Hemocompatibile Thin Films Assessed under Blood Flow Shear Forces
Abstract
1. Introduction
2. Materials and Methods
2.1. Surface Modification
2.2. Fundamental Research Material Evaluation
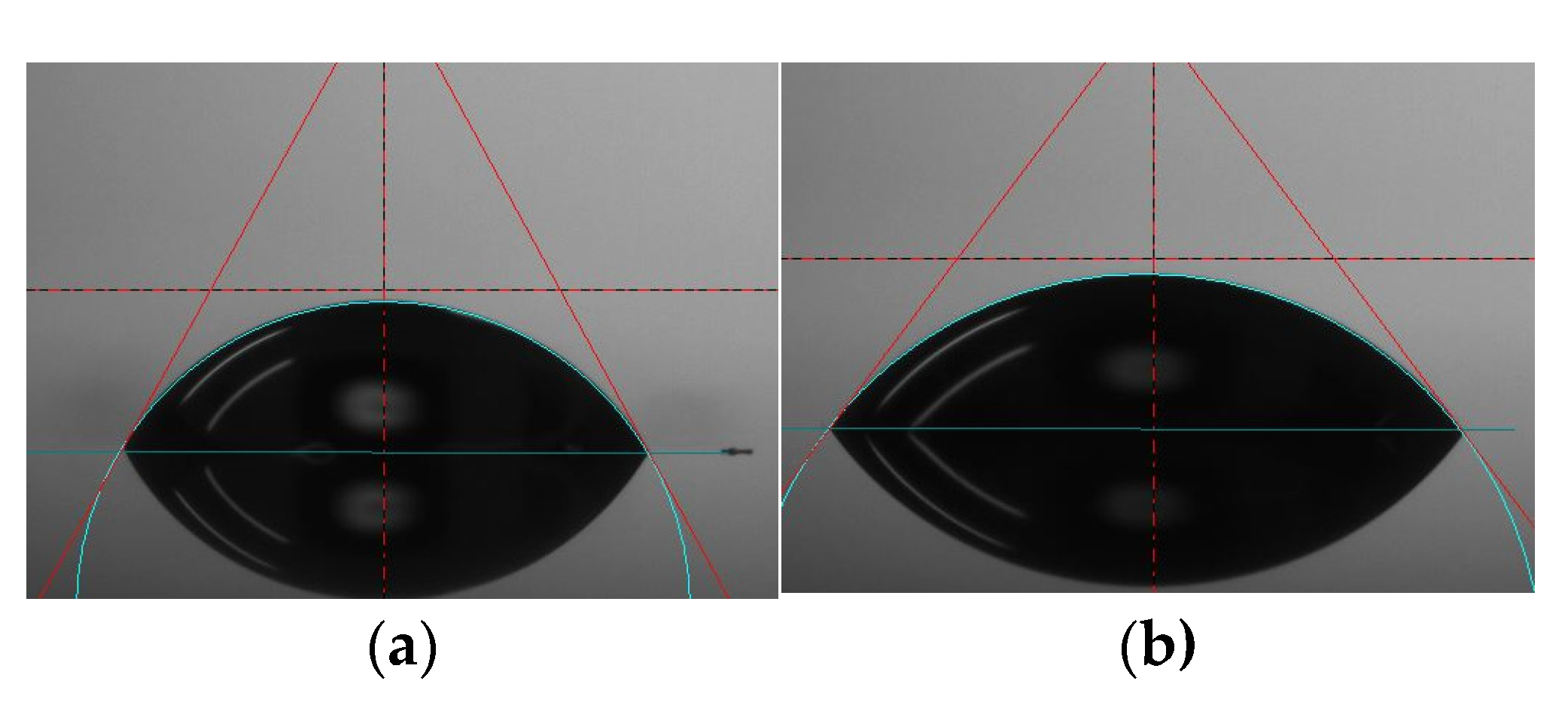
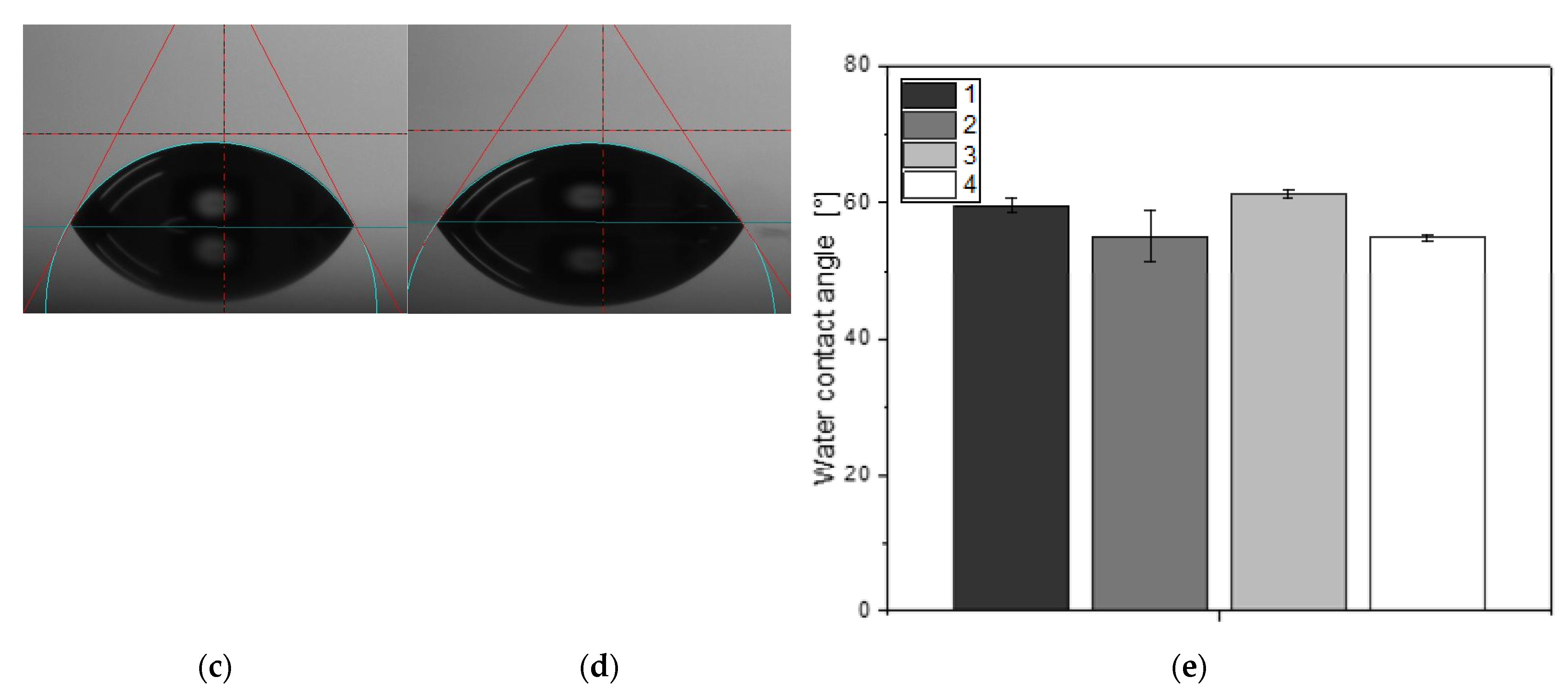
2.2.1. Surface Wettability
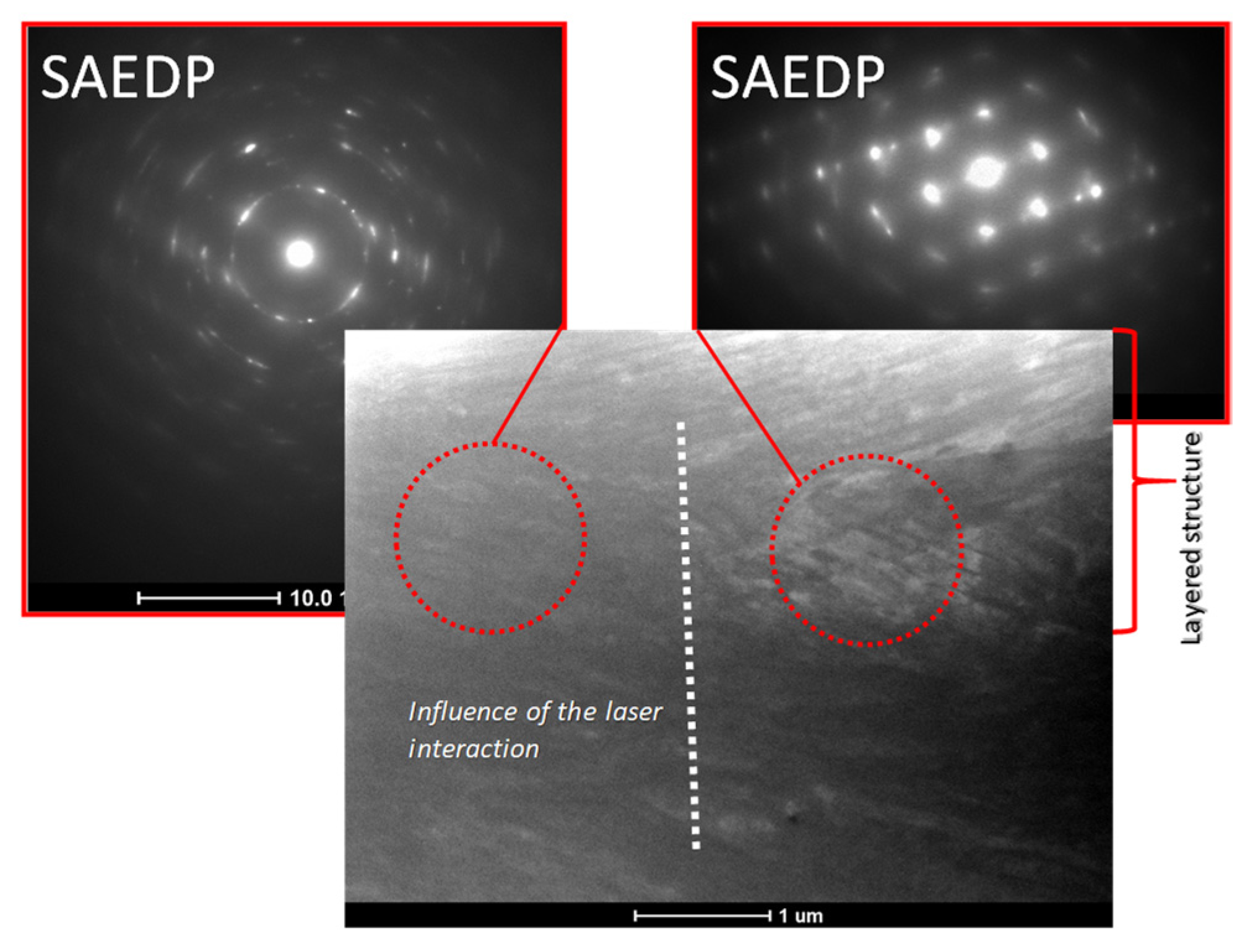
2.2.2. Microstructure Analysis
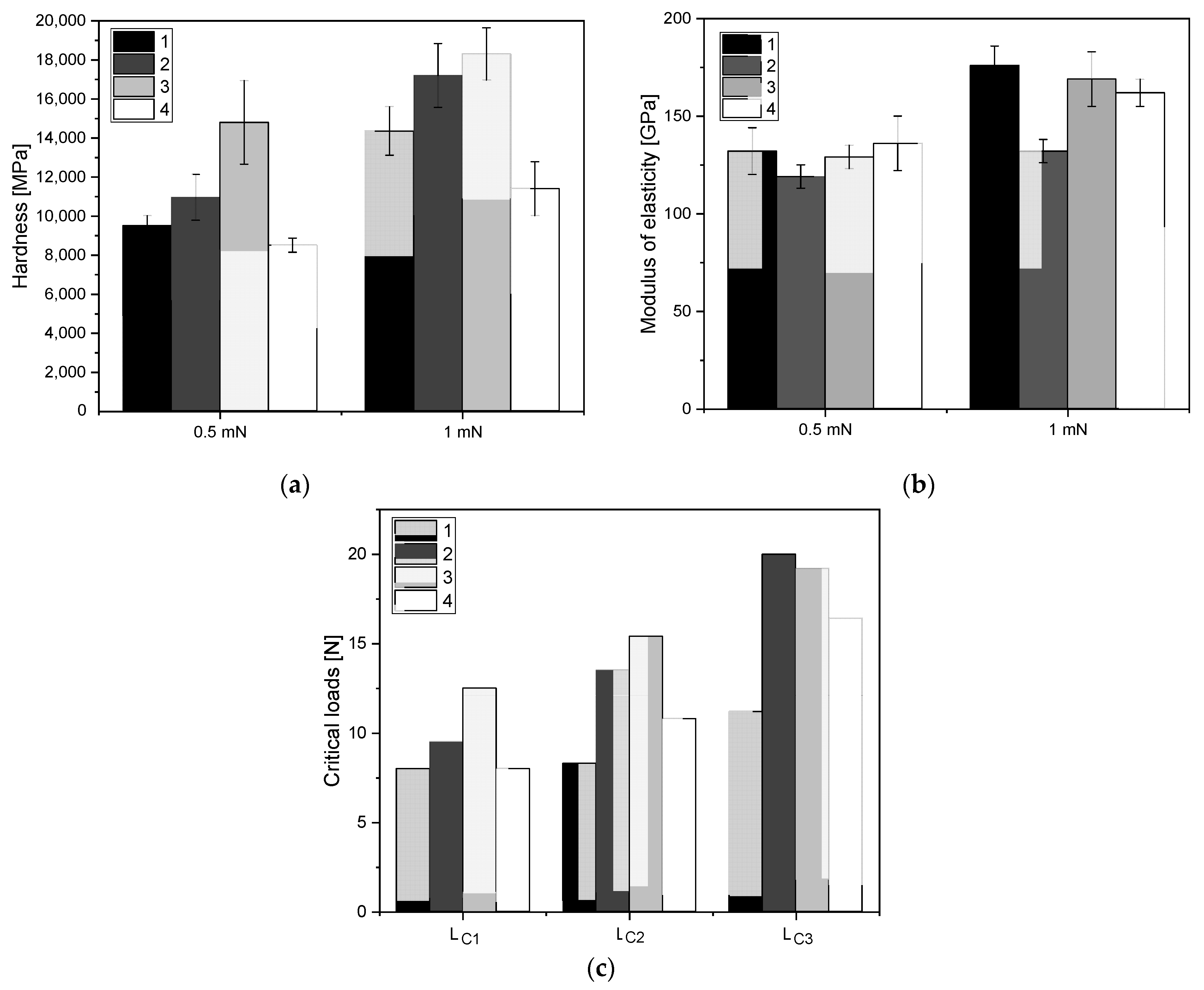
2.2.3. Mechanical Evaluation of Thin-Film Materials
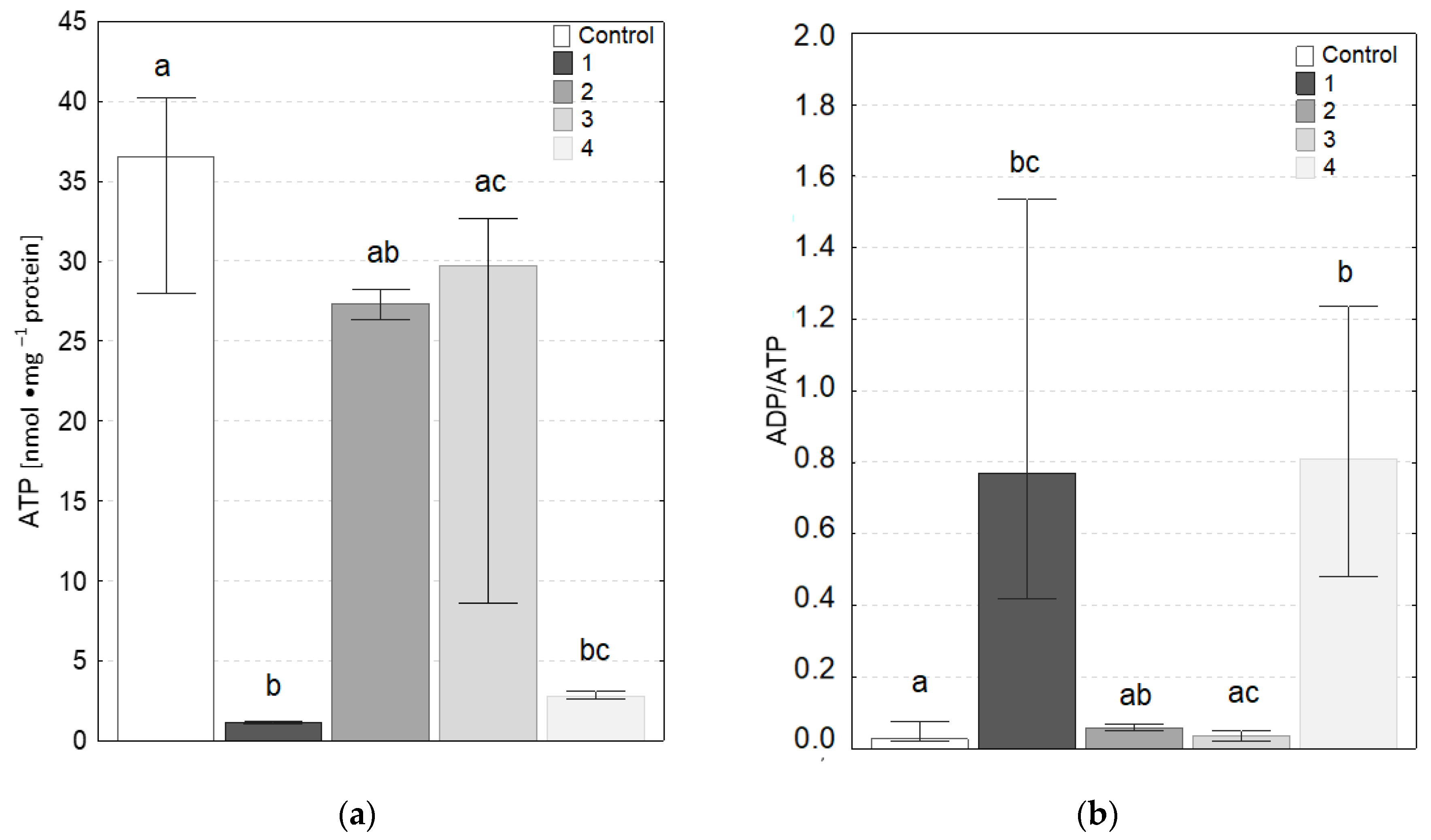
2.2.4. Adenosine Triphosphate (ATP) Level and Adenosine Diphosphate/Adenosine Triphosphate (ADP/ATP) Ratio
2.3. Applied Research Material Evaluation Based on Hemocompatibility Assessed in Dynamic Conditions
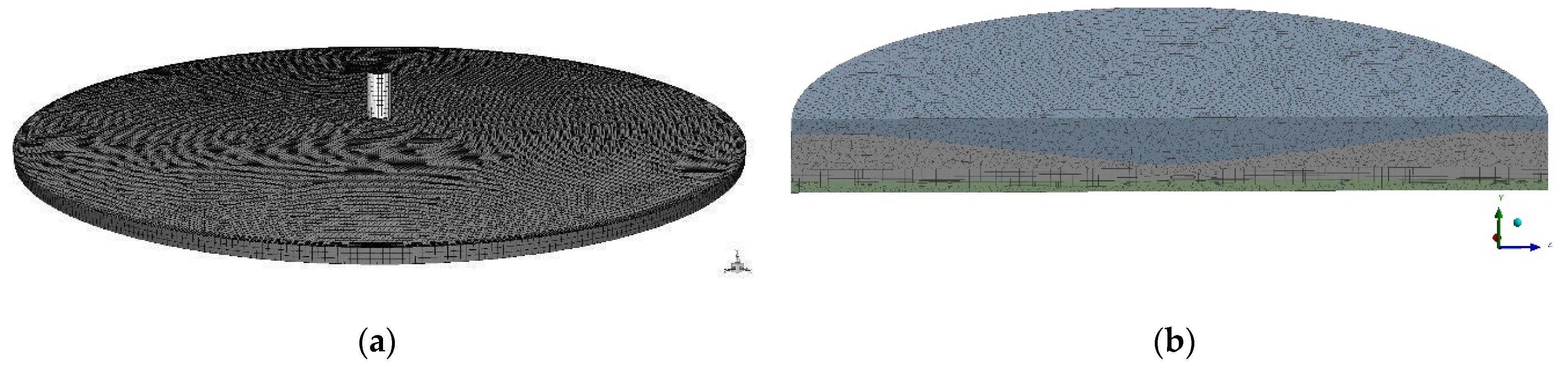
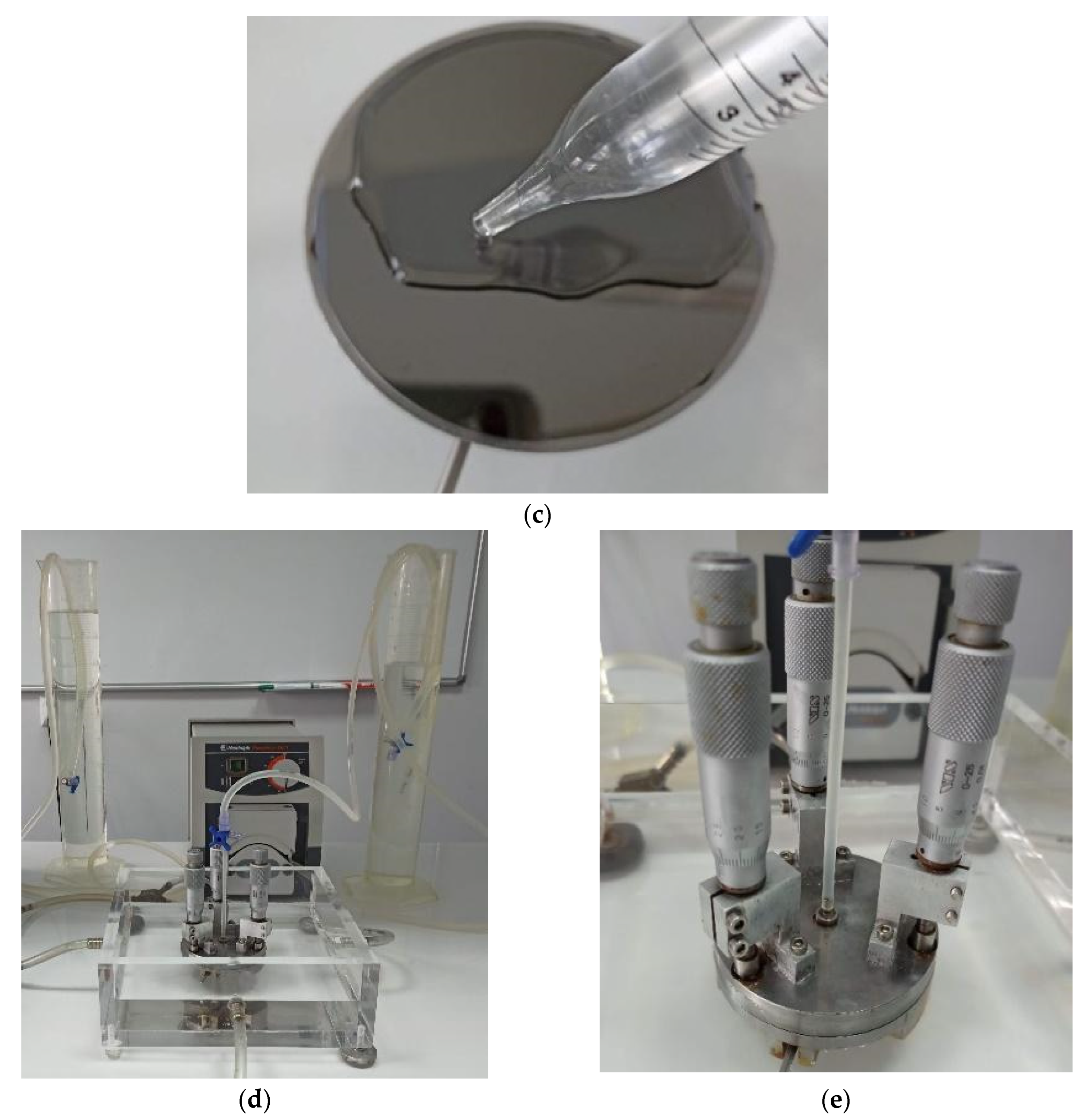
2.3.1. Models of Blood–Material Adhesion Tests: Radial Flow and Impact-R
2.3.2. Blood Cell Detachment under Shear Flow
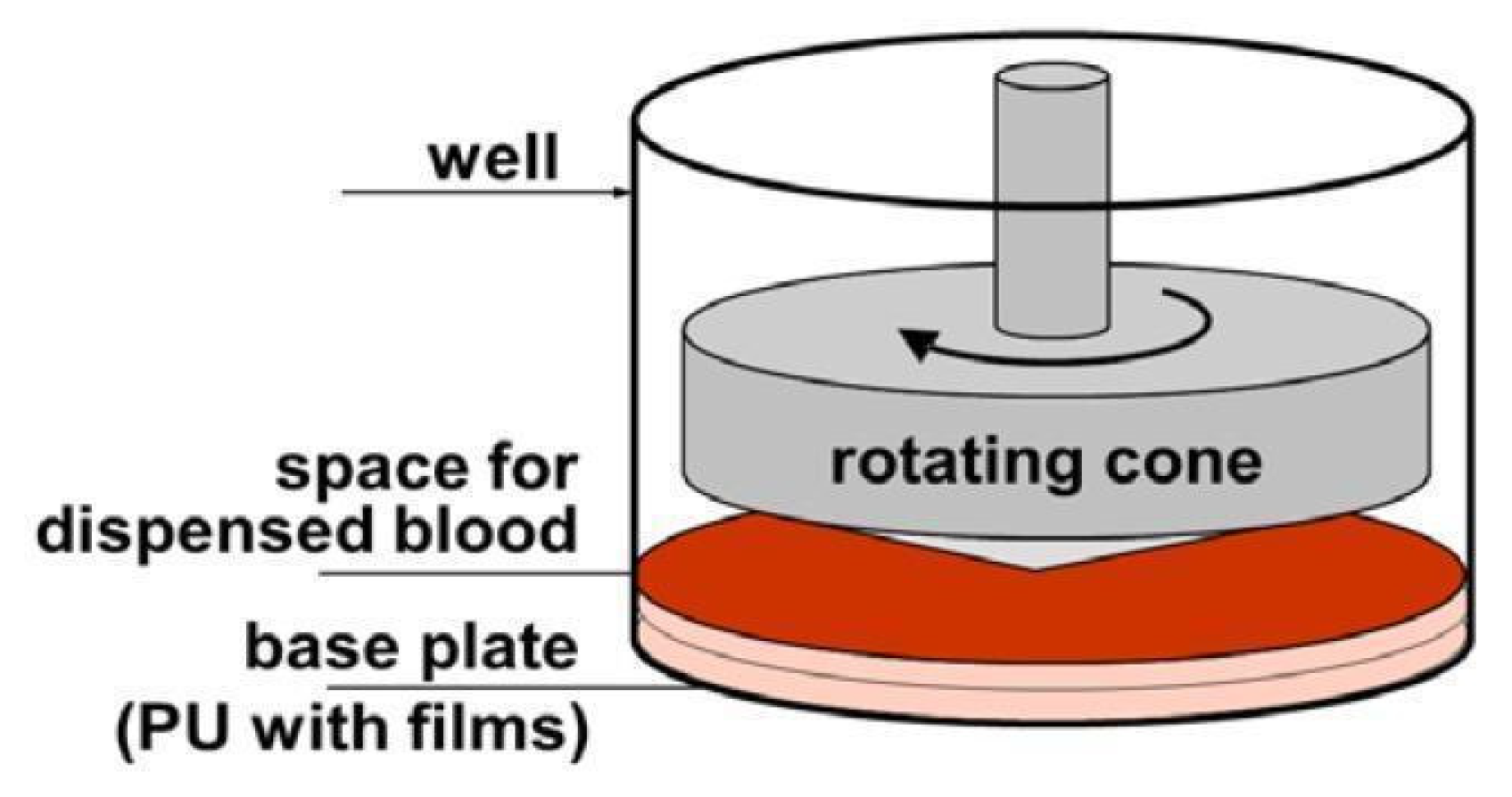
2.3.3. A Simplified Method for Assessing Hemocompatibility under High Shear Stresses
- Lower donor blood requirements for the test—only 130 μL of blood per test,
- Availability of a commercial testing device,
- The accepted principle of a rotating cone and plate as a source of shear stress, as in rheometers or viscometers.
3. Results
3.1. Surface Wetttability
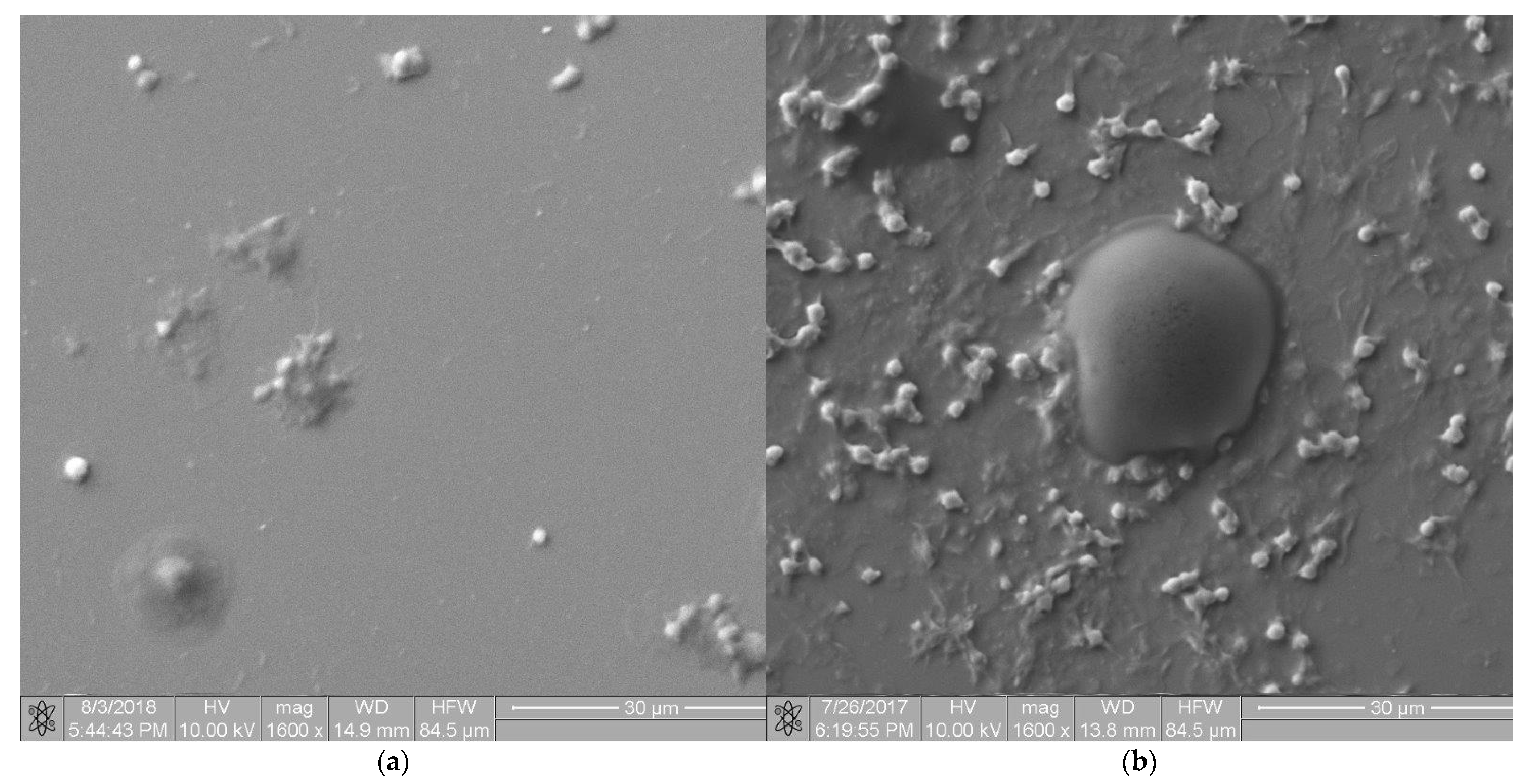
3.2. Microstructure Analysis
3.3. Mechanical Evaluation of Thin-Film Materials
3.4. ATP Level and ADP/ATP Ratio
3.5. Mechanical Evaluation of Thin-Film Materials
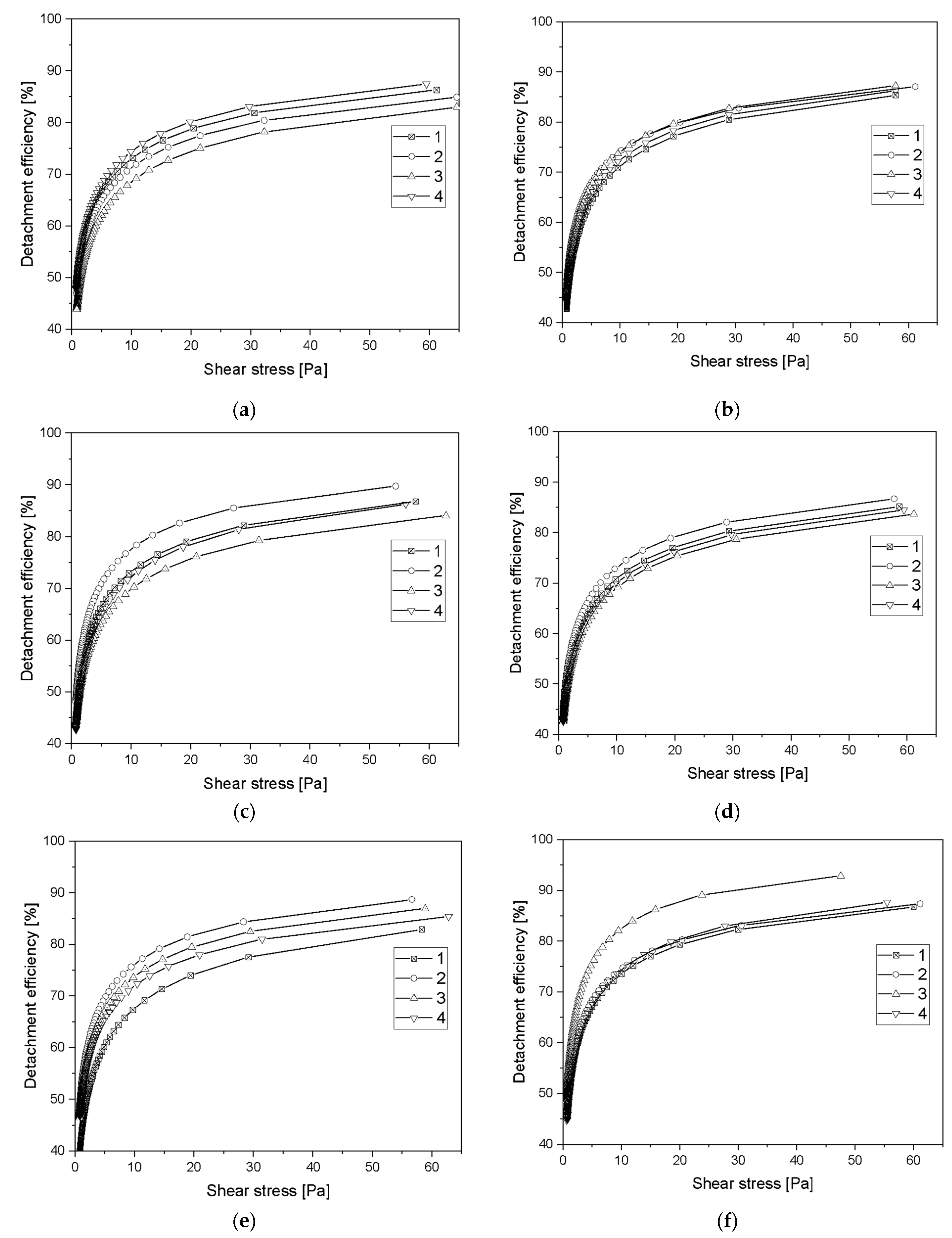
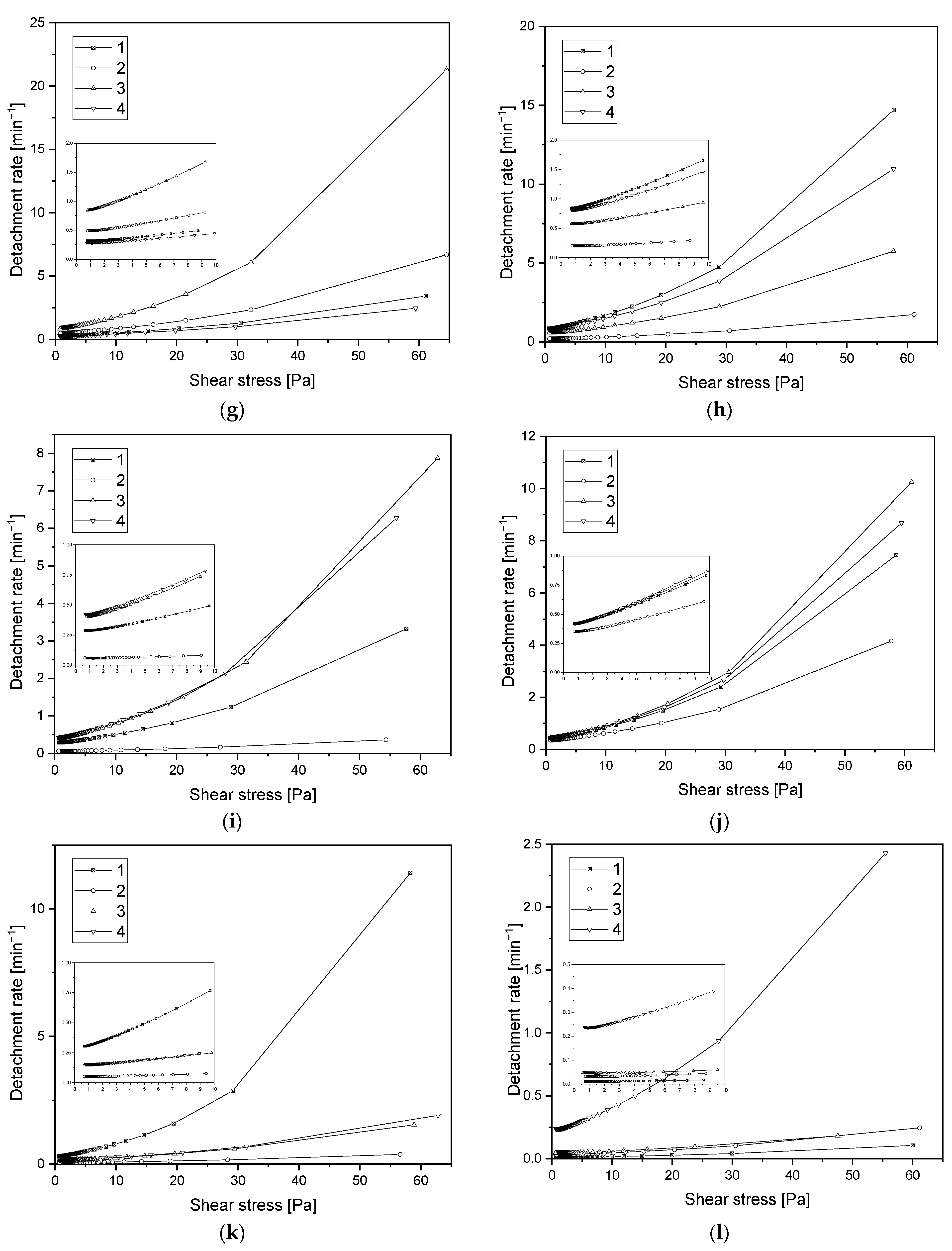
3.6. Assessment of Hemocompatibility of Materials with Arterial Blood Flow
3.7. Assessment of Hemocompatibility of Materials with Arterial Blood Flow
4. Discussion
5. Conclusions
- The ALS coatings exhibit higher hardness values compared to the SPU coatings. Samples obtained by the ALS method also have a high Young’s modulus and are resistant to high critical loads.
- Hydrophilic properties influence cell adhesion. On the basis of the obtained test results, the hydrophilic properties of the developed materials were found, but without the risk of excessive activation of platelets.
- Based on the basic research test, based on radial flow chamber, the highest detachment efficiency obtained for ALS samples was observed. Samples obtained with the SPU method exhibit the lowest detachment rate and the highest critical stress value. Based on the observations obtained, these types of materials show a strong interaction with blood morphotic elements. It can be concluded on this basis that, from the point of view of the objectives of the work, these materials should probably be disqualified. However, the final decision was made on the basis of the clinical blood test. From the point of view of biomaterials engineering, however, this is not a parameter that must ultimately eliminate this type of material. The surface could strongly influence on the cell adhesion, but this does not at all indicate that the cells must be strongly activated.
- A decrease in ATP concentration and an increase in ADP/ATP ratio, compared with control, were observed in fibroblasts after an exposure to contact with coatings 1 and 4. Moreover, the coatings 2 and 3 showed no difference compared to the control.
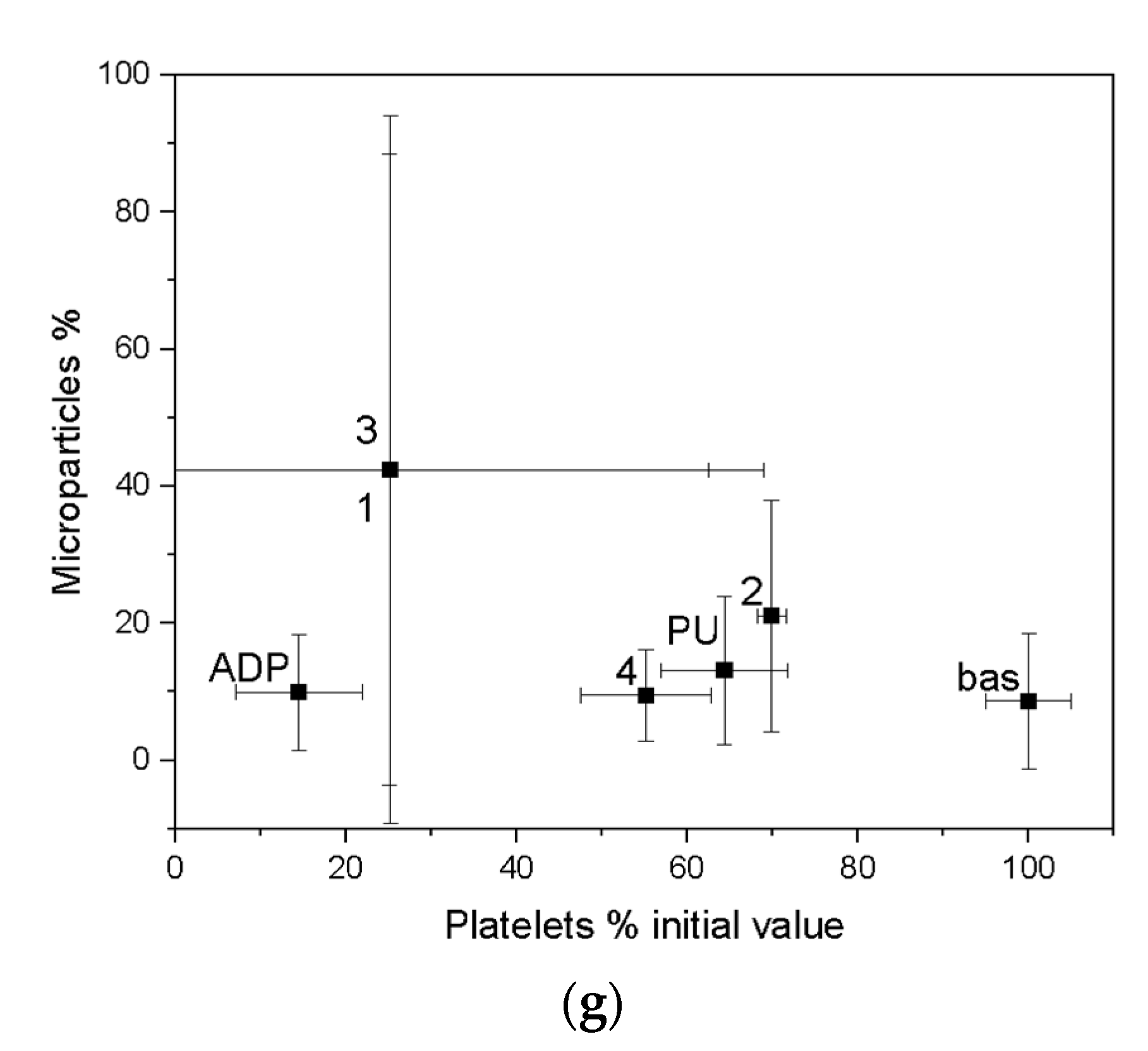
- The observation resulting from the clinical whole-blood test showed the case mentioned in the previous application. The materials labelled 2, which showed strong interactions with blood morphotic elements in the primary test, showed at the same time the best hemocompatibility properties in whole blood tests.
- Materials marked as 2 (SPU) had the lowest platelet consumption, which ultimately ranked them as the best of the group of materials tested. Platelet-derived microparticles analyzed by Anexin V are another important indicator for material classification. Materials labelled with 2 showed the lowest risk of microparticle formation, which can be summarized as having the least impact on platelet destruction.
- For sample 4 (SPU), the concentration of platelet microparticles slightly exceeded the human reference values while coating 3, manufactured using ALS method, showed the highest platelet activation under shear forces.
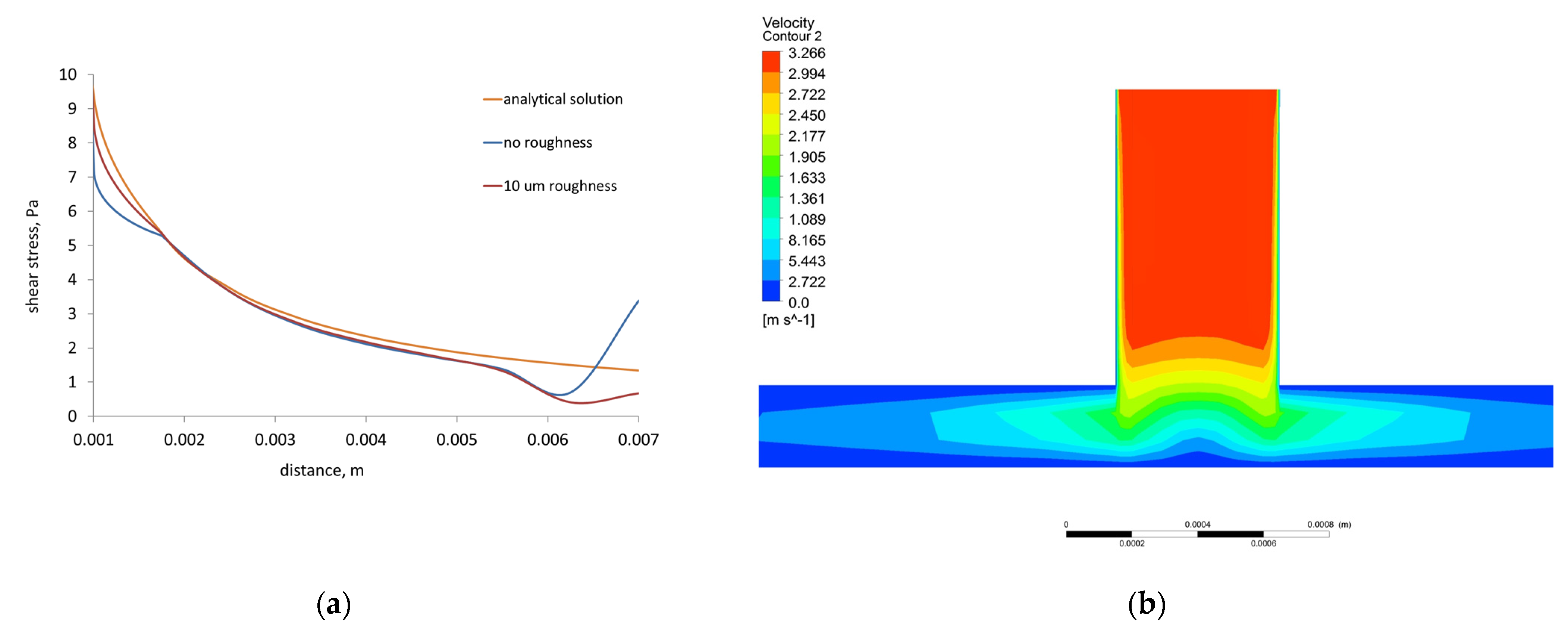
- The shear stress computed by the 3D FVM model of the radial flow test is in good qualitative and quantitative agreement with the shear stress calculated using analytical equation. The differences were caused by implementing roughness, turbulence, and 3D solution in the former.
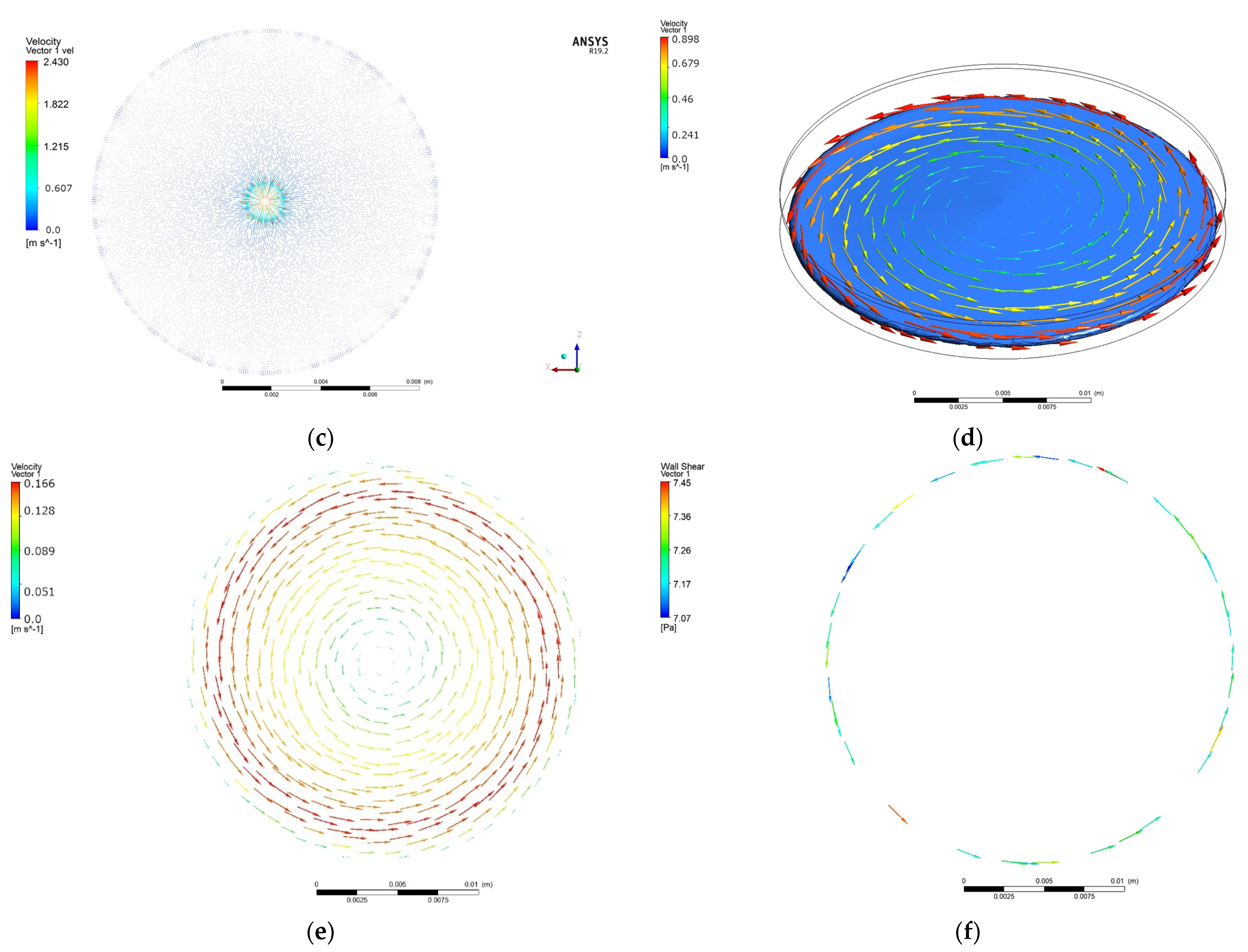
- The cone and plate test model developed in the present study comprising roughness of the sample and blood flow turbulence, gives a better approximation of the blood–material interaction than the other attempts in this area.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Kyriakopoulos, C.P.; Kapelios, C.J.; Stauder, E.L.; Taleb, I.; Hamouche, R.; Sideris, K.; Koliopoulou, A.G.; Bonios, M.J.; Drakos, S.G. LVAD as a Bridge to Remission from Advanced Heart Failure: Current Data and Opportunities for Improvement. J. Clin. Med. 2022, 11, 3542. [Google Scholar] [CrossRef]
- van Hagen, I.M.; Roos-Hesselink, J.W.; Ruys, T.P.E.; Merz, W.M.; Goland, S.; Gabriel, H.; Lelonek, M.; Trojnarska, O.; al Mahmeed, W.A.; Balint, H.O.; et al. Pregnancy in Women With a Mechanical Heart Valve. Circulation 2015, 132, 132–142. [Google Scholar] [CrossRef]
- Chikwe, J.; Egorova, N.N.; Adams, D.H. Age Cutoffs for Bioprosthetic vs Mechanical Aortic Valve Replacement—Reply. JAMA 2015, 313, 523. [Google Scholar] [CrossRef]
- Head, S.J.; Kappetein, A.P. Aortic Valve Replacement in Younger Adults: A Biological Valve Is Not the Logical Choice. Eur. Heart J. 2016, 37, 2668–2670. [Google Scholar] [CrossRef]
- Aortic Stenosis. Available online: https://www.msdmanuals.com/professional/cardiovascular-disorders/valvular-disorders/aortic-stenosis (accessed on 26 August 2022).
- Indications for Valve Replacement for High Gradient Aortic Stenosis in Adults. Available online: https://www.uptodate.com/contents/indications-for-valve-replacement-for-high-gradient-aortic-stenosis-in-adults (accessed on 26 August 2022).
- Braverman, A.C. Aortic replacement for bicuspid aortic valve aortopathy: When and why? J. Thorac. Cardiovasc. Surg. 2019, 157, 520–525. [Google Scholar] [CrossRef]
- Marco, R.; Maurizio, T.; Andrea, G.; Alberto, P.; Fabian, N.; Von Segesser, L.; Francesco, M. The Evolution of Surgical Valves. Cardiovasc. Med. 2017, 20, 285–292. [Google Scholar] [CrossRef]
- Park, M.H.; Zhu, Y.; Imbrie-Moore, A.M.; Wang, H.; Marin-Cuartas, M.; Paulsen, M.J.; Woo, Y.J. Heart Valve Biomechanics: Th Frontiers of Modeling Modalities and the Expansive Capabilities of Ex Vivo Heart Simulation. Front. Cardiovasc. 2021, 8, 1–10. [Google Scholar] [CrossRef]
- Goubergrits, L.; Affeld, K.; Kertzscher, U. Innovative Developments of the Heart Valves Designed for Use in Ventricular Assist Devices. Expert Rev. Med. Devices 2005, 2, 61–71. [Google Scholar] [CrossRef]
- Lee, C.S.; Chandran, K.B.; Chen, L.D. Cavitation Dynamics of Medtronic Hall Mechanical Heart Valve Prosthesis: Fluid Squeezing Effect. J. Biomech. Eng. 1996, 118, 97–105. [Google Scholar] [CrossRef]
- Fiedler, A.G.; Tolis, G. Surgical Treatment of Valvular Heart Disease: Overview of Mechanical and Tissue Prostheses, Advantages, Disadvantages, and Implications for Clinical Use. Curr. Treat. Options Cardiovasc. Med. 2018, 20, 7. [Google Scholar] [CrossRef]
- Newer Heart Valve Surgery Options. Available online: https://www.heart.org/en/health-topics/heart-valve-problems-and-disease/understanding-your-heart-valve-treatment-options/newer-heart-valve-surgery-options (accessed on 26 August 2022).
- Lahpor, J. State of the Art: Implantable Ventricular Assist Devices. Curr. Opin. Organ Transplant. 2009, 14, 554–559. [Google Scholar] [CrossRef]
- Aaronson, K.D.; Slaughter, M.S.; Miller, L.W.; McGee, E.C.; Cotts, W.G.; Acker, M.A.; Jessup, M.L.; Gregoric, I.D.; Loyalka, P.; Frazier, O.H.; et al. Use of an Intrapericardial, Continuous-Flow, Centrifugal Pump in Patients Awaiting Heart Transplantation. Circulation 2012, 125, 3191–3200. [Google Scholar] [CrossRef]
- Carrel, T.; Englberger, L.; Martinelli, M.; Takala, J.; Boesch, C.; Sigurdadottir, V.; Gygax, E.; Kadner, A.; Mohacsi, P. Continuous Flow Left Ventricular Assist Devices: A Valid Option for Heart Failure Patients. Swiss Med. Wkly. 2012, 142, w17301. [Google Scholar] [CrossRef]
- Lauer, M.S.; Kiley, J.P.; Mockrin, S.C.; Mensah, G.A.; Hoots, W.K.; Patel, Y.; Cook, N.L.; Patterson, A.P.; Gibbons, G.H. National Heart, Lung, and Blood Institute (NHLBI) Strategic Visioning Setting an Agenda Together for the NHLBI of 2025. Circulation 2015, 131, 1106–1109. [Google Scholar] [CrossRef]
- Annual Report 2019, Eurotransplant. Available online: https://www.eurotransplant.org/wp-content/uploads/2020/06/Annual-Report-2019.pdf (accessed on 26 August 2022).
- INTERMACS Interagency Registry for Mechanically Assisted Circulatory Support, Quarterly Statistical Report, Centers for Medicare & Medicaid Services, 2012 Q4. Available online: https://www.uab.edu/medicine/intermacs/images/CMS/CMS_Report_2012_Q4.pdf (accessed on 26 August 2022).
- Kannojiya, V.; Das, A.K.; Das, P.K. Comparative Assessment of Different Versions of Axial and Centrifugal LVADs: A Review. Artif. Organs 2021, 45, 665–681. [Google Scholar] [CrossRef]
- Mariani, S.; Hanke, J.S.; Li, T.; Merzah, A.S.; Chatterjee, A.; Deniz, E.; Haverich, A.; Schmitto, J.D.; Dogan, G. Device Profile of the Heartware HVAD System as a Bridge-to-Transplantation in Patients with Advanced Heart Failure: Overview of Its Safety and Efficacy. Expert Rev. Med. Devices 2019, 16, 1003–1015. [Google Scholar] [CrossRef]
- Cestari, V.; Pessoa, V.L.; de Souza Neto, J.D.; Moreira, T.; Florêncio, R.; de Vasconcelos, G.G.; Souza, L.; Braga, A.; Sobral, M.G. Clinical Evolution of Patients Using Ventricular Assist Devices as a Bridge for Transplantation. Transplant. Proc. 2018, 50, 796–803. [Google Scholar] [CrossRef]
- Brunette, D.M.; Tengvall, P.; Textor, M.; Thomsen, P. Titanium in Medicine; Engineering Materials; Springer: Berlin/Heidelberg, Germany, 2001. [Google Scholar]
- Adachi, I.; Fraser, C.D. Mechanical Circulatory Support for Infants and Small Children. Semin. Thorac. Cardiovasc. Surg. Pediatric Card. Surg. Annu. 2011, 14, 38–44. [Google Scholar] [CrossRef]
- Dominik, J.; Zacek, P.; Vojacek, J. Aortic Valve Replacement. In Aortic Regurgitation; Springer International Publishing: Cham, Switherlands, 2018; pp. 123–152. [Google Scholar]
- Vieira, J.L.; Ventura, H.O.; Mehra, M.R. Mechanical Circulatory Support Devices in Advanced Heart Failure: 2020 and Beyond. Prog. Cardiovasc. Dis. 2020, 63, 630–639. [Google Scholar] [CrossRef] [PubMed]
- Macaluso, G.P.; Pagani, F.D.; Slaughter, M.S.; Milano, C.A.; Feller, E.D.; Tatooles, A.J.; Rogers, J.G.; Wieselthaler, G.M. Time in Therapeutic Range Significantly Impacts Survival and Adverse Events in Destination Therapy Patients. ASAIO J. 2022, 68, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Tycińska, A.; Grygier, M.; Biegus, J.; Czarnik, T.; Dąbrowski, M.; Depukat, R.; Gierlotka, M.; Gil, M.; Hawranek, M.; Hirnle, T.; et al. Mechanical circulatory support. An expert opinion of the Association of Intensive Cardiac Care and Association of Cardiovascular Interventions of the Polish Cardiac Society. Kardiol. Pol. 2021, 79, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Vašků, J.; Urbánek, P. Constructional and Functional Characteristics of Recent Total Artificial Heart Models TNS Brno VII, VIII, and IX. Artif. Organs 1995, 19, 535–543. [Google Scholar] [CrossRef]
- Li, R.L.; Russ, J.; Paschalides, C.; Ferrari, G.; Waisman, H.; Kysar, J.W.; Kalfa, D. Mechanical Considerations for Polymeric Heart Valve Development: Biomechanics, Materials, Design and Manufacturing. Biomaterials 2019, 225, 119493. [Google Scholar] [CrossRef]
- Dabagh, M.; Abdekhodaie, M.J.; Khorasani, M.T. Effects of Polydimethylsiloxane Grafting on the Calcification, Physical Properties, and Biocompatibility of Polyurethane in a Heart Valve. J. Appl. Polym. Sci. 2005, 98, 758–766. [Google Scholar] [CrossRef]
- Santerre, J.P.; Woodhouse, K.; Laroche, G.; Labow, R.S. Understanding the Biodegradation of Polyurethanes: From Classical Implants to Tissue Engineering Materials. Biomaterials 2005, 26, 7457–7470. [Google Scholar] [CrossRef]
- Paturej, M.; el Fray, M. Syntheses of New Poly(Ester-Carbonate-Urethane)s Based on Trimethylene Carbonate (TMC) and Polyester Polyol Derived from Dimerized Fatty Acid. Polimery 2009, 54, 611–617. [Google Scholar] [CrossRef]
- Steiner, G. Dreidimensional Endlos Spritzgießen. Osterr. Kunstst. Z. 2012, 43, 8–10. [Google Scholar]
- Steiner, G.; Gerndorf, R. Kunststoff-Metall-Hybride Der Neuen Generation. Kunststoffe 2004, 7, 83–87. [Google Scholar]
- Steiner, G. Herstellung von Spritzgussteilen Mit Hochwertigen Oberflächen. VLK-News 2005, 3, 7–10. [Google Scholar]
- Steiner, G.; Eichler, H. EXJECTION®: Streets Ahead of Injection Moulding. Kunstst. Int. 2008, 4, 24–28. [Google Scholar]
- Steiner, G. Mobile Botschaften. Kunststoffe 2011, 4, 80–84. [Google Scholar]
- Mazurek, M.M.; Rokicki, G. Investigations on the Synthesis and Properties of Biodegradable Poly(Ester-Carbonate-Urea-Urethane)s. Pol. J. Chem. Technol. 2013, 15, 80–88. [Google Scholar] [CrossRef]
- Heinzler, F. Nutzbare Daten Aus Der Spritzgiessmaschine. Kunststoff 2014, 2, 52–56. [Google Scholar]
- Honkanen, M. Injection-Molded Hybrids-Characterization of Metal-Plastic Interfacial Features. Ph.D. Thesis, Tampere University of Technology, Tampere, Finland, 11 November 2011. [Google Scholar]
- Pisanu, L.; Santiago, L.C.; Barbosa, J.D.V.; Beal, V.E.; Nascimento, M.L.F. Effect of the Process Parameters on the Adhesive Strength of Dissimilar Polymers Obtained by Multicomponent Injection Molding. Polymers 2021, 13, 1039. [Google Scholar] [CrossRef] [PubMed]
- Yeh, R.-Y.; Hsu, R.-Q. Application of Porous Oxide Layer in Plastic/Metal Direct Adhesion by Injection Molding. J. Adhes. Sci. Technol. 2015, 29, 1617–1627. [Google Scholar] [CrossRef]
- Chen, J.-Y.; Hwang, S.-J. Application of Surface Treatments on Releasing Adhesion Force during Thermoplastic Polyurethane Injection Molding Process. Polym. Eng. Sci. 2017, 57, 299–305. [Google Scholar] [CrossRef]
- Major, R.; Plutecka, H.; Gruszczynska, A.; Lackner, J.M.; Major, B. Effects of the surface modification of polyurethane substrates on genotoxicity and blood activation processes. Mater Sci Eng C Mater Biol Appl 2017, 79, 756–762. [Google Scholar] [CrossRef]
- ISO. Metallic Materials—Instrumented Indentation Test for Hardness and Material Parameters—Part 1: Test Method; ISO: Geneva, Switzerland, 2015. [Google Scholar]
- Fischer-Cripps, A.C. Critical Review of Analysis and Interpretation of Nanoindentation Test Data. Surf. Coat. Technol. 2006, 200, 4153–4165. [Google Scholar] [CrossRef]
- Kot, M.; Lacki, P. Contact Mechanics of Coating-Substrate Systems. I. Methods of Analysis and FEM Modelling of Nanoindentation Tests. J. Balk. Tribol. Assoc. 2012, 18, 598–614. [Google Scholar]
- ISO. Fine Ceramics (Advanced Ceramics, Advanced Technical Ceramics)—Determination of Adhesion of Ceramic Coatings by Scratch Testing; ISO: Geneva, Switzerland, 2005. [Google Scholar]
- Bradford, M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Décavé, E.; Garrivier, D.; Bréchet, Y.; Fourcade, B.; Bruckert, F. Shear Flow-Induced Detachment Kinetics of Dictyostelium Discoideum Cells from Solid Substrate. Biophys. J. 2002, 82, 2383–2395. [Google Scholar] [CrossRef] [PubMed]
- Trembecka-Wójciga, K.; Kopernik, M.; Surmiak, M.; Major, R.; Gawlikowski, M.; Bruckert, F.; Kot, M.; Lackner, J.M. Effect of the Mechanical Properties of Carbon-Based Coatings on the Mechanics of Cell–Material Interactions. Colloids Surf. B Biointerfaces 2021, 197, 111359. [Google Scholar] [CrossRef] [PubMed]
- Sucosky, P.; Padala, M.; Elhammali, A.; Balachandran, K.; Jo, H.; Yoganathan, A.P. Design of an Ex Vivo Culture System to Investigate the Effects of Shear Stress on Cardiovascular Tissue. J. Biomech. Eng. 2008, 130, 035001. [Google Scholar] [CrossRef] [PubMed]
- Major, R.; Trembecka-Wójciga, K.; Lackner, J.M.; Plutecka, H. In Vitro Hemocompatibility of Thin Films Materials for Direct Blood Contact. Eng. Biomater. 2015, 18, 2–8. [Google Scholar]
- Brown, P.H.; Balbo, A.; Zhao, H.; Ebel, C.; Schuck, P. Density Contrast Sedimentation Velocity for the Determination of Protein Partial-Specific Volumes. PLoS ONE 2011, 6, e26221. [Google Scholar] [CrossRef]
- Lackner, J.M.; Waldhauser, W.; Hartmann, P.; Bruckert, F.; Weidenhaupt, M.; Major, R.; Sanak, M.; Wiesinger, M.; Heim, D. Hemocompatibility of Inorganic Physical Vapor Deposition (PVD) Coatings on Thermoplastic Polyurethane Polymers. J. Funct. Biomater. 2012, 3, 283–297. [Google Scholar] [CrossRef]
- Launder, B.E.; Spalding, D.B. The Numerical Computation of Turbulent Flows. Comput. Methods Appl. Mech. Eng. 1974, 3, 269–289. [Google Scholar] [CrossRef]
- Kopernik, M.; Tokarczyk, P. Development of Multi-Phase Models of Blood Flow for Medium-Sized Vessels with Stenosis. Acta Bioeng. Biomech. 2019, 21, 63–70. [Google Scholar] [CrossRef]
- Walburn, F.J.; Schneck, D.J. A Constitutive Equation for Whole Human Blood. Biorheology 1976, 13, 201–210. [Google Scholar] [CrossRef]
- Nikuradse, J. National Advisory Committee for Aeronautics Technical Memorandum, 1292, Laws of Flow in Rough Pipes; NACA: Washington, DC, USA, 1950.
- Sanak, M.; Jakieła, B.; Wegrzyn, W. Assessment of Hemocompatibility of Materials with Arterial Blood Flow by Platelet Functional Tests. Bull. Pol. Acad. Sci. Tech. Sci. 2010, 58, 317–322. [Google Scholar] [CrossRef][Green Version]
- Bongrand, P. Ligand-Receptor Interactions. Rep. Prog. Phys. 1999, 62, 921–968. [Google Scholar] [CrossRef]
- Albelda, S.M.; Buck, C.A. Integrins and Other Cell Adhesion Molecules. FASEB J. 1990, 4, 2868–2880. [Google Scholar] [PubMed]
- Takeuchi, T. Molecular Structure of the Tyrosinase Gene. Pigment. Cell Res. 2008, 3, 61–66. [Google Scholar] [CrossRef]
- Seyfert, U.T.; Biehl, V.; Schenk, J. In Vitro Hemocompatibility Testing of Biomaterials According to the ISO 10993-4. Biomol. Eng. 2002, 19, 91–96. [Google Scholar] [CrossRef]
- Streller, U.; Sperling, C.; Hübner, J.; Hanke, R.; Werner, C. Design and Evaluation of Novel Blood Incubation Systems for in Vitro Hemocompatibility Assessment of Planar Solid Surfaces. J. Biomed. Mater. Res. Part B Appl. Biomater. 2003, 66, 379–390. [Google Scholar] [CrossRef]
- Otto, M.; Klein, C.L.; Kohler, H.; Wagner, M.; Rohrig, O.; Kirkpatrick, C.J. Dynamic Blood Cell Contact with Biomaterials: Validation of a Flow Chamber System According to International Standards. J. Mater. Sci. Mater. Med. 1997, 8, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Pierrat, S.; Brochard-Wyart, F.; Nassoy, P. Enforced Detachment of Red Blood Cells Adhering to Surfaces: Statics and Dynamics. Biophys. J. 2004, 87, 2855–2869. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dahl, J.B.; Lin, J.-M.G.; Muller, S.J.; Kumar, S. Microfluidic Strategies for Understanding the Mechanics of Cells and Cell-Mimetic Systems. Annu. Rev. Chem. Biomol. Eng. 2015, 6, 293–317. [Google Scholar] [CrossRef]
- Yamamoto, S.; Kawana, A.; Ichimura, H.; Masuda, C. Relationship between Tribological Properties and Sp3/Sp2 Structure of Nitrogenated Diamond-like Carbon Deposited by Plasma CVD. Surf. Coat. Technol. 2012, 210, 1–9. [Google Scholar] [CrossRef]
- Herricks, T.; Avril, M.; Janes, J.; Smith, J.D.; Rathod, P.K. Clonal Variants of Plasmodium Falciparum Exhibit a Narrow Range of Rolling Velocities to Host Receptor CD36 under Dynamic Flow Conditions. Eukaryot. Cell 2013, 12, 1490–1498. [Google Scholar] [CrossRef]
- Song, J.W.; Cavnar, S.P.; Walker, A.C.; Luker, K.E.; Gupta, M.; Tung, Y.-C.; Luker, G.D.; Takayama, S. Microfluidic Endothelium for Studying the Intravascular Adhesion of Metastatic Breast Cancer Cells. PLoS ONE 2009, 4, e5756. [Google Scholar] [CrossRef]
- Schaff, U.Y.; Xing, M.M.Q.; Lin, K.K.; Pan, N.; Jeon, N.L.; Simon, S.I. Vascular Mimetics Based on Microfluidics for Imaging the Leukocyte–Endothelial Inflammatory Response. Lab Chip 2007, 7, 448–456. [Google Scholar] [CrossRef]
- Gutierrez, E.; Petrich, B.G.; Shattil, S.J.; Ginsberg, M.H.; Groisman, A.; Kasirer-Friede, A. Microfluidic Devices for Studies of Shear-Dependent Platelet Adhesion. Lab Chip 2008, 8, 1486. [Google Scholar] [CrossRef]
- Kucukal, E.; Little, J.A.; Gurkan, U.A. Shear Dependent Red Blood Cell Adhesion in Microscale Flow. Integr. Biol. 2018, 10, 194–206. [Google Scholar] [CrossRef]
- Hannan, R.T.; Peirce, S.M.; Barker, T.H. Fibroblasts: Diverse Cells Critical to Biomaterials Integration. ACS Biomater. Sci. Eng. 2018, 4, 1223–1232. [Google Scholar] [CrossRef]
- Ma, C.; Kuzma, M.L.; Bai, X.; Yang, J. Biomaterial-Based Metabolic Regulation in Regenerative Engineering. Adv. Sci. 2019, 6, 1900819. [Google Scholar] [CrossRef]
- Jansen, J.; von Recum, J. Textured and Porous Materials: Biomaterials Science: An Introduction to Materials in Medicine; Elsevier Science & Technology Books: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Rahmati, M.; Silva, E.A.; Reseland, J.E.; Heyward, C.A.; Haugen, H.J. Biological Responses to Physicochemical Properties of Biomaterial Surface. Chem. Soc. Rev. 2020, 49, 5178–5224. [Google Scholar] [CrossRef]
- Major, R.; Gawlikowski, M.; Plutecka, H.; Surmiak, M.; Kot, M.; Dyner, M.; Lackner, J.M.; Major, B. Biocompatibility Testing of Composite Biomaterial Designed for a New Petal Valve Construction for Pulsatile Ventricular Assist Device. J. Mater. Sci. Mater. Med. 2021, 32, 118. [Google Scholar] [CrossRef]
- Yoshimoto, Y.; Hasebe, T.; Nagashima, S.; Kamijo, A.; Nakatani, T.; Yamagami, T.; Kitamura, N.; Kitagawa, T.; Hotta, A.; Takahashi, K.; et al. Human Umbilical Vein Endothelial Cell Interaction with Fluorine-Incorporated Amorphous Carbon Films. Jpn. J. Appl. Phys. 2012, 51, 090129. [Google Scholar] [CrossRef]
- Derakhti, S.; Safiabadi-Tali, S.H.; Amoabediny, G.; Sheikhpour, M. Attachment and Detachment Strategies in Microcarrier-Based Cell Culture Technology: A Comprehensive Review. Mater. Sci. Eng. C 2019, 103, 109782. [Google Scholar] [CrossRef]
- Siddique, A.; Meckel, T.; Stark, R.W.; Narayan, S. Improved Cell Adhesion under Shear Stress in PDMS Microfluidic Devices. Colloids Surf. B Biointerfaces 2017, 150, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Buschmann, M.H.; Dieterich, P.; Adams, N.A.; Schnittler, H.-J. Analysis of Flow in a Cone-and-Plate Apparatus with Respect to Spatial and Temporal Effects on Endothelial Cells. Biotechnol. Bioeng. 2005, 89, 493–502. [Google Scholar] [CrossRef]
- Spruell, C.; Baker, A.B. Analysis of a High-Throughput Cone-and-Plate Apparatus for the Application of Defined Spatiotemporal Flow to Cultured Cells. Biotechnol. Bioeng. 2013, 110, 1782–1793. [Google Scholar] [CrossRef] [PubMed]


| Sample | Material | Coating Thickness [nm] | Gas Flow [sccm] | Deposition Method | |
|---|---|---|---|---|---|
| 1 | Si-a-C:H | 15.0 | 24.0:6.0 | Ar:C2H2 | SPU (PULSED) |
| 2 | a-C:H | 94.7 | 24.0:6.0:0 | Ar:C2H2:N2 | SPU (PULSED) |
| 3 | a-C:H:N | 110.3 | 17.5:2.5 | C2H2:N2 | ALS |
| 4 | Si-a-C:H | 124.3 | 28.2:1.8 | Ar:C2H2 | SPU (PULSED) |
| Sample | Time [min] | Critical Tress [Pa] | Spontaneous Detachment Rate [min−1] | Detachment Efficiency [%] | |||
|---|---|---|---|---|---|---|---|
| Erythrocytes | Platelets | Erythrocytes | Platelets | Erythrocytes | Platelets | ||
| 1 | 2.5 | 1.00 | 1.37 | 0.307 | 0.830 | 75.85 | 73.83 |
| 5.0 | 1.10 | 1.36 | 0.279 | 0.415 | 75.85 | 73.71 | |
| 7.5 | 1.94 | 0.98 | 0.306 | 0.010 | 70.35 | 76.30 | |
| 2 | 2.5 | 1.08 | 0.87 | 0.477 | 0.195 | 74.44 | 77.02 |
| 5.0 | 0.79 | 1.11 | 0.056 | 0.344 | 79.72 | 75.77 | |
| 7.5 | 0.84 | 0.83 | 0.050 | 0.030 | 78.53 | 77.41 | |
| 3 | 2.5 | 1.47 | 1.01 | 0.837 | 0.557 | 71.81 | 76.61 |
| 5.0 | 1.34 | 1.53 | 0.394 | 0.414 | 72.95 | 72.10 | |
| 7.5 | 1.00 | 0.65 | 0.147 | 0.043 | 76.41 | 83.68 | |
| 4 | 2.5 | 1.06 | 1.20 | 0.273 | 0.791 | 74.97 | 75.06 |
| 5.0 | 1.30 | 1.45 | 0.413 | 0.415 | 74.71 | 72.93 | |
| 7.5 | 0.90 | 1.07 | 0.147 | 0.229 | 77.17 | 76.68 | |
| Sample | Coating Thickness [nm] | Water Contact Angle | Hardness | Modulus of Elasticity | Critical Loads | Nucleotide Labeling | Critical Stress | Hemocompatibility |
|---|---|---|---|---|---|---|---|---|
| 1 | 15 | Average properties | Average properties | Average properties | Average properties | Average properties | Average properties | Average properties |
| 2 | 94.7 | Low value | The highest value among the SPU method | The lowest value | The best quality of the layer-substrate joint by the SPU method | The best properties | The lowest critical stress | The best properties |
| 3 | 110.3 | Average properties | The highest value among all methods | Average properties | The best quality of the layer-substrate joint among all methods | The best properties | The highest detachment efficiency | Average properties |
| 4 | 124.3 | The lowest value | Average properties | Average properties | Average properties | Average properties | Average properties | Average properties |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Major, R.; Wilczek, G.; Więcek, J.; Gawlikowski, M.; Plutecka, H.; Kasperkiewicz, K.; Kot, M.; Pomorska, M.; Ostrowski, R.; Kopernik, M. Hemocompatibile Thin Films Assessed under Blood Flow Shear Forces. Molecules 2022, 27, 5696. https://doi.org/10.3390/molecules27175696
Major R, Wilczek G, Więcek J, Gawlikowski M, Plutecka H, Kasperkiewicz K, Kot M, Pomorska M, Ostrowski R, Kopernik M. Hemocompatibile Thin Films Assessed under Blood Flow Shear Forces. Molecules. 2022; 27(17):5696. https://doi.org/10.3390/molecules27175696
Chicago/Turabian StyleMajor, Roman, Grażyna Wilczek, Justyna Więcek, Maciej Gawlikowski, Hanna Plutecka, Katarzyna Kasperkiewicz, Marcin Kot, Małgorzata Pomorska, Roman Ostrowski, and Magdalena Kopernik. 2022. "Hemocompatibile Thin Films Assessed under Blood Flow Shear Forces" Molecules 27, no. 17: 5696. https://doi.org/10.3390/molecules27175696
APA StyleMajor, R., Wilczek, G., Więcek, J., Gawlikowski, M., Plutecka, H., Kasperkiewicz, K., Kot, M., Pomorska, M., Ostrowski, R., & Kopernik, M. (2022). Hemocompatibile Thin Films Assessed under Blood Flow Shear Forces. Molecules, 27(17), 5696. https://doi.org/10.3390/molecules27175696







