An Investigation of the Anti-Depressive Properties of Phenylpropanoids and Flavonoids in Hemerocallis citrina Baroni
Abstract
:1. Introduction
2. Results and Discussion
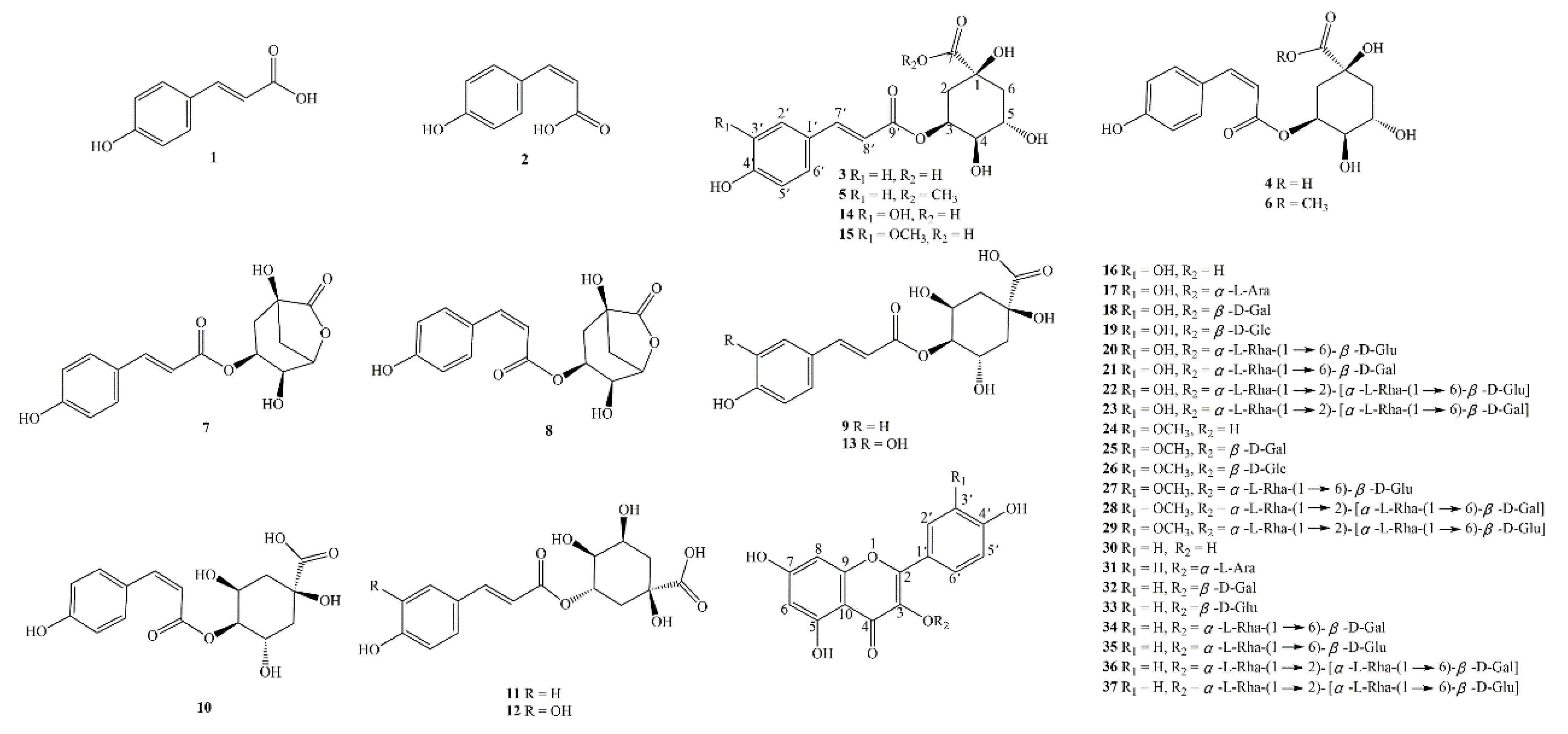
2.1. Structure Elucidation of Novel Compound
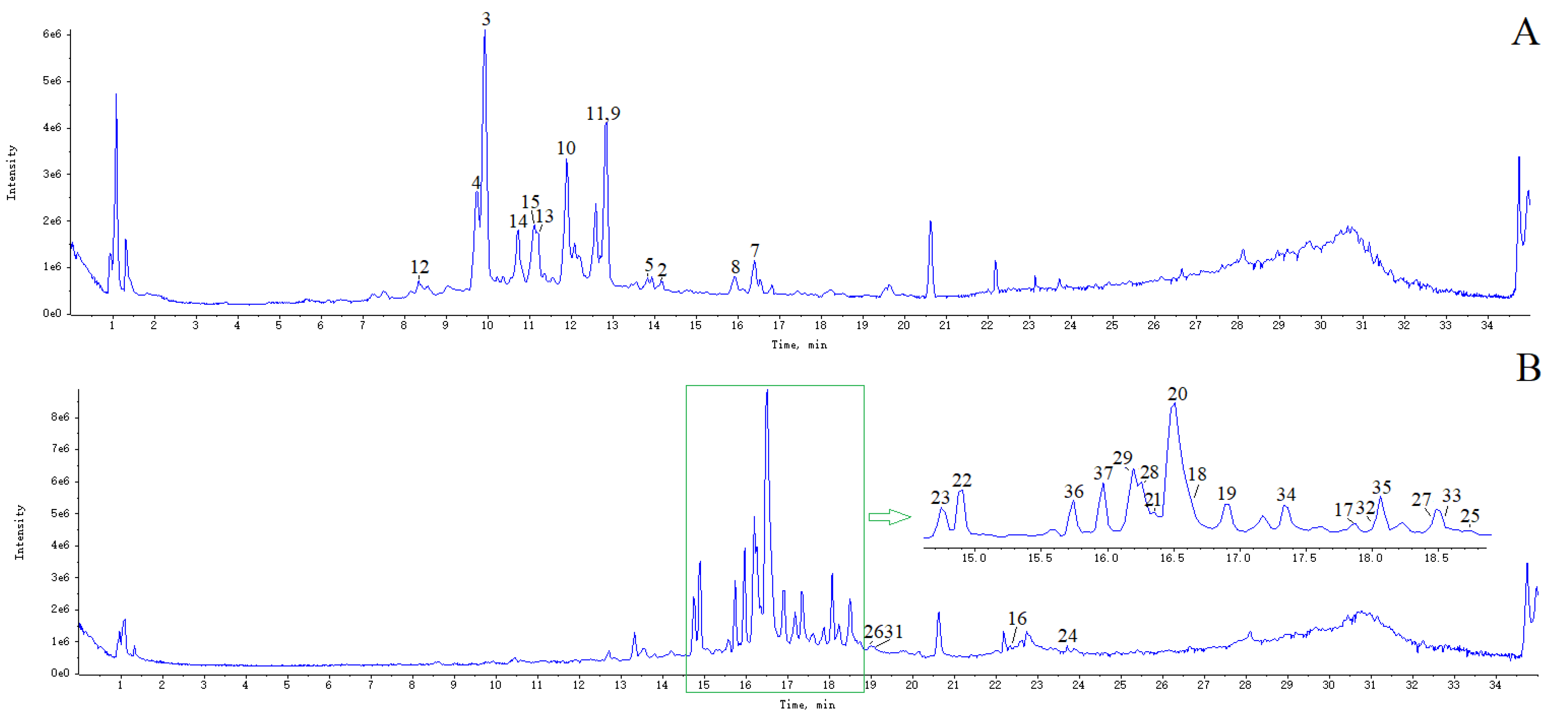
2.2. Enrichment of Phenylpropanoids and Flavonoids
2.3. Antidepressant Activity of HFPE and HFFE
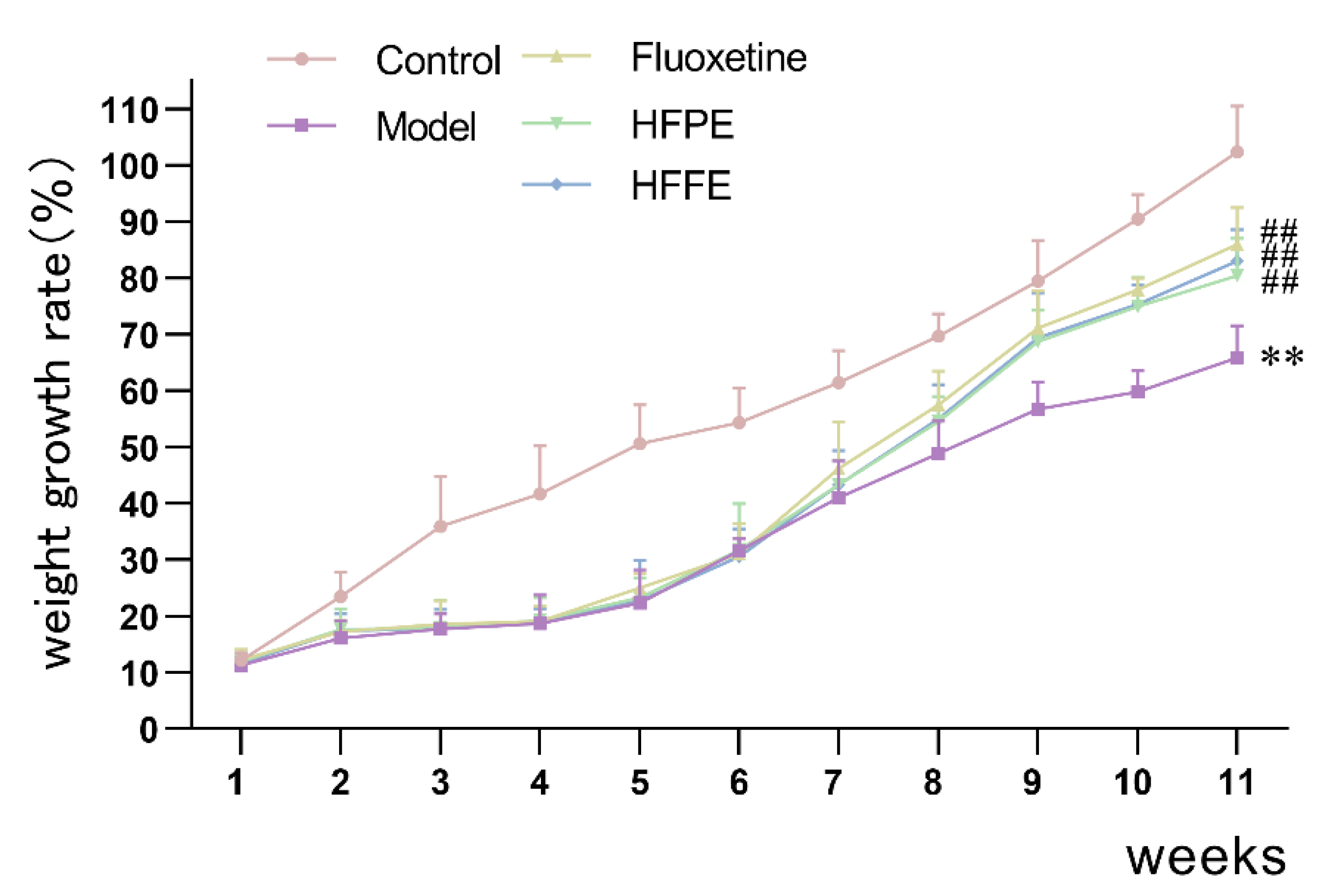
2.3.1. Body Weight
2.3.2. Effects of HFPE and HFFE on Sucrose Preference Test (SPT)
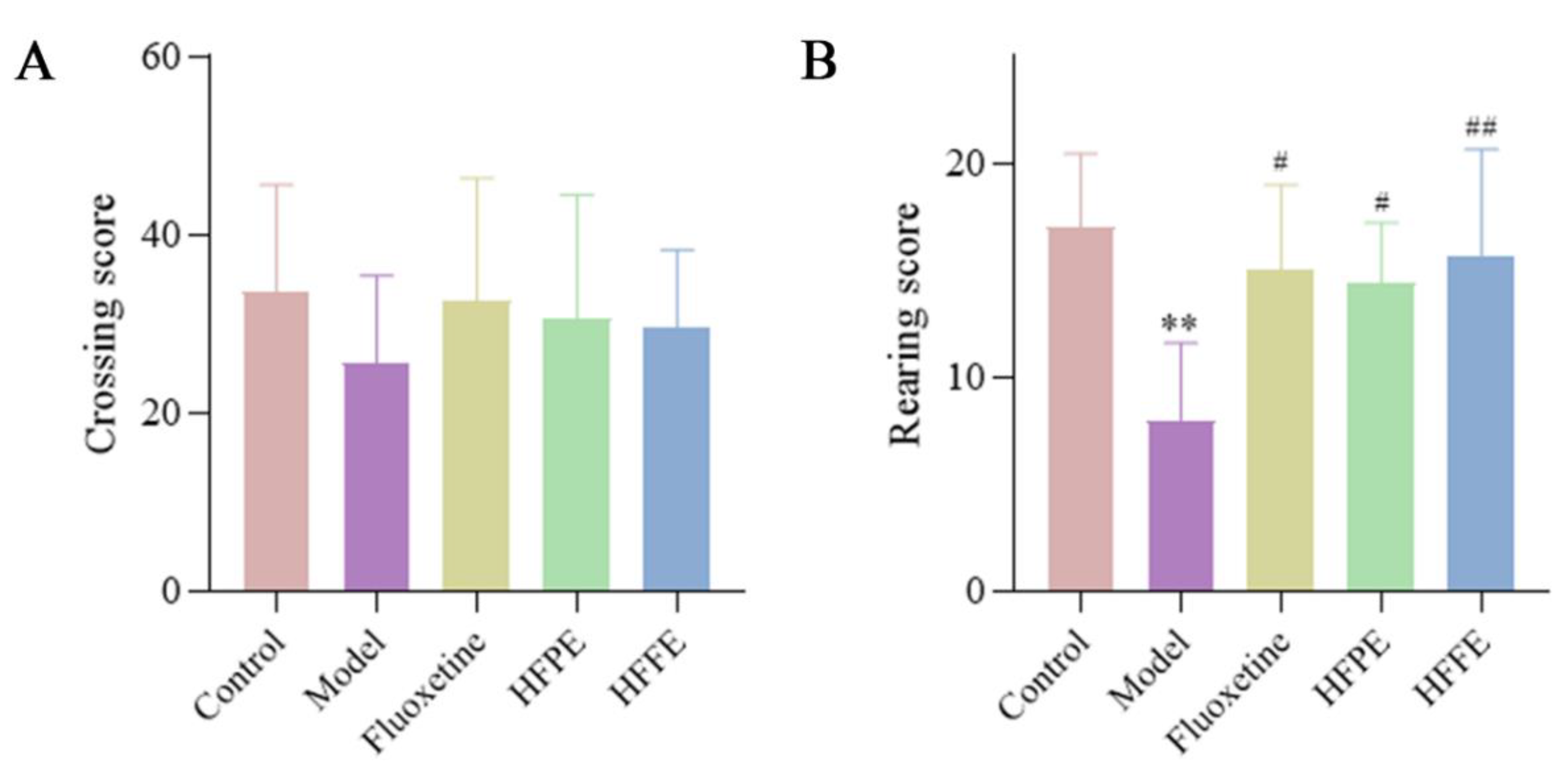
2.3.3. Effects of HFPE and HFFE on Open-Field Test (OFT)
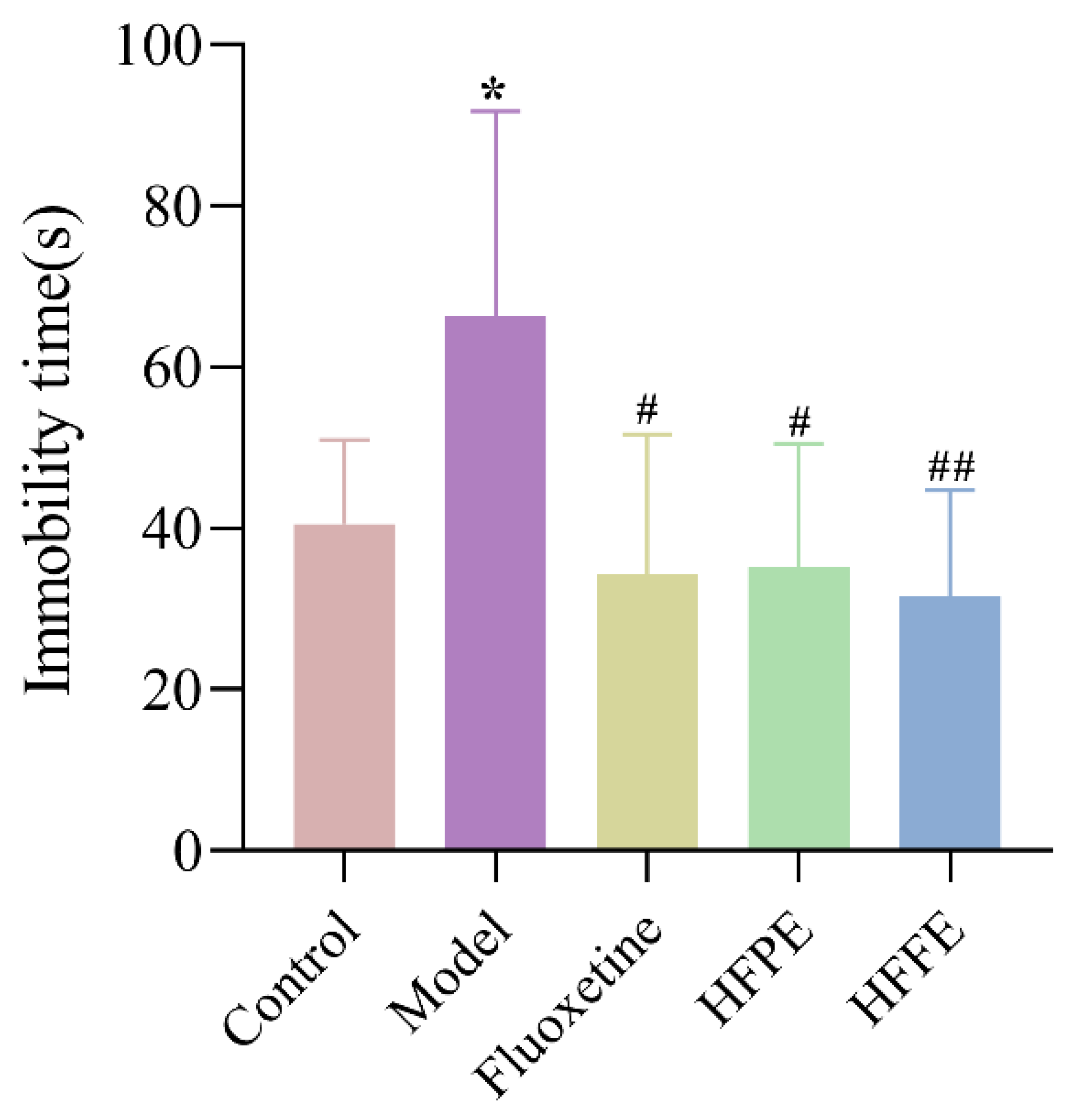
2.3.4. Effects of HFPE and HFFE on the Forced Swimming Test (FST)
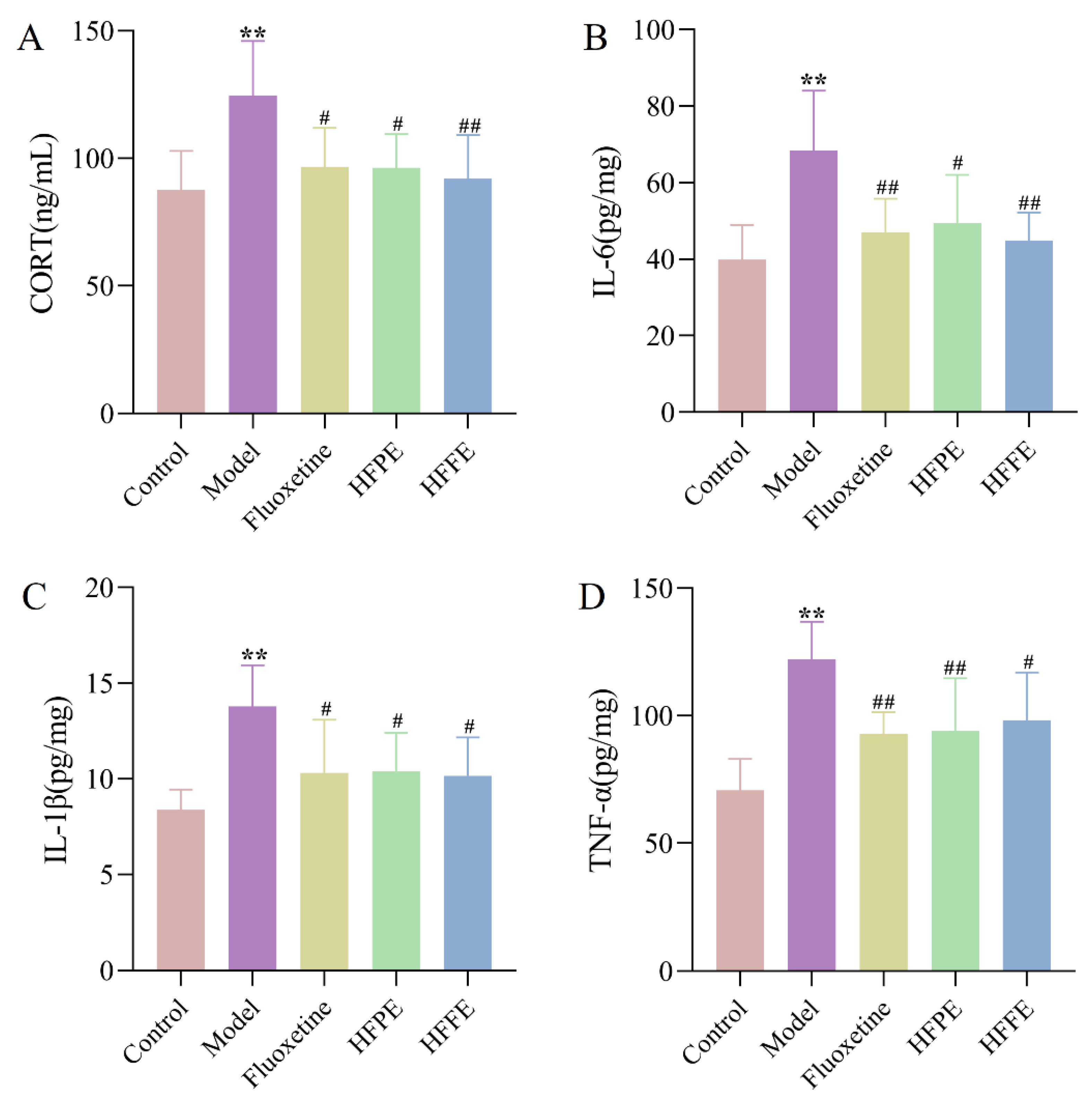
2.3.5. Effects of HFPE and HFFE on Serum Corticosterone (CORT) Level and the Inflammatory Level in Hippocampus
2.4. Anti-Neuroinflammatory Activity and Structure–Activity Relationship
2.4.1. Role of Quinic Acid or Quinide Group of Phenylpropanoids
2.4.2. Role of Sugar Moieties at C-3 of Flavonoids
2.4.3. Role of Substituent Group at C-3′ of Flavonoids
3. Materials and Methods
3.1. Plant Material
3.2. Apparatus and Reagents
3.3. Extraction and Isolation
3.4. Nuclear Magnetic Resonance Spectrometry (NMR)
3.5. Enrichment of HFPE and HFFE of H. citrina Flower Buds
3.6. UHPLC-MS Detection and Data Analysis
3.7. Antidepressant-Like Effects of HFPE and HFFE
3.7.1. Animals
3.7.2. Groups and Drug Administration
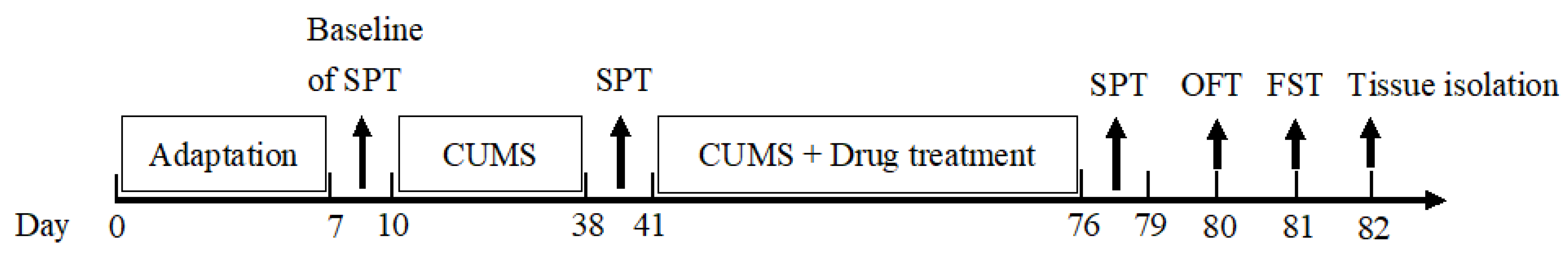
3.7.3. Body Weight and CUMS Procedure
3.7.4. Sucrose Preference Testing
3.7.5. Open-Field Test
3.7.6. Forced Swimming Test
3.7.7. Determination of Serum CORT Level and IL-6, IL-1β, and TNF-α Level in the Hippocampus
3.8. Anti-Neuroinflammatory Activity
3.8.1. Cellular Culture
3.8.2. CCK8 Cytotoxic Activity
3.8.3. Inhibition of NO Production
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Wang, J.; Hu, D.M.; Hou, J.; Li, S.S.; Wang, W.P.; Li, J.; Bai, J. Ethyl acetate fraction of Hemerocallis citrina Baroni decreases tert-butyl hydroperoxide-induced oxidative stress damage in BRL-3A Cells. Oxidative Med. Cell. Longev. 2018, 13, 2198–2206. [Google Scholar] [CrossRef]
- Du, B.; Tang, X.; Liu, F.; Zhang, C.; Zhao, G.; Ren, F.; Leng, X. Antidepressant-like effects of the hydroalcoholic extracts of Hemerocallis citrina and its potential active components. BMC. Complement. Altern. Med. 2014, 14, 326. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Liu, Y.J.; Wang, Y.B.; Yi, L.T. Role for monoaminergic systems in the antidepressant-like effect of ethanol extracts from Hemerocallis citrina. J. Ethnopharmacol. 2012, 139, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Kao, F.J.; Chiang, W.D.; Liu, H.M. Inhibitory effect of daylily buds at various stages of maturity on nitric oxide production and the involved phenolic compounds. LWT-Food Sci. Technol. 2015, 61, 130–137. [Google Scholar] [CrossRef]
- Shen, N.; Huang, X.D.; Li, Z.W.; Wang, Y.C.; Qi, L.; An, Y.; Liu, T.T. Effects of Hemerocallis citrine baroni flavonids on CCl4-induced liver fibrosis of rats. Acta Pharm. Sin. 2015, 50, 547–551. [Google Scholar]
- Cichewicz, R.H.; Zhang, Y.J.; Seeram, N.P.; Nair, M.G. Inhibition of human tumor cell proliferation by novel anthraquinones from daylilies. Life Sci. 2004, 74, 1791–1799. [Google Scholar] [CrossRef]
- Matsumoto, T.; Nakamura, S.; Nakashima, S.; Ohta, T.; Yano, M.; Tsujihata, J.; Tsukioka, J.; Ogawa, K.; Fukaya, M.; Yoshikawa, M.; et al. gamma-Lactam alkaloids from the flower buds of daylily. J. Nat. Med. 2016, 70, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.Y.; Chang, L.Y.; Chou, S.S.; Hsiao, Y.L.; Chien, Y.W. Studies on the antioxidant components and activities of the methanol extracts of commercially grown Hemerocallis Fulva L. (Daylily) in TAIWAN. J. Food Biochem. 2010, 34, 90–104. [Google Scholar] [CrossRef]
- Yang, Z.D.; Chen, H.; Li, Y.C. A new glycoside and a novel-type diterpene from Hemerocallis fulva (L.) L. Helv. Chim. Acta 2003, 86, 3305–3309. [Google Scholar] [CrossRef]
- Konishi, T.; Fujiwara, Y.; Konoshima, T.; Kiyosawa, S.; Nishi, M.; Miyahara, K. Steroidal saponins from Hemerocallis fulva var. kwanso. Chem. Pharm. Bull. 2001, 49, 318–320. [Google Scholar] [CrossRef]
- Cichewicz, R.H.; Nair, M.G. Isolation and characterization of stelladerol, a new antioxidant naphthalene glycoside, and other antioxidant glycosides from edible daylily (Hemerocallis) flowers. J. Agric. Food Chem. 2002, 50, 87–91. [Google Scholar] [CrossRef]
- Xu, P.; Wang, K.Z.; Lu, C.; Dong, L.M.; Zhai, J.L.; Liao, Y.H.; Aibai, S.; Yang, Y.; Liu, X.M. Antidepressant-like effects and cognitive enhancement of the total phenols extract of Hemerocallis citrina Baroni in chronic unpredictable mild stress rats and its related mechanism. J. Ethnopharmacol. 2016, 194, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Duman, R.S.; Aghajanian, G.K. Synaptic dysfunction in depression: Potential therapeutic targets. Science 2012, 338, 68–72. [Google Scholar] [CrossRef]
- Wang, J.; Cheng, C.; Xin, C.; Wang, Z. The Antidepressant-like effect of flavonoids from Trigonella Foenum-Graecum seeds in chronic restraint stress mice via modulation of monoamine regulatory pathways. Molecules 2019, 24, 1105. [Google Scholar] [CrossRef]
- Murray, C.J.; Lopez, A.D. Evidence-based health policy—Lessons from the global burden of disease study. Science 1996, 274, 740–743. [Google Scholar] [CrossRef]
- Majd, M.; Saunders, E.F.H.; Engeland, C.G. Inflammation and the dimensions of depression: A review. Front. Neuroendocrinol. 2020, 56, 100800. [Google Scholar] [CrossRef] [PubMed]
- Drevets, W.C.; Wittenberg, G.M.; Bullmore, E.T.; Manji, H.K. Immune targets for therapeutic development in depression: Towards precision medicine. Nat. Rev. Drug Discov. 2022, 21, 224–244. [Google Scholar] [CrossRef] [PubMed]
- Abiega, O.; Beccari, S.; Diaz-Aparicio, I.; Nadjar, A.; Laye, S.; Leyrolle, Q.; Gomez-Nicola, D.; Domercq, M.; Perez-Samartin, A.; Sanchez-Zafra, V.; et al. Neuronal hyperactivity disturbs ATP microgradients, impairs microglial motility, and reduces phagocytic receptor expression triggering apoptosis/microglial phagocytosis uncoupling. PLoS Biol. 2016, 14, e1002466. [Google Scholar] [CrossRef] [PubMed]
- Block, M.L.; Zecca, L.; Hong, J.-S. Microglia-mediated neurotoxicity: Uncovering the molecular mechanisms. Nat. Rev. Neurosci. 2007, 8, 57–69. [Google Scholar] [CrossRef]
- Lawson, L.J.; Perry, V.H.; Dri, P.; Gordon, S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience 1990, 39, 151–170. [Google Scholar] [CrossRef]
- Luo, L.; Wang, Y.; Wang, Y.; Xu, J.; He, X. Potential in vitro anti-neuroinflammatory sterols from mango fruits (Mangifera indica L.). J. Funct. Food 2021, 84, 104846. [Google Scholar] [CrossRef]
- Tambuyzer, B.R.; Ponsaerts, P.; Nouwen, E.J. Microglia: Gatekeepers of central nervous system immunology. J. Leukoc. Biol. 2009, 85, 352–370. [Google Scholar] [CrossRef]
- Salter, M.W.; Beggs, S. Sublime Microglia: Expanding Roles for the Guardians of the CNS. Cell 2014, 158, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Nestler, E.J.; Barrot, M.; DiLeone, R.J.; Eisch, A.J.; Gold, S.J.; Monteggia, L.M. Neurobiology of depression. Neuron 2002, 34, 13–25. [Google Scholar] [CrossRef]
- Goetz, G.; Fkyerat, A.; Metais, N.; Kunz, M.; Tabacchi, R.; Pezet, R.; Pont, V. Resistance factors to grey mould in grape berries: Identification of some phenolics inhibitors of Botrytis cinerea stilbene oxidase. Phytochemistry 1999, 52, 59–767. [Google Scholar] [CrossRef]
- Zhou, H.Y.; Li, S.M. Study on constituents from leaves of Phyllostachys pubescens. Chin. Pharm. J. 2006, 41, 662–663. [Google Scholar]
- Kuczkowiak, U.; Petereit, F.; Nahrstedt, A. Hydroxycinnamic acid derivatives obtained from a commercial crataegus extract and from authentic crataegus spp. Sci. Pharm. 2014, 82, 835–846. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Lee, S.; Jang, H.J.; Su, X.D.; Wang, H.S.; Kim, Y.H.; Yang, S.Y. Inhibition potential of phenolic constituents from the aerial parts of Tetrastigma hemsleyanum against soluble epoxide hydrolase and nitric oxide synthase. J. Enzym. Inhib. Med. Chem. 2019, 34, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Sadhu, S.K.; Okuyama, E.; Fujimoto, H.; Ishibashi, M.; Yesilada, E. Prostaglandin inhibitory and antioxidant components of Cistus laurifolius, a Turkish medicinal plant. J. Ethnopharmacol. 2006, 108, 371–378. [Google Scholar] [CrossRef]
- Lee, Y.G.; Cho, J.Y.; Kim, C.M.; Lee, S.H.; Kim, W.S.; Jeon, T.I.; Park, K.H.; Moon, J.H. Coumaroyl quinic acid derivatives and flavonoids from immature pear (Pyrus pyrifolia nakai) fruit. Food Sci. Biotechnol. 2013, 22, 803–810. [Google Scholar] [CrossRef]
- Zhao, X.C. Studies on the Chemical Constituents of the Flowers of Hemerocallis Minor Mill. Master’s Thesis, Shanghai Jiaotong University, Shanghai, China, 2017; p. 64. [Google Scholar]
- Will, F.; Zessner, H.; Becker, H.; Dietrich, H. Semi-preparative isolation and physico-chemical characterization of 4-coumaroylquinic acid and phloretin-2’-xylogluco side from laccase-oxidized apple juice. LWT-Food. Sci. Technol. 2007, 40, 1344–1351. [Google Scholar] [CrossRef]
- Lu, Y.R.; Sun, Y.; Foo, L.Y.; McNabb, W.C.; Molan, A.L. Phenolic glycosides of forage legume Onobrychis viciifolia. Phytochemistry 2000, 55, 67–75. [Google Scholar] [CrossRef]
- Wong, S.K.; Lim, Y.Y.; Ling, S.K.; Chan, E.W.C. Caffeoylquinic acids in leaves of selected Apocynaceae species: Their isolation and content. Pharmacogn. Res. 2014, 6, 67–72. [Google Scholar]
- Janda, B.; Stochmal, A.; Montoro, P.; Piacente, S.; Oleszek, W. Phenolics in aerial parts of persian clover trifolium resupinatum. Nat. Prod. Commun. 2009, 4, 1661–1664. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Kim, J.S.; Kim, H.P.; Lee, J.H.; Kang, S.S. Phenolic constituents from the flower buds of Lonicera japonica and their 5-lipoxygenase inhibitory activities. Food Chem. 2010, 120, 134–139. [Google Scholar] [CrossRef]
- Dokli, I.; Navarini, L.; Hamersak, Z. Syntheses of 3-, 4-, and 5-O-feruloylquinic acids. Tetrahedron Asymmetr. 2013, 24, 785–790. [Google Scholar] [CrossRef]
- Wu, T.; Abdulla, R.; Yang, Y.; Aisa, H.A. Flavonoids from gossypium hirsutum flowers. Chem. Nat. Compd. 2008, 44, 370–371. [Google Scholar] [CrossRef]
- Yoshida, T.; Maruyama, T.; Nitta, A.; Okuda, T. Eucalbanin-a, Eucalbanin-b and eucalbanin-c, monomeric and dimeric hydrolyzable tannins from Eucalyptus-alba reinw. Chem. Pharm. Bull. 1992, 40, 1750–1754. [Google Scholar] [CrossRef]
- Kim, J.; Kang, S.S.; Ro, L.K. Flavonol glycosides from the aerial parts of Metaplexis japonica. Korean J. Pharm. 2012, 43, 206–212. [Google Scholar]
- Kazuma, K.; Noda, N.; Suzuki, M. Malonylated flavonol glycosides from the petals of Clitoria ternatea. Phytochemistry 2003, 62, 229–237. [Google Scholar] [CrossRef]
- Hassan, A.R.; Amer, K.F.; El-Toumy, S.A.; Nielsen, J.; Christensen, S.B. A new flavonol glycoside and other flavonoids from the aerial parts of Taverniera aegyptiaca. Nat. Prod. Res. 2019, 33, 1135–1139. [Google Scholar] [CrossRef]
- Hirose, Y.; Fujita, T.; Ishii, T.; Ueno, N. Antioxidative properties and flavonoid composition of Chenopodium quinoa seeds cultivated in Japan. Food Chem. 2010, 119, 1300–1306. [Google Scholar] [CrossRef]
- Jiang, C.L.; Tsai, S.F.; Lee, S.S. Flavonoids from Curcuma longa Leaves and their NMR Assignments. Nat. Prod. Commun. 2015, 10, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Su, X.C.; Chen, L.; Aisa, H.A. Flavonoids and sterols from Alhagi sparsifolia. Chem. Nat. Compd. 2008, 44, 365. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Tan, N.H. Chemical constituents of taxodium mucronatum. Nat. Prod. Res. Dev. 2007, 19, 801–821. [Google Scholar]
- Beck, M.A.; Haberlein, H. Flavonol glycosides from Eschscholtzia californica. Phytochemistry 1999, 50, 329–332. [Google Scholar] [CrossRef]
- Zhang, M.L.; Huo, C.H.; Dong, M.; Liang, C.H.; Gu, Y.C.; Shi, Q.W. Non-taxoid chemical constituents from leaves of Taxus mairei. China J. Chin. Mater. Med. 2007, 32, 1421–1425. [Google Scholar]
- Min, B.S.; Tomiyama, M.; Ma, C.M.; Nakamura, N.; Hattori, M. Kaempferol acetylrhamnosides from the rhizome of Dryopteris crassirhizoma and their inhibitory effects on three different activities of human immunodeficiency virus-1 reverse transcriptase. Chem. Pharm. Bull. 2001, 49, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Yao, H.; Sun, B. Isolation and identification of chemical constituents from Acer paxii Franch. J. Shenyang Pharm. Univ. 2016, 33, 531–536. [Google Scholar]
- Hou, W.C.; Lin, R.D.; Lee, T.H.; Huang, Y.H.; Hsu, F.L.; Lee, M.H. The phenolic constituents and free radical scavenging activities of Gynura formosana Kiamnra. J. Sci. Food Agric. 2005, 85, 615–621. [Google Scholar] [CrossRef]
- Feng, W.S.; Hao, Z.Y.; Zheng, X.K.; Kuang, H.X. Chemical constituents from leaves of celastrus gemmatus Loes. Acta Pharm. Sin. 2007, 42, 625–630. [Google Scholar]
- Amen, Y.M.; Marzouk, A.M.; Zaghloul, M.G.; Afifi, M.S. A new acylated flavonoid tetraglycoside with anti-inflammatory activity from Tipuana tipu leaves. Nat. Prod. Res. 2015, 29, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhou, C. Corticosterone reduces brain mitochondrial function and expression of mitofusin, BDNF in depression-like rodents regardless of exercise preconditioning. Psychoneuroendocrinology 2012, 37, 1057–1070. [Google Scholar] [CrossRef] [PubMed]
- Marin, M.T.; Cruz, F.C.; Planeta, C.S. Chronic restraint or variable stresses differently affect the behavior, corticosterone secretion and body weight in rats. Physiol. Behav. 2007, 90, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Willner, P. Validity, reliability and utility of the chronic mild stress model of depression: A 10-year review and evaluation. Psychopharmacology 1997, 134, 319–329. [Google Scholar] [CrossRef]
- Li, S.; Li, T.; Jin, Y.; Qin, X.; Tian, J.; Zhang, L. Antidepressant-like effects of coumaroylspermidine extract from Safflower injection residues. Front. Pharmacol. 2020, 11, 713. [Google Scholar] [CrossRef]
- Dong, X.; Lu, K.; Lin, P.; Che, H.; Li, H.; Song, L.; Yang, X.; Xie, W. Saccharina japonica ethanol extract ameliorates depression/anxiety-like behavior by inhibiting Inflammation, oxidative stress, and apoptosis in dextran sodium sulfate induced ulcerative colitis mice. Front. Nutr. 2021, 16, 784532. [Google Scholar] [CrossRef]
- Hu, Y.; Zhao, M.; Zhao, T.; Qi, M.; Yao, G.; Dong, Y. The protective effect of pilose antler peptide on cums-induced depression through AMPK/Sirt1/NF-κB/NLRP3-mediated pyroptosis. Front. Pharmacol. 2022, 13, 815413. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.P.; Cullinan, W.E. Neurocircuitry of stress: Central control of the hypothalamo-pituitary-adrenocortical axis. Trends. Neurosci. 1997, 20, 78–84. [Google Scholar] [CrossRef]
- Anacker, C.; Zunszain, P.A.; Carvalho, L.A.; Pariante, C.M. The glucocorticoid receptor: Pivot of depression and of antidepressant treatment? Psychoneuroendocrinology 2011, 36, 415–425. [Google Scholar] [CrossRef]
- Yi, L.T.; Li, J.; Li, H.C.; Zhou, Y.; Su, B.F.; Yang, K.F.; Jiang, M.; Zhang, Y.T. Ethanol extracts from Hemerocallis citrina attenuate the decreases of brain-derived neurotrophic factor, TrkB levels in rat induced by corticosterone administration. J. Ethnopharmacol. 2012, 144, 328–334. [Google Scholar] [CrossRef]
- Strenn, N.; Suchankova, P.; Nilsson, S.; Fischer, C.; Wegener, G.; Mathe, A.A.; Ekman, A. Expression of inflammatory markers in a genetic rodent model of depression. Behav. Brain. Res. 2015, 281, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Chen, H.; Tang, X.; He, B.; Gu, L.; Feng, H. Total Saikosaponins Attenuates Depression-Like Behaviors induced by chronic unpredictable mild stress in rats by regulating the PI3K/AKT/NF-kappa B signaling axis. Evid.-Based Complement. Altern. 2022, 2022, 4950414. [Google Scholar] [CrossRef]
- Rotelli, A.E.; Guardia, T.; Juarez, A.O.; de la Rocha, N.E.; Pelzer, L.E. Comparative study of flavonoids in experimental models of inflammation. Pharmacol. Res. 2003, 48, 601–606. [Google Scholar] [CrossRef]
- Qin, X.L.; Sun, H.Y.; Yang, W.; Li, Y.J.; Zheng, L.; Liu, T.; Huang, Y. Analysis of metabolites of quercitrin in rat intestinal flora by using UPLC-ESI-Q-TOF-MS /MS. China J. Chin. Mater. Med. 2017, 42, 357–362. [Google Scholar]
- Willner, P. Chronic mild stress (CMS) revisited: Consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology 2005, 52, 90–110. [Google Scholar] [CrossRef] [PubMed]
- Konkle, A.T.M.; Baker, S.L.; Kentner, A.C.; Barbagallo, L.S.M.; Merali, Z.; Bielajew, C. Evaluation of the effects of chronic mild stressors on hedonic and physiological responses: Sex and strain compared. Brain Res. 2003, 992, 227–238. [Google Scholar] [CrossRef]
- Ma, Z.; Ji, W.; Qu, R.; Wang, M.; Yang, W.; Zhan, Z.; Fu, Q.; Ma, S. Metabonomic Study on the Antidepressant-Like Effects of Banxia Houpu Decoction and Its Action Mechanism. Evid.-Based Complement. Altern. 2013, 2013, 213739. [Google Scholar] [CrossRef]
- Tang, J.; Xue, W.; Xia, B.; Ren, L.; Tao, W.; Chen, C.; Zhang, H.; Wu, R.; Wang, Q.; Wu, H.; et al. Involvement of normalized NMDA receptor and mTOR-related signaling in rapid antidepressant effects of Yueju and ketamine on chronically stressed mice. Sci. Rep. 2015, 5, 13573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Hu, Q.; Jiang, S.; Li, F.; Lin, J.; Han, L.; Hong, Y.; Lu, W.; Gao, Y.; Chen, D. Flos Chrysanthemi Indici protects against hydroxyl-induced damages to DNA and MSCs via antioxidant mechanism. J. Saudi Chem. Soc. 2015, 19, 454–460. [Google Scholar] [CrossRef] [Green Version]


| Position | 8 | |
|---|---|---|
| δH | δC | |
| 1 | 73.0 | |
| 2a | 2.03 (1H, t, J = 11.8 Hz) | 36.8 |
| 2b | 2.12 (1H, ddd, J = 2.7, 6.9, 11.5 Hz) | |
| 3 | 4.88 (1H, m) | 70.0 |
| 4 | 4.28 (1H, t, J = 4.6 Hz) | 64.7 |
| 5 | 4.72 (1H, t, J = 5.4 Hz) | 77.7 |
| 6a | 2.53 (1H, d, J = 11.8 Hz) | 37.8 |
| 6b | 2.29 (1H, ddd, J = 2.8, 6.0, 11.5 Hz) | |
| 7 | 178.9 | |
| 1′ | 127.5 | |
| 2′ | 134.0 | |
| 3′ | 6.76 (1H, br d, J = 8.7 Hz) | 115.8 |
| 4′ | 7.68 (1H, br d, J = 8.4 Hz) | 134.1 |
| 5′ | 6.76 (1H, br d, J = 8.7 Hz) | 116.0 |
| 6′ | 7.68 (1H, br d, J = 8.4 Hz) | 160.3 |
| 7′ | 6.90 (1H, d, J = 12.8 Hz) | 146.1 |
| 8′ | 5.82 (1H, d, J = 12.8 Hz) | 116.1 |
| 9′ | 166.9 | |
| Compounds or Extract | IC50 (μM or ug/mL) a,b | Compounds | IC50 (μM) a,b | ||
|---|---|---|---|---|---|
| NO Inhibitory | Cell Viability | NO Inhibitory | Cell Viability | ||
| HFE | 497.01 ± 20.45 | >100 | 19 | >100 | >100 |
| HFPE | 25.75 ± 5.67 | >100 | 20 | >100 | >100 |
| HFFE | 168.52 ± 16.35 | >100 | 21 | >100 | >100 |
| 1 | >100 | >100 | 22 | >100 | >100 |
| 2 | >100 | >100 | 23 | >100 | >100 |
| 3 | 78.52 ± 8.23 | >100 | 24 | 13.56 ± 0.66 | >100 |
| 4 | 70.44 ± 5.86 | >100 | 25 | 48.67 ± 3.75 | >100 |
| 5 | 95.77 ± 7.18 | >100 | 26 | >100 | >100 |
| 6 | >100 | >100 | 27 | >100 | >100 |
| 7 | 94.56 ± 5.62 | >100 | 28 | 90.66 ± 10.37 | >100 |
| 8 | 36.04 ± 2.78 | >100 | 29 | >100 | >100 |
| 9 | >100 | >100 | 30 | 21.99 ± 2.81 | >100 |
| 10 | >100 | >100 | 31 | >100 | >100 |
| 11 | >100 | >100 | 32 | >100 | >100 |
| 12 | >100 | >100 | 33 | >100 | >100 |
| 13 | 74.43 ± 6.53 | >100 | 34 | >100 | >100 |
| 14 | >100 | >100 | 35 | >100 | >100 |
| 15 | >100 | >100 | 36 | >100 | >100 |
| 16 | 17.48 ± 3.25 | >100 | 37 | 96.11 ± 11.55 | >100 |
| 17 | >100 | >100 | Indomethacin c | 52.56 ± 4.58 | >100 |
| 18 | >100 | >100 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, T.; Sun, Y.; Wang, L.; Wang, J.; Wu, B.; Yan, T.; Jia, Y. An Investigation of the Anti-Depressive Properties of Phenylpropanoids and Flavonoids in Hemerocallis citrina Baroni. Molecules 2022, 27, 5809. https://doi.org/10.3390/molecules27185809
Ma T, Sun Y, Wang L, Wang J, Wu B, Yan T, Jia Y. An Investigation of the Anti-Depressive Properties of Phenylpropanoids and Flavonoids in Hemerocallis citrina Baroni. Molecules. 2022; 27(18):5809. https://doi.org/10.3390/molecules27185809
Chicago/Turabian StyleMa, Tiancheng, Yu Sun, Lida Wang, Jinyu Wang, Bo Wu, Tingxu Yan, and Ying Jia. 2022. "An Investigation of the Anti-Depressive Properties of Phenylpropanoids and Flavonoids in Hemerocallis citrina Baroni" Molecules 27, no. 18: 5809. https://doi.org/10.3390/molecules27185809
APA StyleMa, T., Sun, Y., Wang, L., Wang, J., Wu, B., Yan, T., & Jia, Y. (2022). An Investigation of the Anti-Depressive Properties of Phenylpropanoids and Flavonoids in Hemerocallis citrina Baroni. Molecules, 27(18), 5809. https://doi.org/10.3390/molecules27185809