Ultrasound-Assisted Extraction of Flavonoids from Potentilla fruticosa L. Using Natural Deep Eutectic Solvents
Abstract
:1. Introduction
2. Experimental Methods
2.1. Chemicals and Plant Material
2.2. Synthesis of NADESs
2.3. Ultrasonic Extraction and Microstructure of Plant Material
2.4. HPLC Analysis
2.5. Response Surface Methodology
2.6. Determination of Antioxidant Capacity
2.7. Determination of Hypoglycemic Capacity
3. Results and Discussion
3.1. Preparation and Physicochemical Properties of DES
3.2. Extraction of Flavonoids Using DES Aqueous Solution
3.3. Effects of Water Content and Ultrasound-Assisted Extraction Conditions
3.4. Response Surface Methodology
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Xu, X.Y.; Qin, Y.Y.; Cao, J.J. Variation characteristics of Potentilla bifurca leaf stochiometry along the elevation gradient on the northeastern Qinghai-Tibetan Plateau. Acta Ecol. Sin. 2019, 39, 9044–9051. [Google Scholar]
- Li, X.; Li, Y. The complete chloroplast genome sequence of Potentilla bifurca L. Mitochondrial DNA Part B 2022, 7, 753–754. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Sun, Y.X.; Meng, X.H.; Yu, T.; Zhu, H.T.; Zhang, Y.J. A new methylene bisflavan-3-ol from the branches and leaves of Potentilla fruticose. Nat. Prod. Res. 2020, 34, 1238–1245. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, Z.; Guan, X.; Hu, Z.; Zhang, Z.; Zheng, J.; Lu, Y. Proteomic analysis of Potentilla fruticosa L. leaves by iTRAQ reveals responses to heat stress. PLoS ONE 2017, 12, e0182917. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.H.; Wang, D.M.; Fan, S.F.; Li, D.W.; Luo, Z.W. Synergistic effects and related bioactive mechanism of Potentilla fruticosa L. leaves combined with Ginkgo biloba extracts studied with microbial test system (MTS). BMC Complement. Altern. Med. 2016, 16, 495. [Google Scholar] [CrossRef]
- Deng, X.; Shang, H.; Chen, J.; Wu, J.; Wang, T.; Wang, Y.; Zhu, C.; Sun, W. Metabolomics Combined with Proteomics Provide a Novel Interpretation of the Changes in Flavonoid Glycosides during White Tea Processing. Foods 2022, 11, 1226. [Google Scholar] [CrossRef]
- Bruno, M.R.; Russo, D.; Cetera, P.; Faraone, I.; Lo Giudice, V.; Milella, L.; Todaro, L.; Sinisgalli, C.; Fritsch, C.; Dumarçay, S.; et al. Chemical analysis and antioxidant properties of orange-tree (Citrus sinensis L.) biomass extracts obtained via different extraction techniques. Biofuels Bioprod. Biorefining 2020, 14, 509–520. [Google Scholar] [CrossRef]
- Faraone, I.; Russo, D.; D’Auria, M.; Bruno, M.R.; Cetera, P.; Todaro, L.; Milella, L. Influence of thermal modification and extraction techniques on yield, antioxidant capacity and phytochemical profile of chestnut (Castanea sativa Mill.) wood. Holzforschung 2021, 75, 260–268. [Google Scholar] [CrossRef]
- Bruno, M.R.; Cetera, P.; Giudice, V.L.; Gerardin, P. Bioactive compounds achieved from residues of the apricot tree (Prunus armeniaca L.) and olive tree (Olea europaea L.): New prospective in the industrial sector. In Proceedings of the 29th European Biomass Conference and Exhibition, Marseille, France, 26–29 April 2021; pp. 186–190. [Google Scholar]
- Gavahian, M.; Farahnaky, A.; Farhoosh, R.; Javidnia, K.; Shahidi, F. Extraction of essential oils from Mentha piperita using advanced techniques: Microwave versus ohmic assisted hydrodistillation. Food Bioprod. Process. 2015, 94, 50–58. [Google Scholar] [CrossRef]
- Das, A.K.; Islam, N.; Faruk, O.; Ashaduzzaman; Dungani, R. Review on tannins: Extraction processes, applications and possibilities. South Afr. J. Bot. 2020, 135, 58–70. [Google Scholar] [CrossRef]
- Shi, X.D.; Nie, S.P.; Yin, J.Y.; Que, Z.Q.; Zhang, L.J.; Huang, X.J. Polysaccharide from leaf skin of Aloe barbadensis Miller: Part, I. Extraction, fractionation, physicochemical properties and structural characterization. Food Hydrocolloid 2017, 73, 176–183. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, J.; Zhang, H.; Dzah, C.S.; Zandile, M.; Duan, Y.; Ma, H.; Luo, X. Advances in ultrasound assisted extraction of bioactive compounds from cash crops—A review. Ultrason. Sonochem. 2018, 48, 538–549. [Google Scholar] [CrossRef]
- Wang, L.; Lu, W.; Li, J.; Hu, J.; Ding, R.; Lv, M.; Wang, Q. Optimization of Ultrasonic-Assisted Extraction and Purification of Zeaxanthin and Lutein in Corn Gluten Meal. Molecules 2019, 24, 2994. [Google Scholar] [CrossRef]
- Aryanti, N.; Wardhani, D.H.; Wasi, A.; Al Ramadhan, G.; Purbasari, A. Extraction Characteristics of Anthocyanin from Roselle (Hibiscus sabdariffa L.) Calyces by Ultrasound-Assisted Extraction. Adv. Sci. Lett. 2017, 23, 5626–5628. [Google Scholar] [CrossRef]
- Tao, Y.; Sun, D.-W. Enhancement of Food Processes by Ultrasound: A Review. Crit. Rev. Food Sci. Nutr. 2014, 55, 570–594. [Google Scholar] [CrossRef] [PubMed]
- Boltic, Z.; Ruzic, N.; Jovanovic, M.; Savic, M.; Jovanovic, J.; Petrovic, S. Cleaner production aspects of tablet coating process in pharmaceutical industry: Problem of VOCs emission. J. Clean. Prod. 2013, 44, 123–132. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Unconventional Deep Eutectic Solvents: Aqueous Salt Hydrates. Chem. Commun. 2003, 1, 70–71. [Google Scholar] [CrossRef]
- Zhang, Q.; Vigier, K.D.; Royer, S.; Jérôme, F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef]
- Ge, X.; Gu, C.; Wang, X.; Tu, J. Deep eutectic solvents (DESs)-derived advanced functional materials for energy and environmental applications: Challenges, opportunities, and future vision. J. Mater. Chem. A 2017, 5, 8209–8229. [Google Scholar] [CrossRef]
- Hou, Y.; Yao, C.; Wu, W. Deep Eutectic Solvents: Green Solvents for Separation Applications. Acta Physico-Chim. Sin. 2018, 34, 873–885. [Google Scholar] [CrossRef]
- Florindo, C.; Branco, L.; Marrucho, I. Development of hydrophobic deep eutectic solvents for extraction of pesticides from aqueous environments. Fluid Phase Equilibria 2017, 448, 135–142. [Google Scholar] [CrossRef]
- Li, X.X.; Kyung, H.R. Development of deep eutectic solvents applied in extraction and separation. J. Sep. Sci. 2016, 39, 3505–3520. [Google Scholar] [CrossRef]
- Hayyan, M.; Hashim, M.A.; Hayyan, A.; Al-Saadi, M.A.; AlNashef, I.M.; Mirghani, M.E.; Saheed, O.K. Are deep eutectic solvents benign or toxic? Chemosphere 2013, 90, 2193–2195. [Google Scholar] [CrossRef]
- Hayyan, M.; Hashim, M.A.; Al-Saadi, M.A.; Hayyan, A.; AlNashef, I.M.; Mirghani, M.E. Assessment of cytotoxicity and toxicity for phosphonium-based deep eutectic solvents. Chemosphere 2013, 93, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K.M.; Lee, M.S.; Nam, M.W.; Zhao, J.; Jin, Y.; Lee, D.-K.; Kwon, S.W.; Jeong, J.H.; Lee, J. Tailoring and recycling of deep eutectic solvents as sustainable and efficient extraction media. J. Chromatogr. A 2015, 1424, 10–17. [Google Scholar] [CrossRef]
- Li, Y.; Pan, Z.; Wang, B.; Yu, W.; Song, S.; Feng, H.; Zhao, W.; Zhang, J. Ultrasound-assisted extraction of bioactive alkaloids from Phellodendri amurensis cortex using deep eutectic solvent aqueous solutions. New J. Chem. 2020, 44, 9172–9178. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Morais, E.S.; Leite, A.C.; Mohamadou, A.; Holmbom, B.; Holmbom, T.; Neves, B.M.; Coutinho, J.A.P.; Freire, M.G.; Silvestre, A.J.D. Enhanced extraction and biological activity of 7-hydroxymatairesinol obtained from Norway spruce knots using aqueous solutions of ionic liquids. Green Chem. 2017, 19, 2626–2635. [Google Scholar] [CrossRef]
- Kosanić, M.; Ranković, B.; Vukojević, J. Antioxidant properties of some lichen species. J. Food Sci. Tech. 2011, 48, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Vieira, V.; Prieto, M.A.; Barros, L.; Coutinho, J.A.; Ferreira, I.C.; Ferreira, O. Enhanced extraction of phenolic compounds using choline chloride based deep eutectic solvents from Juglans regia L. Ind. Crop. Prod. 2018, 115, 261–271. [Google Scholar] [CrossRef]
- Patil, J.; Ghodke, S.; Jain, R.; Dandekar, P. Extraction of Vitamin D from Button Mushroom (Agaricus bisporus) Using Deep Eutectic Solvent and Ultrasonication. ACS Sustain. Chem. Eng. 2018, 6, 10578–10586. [Google Scholar] [CrossRef]
- Li, Y.; Hsieh, Y.-H.; Pan, Z.; Zhang, L.; Yu, W.; Wang, B.; Zhang, J. Extraction of Alkaloids from Coptidis Rhizoma via Betaine-Based Deep Eutectic Solvents. ChemistrySelect 2020, 5, 4973–4978. [Google Scholar] [CrossRef]
- Cao, J.; Yang, M.; Cao, F.; Wang, J.; Su, E. Well-Designed Hydrophobic Deep Eutectic Solvents As Green and Efficient Media for the Extraction of Artemisinin from Artemisia annua Leaves. ACS Sustain. Chem. Eng. 2017, 5, 3270–3278. [Google Scholar] [CrossRef]
- Qi, X.-L.; Peng, X.; Huang, Y.-Y.; Li, L.; Wei, Z.-F.; Zu, Y.-G.; Fu, Y.-J. Green and efficient extraction of bioactive flavonoids from Equisetum palustre L. by deep eutectic solvents-based negative pressure cavitation method combined with macroporous resin enrichment. Ind. Crop. Prod. 2015, 70, 142–148. [Google Scholar] [CrossRef]
- Lores, H.; Romero, V.; Costas, I.; Bendicho, C.; Lavilla, I. Natural deep eutectic solvents in combination with ultrasonic energy as a green approach for solubilisation of proteins: Application to gluten determination by immunoassay. Talanta 2017, 162, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Duan, M.-H.; Yao, X.-H.; Zhang, Y.-H.; Zhao, C.-J.; Zu, Y.-G.; Fu, Y.-J. Green extraction of five target phenolic acids from Lonicerae japonicae Flos with deep eutectic solvent. Sep. Purif. Technol. 2016, 157, 249–257. [Google Scholar] [CrossRef]
- Espino, M.; de los Ángeles Fernández, M.; Gomez, F.J.; Silva, M.F. Natural designer solvents for greening analytical chemistry. Trends Anal. Chem. 2016, 76, 126–136. [Google Scholar] [CrossRef]
- Hsieh, Y.-H.; Li, Y.; Pan, Z.; Chen, Z.; Lu, J.; Yuan, J.; Zhu, Z.; Zhang, J. Ultrasonication-assisted synthesis of alcohol-based deep eutectic solvents for extraction of active compounds from ginger. Ultrason. Sonochemistry 2019, 63, 104915. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Y.; Wang, M.; Bi, W.; Li, H.; Chen, D.D.Y. High-Throughput Analysis for Artemisinins with Deep Eutectic Solvents Mechanochemical Extraction and Direct Analysis in Real Time Mass Spectrometry. Anal. Chem. 2018, 90, 3109–3117. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, B.; Parkinson, C.; Miquel, M. Deterpenation of Citrus Essential Oils Using Glycerol-Based Deep Eutectic Solvents. Sep. Purif. Technol. 2018, 206, 1–13. [Google Scholar] [CrossRef]
- Kou, X.; Ke, Y.; Wang, X.; Rahman, R.T.; Xie, Y.; Chen, S.; Wang, H. Simultaneous extraction of hydrophobic and hydrophilic bioactive compounds from ginger (Zingiber officinale Roscoe). Food Chem. 2018, 257, 223–229. [Google Scholar] [CrossRef]
- Huang, J.; Guo, X.; Xu, T.; Fan, L.; Zhou, X.; Wu, S. Ionic deep eutectic solvents for the extraction and separation of natural products. J. Chromatogr. A 2019, 1598, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Van Osch, D.J.; Dietz, C.H.; Van Spronsen, J.; Kroon, M.C.; Gallucci, F.; van Sint Annaland, M.; Tuinier, R. A search for natural hydrophobic deep eutectic solvents based on natural components. ACS Sustain. Chem. Eng. 2019, 7, 2933–2942. [Google Scholar] [CrossRef]
- Manukovskaya, M.V.; Shchetilina, I.P.; Pisklyukova, Y.N.; Klimova, E.A.; Korystin, M.I. The use of ultrasonic extraction in the technology of functional drinks based on plant raw materials. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2021; Volume 845, p. 012114. [Google Scholar] [CrossRef]
- Liu, J.; Li, C.; Ding, G.; Quan, W. Artificial Intelligence Assisted Ultrasonic Extraction of Total Flavonoids from Rosa sterilis. Molecules 2021, 26, 3835. [Google Scholar] [CrossRef]
- Dorta, E.; Lobo, M.G.; González, M. Using drying treatments to stabilise mango peel and seed: Effect on antioxidant activity. LWT 2012, 45, 261–268. [Google Scholar] [CrossRef]
- González-Montelongo, R.; Lobo, M.G.; González, M. The effect of extraction temperature, time and number of steps on the antioxidant capacity of methanolic banana peel extracts. Sep. Purif. Technol. 2010, 71, 347–355. [Google Scholar] [CrossRef]
- Şahin, S.; Aybastıer, Ö.; Işık, E. Optimisation of ultrasonic-assisted extraction of antioxidant compounds from Artemisia absinthium using response surface methodology. Food Chem. 2013, 141, 1361–1368. [Google Scholar] [CrossRef]
- Duan, L.; Dou, L.-L.; Guo, L.; Li, P.; Liu, E.-H. Comprehensive Evaluation of Deep Eutectic Solvents in Extraction of Bioactive Natural Products. ACS Sustain. Chem. Eng. 2016, 4, 2405–2411. [Google Scholar] [CrossRef]
- Xu, J.; Wang, W.; Liang, H.; Zhang, Q.; Li, Q. Optimization of ionic liquid based ultrasonic assisted extraction of antioxidant compounds from Curcuma longa L. using response surface methodology. Ind. Crop. Prod. 2015, 76, 487–493. [Google Scholar] [CrossRef]
- Zhao, D.; Kai, J.; Bin Zhao, T.; Qiao, C. Study on Optimization of extraction process of Lycium barbarum L. polyphenols by Response Surface Methodology. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2020; Volume 531, p. 012069. [Google Scholar] [CrossRef]
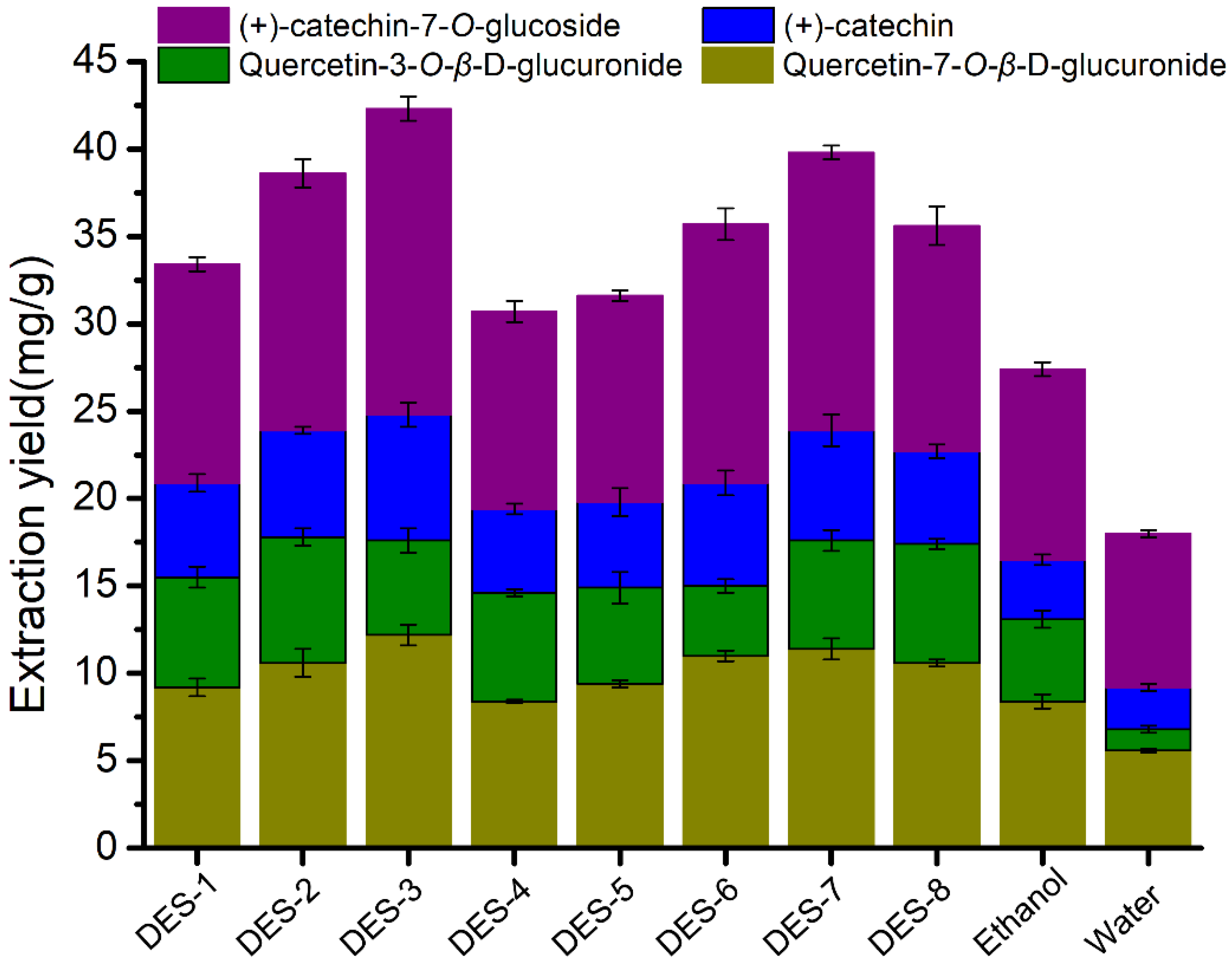

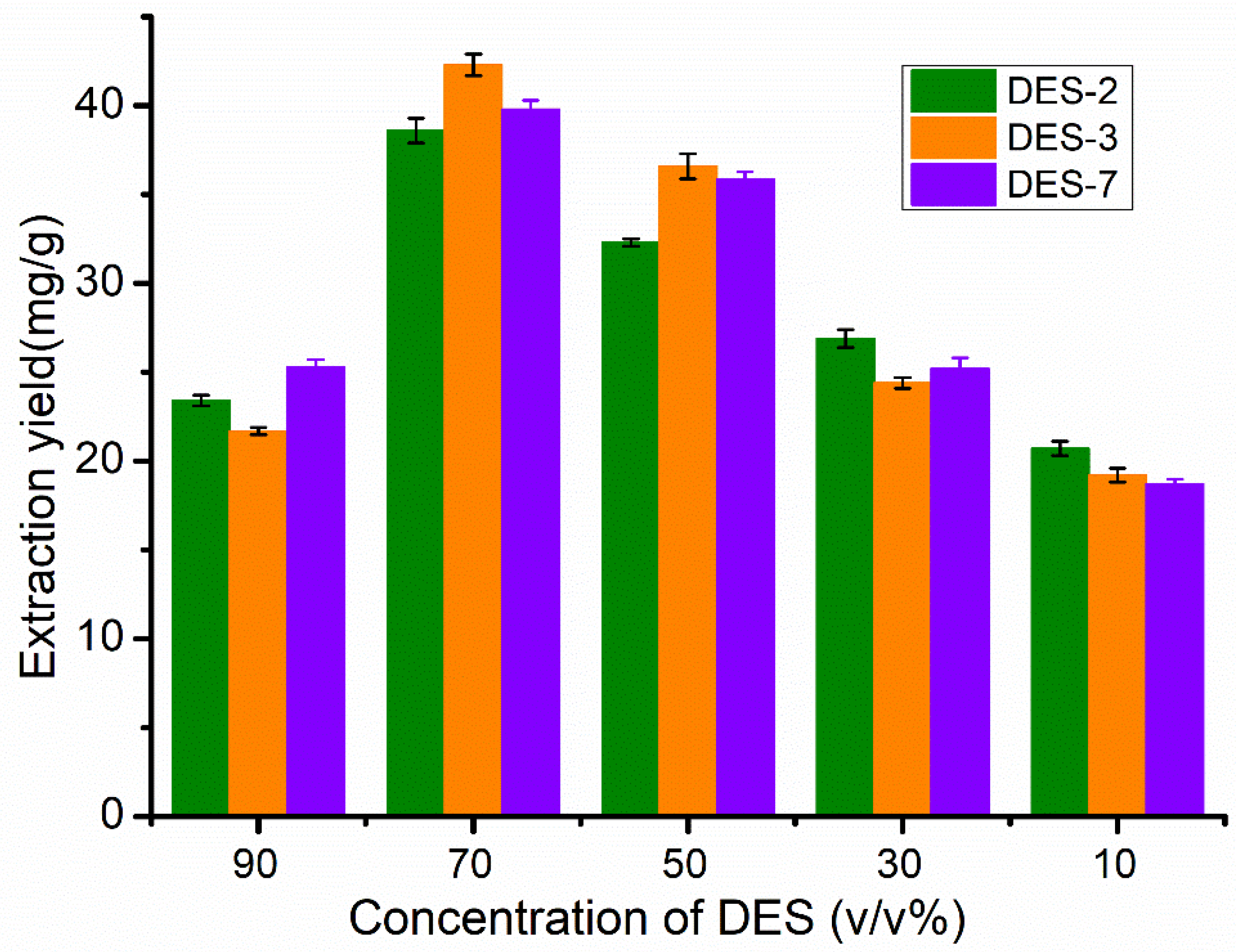
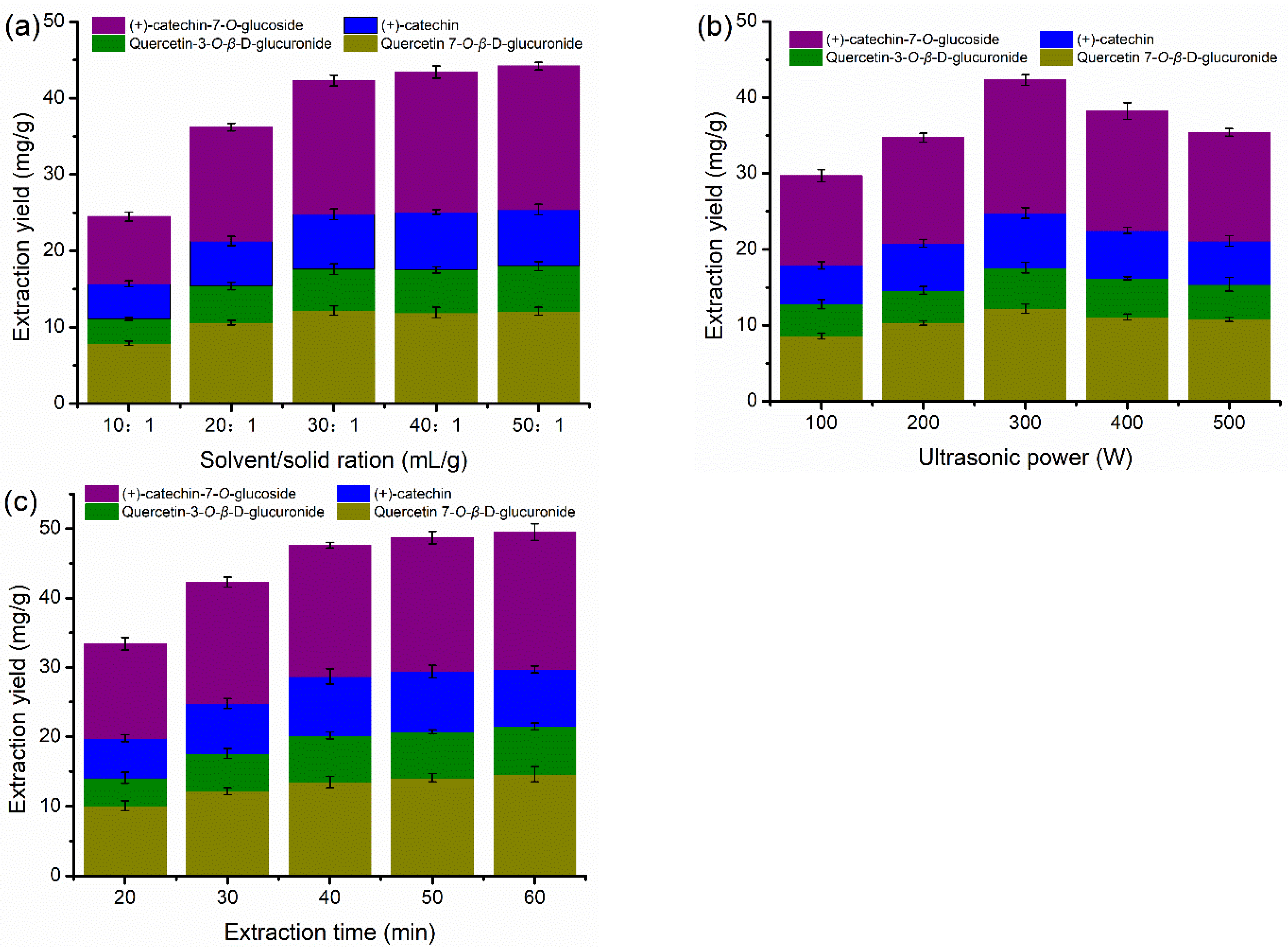
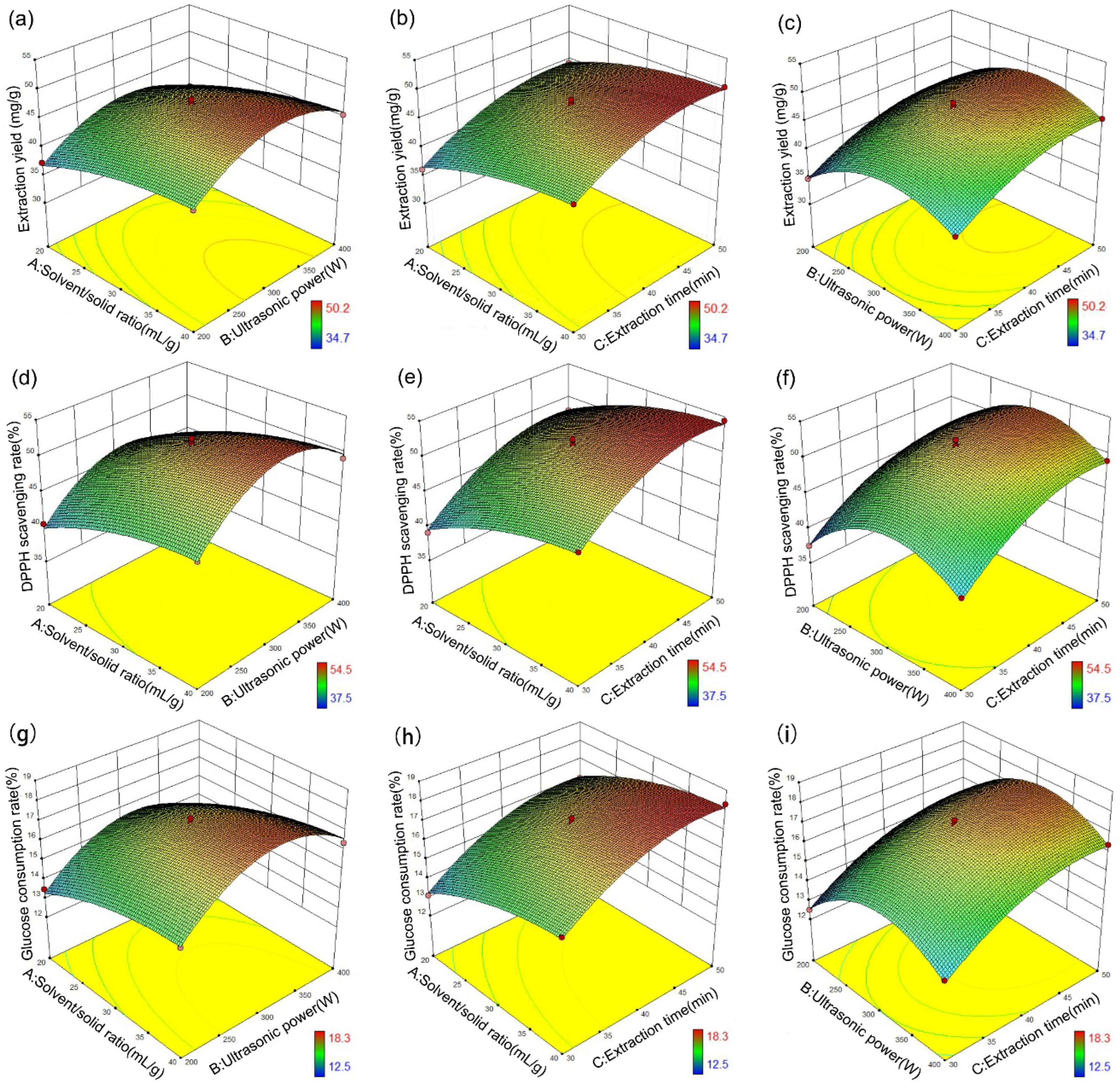
| DESs | HBA | HBD | Mole Ratio | Viscosity a (mPa*s) | pH a |
|---|---|---|---|---|---|
| DES-1 | Choline chloride | DL-Malic acid | 1:1 | 1.621 | 1.74 |
| DES-2 | Choline chloride | L-(+)tartaric acid | 2:1 | 1.732 | 1.66 |
| DES-3 | Choline chloride | Citric acid | 1:1 | 1.843 | 1.58 |
| DES-4 | Choline chloride | DL-Malic acid/proline | 1:1:1 | 1.966 | 1.61 |
| DES-5 | Betaine | DL-Malic acid | 1:1 | 1.586 | 1.63 |
| DES-6 | Betaine | L-(+)tartaric acid | 2:1 | 1.815 | 1.57 |
| DES-7 | Betaine | Citric acid | 1:1 | 1.786 | 1.82 |
| DES-8 | Betaine | DL-Malic acid/proline | 1:1:1 | 1.834 | 1.72 |
| Run | A (mL/g) | B (W) | C (min) | Total Yield of Flavonoids (mg/g) | DPPH Scavenging Rate (%) | Glucose Consumption Rate (%) |
|---|---|---|---|---|---|---|
| 1 | 30 | 300 | 40 | 47.60 | 51.5 | 17.2 |
| 2 | 20 | 400 | 40 | 39.64 | 42.8 | 14.3 |
| 3 | 30 | 300 | 40 | 47.53 | 51.4 | 17.2 |
| 4 | 20 | 300 | 50 | 44.31 | 48.0 | 16.0 |
| 5 | 20 | 200 | 40 | 37.35 | 40.5 | 13.5 |
| 6 | 20 | 300 | 30 | 36.20 | 39.1 | 13.1 |
| 7 | 40 | 300 | 50 | 50.24 | 54.5 | 18.3 |
| 8 | 30 | 400 | 30 | 38.20 | 41.3 | 13.8 |
| 9 | 30 | 200 | 30 | 34.70 | 37.5 | 12.5 |
| 10 | 30 | 300 | 40 | 48.21 | 52.2 | 17.4 |
| 11 | 40 | 200 | 40 | 42.35 | 45.9 | 15.3 |
| 12 | 30 | 400 | 50 | 45.27 | 49.0 | 16.4 |
| 13 | 40 | 300 | 30 | 43.40 | 47.0 | 15.7 |
| 14 | 30 | 200 | 50 | 41.72 | 45.1 | 15.1 |
| 15 | 40 | 400 | 40 | 45.43 | 49.2 | 16.4 |
| 16 | 30 | 300 | 40 | 47.44 | 51.4 | 17.2 |
| 17 | 30 | 300 | 40 | 46.72 | 50.5 | 16.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, H.; Li, J.; Wang, G.; Zuo, W.; Zeng, Y.; Liu, L. Ultrasound-Assisted Extraction of Flavonoids from Potentilla fruticosa L. Using Natural Deep Eutectic Solvents. Molecules 2022, 27, 5794. https://doi.org/10.3390/molecules27185794
Xue H, Li J, Wang G, Zuo W, Zeng Y, Liu L. Ultrasound-Assisted Extraction of Flavonoids from Potentilla fruticosa L. Using Natural Deep Eutectic Solvents. Molecules. 2022; 27(18):5794. https://doi.org/10.3390/molecules27185794
Chicago/Turabian StyleXue, He, Jinping Li, Guiyao Wang, Wenming Zuo, Yang Zeng, and Likuan Liu. 2022. "Ultrasound-Assisted Extraction of Flavonoids from Potentilla fruticosa L. Using Natural Deep Eutectic Solvents" Molecules 27, no. 18: 5794. https://doi.org/10.3390/molecules27185794





