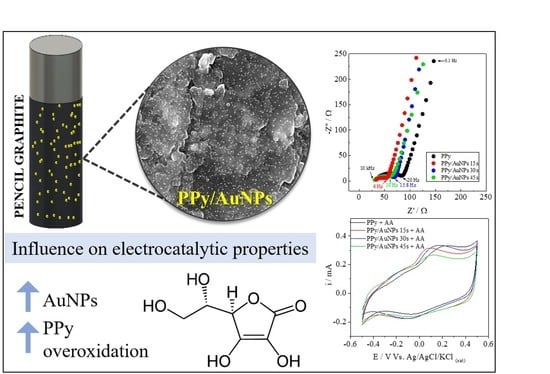
Interfacial Characterization of Polypyrrole/AuNP Composites towards Electrocatalysis of Ascorbic Acid Oxidation
Abstract
:1. Introduction
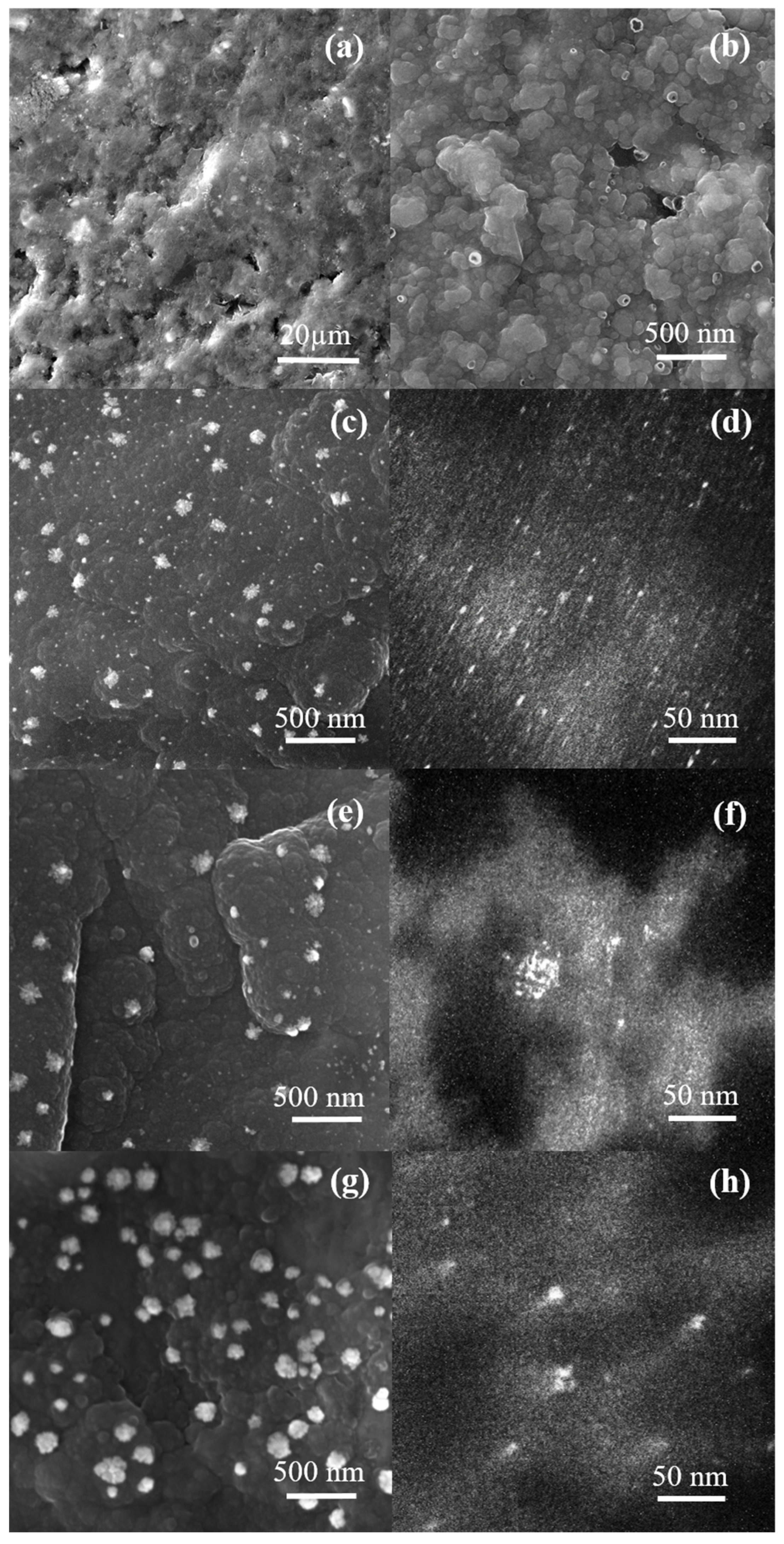
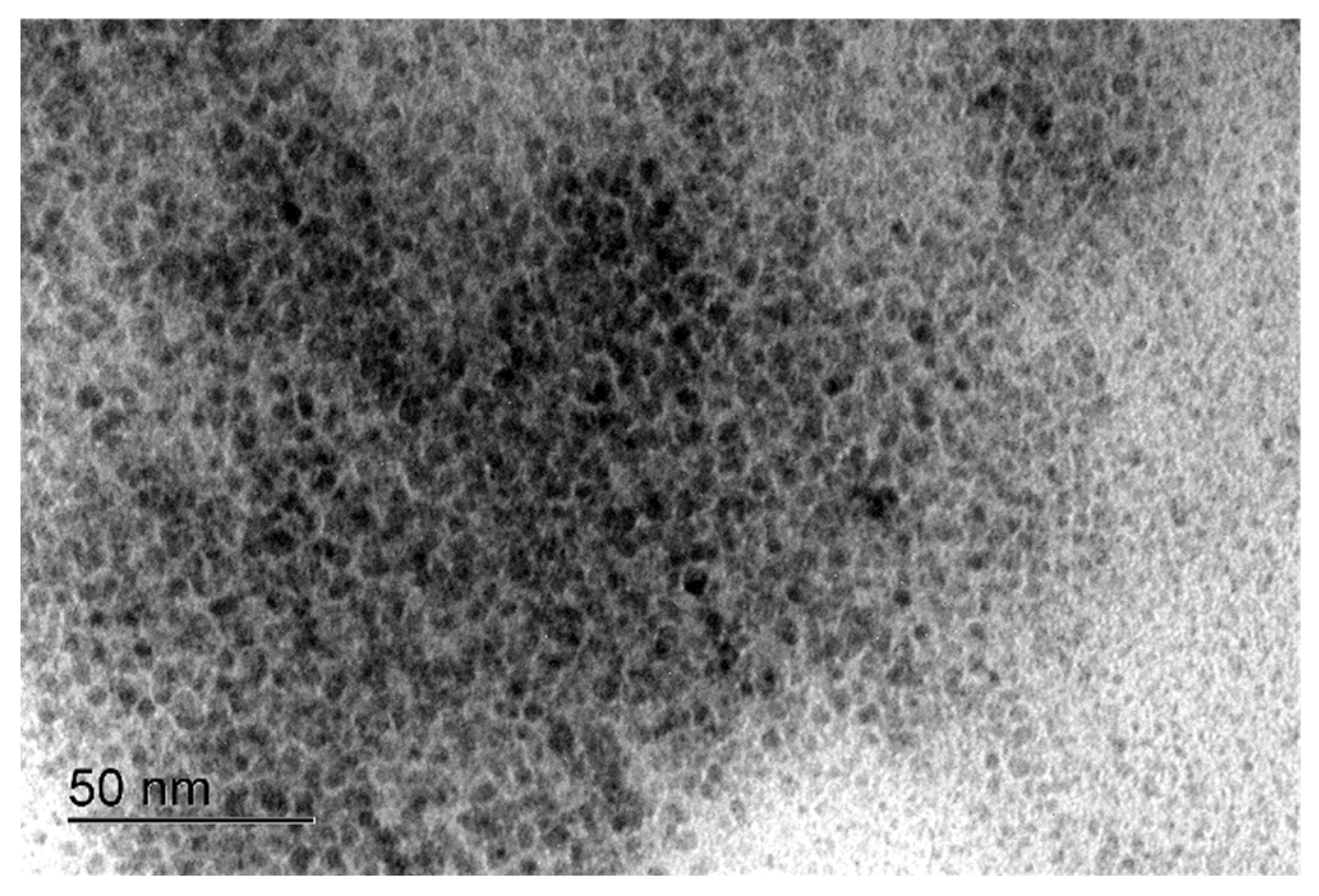
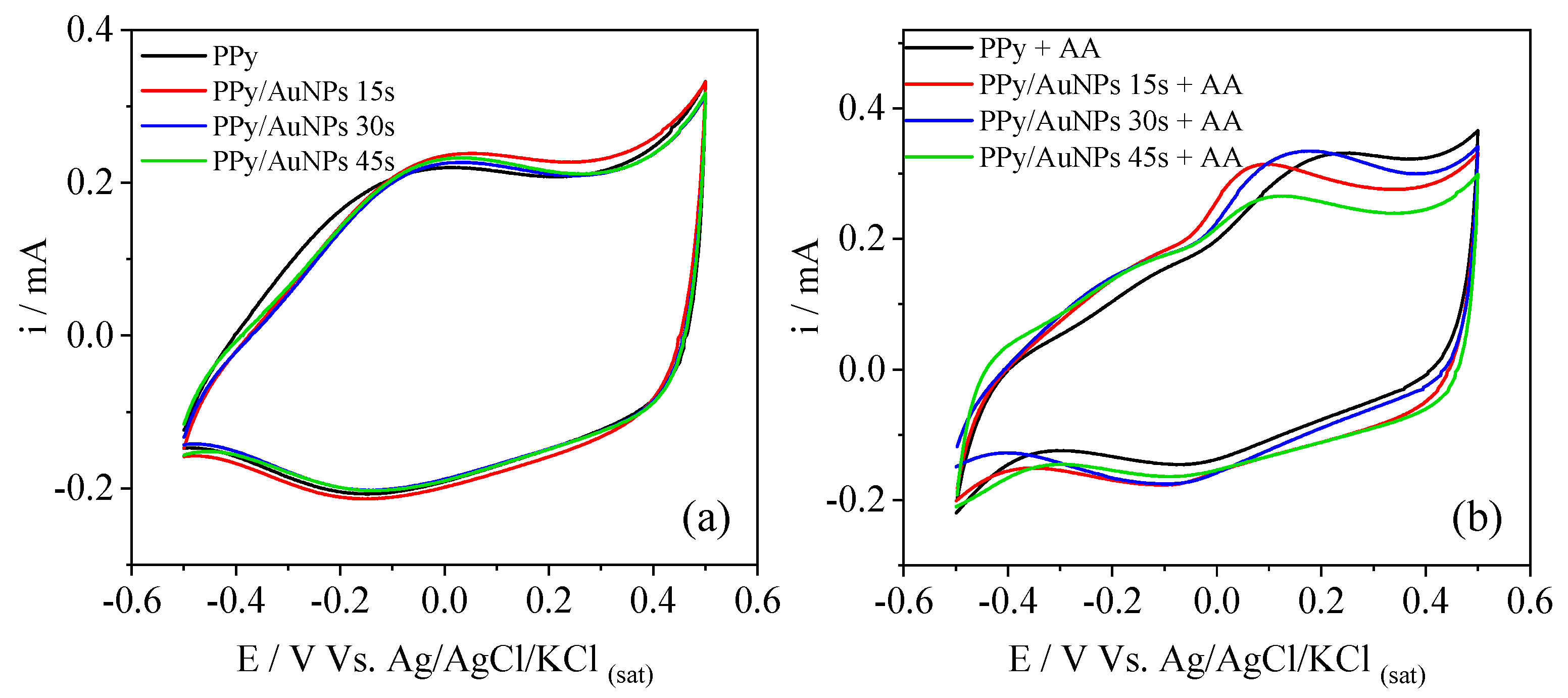
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Electrochemical Synthesis and Gold Reduction
3.3. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Namsheer, K.; Rout, C.S. Conducting Polymers: A Comprehensive Review on Recent Advances in Synthesis, Properties and Applications. RSC Adv. 2021, 11, 5659–5697. [Google Scholar] [CrossRef]
- Heeger, A.J. Semiconducting and Metallic Polymers: The Fourth Generation of Polymeric Materials. J. Phys. Chem. B 2001, 105, 8476–8491. [Google Scholar] [CrossRef]
- Bredas, J.L.; Street, G.B. Polarons, Bipolarons, and Solitons in Conducting Polymers. Acc. Chem. Res. 1985, 18, 309–315. [Google Scholar] [CrossRef]
- Wang, J.; Hui, N. Electrochemical Functionalization of Polypyrrole Nanowires for the Development of Ultrasensitive Biosensors for Detecting MicroRNA. Sens. Actuators B Chem. 2019, 281, 478–485. [Google Scholar] [CrossRef]
- Wang, T.; Farajollahi, M.; Choi, Y.S.; Lin, I.-T.; Marshall, J.E.; Thompson, N.M.; Kar-Narayan, S.; Madden, J.D.W.; Smoukov, S.K. Electroactive Polymers for Sensing. Interface Focus 2016, 6, 20160026. [Google Scholar] [CrossRef]
- Ibanez, J.G.; Rincón, M.E.; Gutierrez-Granados, S.; Chahma, M.; Jaramillo-Quintero, O.A.; Frontana-Uribe, B.A. Conducting Polymers in the Fields of Energy, Environmental Remediation, and Chemical–Chiral Sensors. Chem. Rev. 2018, 118, 4731–4816. [Google Scholar] [CrossRef]
- Xia, L.; Wei, Z.; Wan, M. Conducting Polymer Nanostructures and Their Application in Biosensors. J. Colloid Interface Sci. 2010, 341, 1–11. [Google Scholar] [CrossRef]
- Vidotti, M.; Dall’Antonia, L.H.; Córdoba de Torresi, S.I.; Bergamaski, K.; Nart, F.C. “On Line” Mass Spectrometric Detection of Ammonia Oxidation Products Generated by Polypyrrole Based Amperometric Sensors. Anal. Chim. Acta 2003, 489, 207–214. [Google Scholar] [CrossRef]
- Pandey, R.K.; Lakshminarayanan, V. Ethanol Electrocatalysis on Gold and Conducting Polymer Nanocomposites: A Study of the Kinetic Parameters. Appl. Catal. B Environ. 2012, 125, 271–281. [Google Scholar] [CrossRef]
- Li, W.; Wang, C.; Lu, X. Conducting Polymers-Derived Fascinating Electrocatalysts for Advanced Hydrogen and Oxygen Electrocatalysis. Coord. Chem. Rev. 2022, 464, 214555. [Google Scholar] [CrossRef]
- Hryniewicz, B.M.; Orth, E.S.; Vidotti, M. Enzymeless PEDOT-Based Electrochemical Sensor for the Detection of Nitrophenols and Organophosphates. Sens. Actuators B Chem. 2018, 257, 570–578. [Google Scholar] [CrossRef]
- Liu, F.; Yuan, Y.; Li, L.; Shang, S.; Yu, X.; Zhang, Q.; Jiang, S.; Wu, Y. Synthesis of Polypyrrole Nanocomposites Decorated with Silver Nanoparticles with Electrocatalysis and Antibacterial Property. Compos. Part B Eng. 2015, 69, 232–236. [Google Scholar] [CrossRef]
- Sapurina, I.; Stejskal, J.; Šeděnková, I.; Trchová, M.; Kovářová, J.; Hromádková, J.; Kopecká, J.; Cieslar, M.; Abu El-Nasr, A.; Ayad, M.M. Catalytic Activity of Polypyrrole Nanotubes Decorated with Noble-Metal Nanoparticles and Their Conversion to Carbonized Analogues. Synth. Met. 2016, 214, 14–22. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, K.; Wei, G.; Su, Z. Fabrication of Polypyrrole Nanoplates Decorated with Silver and Gold Nanoparticles for Sensor Applications. RSC Adv. 2015, 5, 69745–69752. [Google Scholar] [CrossRef]
- Xu, J.; Hu, J.; Ouan, B.; Wei, Z. Decorating Polypyrrole Nanotubes with Au Nanoparticles by an in Situ Reduction Process. Macromol. Rapid Commun. 2009, 30, 936–940. [Google Scholar] [CrossRef] [PubMed]
- Dursun, S.; Yavuz, E.; Çetinkaya, Z. In Situ Reduction of Chloroauric Acid (HAuCl4) for Generation of Catalytic Au Nanoparticle Embedded Triazine Based Covalent Organic Polymer Networks. RSC Adv. 2019, 9, 38538–38546. [Google Scholar] [CrossRef]
- Amatore, C.; Arbault, S.; Ferreira, D.C.M.; Tapsoba, I.; Verchier, Y. Vitamin C Stimulates or Attenuates Reactive Oxygen and Nitrogen Species (ROS, RNS) Production Depending on Cell State: Quantitative Amperometric Measurements of Oxidative Bursts at PLB-985 and RAW 264.7 Cells at the Single Cell Level. J. Electroanal. Chem. 2008, 615, 34–44. [Google Scholar] [CrossRef]
- Kaźmierczak-Barańska, J.; Boguszewska, K.; Adamus-Grabicka, A.; Karwowski, B.T. Two Faces of Vitamin c—Antioxidative and pro-Oxidative Agent. Nutrients 2020, 12, 1501. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Serban, A.I.; Fafaneata, C. Electrochemical Methods for Ascorbic Acid Determination. Electrochim. Acta 2014, 121, 443–460. [Google Scholar] [CrossRef]
- Malinauskas, A.; Garjonyte, R.; Mažeikiene, R.; Jurevičiute, I. Electrochemical Response of Ascorbic Acid at Conducting and Electrogenerated Polymer Modified Electrodes for Electroanalytical Applications: A Review. Talanta 2004, 64, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Madrakian, T.; Haghshenas, E.; Afkhami, A. Simultaneous Determination of Tyrosine, Acetaminophen and Ascorbic Acid Using Gold Nanoparticles/Multiwalled Carbon Nanotube/Glassy Carbon Electrode by Differential Pulse Voltammetric Method. Sens. Actuators B Chem. 2014, 193, 451–460. [Google Scholar] [CrossRef]
- Curulli, A. Nanomaterials in Electrochemical Sensing Area: Applications and Challenges in Food Analysis. Molecules 2020, 25, 5759. [Google Scholar] [CrossRef] [PubMed]
- Phong, N.H.; Toan, T.T.T.; Tinh, M.X.; Tuyen, T.N.; Mau, T.X.; Khieu, D.Q. Simultaneous Voltammetric Determination of Ascorbic Acid, Paracetamol, and Caffeine Using Electrochemically Reduced Graphene-Oxide-Modified Electrode. J. Nanomater. 2018, 2018, 5348016. [Google Scholar] [CrossRef]
- Maouche, N.; Nessark, B.; Bakas, I. Platinum Electrode Modified with Polyterthiophene Doped with Metallic Nanoparticles, as Sensitive Sensor for the Electroanalysis of Ascorbic Acid (AA). Arab. J. Chem. 2019, 12, 2556–2562. [Google Scholar] [CrossRef]
- Demirkan, B.; Bozkurt, S.; Cellat, K.; Arıkan, K.; Yılmaz, M.; Şavk, A.; Çalımlı, M.H.; Nas, M.S.; Atalar, M.N.; Alma, M.H.; et al. Palladium Supported on Polypyrrole/Reduced Graphene Oxide Nanoparticles for Simultaneous Biosensing Application of Ascorbic Acid, Dopamine, and Uric Acid. Sci. Rep. 2020, 10, 2946. [Google Scholar] [CrossRef] [PubMed]
- Özcan, L.; Şahin, M.; Şahin, Y. Electrochemical Preparation of a Molecularly Imprinted Polypyrrole-Modified Pencil Graphite Electrode for Determination of Ascorbic Acid. Sensors 2008, 8, 5792–5805. [Google Scholar] [CrossRef]
- Ghanbari, K.; Hajian, A. Electrochemical Characterization of Au/ZnO/PPy/RGO Nanocomposite and Its Application for Simultaneous Determination of Ascorbic Acid, Epinephrine, and Uric Acid. J. Electroanal. Chem. 2017, 801, 466–479. [Google Scholar] [CrossRef]
- Ghanbari, K.; Bonyadi, S. Modified Glassy Carbon Electrode with Polypyrrole Nanocomposite for the Simultaneous Determination of Ascorbic Acid, Dopamine, Uric Acid, and Folic Acid. J. Electrochem. Sci. Technol. 2020, 11, 68–83. [Google Scholar] [CrossRef]
- Vidotti, M.; Dall’Antonia, L.H.; Cintra, E.P.; Córdoba de Torresi, S.I. Reduction of Interference Signal of Ascorbate and Urate in Poly(Pyrrole)-Based Ammonia Sensors in Aqueous Solutions. Electrochim. Acta 2004, 49, 3665–3670. [Google Scholar] [CrossRef]
- Zakerian, R.; Bahar, S. Electrochemical Exfoliation of Pencil Graphite for Preparation of Graphene Coating as a New Versatile SPME Fiber for Determination of Polycyclic Aromatic Hydrocarbons by Gas Chromatography. Microchim. Acta 2019, 186, 861. [Google Scholar] [CrossRef] [PubMed]
- Uygun, Z.O.; Dilgin, Y. A Novel Impedimetric Sensor Based on Molecularly Imprinted Polypyrrole Modified Pencil Graphite Electrode for Trace Level Determination of Chlorpyrifos. Sens. Actuators B Chem. 2013, 188, 78–84. [Google Scholar] [CrossRef]
- Christensen, P.A.; Hamnett, A. In Situ Spectroscopic Investigations of the Growth, Electrochemical Cycling and Overoxidation of Polypyrrole in Aqueous Solution. Electrochim. Acta 1991, 36, 1263–1286. [Google Scholar] [CrossRef]
- Li, Y.; Qian, R. Electrochemical Overoxidation of Conducting Polypyrrole Nitrate Film in Aqueous Solutions. Electrochim. Acta 2000, 45, 1727–1731. [Google Scholar] [CrossRef]
- Maruthamuthu, S.; Chandrasekaran, J.; Manoharan, D.; Magesh, R. Conductivity and Dielectric Analysis of Nanocolloidal Polypyrrole Particles Functionalized with Higher Weight Percentage of Poly(Styrene Sulfonate) Using the Dispersion Polymerization Method. J. Polym. Eng. 2017, 37, 481–492. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Liu, Z.; Robinson, C. Inorganic/Organic Mesostructure Directed Synthesis of Wire/Ribbon-like Polypyrrole Nanostructures. Chem. Commun. 2004, 1852–1853. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Peng, H.; Wang, W.; Liu, T. Synthesis and Characterization of Polypyrrole Composites for Corrosion Protection of Steel. J. Appl. Polym. Sci. 2010, 116, 1524–1537. [Google Scholar] [CrossRef]
- Jang, J.; Yoon, H. Facile Fabrication of Polypyrrole Nanotubes Using Reverse Microemulsion Polymerization. Chem. Commun. 2003, 6, 720–721. [Google Scholar] [CrossRef]
- Lyons, M.E.G.; Breen, W.; Cassidy, J. Ascorbic acid oxidation at polypyrrole-coated electrodes. J. Chem. Soc. Faraday Trans. 1991, 87, 115–123. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Wang, Z.; Wang, S. Electrocatalytic Oxidation of Ascorbic Acid at Polypyrrole Nanowire Modified Electrode. Synth. Met. 2006, 156, 610–613. [Google Scholar] [CrossRef]
- Hughes, D.E. Irreversible Reaction Kinetics of the Aerobic Oxidation of Ascorbic Acid. Anal. Chem. 1985, 57, 555–558. [Google Scholar] [CrossRef]
- Sivanesan, A.; Kannan, P.; Abraham John, S. Electrocatalytic Oxidation of Ascorbic Acid Using a Single Layer of Gold Nanoparticles Immobilized on 1,6-Hexanedithiol Modified Gold Electrode. Electrochim. Acta 2007, 52, 8118–8124. [Google Scholar] [CrossRef]
- Shakkthivel, P.; Chen, S.M. Simultaneous Determination of Ascorbic Acid and Dopamine in the Presence of Uric Acid on Ruthenium Oxide Modified Electrode. Biosens. Bioelectron. 2007, 22, 1680–1687. [Google Scholar] [CrossRef] [PubMed]
- Max, J.J.; Chapados, C. Infrared Spectroscopy of Aqueous Carboxylic Acids: Comparison between Different Acids and Their Salts. J. Phys. Chem. A 2004, 108, 3324–3337. [Google Scholar] [CrossRef]
- García-Hernández, C.; García-Cabezón, C.; Medina-Plaza, C.; Martín-Pedrosa, F.; Blanco, Y.; de Saja, J.A.; Rodríguez-Méndez, M.L. Electrochemical Behavior of Polypyrrol/AuNP Composites Deposited by Different Electrochemical Methods: Sensing Properties towards Catechol. Beilstein J. Nanotechnol. 2015, 6, 2052–2061. [Google Scholar] [CrossRef] [PubMed]
- Kannan, A.; Radhakrishnan, S. Fabrication of an Electrochemical Sensor Based on Gold Nanoparticles Functionalized Polypyrrole Nanotubes for the Highly Sensitive Detection of L-Dopa. Mater. Today Commun. 2020, 25, 101330. [Google Scholar] [CrossRef]
- Bisquert, J. Theory of the Impedance of Electron Diffusion and Recombination in a Thin Layer. J. Phys. Chem. B 2002, 106, 325–333. [Google Scholar] [CrossRef]
- Garcia-Belmonte, G.; Bisquert, J. Impedance Analysis of Galvanostatically Synthesized Polypyrrole Films. Correlation of Ionic Diffusion and Capacitance Parameters with the Electrode Morphology. Electrochim. Acta 2002, 47, 4263–4272. [Google Scholar] [CrossRef]
- Marchesi, L.F.; Jacumasso, S.C.; Quintanilha, R.C.; Winnischofer, H.; Vidotti, M. The Electrochemical Impedance Spectroscopy Behavior of Poly(Aniline) Nanocomposite Electrodes Modified by Layer-by-Layer Deposition. Electrochim. Acta 2015, 174, 864–870. [Google Scholar] [CrossRef]
- Soares, A.L.; Zamora, M.L.; Marchesi, L.F.; Vidotti, M. Adsorption of Catechol onto PEDOT Films Doped with Gold Nanoparticles: Electrochemical and Spectroscopic Studies. Electrochim. Acta 2019, 322, 134773. [Google Scholar] [CrossRef]
- Hryniewicz, B.M.; Lima, R.V.; Wolfart, F.; Vidotti, M. Influence of the PH on the Electrochemical Synthesis of Polypyrrole Nanotubes and the Supercapacitive Performance Evaluation. Electrochim. Acta 2019, 293, 447–457. [Google Scholar] [CrossRef]


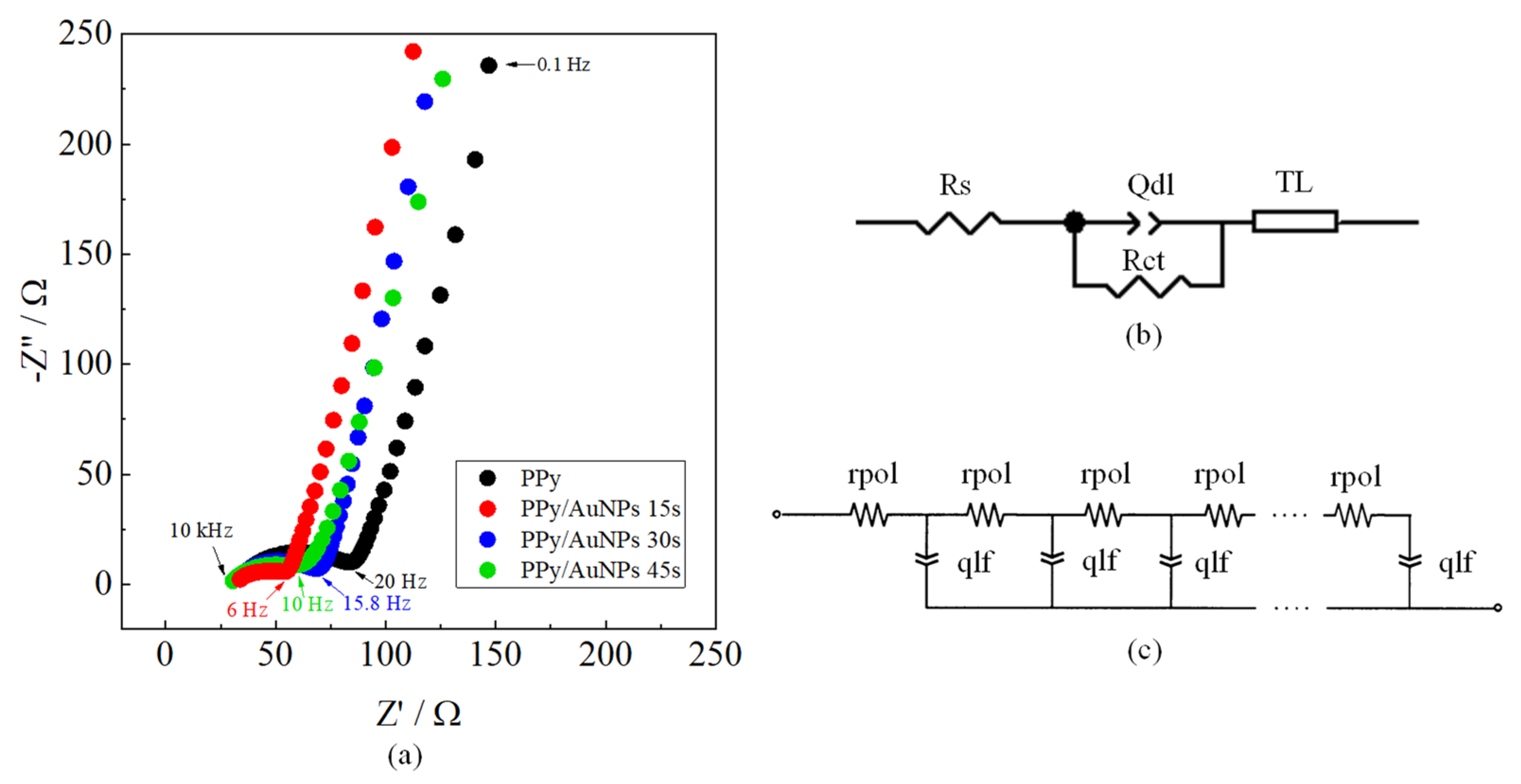
| Electrode | Rs/Ω | Rct/Ω | Qdl/mF sn−1 | Ndl | Rpol/Ω | Qlf/mF sn−1 | Nlf |
|---|---|---|---|---|---|---|---|
| PPy | 31.73 | 47.93 | 0.170 | 0.64 | 23.98 | 6.09 | 0.827 |
| PPy/AuNPs 15 s | 30.69 | 24.38 | 0.816 | 0.514 | 8.176 | 6.00 | 0.848 |
| PPy/AuNPs 30 s | 30.88 | 34.36 | 0.154 | 0.645 | 21.31 | 6.65 | 0.861 |
| PPy/AuNPs 45 s | 29.18 | 24.12 | 0.487 | 0.634 | 39.97 | 6.38 | 0.832 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pesqueira, C.; Hryniewicz, B.M.; Bach-Toledo, L.; Tenório, L.N.; Marchesi, L.F.; Mazon, T.; Vidotti, M. Interfacial Characterization of Polypyrrole/AuNP Composites towards Electrocatalysis of Ascorbic Acid Oxidation. Molecules 2022, 27, 5776. https://doi.org/10.3390/molecules27185776
Pesqueira C, Hryniewicz BM, Bach-Toledo L, Tenório LN, Marchesi LF, Mazon T, Vidotti M. Interfacial Characterization of Polypyrrole/AuNP Composites towards Electrocatalysis of Ascorbic Acid Oxidation. Molecules. 2022; 27(18):5776. https://doi.org/10.3390/molecules27185776
Chicago/Turabian StylePesqueira, Camila, Bruna M. Hryniewicz, Larissa Bach-Toledo, Luciane Novaes Tenório, Luís F. Marchesi, Talita Mazon, and Marcio Vidotti. 2022. "Interfacial Characterization of Polypyrrole/AuNP Composites towards Electrocatalysis of Ascorbic Acid Oxidation" Molecules 27, no. 18: 5776. https://doi.org/10.3390/molecules27185776
APA StylePesqueira, C., Hryniewicz, B. M., Bach-Toledo, L., Tenório, L. N., Marchesi, L. F., Mazon, T., & Vidotti, M. (2022). Interfacial Characterization of Polypyrrole/AuNP Composites towards Electrocatalysis of Ascorbic Acid Oxidation. Molecules, 27(18), 5776. https://doi.org/10.3390/molecules27185776