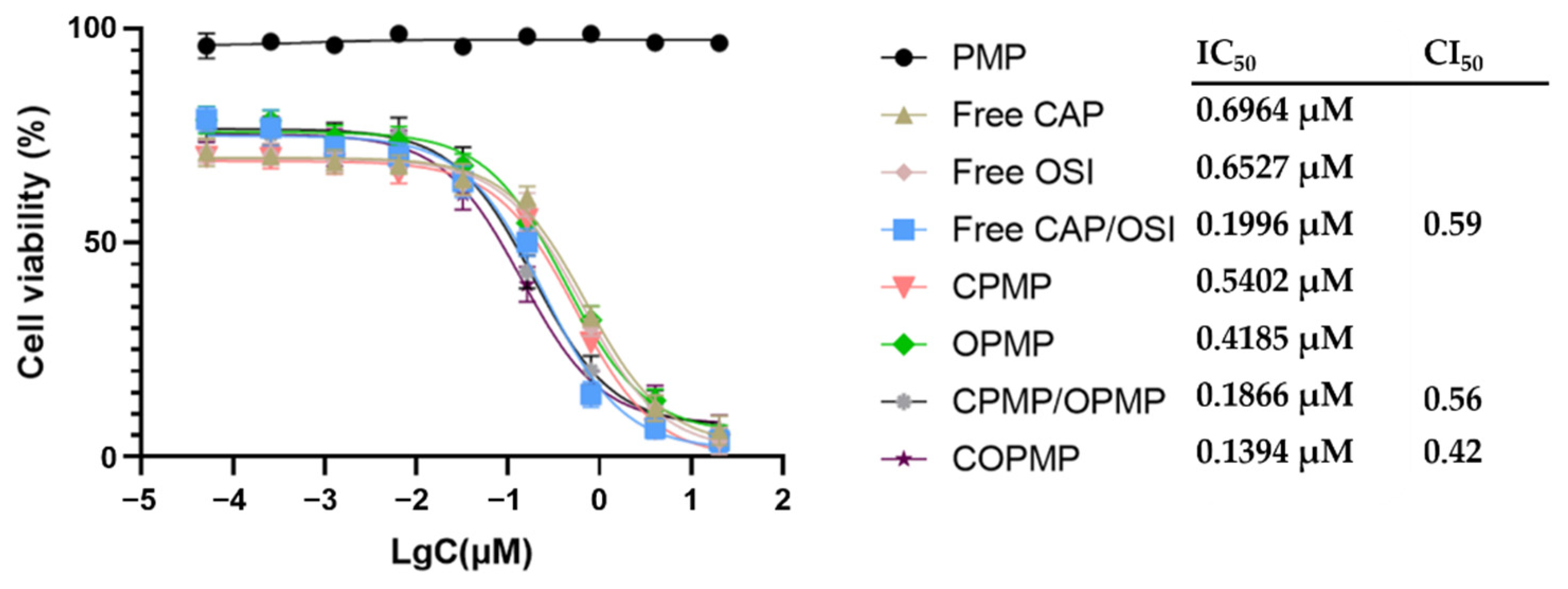
2.4. Cellular Growth Inhibition
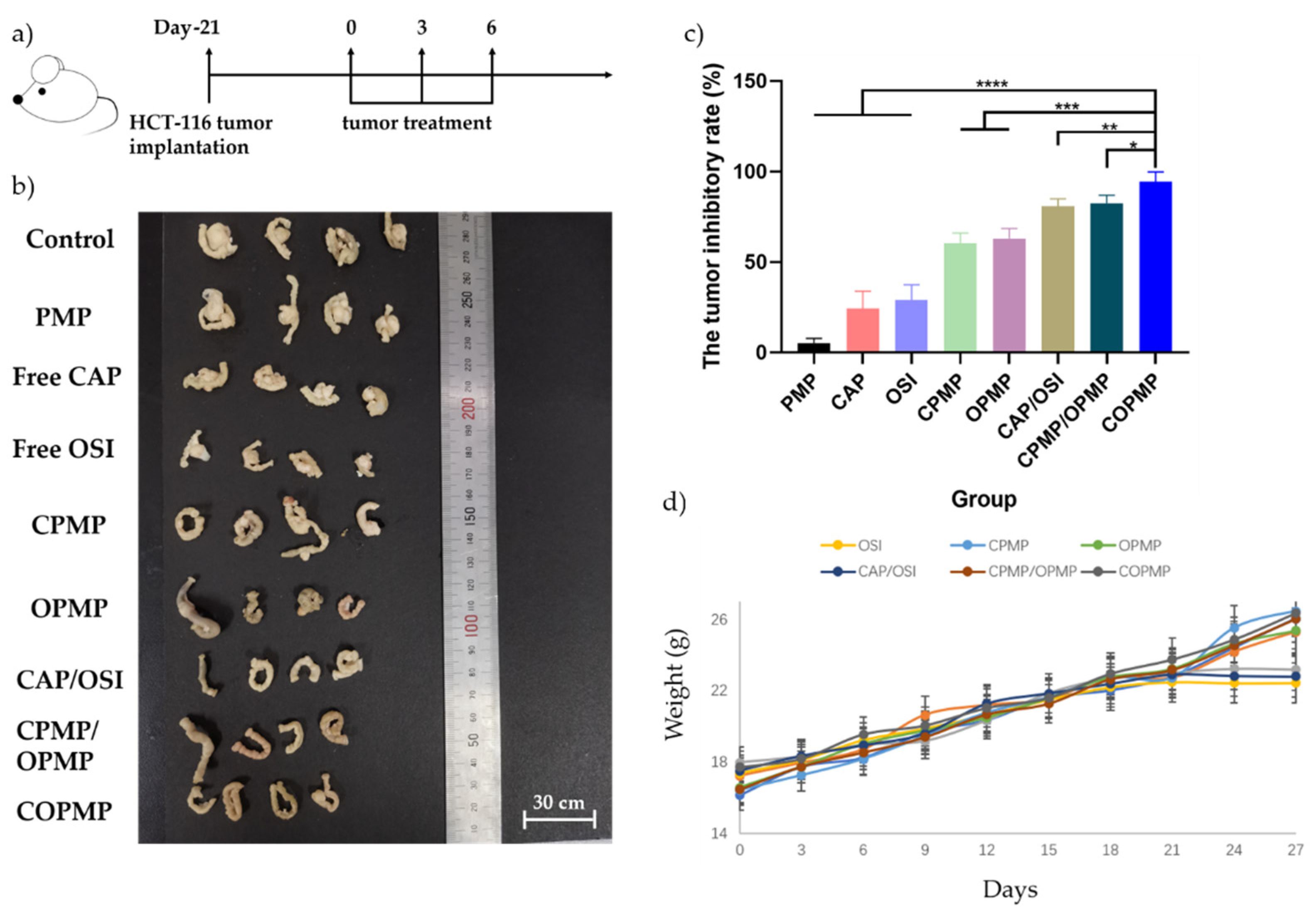
2.5. In Vivo Antitumor Effects of Microparticles
3. Materials and Methods
3.1. Materials
3.2. Fabrication of the Eudragit S100/CAP-OSI-PLGA Microparticles (COPMP), Eudragit S100/CAP-PLGA Microparticles (CPMP), Eudragit S100/OSI-PLGA Microparticles (OPMP), and Eudragit S100/PLGA Microparticles (PMP)
3.3. Structural Morphology and Size Distribution
3.4. Drug Loading (DL) and Encapsulation Efficiency (EE)
3.5. Physical Characterization
3.6. In Vitro Release
3.7. Cytotoxicity Assay
3.7.1. Formula Ratio
3.7.2. Cellular Growth Inhibition
3.8. In Vivo Antitumor Study
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Banerjee, A.; Pathak, S.; Subramanium, V.D.; Dharanivasan, G.; Murugesan, R.; Verma, R.S. Strategies for targeted drug delivery in treatment of colon cancer: Current trends and future perspectives. Drug Discov. Today 2017, 22, 1224–1232. [Google Scholar] [CrossRef] [PubMed]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef]
- Mortezaee, K.; Parwaie, W.; Motevaseli, E.; Mirtavoos-Mahyari, H.; Musa, A.E.; Shabeeb, D.; Esmaely, F.; Najafi, M.; Farhood, B. Targets for improving tumor response to radiotherapy. Int. Immunopharmacol. 2019, 76, 105847. [Google Scholar] [CrossRef]
- Najafi, M.; Salehi, E.; Farhood, B.; Nashtaei, M.S.; Hashemi, G.N.; Khanlarkhani, N.; Namjoo, Z.; Mortezaee, K. Adjuvant chemotherapy with melatonin for targeting human cancers: A review. J. Cell. Physiol. 2019, 234, 2356–2372. [Google Scholar] [CrossRef]
- Mortezaee, K.; Najafi, M. Immune system in cancer radiotherapy: Resistance mechanisms and therapy perspectives. Crit. Rev. Oncol./Hematol. 2021, 157, 3180. [Google Scholar] [CrossRef]
- Foxtrot Collaborative Group. Feasibility of preoperative chemotherapy for locally advanced, operable colon cancer: The pilot phase of a randomised controlled trial. Lancet Oncol. 2012, 13, 1152–1160. [Google Scholar] [CrossRef]
- Rödel, C. Preoperative chemoradiotherapy for rectal cancer. Nat. Rev. Clin. Oncol. 2010, 7, 129–130. [Google Scholar] [CrossRef]
- Yang, X.H.; Li, K.G.; Wei, J.B.; Wu, C.H.; Liang, S.X.; Mo, X.W.; Chen, J.S.; Tang, W.Z.; Qu, S. Retrospective study of preoperative chemoradiotherapy with capecitabine versus capecitabine plus oxaliplatin for locally advanced rectal cancer. Sci. Rep. 2020, 10, 12539. [Google Scholar] [CrossRef]
- Figueroa-Magalhães, M.C.; Jelovac, D.; Connolly, R.; Wolff, A.C. Treatment of HER2-positive breast cancer. Breast 2014, 23, 128–136. [Google Scholar] [CrossRef]
- Hejmady, S.; Pradhan, R.; Alexander, A.; Agrawal, M.; Singhvi, G.; Gorain, B.; Tiwari, S.; Kesharwani, P.; Dubey, S.K. Recent advances in targeted nanomedicine as promising antitumor therapeutics. Drug Discov. Today 2020, 25, 2227–2244. [Google Scholar] [CrossRef]
- Adityan, S.; Tran, M.; Bhavsar, C.; Wu, S.Y. Nano-therapeutics for modulating the tumour microenvironment: Design, development, and clinical translation. J. Control. Release 2020, 327, 512–532. [Google Scholar] [CrossRef] [PubMed]
- Nazeri, M.T.; Javanbakht, S.; Shaabani, A.; Ghorbani, M. 5-aminopyrazole-conjugated gelatin hydrogel: A controlled 5-fluorouracil delivery system for rectal administration. J. Drug Deliv. Sci. Technol. 2020, 57, 101669. [Google Scholar] [CrossRef]
- Melo, M.; Nunes, R.; Sarmento, B.; Neves, J.D. Rectal administration of nanosystems: From drug delivery to diagnostics. Mater. Today Chem. 2018, 10, 128–141. [Google Scholar] [CrossRef]
- Iurciuc-Tincu, C.-E.; Atanase, L.I.; Ochiuz, L.; Jérôme, C.; Sol, V.; Martin, P.; Popa, M. Curcumin-loaded polysaccharides-based complex particles obtained by polyelectrolyte complexation and ionic gelation. I-Particles obtaining and characterization. Int. J. Biol. Macromol. 2020, 147, 629–642. [Google Scholar] [CrossRef] [PubMed]
- Iurciuc, C.-E.; Atanase, L.I.; Jérôme, C.; Sol, V.; Martin, P.; Popa, M.; Ochiuz, L. Polysaccharides-Based Complex Particles’ Protective Role on the Stability and Bioactivity of Immobilized Curcumin. Int. J. Mol. Sci. 2021, 22, 3075. [Google Scholar] [CrossRef]
- Dudhipala, N.; Puchchakayala, G. Capecitabine lipid nanoparticles for anti-colon cancer activity in 1, 2-dimethylhydrazine induced colon cancer: Preparation, cytotoxic, pharmacokinetic and pathological evaluation. Drug Dev. Ind. Pharm. 2018, 44, 1572–1582. [Google Scholar] [CrossRef]
- Mikhail, S.E.; Sun, J.F.; Marshall, J.L. Safety of capecitabine: A review. Expert Opin. Drug Saf. 2010, 9, 831–841. [Google Scholar] [CrossRef]
- Lam, S.W.; Guchelaar, H.J.; Boven, E. The role of pharmacogenetics in capecitabine efficacy and toxicity. Cancer Treat. Rev. 2016, 50, 9–22. [Google Scholar] [CrossRef]
- Li, T.; Liu, D.; Lei, X.; Jiang, Q. Par3L enhances colorectal cancer cell survival by inhibiting Lkb1/AMPK signaling pathway. Biochem. Biophys. Res. Commun. 2017, 482, 1037–1041. [Google Scholar] [CrossRef]
- Herbst, R.S. Review of epidermal growth factor receptor biology. Int. J. Radiat. Oncol. Biol. Phys. 2004, 59, 21–26. [Google Scholar] [CrossRef]
- Mayor, S. Osimertinib effective in EGFR T790M-positive lung cancer. Lancet Oncol. 2017, 18, e9. [Google Scholar] [CrossRef]
- Jin, P.; Jiang, J.; Xie, N.; Zhou, L.; Huang, Z.; Zhang, L.; Qin, S.; Fu, S.; Peng, L.; Gao, W.; et al. MCT1 relieves osimertinib-induced CRC suppression by promoting autophagy through the LKB1/AMPK signaling. Cell Death Dis. 2019, 10, 615. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Chen, Y.; Xu, M.; Chen, L.; Zhang, X.; To, K.K.; Zhao, H.; Wang, F.; Xia, Z.; Chen, X.; et al. Osimertinib (AZD9291) Enhanced the Efficacy of Chemotherapeutic Agents in ABCB1- and ABCG2-Overexpressing Cells In Vitro, In Vivo, and Ex Vivo. Mol. Cancer Ther. 2016, 15, 1845–1858. [Google Scholar] [CrossRef]
- Shen, H.; Yang, J.; Huang, Q.; Jiang, M.J.; Tan, Y.N.; Fu, J.F.; Zhu, L.Z.; Fang, X.F.; Yuan, Y. Different treatment strategies and molecular features between right-sided and left-sided colon cancers. World J. Gastroenterol. 2015, 21, 6470–6478. [Google Scholar] [CrossRef] [PubMed]
- Gad, H.A.; Kamel, A.O.; Ezzat, O.M.; El Dessouky, H.F.; Sammour, O.A. Doxycycline hydrochloride-metronidazole solid lipid microparticles gels for treatment of periodontitis: Development, in-vitro and in-vivo clinical evaluation. Expert Opin. Drug Deliv. 2017, 14, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.G.; Li, J.J.; Williams, G.R.; Zhao, M. Electrospun amorphous solid dispersions of poorly water-soluble drugs: A review. J. Control. Release 2018, 292, 91–110. [Google Scholar] [CrossRef]
- Boda, S.K.; Li, X.; Xie, J. Electrospraying an enabling technology for pharmaceutical and biomedical applications: A review. J. Aerosol Sci. 2018, 125, 164–181. [Google Scholar] [CrossRef]
- Hao, S.; Wang, B.; Wang, Y.; Xu, Y.Q. Enteric-coated sustained-release nanoparticles by coaxial electrospray: Preparation, characterization, and in vitro evaluation. J. Nanopartic. Res. 2014, 16, 2204. [Google Scholar] [CrossRef]
- Hitomi, K.; Kaoru, N.; Akihiro, W. Size-selected synthesis of metal nanoparticles by using electrospray in a liquid medium. Colloids Surf. A Physicochem. Eng. Asp. 2019, 581, 123836. [Google Scholar]
- Zhang, N.; Fu, J.N.; Chou, T.C. Synergistic combination of microtubule targeting anticancer fludelone with cytoprotective panaxytriol derived from panax ginseng against MX-1 cells in vitro: Experimental design and data analysis using the combination index method. Am. J. Cancer Res. 2015, 6, 97–104. [Google Scholar]
- Zhang, L.; Huang, J.; Si, T.; Xu, R.X. Coaxial electrospray of microparticles and nanoparticles for biomedical applications. Expert Rev. Med. Devices 2012, 9, 595–612. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Liu, W.; Jiang, P.; Hong, T. Coaxial Electrohydrodynamic Atomization for the Production of Drug-Loaded Micro/Nanoparticles. Micromachines 2019, 10, 125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morais, A.Í.S.; Vieira, E.G.; Afewerki, S.; Sousa, R.B.; Honorio, L.M.C.; Cambrussi, A.N.C.O.; Santos, J.A.; Bezerra, R.D.S.; Furtini, J.A.O.; Silva-Filho, E.C.; et al. Fabrication of Polymeric Microparticles by Electrospray: The Impact of Experimental Parameters. J. Funct. Biomater. 2020, 11, 4. [Google Scholar] [CrossRef]
- Mitra, A.; Nedasadat, S.A.; Navid, Y.M. Utilization of supercritical CO2 gas antisolvent (GAS) for production of Capecitabine nanoparticles as anti-cancer drug: Analysis and optimization of the process conditions. J. CO2 Util. 2021, 46, 101465. [Google Scholar]
- Patel, P.; Barot, T.; Kulkarni, P. Formulation, Characterization and In-vitro and In-vivo Evaluation of Capecitabine Loaded Niosomes. Curr. Drug Deliv. 2020, 17, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Faraji, D.B.; Hasani, A.M.; Sheisi, N.; Goleij, P.; Mirmajidi, T.; Chogan, F.; Irani, M.; Sharafian, F. Synthesis of PLGA/chitosan/zeolites and PLGA/chitosan/metal organic frameworks nanofibers for targeted delivery of Paclitaxel toward prostate cancer cells death. Int. J. Biol. Macromol. 2020, 164, 1461–1474. [Google Scholar] [CrossRef] [PubMed]

| CAP:OSI | IC50,CAP (μM) | IC50,OSI (μM) | CI50 |
|---|---|---|---|
| Free CAP | 0.6990 | ||
| CAP:OSI = 5:1 | 0.1651 | 0.3314 | 0.76 |
| CAP:OSI = 2:1 | 0.1771 | 0.2851 | 0.70 |
| CAP:OSI = 1:1 | 0.1901 | 0.1901 | 0.57 |
| CAP:OSI = 1:2 | 0.3257 | 0.1643 | 0.72 |
| CAP:OSI = 1:5 | 0.3571 | 0.1433 | 0.74 |
| Free OSI | 0.6373 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, R.; Zhai, R.; Wang, C.; Liang, S.; Wang, J.; Liu, Z.; Li, W. Compound Capecitabine Colon-Targeted Microparticle Prepared by Coaxial Electrospray for Treatment of Colon Tumors. Molecules 2022, 27, 5690. https://doi.org/10.3390/molecules27175690
Chen R, Zhai R, Wang C, Liang S, Wang J, Liu Z, Li W. Compound Capecitabine Colon-Targeted Microparticle Prepared by Coaxial Electrospray for Treatment of Colon Tumors. Molecules. 2022; 27(17):5690. https://doi.org/10.3390/molecules27175690
Chicago/Turabian StyleChen, Ruiqi, Ruidong Zhai, Chao Wang, Shulong Liang, Jing Wang, Zhepeng Liu, and Wenlin Li. 2022. "Compound Capecitabine Colon-Targeted Microparticle Prepared by Coaxial Electrospray for Treatment of Colon Tumors" Molecules 27, no. 17: 5690. https://doi.org/10.3390/molecules27175690





