Abstract
The purpose of this paper is to review the scientific results and summarise the emerging topic of the effects of statistic magnetic field on the structure, biochemical activity, and gene expression of plants. The literature on the subject reports a wide range of possibilities regarding the use of the magnetic field to modify the properties of plant cells. MFs have a significant impact on the photosynthesis efficiency of the biomass and vigour accumulation indexes. Treating plants with SMFs accelerates the formation and accumulation of reactive oxygen species. At the same time, the influence of MFs causes the high activity of antioxidant enzymes, which reduces oxidative stress. SMFs have a strong influence on the shape of the cell and the structure of the cell membrane, thus increasing their permeability and influencing the various activities of the metabolic pathways. The use of magnetic treatments on plants causes a higher content of proteins, carbohydrates, soluble and reducing sugars, and in some cases, lipids and fatty acid composition and influences the uptake of macro- and microelements and different levels of gene expression. In this study, the effect of MFs was considered as a combination of MF intensity and time exposure, for different varieties and plant species. The following article shows the wide-ranging possibilities of applying magnetic fields to the dynamics of changes in the life processes and structures of plants. Thus far, the magnetic field is not widely used in agricultural practice. The current knowledge about the influence of MFs on plant cells is still insufficient. It is, therefore, necessary to carry out detailed research for a more in-depth understanding of the possibilities of modifying the properties of plant cells and achieving the desired effects by means of a magnetic field.
1. Introduction
The Earth’s magnetic field (geomagnetic field, GMF) is a natural component of the environment for all living organisms. The magnetic field is the primary environmental factor for plants on Earth. The study of the influence of static magnetic fields (SMFs) on biological systems has been of great interest for many years. The SMF is characterised by low unstable parameters relative to the other types of MFs, which facilitate its application in biological systems [1,2,3,4,5]. Researchers are focusing on finding a mechanism explaining such interaction and developing a technique by which it is possible to shape the biological activity of a cell. Due to their nature, MFs can easily penetrate tissues and directly affect cell function [6,7]. Different cellular components and organelles, including mitochondria, cell membranes, protein, and DNA, change their electromagnetic behaviour under SMFs, hence affecting various physiological and biochemical responses in the cells [6]. A weak field with ‘very small forces’ can change the speed of electron movement. The rotations of the molecules carrying the magnetic moment precession can significantly initiate the subsequent biophysical processes. They are involved in non-specific responses to MFs that are seen in systems with processes involving cell growth and gene expression in plants. The plasma membrane is the principal structural element of the cell directly exposed to the MF. Thus induced structural changes in the cell membrane may affect cell properties such as changes in the shape and size of cells, ion activation, and dipole polarisation in living cells [8,9,10,11,12]. The effects of the magnetic field (MF) on plants, fungi, and microbes can be elucidated by ion cyclotron resonance (ICR) and the radical-pair model. These two mechanisms also play essential roles in the magnetoreception of organisms [1]. Studies in the literature on the subject [13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28] report that MFs influence the physiological and biochemical processes of plants, enzymatic activity, cell production, protein biosynthesis, photochemical activity, and the content of bioactive components. In this way, this field affects metabolism and cell division, activates plant growth, and shapes the quality characteristics of plants. MFs may also play vital roles in the nutrient uptake capacity of plants [29,30,31,32,33,34,35,36,37,38]. They may affect the reduction in toxins in plants, thus increasing health safety. MFs influence the diffusion of biological particles in solutions through the Lorentz force mechanism or the Maxwell stress, as well as the biochemical processes involving free radicals [39,40,41,42]. Other studies also report a positive effect of MFs on the content of photosynthetic pigments, photosystem II performance (PSII), and an efficiency index based on light energy absorption [18,43,44,45,46,47,48,49,50,51,52]. MFs can also influence the content of reactive oxygen and nitrogen species as the molecules formed in many biological processes, and they can also increase antioxidant activity and thus reduce oxidative damage to plant cells [4,40,52,53,54,55,56,57,58,59]. The influence of MFs on the expression of plant genes has been documented by many researchers [27,42,57,60,61,62,63,64]. They found significant changes in the expression of genes that play essential roles in regulating metabolism, biosynthesis, and the cellular stress response. As part of the research, attempts have also been made to explain the mechanisms of MF interaction with biostructures. Thus far, such a mechanism has not been experimentally verified. Several solutions have been proposed in this regard, including the radical-pair mechanism, the tripartite mechanism, and the level-mixing mechanism based on quantum biophysics [65,66,67,68,69].
In contrast, Barbic [6] proposed several other possible mechanisms for activating ion channels based on the magnetocaloric effect, the mechanical deformation of the cell membrane by diamagnetic forces, and the Einstein–de Haas effect.
In recent years, scientists have increasingly researched the use of MFs to improve plant growth and overall productivity. The MF is a technique that can ecologically and cheaply induce new properties in plants and is also useful in terms of their use in food and pharmacology. It can also shape plant resistance to diseases and pests to increase productivity. Despite numerous studies, it is still an innovative area of research focused on laboratory tests [70]. This review provides a ranking of the existing knowledge and the latest reports on the impact and possibilities of using the MF. It presents numerous scientific achievements on the impact of the MF on photosynthesis, cryptochromes, biomass productivity, reactive oxygen species, nitric oxide content, enzyme activity, structure and cell growth, plant components, and gene expression.
2. Effect of MF on Photosynthesis, Cryptochromes, and Biomass Productivity
Photosynthesis is the basis of life on Earth, leading to the production of biomass by converting CO2 from the atmosphere and sunlight to release oxygen. It is equated with a high-energy process and is also associated with the high efficiency of energy transfer. Photosynthesis can provide multiple parameters related to the activity and productivity of plants [71]. Sunlight captured by the plant splits the water and extracts the electrons, boosting an electron to a high energy level in photosystem II (PSII). Subsequently, the electrons travel through the chloroplast’s electron transport chain to photosystem I (PSI). At the end of the chain, the electron is passed to NADP+ to create NADPH. A share of the released energy is used to pump hydrogen ions driving ATP chemical energy production [65,72,73]. The research conducted, among others, by Deamici et al. 2019 [46], Thomas et al. 2019 [36], and Sarraf et al. 2021 [74] showed a positive effect of MFs on the photosynthesis apparatus. Photosynthesis parameters in soybean seedlings, such as the maximum quantum yield Fv/Fm, the quantum yield of electron transport ϕ Eo = ETo/ABS, the relative amplitude of the I–P phase ∆ V (I–P), PIABS, the rate of photosynthesis Pn, and the performance index PI, increased under the influence of the magnetic field, which contributed to a higher level of light absorption efficiency [48]. The performance index (PI) provides information on the structure and function of PSII and the performance of specific membrane electron transport reactions [75]. Shine et al. [43] showed that the treatment of MF soybeans (150 and 200 mT) resulted in a significant increase in quantum yields and the performance index compared with control plants. The pre-treatment of the SMF also increased the concentration of active PSII reaction centres. These results are consistent with previous studies after the magnetopriming of soybean plants [43,52]. A study by Baby et al. (2011) [76] also showed an increase in the performance index influenced by the density of reaction centres in the chlorophyll, the exciton trapped on the absorbed photon, and the efficiency with which the trapped exciton can transfer the electron to the transport chain. As a result, the plants showed high efficiency in the photosynthesis process.
Cryptochromes (CRY1 and CRY2) are photoreceptors through which photosynthetic organisms receive blue light (B, 400–499 nm). They are involved in many aspects of plant growth and development, such as stem elongation inhibition, leaf unfolding, chlorophyll production and initiation of photosynthesis, and stress response [77,78,79,80]. Phosphorylation is a metabolic pathway in which, under the influence of blue light, energy is released upon the oxidation of reduced nucleotides and converted into ATP energy [77,81,82]. Activation of cryptochromes is observed in periods of illumination alternating with darkness. Hore and Mouritsen (2016) [83] explained the biological photoreceptor as a magnetoreceptor function based on the radical-pair hypothesis. The produced unpaired radicals interact with magnetic fields, resulting in a change in the interconversion of the flavin redox state, and thus, the biological activity of the plant may change. The studies by Ahmad et al. (2007) [84], Xu et al. (2014, 2015) [85,86], and Pooam et al. (2019) [87] showed that an MF (500 µT) enhanced the biological response of cryptochromes to the applied MF. Using pulsed lighting conditions, they observed the reactions of cryptochromes to the applied MF. They documented that the Arabidopsis seedling growth was inhibited by activating cryptochromes in response to blue light. The greater the biological activity of cryptochromes, the shorter the seedling hypocotyl was recorded in response to blue light. The MF could change the ratio of different redox states of cryptochromes and thus change their biological activity. Other researchers such as Yu et al. (2007) [88] and Burney et al. (2009) [89] confirmed that MFs influenced the activation of cryptochrome by blue light. A 500 µT MF enhanced the blue-light-dependent phosphorylation of CRY1 and CRY2, while a nearly zero MF reduced the phosphorylation. In contrast, MFs attenuated CRY1 and CRY2 dephosphorylation in the dark, while a nearly null MF enhanced dephosphorylation in the dark. The modification of the levels of Arabidopsis cryptochrome phosphorylation via magnetic fields to some extent influenced the functions of the cryptochromes.
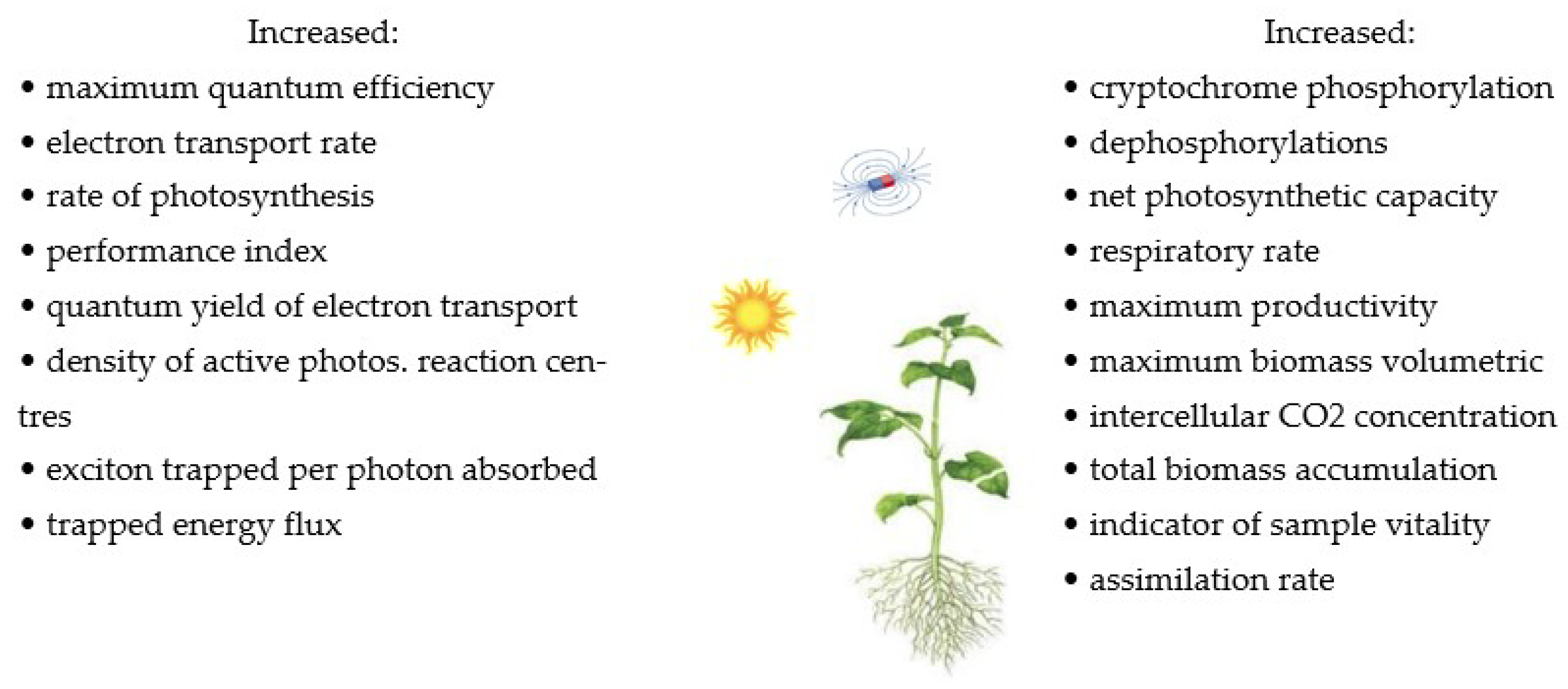
The influence of MFs on cell growth and biomass concentration in plants was investigated [22,25,33,34,61,90]. The results of these studies showed that MFs increase biomass accumulation and vigour indicators, parameters related to germination, such as germination rate, water uptake, and seedling length. MFs cause cellular stress that can affect the growth and production of biomass. On the other hand, the continuous exposure of cells to MFs may promote the adaptation of cells to MF-induced stress, resulting in lower biomass production. The influence of MFs on biological systems can be treated as stimulating, in which two parameters play important roles in biomass productivity, exposure time (24 h/d and 1 h/d) and MF intensity (20, 60 mT, 250 mT). The methods using MFs and their effects on several plants in terms of photosynthesis, cryptochromes, and biomass productivity are summarised in Table 1 and Figure 1.

Table 1.
Effects of MFs on photosynthesis and cryptochrome and biomass productivity.
Figure 1.
The summary of the effects of MFs on photosynthesis, cryptochromes, and biomass productivity [46,49]. Copyright Bioresour. Technol. 2019, 292, 121945. Copyright Int. J. Mol. Sci. 2021, 22, 9353.
3. Effect of MFs at a Molecular Level
MFs may cause changes in the parameters of biochemical processes. They can regulate overall plant growth by influencing enzyme activity, metabolite transport, growth regulators, ions, and water. A lower concentration of MFs may stimulate the transport of carbohydrates and plant growth hormones to the distant growth zones of individual plant organs. The literature reports that MFs showed a positive effect on photosynthesis and the content of chlorophyll [2]. Geomagnetic fields can affect various enzymes. The activities of Ca2+/calmodulin-dependent cyclic nucleotide phosphodiesterase (20 μT) and cytochrome C oxidase (50 Hz) were altered by MFs [1,3]. MFs can also influence biological processes involving photochemical reactions. Scientists have identified the mechanisms of some changes in enzyme activity during exposure to MFs. MF effects are exerted by the inter-conversion of singlet and triplet rotatory states of the radical pair of biomolecules. Some enzyme reactions are sensitive, and their kinetics are affected by MFs [1]. MFs increase the content of auxins and the activity of enzymes that regulate the elongation of the plant cell wall [7]. They lead to an increase in catalase and peroxidase enzymes, the stimulation of reactive oxygen species, and changes in the activity of amylase and nitrate reductase in seeds [14]. An extremely low MF (0.2–0.3 μT) stimulated the activity of Na and K-ATPases, whereas a weak but moderate MF influenced the redox activity of cytochrome C oxidase. The treatment of 30 mT increased the esterase activity, whereas a 1 mT MF influenced the activity of horseradish peroxidase, and a strong MF (6 T) reduced the L-glutamate dehydrogenase and catalase activity, but 2 T substantially enhanced the activity of carboxydismutase. The strong MF also enhanced the activity of trypsin and ornithine decarboxylase [1]. MF affects the membranes and Ca2+ signalling in plant cells, and many magnetic effects in living organisms are probably due to the alterations in membrane-associated Ca2+ flux. Na channels are less affected than Ca2+ channels, and due to the changes in Ca2+ channels, the Ca content might be reduced in MF-treated plants. MF treatment in seeds induces changes in the protein and lipid profiles of harvested seeds [15].
4. Effect of MFs on Reactive Oxygen Species, Nitric Oxide Content, and Enzyme Activity
Many biochemical processes, such as photosynthesis and metabolism, are accompanied by the formation of radicals or radical anions, which are dependent on the magnetic field because they have unpaired electrons [39]. These radicals are also formed under the conditions of abiotic and biotic stress. They can damage cellular components such as lipids, proteins, and nucleic acids [97]. The accumulation of radicals can also lead to disturbances in gene expression, changes in the activity of certain enzymes, damage to membranes, and a reduction in the level of antioxidant hormones [57,98,99]. Studies have shown the effect of static magnetic field on the accumulation of reactive oxygen species (ROS) [17,40,41,42,45,51,57,58,63,64,98,100]. Oxidative stress has been investigated in several plant species such as cherry tomato, cucumber, lettuce, maize soybean, tobacco, and tomato. There was a significant increase in superoxide radicals and hydrogen peroxide after plant exposure to a magnetic field of 20–250 mT for an exposure time of 0.5–12 h depending on plant species. Studies have shown an increase in superoxide radicals from 35% to 100% and hydrogen peroxide from 8% to 104% depending on the variety and the used method. The concentration of the hydroxyl radical (.OH) in maize and soybean increased from 16% to 50% with an increase in the intensity of 100–200 mT MF. Generally, the magnetic field increases the mean concentration of radicals, especially in the metabolically active tissues of plant cells that contain free radicals. Other studies [48,51,52,58,76,100] in some cases showed a decrease in the value of ROS under the influence of MFs. This was the case for 30–45-day-old soybean and lettuce exposed to a high MF of 770 mT. The studies by Kataria et al. 2021 [48], Latef et al. 2020 [100], and Chen et al. [95] found that under the influence of MFs, the content of hydrogen peroxide and superoxide radicals decreased, while the content of nitric oxide radicals increased. In other studies [42,59], the simultaneous effect of MFs on the content of hydrogen peroxide and nitric oxide was demonstrated. The enzymes scavenging and protecting against reactive oxygen species (ROS) include superoxide dismutase (SOD), which is involved in the detoxification of ROS and the breakdown of superoxide radicals into oxygen and H2O2; catalase (CAT), which eliminates hydrogen peroxide H2O2 and disintegrates H2O2 into water and oxygen and the peroxidase family (peroxides isozymes); peroxidases (POX), which catalyses the degradation reaction of H2O2; guaiacol peroxidase (GPX), which acts as an active scavenger of reactive intermediate types of radicals and catalyses the reduction of H2O2 and HO2 to water and lipid alcohols; and ascorbate peroxidase (APX), which is the most extensively dispersed antioxidant enzymes using ascorbate as substrate; POD is another peroxidase that utilises guaiacol and pyrogallol as substrates for H2O2 detoxification [91,97,99,101]. In addition, antioxidant enzymes protect the plant which results in higher plant productivity. The main protective role against free radicals is to increase the activity of ROS-capturing enzymes. The first enzymes of the detoxification process are SOD and CAT, the activity of which significantly increased in plants treated with MFs, namely in cherry tomato, cucumber, lentils, maize, microalgae, radish, shallot, soybean, wheat, and lettuce [17,28,40,100,102,103,104,105,106,107]. The registered higher CAT activity was related to SOD, except for the research plant cells of algae and the germinating seeds of the soybean. The increased activity of antioxidant enzymes under the influence of stimulating magnetic treatments may indicate the alleviation of oxidative stress. Studies have shown a higher activity of SOD and CAT in the roots of lentils and maize plants than in their shoots, while the highest activity of these enzymes was recorded in the leaves of shallot and wheat plants [28,104,106,107]. The exposure of wheat seedlings to an SMF (30 mT) increased CAT activity and decreased APX and PO activities, which resulted in a 43% reduction in lipid peroxidation [56,108,109]. Other studies [54] showed an increase in SOD activity in MF-treated suspension tobacco cells (10 and 30 mT), with a simultaneous decrease in the activity of CAT and APX enzymes.
The purification of ROS can also be achieved with non-enzymatic antioxidants. Non-enzymatic antioxidants such as flavonoid anthocyanins and carotenoids, which are abundant in several parts of plants, can contribute to H2O2 removal [97,110]. Flavonoids are a group of naturally occurring compounds showing antioxidant activity as secondary metabolites in plants. Ghanati et al. (2007) [101] and Jouni et al. (2012) showed a reduction in the total amount of phenolic compounds and flavonoids in basil and broad bean plants exposed to SMFs. Other studies showed an increase in ascorbate content in plants treated with an MF of 7 mT and a decrease in the value of this parameter at 150 mT [46,111]. MDA, a lipid peroxidation product, has been recognised as an indicator of oxidative damage. Electrolyte leakage is also generally considered an indirect measure of plant cell membrane damage [112]. Several studies showed the effect of an MF with an intensity of up to 100 mT on the increase in MDA content in plants, while for an MF of 600 mT, the index was significantly reduced [25,61,113,114]. The methods using MFs and their effects on several plants in terms of reactive oxygen species, nitric oxide content, and enzyme activity are summarised in Table 2 and Figure 2.

Table 2.
Effects of MFs on reactive oxygen species, nitric oxide content, and enzyme activity.
Table 2.
Effects of MFs on reactive oxygen species, nitric oxide content, and enzyme activity.
| Variety | Plant Species | Method | Effect | Reference |
|---|---|---|---|---|
| Basil (Ocimum basilicum) | 12-week-old plants | 30 mT SMF for 6 days at 5 h/day to plants | Decreased the activity of polyphenol oxidase (ap.24%) and phenylalanine ammonia-lyase (ap.68%) and phenolic compound content (ap.73%) in shoots; increased the amount of essential oils of methyl chavicol (46%) | [115] |
| Bean (Phaseolus vulgaris L.) | 14-day-old plants | 130 mT SMF within growing plants | Increased the guaiacol peroxidase activity by 44% in leaves but no significant changes in roots and shoots | [19] |
| Broad bean (Vicia faba L.) | Two-leaved plants | 15 mT SMF for 8 days, each 8 h/day of plants | Increased the SOD activity (ap.30%) and the rate of lipid peroxidation (MDA ap.6%); decreased the total flavonoid content (ap.25%), and peroxidase and polyphenol oxidase activity (ap.18%) | [101] |
| Broad bean (Vicia faba L.) | 8-day-old seedlings | 30 mT SMF for 8 h/day of plants | Increased the content of hydrogen peroxide by 75% in the shoot and enzyme activity of CAT by about 100% in root and shoot | [110] |
| Cherry tomato (Lycopersicon esculentum L.) | Germinating seeds | 50–150 mT SMF at 30 min and 1 h to seeds | Increased the radical content of superoxide (ap.100% at 4 h imbibition) and hydrogen peroxide (ap.60% at 24 h imb.) and antioxidant enzyme activities of SOD (ap.26%-36 h imb.), catalase (ap.36%-8 h imb.), POX (ap.78%-4 h imb.), APX (ap.150%-12 h imb) and GR (ap.50%-24 h imb.) | [40] |
| Cucumber (var. Barsati) | 7-day-old seedlings | Pre-treatment of SMF 100 to 250 mT for 1, 2, or 3 h of seeds at imbibition time of | Increased the content of superoxide (40%), hydrogen peroxide (8%) and hydrolytic enzyme activity of b-amylase (51%), protease activities (13%), and the antioxidant enzyme activity of SOD (8%,), GR (77%), and CAT (83%) | [17] |
| Lentils (Lens culinaris L.) | 15-day-old seedlings | Pre-treatment of SMF from 0.06 to 0.36 T for 5, 10, and 20 min to seeds | Increased the enzyme activity of APX by 210% and 350% in shoot and root, respectively (at 0.36 T, 20 min) but no significant changes in SOD | [104] |
| Lentils (10823 (ILL10823) | Shoots and roots of 7 days of plant growing | Pre-treatment of SMF 1–100 mT for 5–30 min to seeds | Increased the enzyme activity of SOD (to 170%), CAT (to280%), and APX (to 270%) in roots depending on the value of MFs; generally decreased the MDA enzyme (to 78%) in roots | [28] |
| Lettuce (Lactuca sativa var. cabitat L.) | 14-week-old plants | Pre-treatment of 0.44, 0.77, 1 T for 1-3 h | Decreased the content of hydrogen peroxide ap.44%), superoxide (ap.44%), and malondialdehyde (31.7%) for 0.77 T at 1–2 h; increased the content of nitric oxide (ap.200%) and the antioxidant enzyme activities of SOD (ap.94%), POD (ap.900%), and GPX (ap.428%) for 0.77 T at 2 h; APX (ap.383%) and CAT (ap.750%) for 0.77 T at 3 h; the non-enzymatic of anthocyanins (257%), ASA (68.3%), GSH (69.7%), and α-tocopherol (165%) for 0.77 T at 3 h; and flavonoids (211%) and phenolics (355%) for 0.77, 1, and 0.44 T at 2–3 h, respectively | [100] |
| Lupin (Lupinus angustifolius L.) | 14-day-old plants | 0.2 mT at16 Hz and 50 Hz MF in growing plants | Increased the guaiacol peroxidase activity by 53% at 50 Hz in roots but no significant changes in shoots | [116] |
| Maize (Zea mays L.) | 7–10-day-old plants | Pre-treatment of SMF 3 and 10 mT for 4 h of seeds | Increased the enzyme activities of SOD (178%, 432%), APX (90%, 100%), and CAT (160%, 468%) for plant (shoot, root) higher value at 3 mT | [104] |
| Maize (Zea mays L.) var. HQPM.1 | 8-day-old seedlings | Pre-treatment of SMF 200 mT for 60 min and 100 mT for 120 min to seeds | Increased the content of superoxide (31–57%), hydroxyl radical (26–39%), hydrogen peroxide (13–48%), and the enzyme activity of POD (10–58%), with a higher value at 200 mT; decreased the SOD enzyme activity (26–64%), with a lower value at 200 mT | [41] |
| Maize (Zea mays) var: HQPM.1 | 30-day-old plants | Pre-treatment of 100 mT for 2 h and 200 mT for 1 h to seeds | Decreased the antioxidant enzymes of SOD (43%) and POD (26%) and reactive oxygen species content of superoxide (26%) and hydroxyl (5%) in leaves at 200 mT | [14] |
| Maize (Zea mays L.) var. JM 216 | 45-day-old seedlings (leaves) | Pre-treatment of SMF 200 mT for 1 h to seeds | Increased the content of superoxide (52%), hydrogen peroxide (12%), α-amylase (76%), and protease activities (ap.3%) of seedlings; decreased the hydrogen peroxide (30%) in leaves | [45] |
| Maize (Zea mays L.) var. JM-216), soybean (Glycine max L.) var. JS-335) | 8-day-old seedlings | Pre-treatment of SMF 200 mT for 1 h to seeds and inhibition time of 96 h | Increased the radical content of superoxide by 81% and 30% hydrogen peroxide −320% and 28%, and enzyme activity of α-amylase (ap. 70%) and 170% for maize and soybean, respectively, and protease s (ap. 6%), depending on inhibition time | [51] |
| Microalga (Chlorella kessleri LEB 113) | Plants with 10 days of cultivation | 30 mT or 60 mT within 10 days of growing, exposure time 24 h or 1 h per day | Increased the antioxidant activity of methanol extracts by 77–217% at 60 mT for 1 h/d depending on the method | [25] |
| Microalgae (Chlorella vulgaris) | Algae cells in culture medium | 10–50 mT SMF for 12 h to plant cells | Increased the antioxidant enzymes activities of SOD (124%), CAT (69%) at 50 mT, and POD (ap.50%) at 10–35 mT | [103] |
| Mung bean (Vigna radiate) | 4-day-old seedlings | Pre-treatment of 600 mT SMF to seeds by conveyer belt | Increased the nitric oxide content (ap.32%, root; 36%, shoot), and the activity of nitric oxide synthase (ap.16, root; 25%, shoot); decreased the concentration of malondialdehyde (ap.56%, root; 8%, leaves), hydrogen peroxide (ap. 13%, leaves) | [95] |
| Parsley (Petroselinum crispum L.) | Plant cells after 6 and 12 h of treatment | 30 mT SMF for 4 h | Increased the activity of CAT (38% at 6 h and 1500% at 12 h), and MDA indicator by ap.16% at 12 h; decreased the activity of APX by 30% and 70% after 6 and 12 h of treatment, respectively | [109] |
| Radish (Raphanus sativus L. var. radicula D.C.) | 5-day-old seedlings | Treatment of 185–325 μT for 14 h to seedlings in light and darkness | Increased the activities of antioxidant enzymes of SOD (up to 135% μT), CAT (up to 135–150%) at 325–650 μT and soluble PO (up to 36–57%) at 185–310 μT, and malondialdehyde content (210%) at 325 μT; lowest value (110%) at 620 μT depending on the test | [117] |
| Shallot (Allium ascalonicum L.) bulbs | Plant of roots and leaves of 8, 12, and 17 days old (symplastic, apoplastic) | 7 mT SMF for 17 days | Increased the antioxidant enzyme activities of GPOD (ap.33% for apoplastic), CAT (ap.40–50% for apoplastic and leaves), SOD (ap.20%, leaves), APX (ap.17%, leaves), the non-enzymatic activity of ascorbate (ap.39%), glutathione (ap.24%), the enzyme activities of glucose-6-PDH (30%), and glutathione reductase (ap. 25%) for leaves of 12–17 d old | [118] |
| Soybean (Glycine max L. Merrill) | Germinating seeds of 1–144 h | 2.9–4.6 mT SMF at 2.2, 19.8, and 33 s to enzyme and seeds | Increased the antioxidant enzyme activities of SOD (130% at 19.8 s for 0–24 h) and CAT (20% at 19.8 s for 24 and 72 h) of root | [102] |
| Soybean (Glycine max L. Merrill J 357) | 28-day-old plants | 2.9–4.6 mT SMF at 2.2 and 19.8 s to seeds | Increased the peroxidase enzyme activity (36% at 19.8 s) and RNA concentrations (111%-2.2 s) for leaves | [119] |
| Soybean (Glycine max L. Merrill) | Approx. 15-day-old plants | 20 and 30 mT SMF for 5 days, 5 h/d of plants | Increased the radical content of hydrogen peroxide (ap. 8–50%) and the enzyme activity of CAT (ap.16% for 2 d) at 30 m, contrary at 20 mT MF | [64] |
| Soybean (from Ayyub Agriculture Research Institute) | Seedling of early growth stage | Pre-treatment of SMF 50, 75, and 100 mT for 3 and 5 min to seeds | Increased the content of MDA (ap.40% at 50 mT for 3 min), ascorbic acid (ap.50–300% at 75 mT-3, 5 min), phenolics (ap.50%), and enzyme activity of PRT, α-AMY, SOD, CAT, and POD in the highest level of 75 mT at 3 and 5 min, and 50 mT and 100 mT at 3 min (over 50%) | [20] |
| Soybean (Glycine max L.) Merr. var: JS-335) | 8-day-old seedlings | Pre-treatment of SMF 150 and 200 mT for 1 h to seeds | Increased the content of superoxide (33–75%), hydroxyl radical (16–50%), hydrogen peroxide (58–30%) in seedlings (embryo hypocotyl), and enzyme of POD (27%, cytosolic; 67%, wall-bound) at 200 mT; decreased ascorbic acid (53%, embryo; 37%, hypocotyl), SOD (12%, cytosolic; 27%, wall-bound), APOX (38%, hypocotyl) at 200 mT | [14] |
| Soybean (Glycine max L.) var. JS-335) | Seedlings growing within 5 days | Pre-treatment of SMF 200 mT for 1 h to seeds | Increased the content of hydrogen peroxide (77%), nitric oxide (42%), superoxide (35%), and enzyme activity of 𝛼-amylase (48%), nitrate reductase (178%), and protease (17%) in roots | [52] |
| Soybean (Glycine max L.) variety JS-335) | 5-day-old seedlings | 200 mT SMF for 1 h to seeds | Increased the radical content of superoxide (43%), hydrogen peroxide (104%), nitric oxide (50%), and enzyme activity of amylase (128%) NOS (75%), and NR (138%) | [42] |
| Soybean (Glycine max L.) Merr. var: JS-335) | 30-day-old plants | Pre-treatment of 200 mT for 1 h and 150 mT for 1 h to seeds | Decreased the superoxide radical content by 16% in leaves at 200 mT | [43] |
| Soybean (Glycine max L. Merrill) var. JS-335 | 45-day-old plants | Pre-treatment of SMF 200 mT for 1 h to seeds | Decreased hydrogen peroxide content by 46%, and activity of antioxidant enzymes of SOD, APX, GR, and POD by 30–300% in leaves Increased α-tocopherol by 36%, ASA/DHA over 30% in leaves, and activity of nitrogenase enzymes in roots by 161% | [51] |
| Soybean (Glycine max) var. JS-335 | 45-day-old plants | Pre-treatment of 200 mT SMF for 1 h to seeds | Increased the activity of carbonic anhydrase (33%) in leaves and nitrogenase (151%) in root and nitric oxide (86%); decreased the content of superoxide (12%), malondialdehyde (14%), and proline (54%) in leaves | [59] |
| Soybean (Glycine max) var. JS-335 | 45-day-old plants | Pre-treatment of 200 mT SMF for 1 h to seeds | Decreased the hydrogen peroxide content (30%), activities of SOD (38%), POD (66%), and GR (60%) in leaves | [51] |
| Soybean (Glycine max L.) variety JS-335) | 45-day-old plants | Pre-treatment of 200 mT SMF for 1 h to seeds | Increased the content of nitric oxide (ap.53%) and nitrate reductase activity (ap.33%); decreased the content of hydrogen peroxide (40%) and α-tocopherol (94%) | [48] |
| Tobacco (Nicotiana tabacum L. cv. Burley 21) | Plant cells | 0.2 m T SMF up to 24 h | Increased the content of NO radical (25–100% for 8 h), hydrogen peroxide (25–108% for 18 h), and salicylic acid (9–30% within 8–24 h) | [63] |
| Tobacco (Nicotiana tabacum L. cv. Burley 21) | Plant cells | 10 mT or 30 mT SMF for 5 days, from day 3 to 7 of subculture | Increased the activity of soluble peroxidase (61% at 10 mT), covalently bound peroxidase (46% at 30 mT), and decreased the ionically peroxidase activity fraction (ap.54% at 10 mT) | [53] |
| Tobacco (Nicotiana tabacum L. cv. Burley 21) | Plant cells | 10 and 30 mT SMF for 5 days, 5 h each day | Increased the enzyme activities of SOD (87% at 30 mT) and decreased activities of CAT (70% at 30 mT) and APX (27% at 10 mT) | [53] |
| Tomato (var. Pusa Rohini) | Germinating seeds of 12 and 24 h | Pre-treatment of 100 mT SMF for 30 min | Increased the content of superoxide (38%), hydrogen peroxide (ap.100%), and antioxidant enzymes activities of catalase (3.7-fold) and ascorbate peroxidase (4.4-fold) at 24 and 12 h of imbibition, respectively | [57] |
| Wheat (Triticum aestivum L. cvs. Tekirdag and Selimiye) | 28-day-old cultivars | Pre-treatment of SMF 2.9–4.7 mT at 2.2–19.8 s | Increased the enzyme activities of SOD (57%, 47%), POX (25%, 202%), APX (160%, 100%), CAT (190%, 100%) and FRAP value (40%, 43%) for Tekirdag cul. (leaf, root) higher value at 19.8 s | [107] |
| Wheat (Triticum aestivum L. cv. Kavir) | Approx. 4-day-old seedlings | 30 mT SMF) for 4 days, each 5 h of germinated seeds | Increased the antioxidant enzyme activity of CAT (ap.70%) and decreased PO activity (ap.24%) | [108] |
| Wheat (Triticum aestivum L. cv. Kavir) | Approx. 3-month-old plants | 30 mT SMF for 4 days, each 5 h of plants before harvest | Increased the activity of CAT (16-fold), radical scavenging capacity (13%) and decreased activity of PO (86%) and rate of lipid peroxidation of membranes (43%) of wheat seeds | [56] |
| Wheat (Triticum aestivum | 100-day-old plants | Pre-treatment of max SMF of 50 mT by seeds or water passing | Increased the phytohormones content of gibberellic acid (76%), indole acetic acid (143%), and benzyl-adenine (212%), and decreased abscisic acid (22%) for seed +water | [69] |
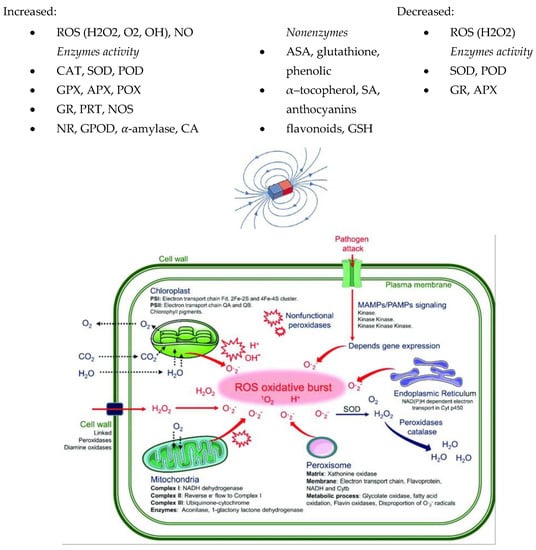
Figure 2.
The summary of the effects of MFs on ROS, NO content, and enzyme activity [120]. Copyright Plants 2021.
Figure 2.
The summary of the effects of MFs on ROS, NO content, and enzyme activity [120]. Copyright Plants 2021.
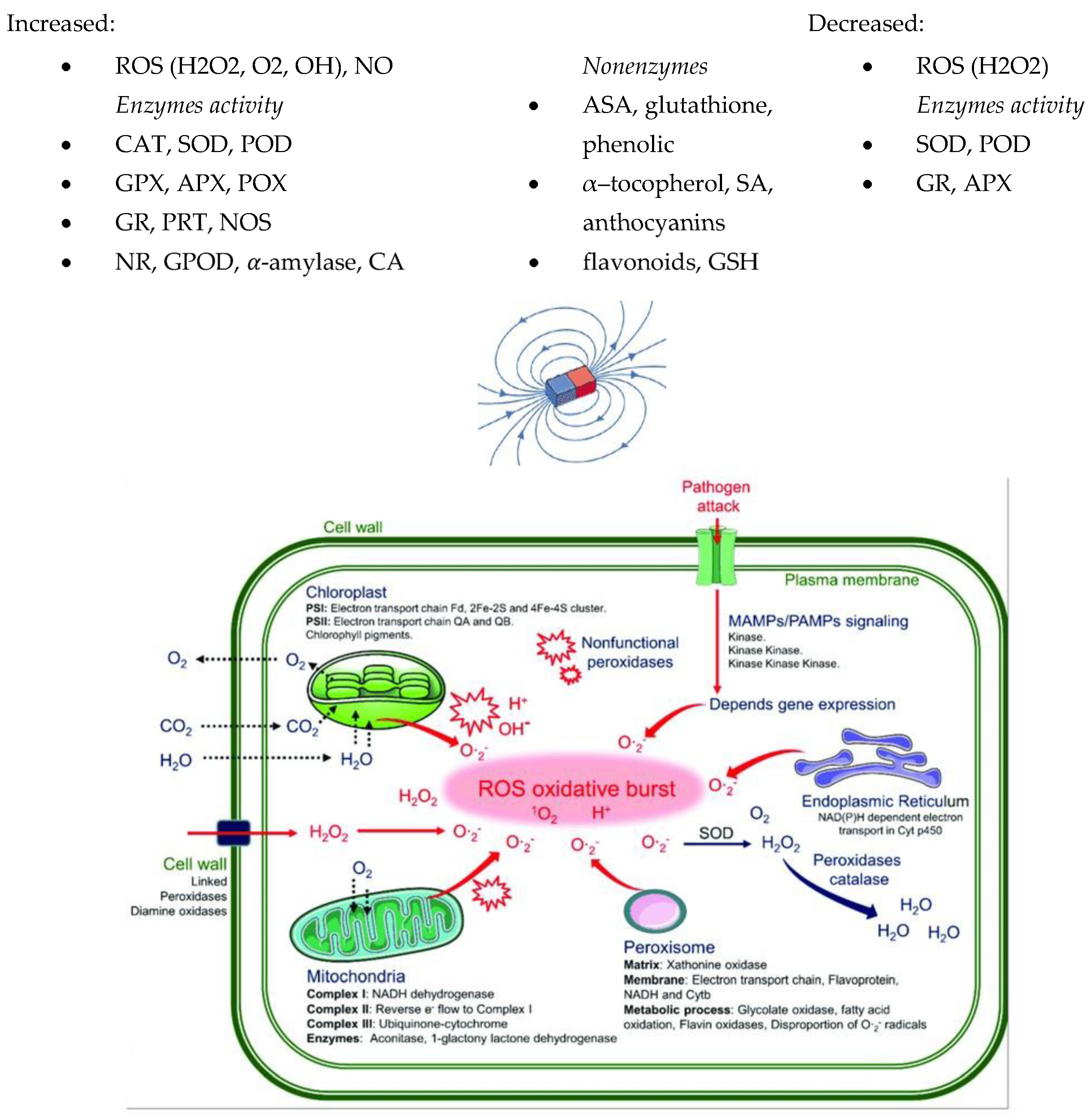
5. Effect of MFs on Structure and Cell Growth
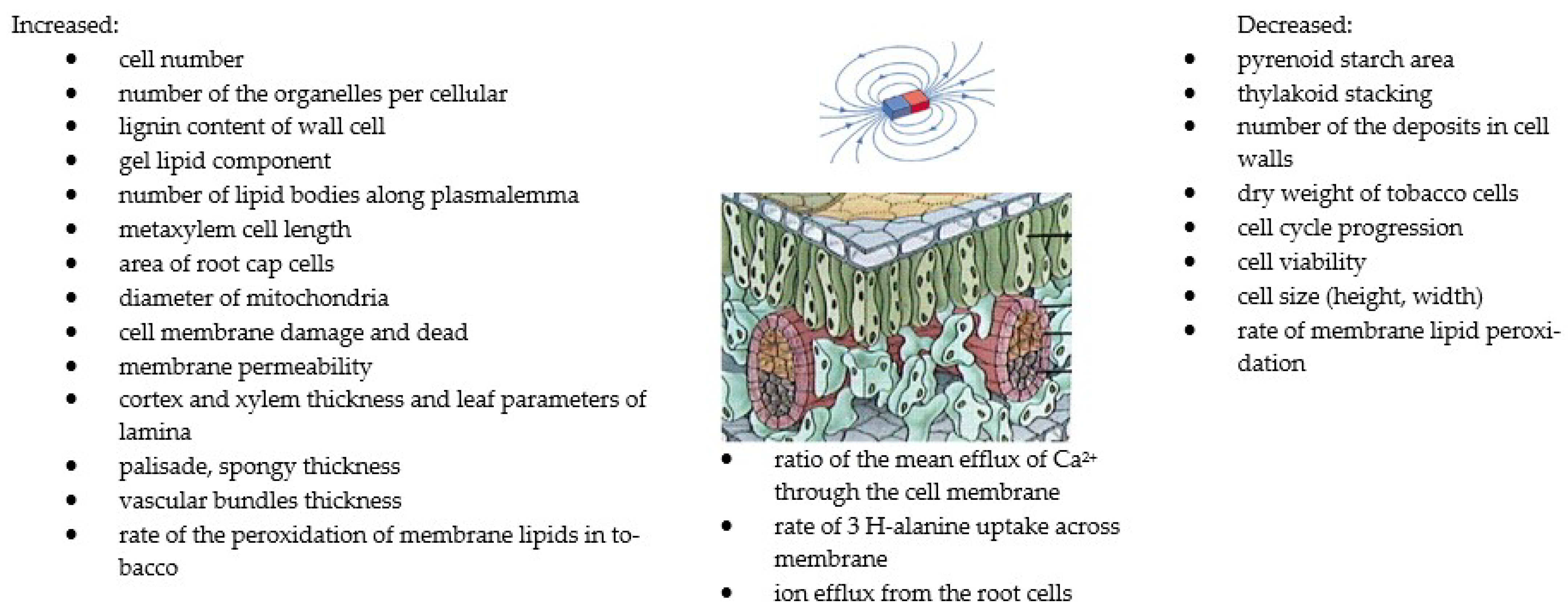
The influence of MFs on the shape of the plasma and the plasma membrane of various biological systems has been studied [35,91,121,122,123]. Medium-intensity SMFs have a strong influence on the shape of the cell and the plasma membrane of various cell types. The extent of cell structure modification in response to SMFs significantly depends on the exposure time. Studies have shown a significant influence of MF plant treatment on biomass concentration [22,25,33]. The highest increases in this parameter, up to 83.2%, were obtained for an MF with an intensity of 60 mT and exposure time of 1 h/d. It was concluded that the application of a higher SMF intensity resulted in a greater amount of obtained biomass. The concentration of biomass results from the increase in the number of cells under the influence of MFs. Mroczek-Zdyrska et al. (2016) [19] showed a maximum cell growth of 79% at 130 mT, depending on the different phases of mitosis in the bean root meristem. Studies [96,124,125] showed a reduction in cell size (length and width) of up to 30% at 30 mT and the induction of longer metaxylem cells at 7 T with a time exposure of 30 h. Belyavskaya (2001) [126] studied the effect of a weak MF on changes in the structure and ultrastructural organisation of some organelles and meristematic cells of pea seedlings. Changes in the volume of the granular component of the nucleus decreased, the nucleolus vacuole appeared, and the shape changed to a more rounded one, compared with the control sample. The degree of alignment of cellular structures under the influence of SMFs depends on the intracellular composition [93]. Minor changes [93] were found in the ultrastructure of C. kessleri cells exposed to a 10 mT MF. Chloroplast and the area and number of starch granules significantly increased. Another study [96] showed internal changes in the stem and leaf structure parameters in tomato plants as affected by magnetised rehydration water and seed treatment with a 50 mT MF. These parameters of cortex xylem and lamina, and spongy and vascular bundles’ thickness increased, and higher values were recorded for magnetised water. Plant cell membranes are primarily exposed to stress, and changes in the membrane structure can cause intracellular modifications (Reszczyńska and Hanaka (2020) [127]). The loss of membrane integrity can alter the composition, structure, and function of plant cells. Selim et al. (2019) [35] found that cell membrane permeability was improved by a 50 mT MF. This parameter increased by about 29,97% when treating plants with magnetised grains, magnetised water, and the combination of magnetised grains and water, respectively, compared with the control. Reduced lipid peroxidation resulted in a decrease in electrolyte leakage and, therefore, reinforcement of membranes. These observations were confirmed by Payez et al. (2012) [108] for 4-day-old plants with a 30 mT MF. By contrast, Sahebjamei et al. (2007) [54] observed an inverse relationship, namely that exposure to MFs significantly increased the level of the peroxidation of membrane lipids of suspension-cultured tobacco cells, compared with the control. Ercan et al. (2022) [91] confirmed that MFs in the range of 20–250 mT caused cell membrane damage in the root tip cell of barley. Other studies [13,128] showed that MFs increased the rate of the efflux of calcium through the cell membrane and ions from the root cell. The magnetic field’s influence on living cells is on the cell cycle [63]. However, the intensity varies depending on cell type, the morphological modifications of cells, and treatment duration. It is assumed that the magnetic field affects the structures of cell membranes, thereby increasing their permeability, which, in turn, influences the various activities of the metabolic pathways. MF treatments can have a significant impact on reducing lipid peroxidation, which reduces electrolyte leakage and thus strengthens the membranes and improves plant growth. The methods using MFs and their effects on several plants in terms of the structure of plants, cell growth, and biomass productivity are summarised in Table 3 and Figure 3.

Table 3.
Effects of MFs on structure and cell growth.
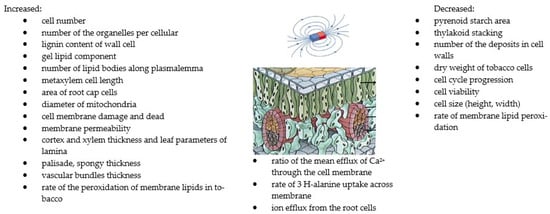
Figure 3.
The summary of the effects of MFs on structure and cell growth [13,27,53,124,126]. Copyright https://brainly.co.id (accessed 23 August 2022).
6. Effect of MFs on Plant Components
Biomass is rich in biologically active compounds such as peptides, polysaccharides, fatty acids, carotenoids, amino acids, etc. For this reason, it can be used in the production of biofuels, pharmaceuticals, and food supplementation [131,132,133].
Chlorophyll is the main photochemically active compound and plays a key role in the growth and adaptation of plants to various environmental conditions. SMF treatments significantly increased the chlorophyll content in barley, canola, chickpea, date palm, maize, maize, microalga, paulownia, soybean, sweet pepper, and wheat [23,31,36,45,48,91,94,134,135,136]. Static magnetic fields in the range of 100 mT and exposures for 240–360 min increased the content of photosynthetic pigments, chlorophyll, and carotenoids above 10% [134]. The combination of MF intensity and exposure time affects the pigment content. Long-term exposure to MFs may reduce pigment content [5,46]. The exposure time of 24 h, compared with 1 h, at the field intensity of 30 mT significantly reduced the content of chlorophyll and carotenoids in the leaves of microalgae. Similarly, an increase in MF activity from 30 to 60 mT resulted in a decrease in pigment content. Other studies [5] showed that the highest content values of chlorophyll and carotenoids in soybean were measured for 3 min 250 mT and were, respectively, almost 80% and 400% higher than those of the control group. On the other hand, an increase in the exposure time and MF intensity decreased the content of chlorophyll by 10% and 14%, and carotenoids by 32% and 57%, respectively. Research [23,32,35,137] has shown that the content of photosynthetic pigments is also significantly influenced by the magnetic water used for irrigation. Selim et al. (2019) [35] found that irrigated magnetic water increased the content of total chlorophyll and carotenoids by 40% and 50%, respectively, compared with the MF treatment of seeds. Moreover, MFs improve the absorption of the necessary elements needed for the formation of chloroplasts and chlorophyll, as characterised by paramagnetic properties [29,138]. The magnetic field stimulates cell growth and influences the biochemical composition of plants depending on factors such as the intensity and duration of exposure. Some studies showed a higher content of protein, carbohydrates, and in some cases, lipids compared with the control [22,25,37,91]. This fact proves that the MF application is a real alternative to the stimulation of lipid synthesis; however, it influences the synthesis of macromolecules in different ways. The use of 30 mT MF for 24 h increased the protein content in microalgae on average by 9%, while the highest carbohydrate content at the level of 45% was observed when using the higher field of 60 mT for 24 h compared with the control [46]. While Bauer et al. (2017) [25] recorded the highest increase in carbohydrate content by 14% for 30 mT, for the field intensity of 60 mT, the tested parameter significantly decreased. Treatment of plants with 30 and 60 mT MFs for 1 h also increased the lipid content by an average of 13% [139], although other studies [46] showed a decrease in the value of this parameter with an application time of 24 h. MFs have significant impacts on the content of other plant components such as proline, soluble sugars, amino acids, ferritin, and fatty acids [20,23,64,100,108]. The highest values of these parameters were recorded at field intensities of 30 and 100 mT, and 0.77 T.
Ferritin is attached to an ion channel and can influence the dynamics of ion transport and the changed movement of ions across the membrane [140]. Hozayn et al. (2016) [23] showed that the magnetic water irrigation of canola increased the content of unsaturated as well as saturated fatty acids and caused the decomposition of oil. In the group of unsaturated acids, linoleic and oleic acids dominated, while in saturated acids, palmitic and stearic acids dominated. Kataria et al. (2020) [58] showed a significant increase in the DNA and RNA content in plants exposed to a magnetic field, which could be caused by an increase in the expression of enzymes that play a role in shoot formation, chlorophyll biosynthesis, and peroxidase biosynthesis. The research of Asghar et al. (2016) [20] showed a higher soybean content of soluble and reducing sugars in the seedlings treated with a magnetic field, compared with the control. Studies have shown the effect of magnetic treatments on the uptake and accumulation of macroelements (N, P, K, Ca, and Mg) and microelements (Fe, Mn, and Cu) in the root and shoot of wheat plants (Selim and Selim) [35]. The maximum increase in uptake of the abovementioned minerals was recorded by the use of magnetised irrigation water compared with the MF seed treatment. Increases in the values of these elements ranged from 152% to 217%. The obtained results are consistent with those mentioned by Taimourya et al. (2017) [32] for strawberries and tomatoes. In contrast, studies on canola with the use of magnetic water showed a reduction in the content of elements such as N, Fe, and Zn and an increase in Mn and Cu compared with the control [23]. MFs may result in better water penetration through plant cell membranes, resulting in increased mineral solubility and better mineral absorption by plant roots [23,141]. Other studies have also shown an increase in the content of essential elements in plants treated with MF. There was an increase in the content of micro- and macroelements in the leaves of date palm plants under the influence of a 100 mT MF [134], while for microalgae, there was a decrease in the content of Fe and Cu microelements and an increase in Zn, Mn, Ni, and Ca for a 10 mT MF [93]. A study by Ercan et al. (2022) [91] showed a very significant reduction in the content of Mg, Ca, K, and P in the roots of the barley plant and rather their stabilisation in the leaves. On the other hand, the content of micronutrients significantly increased both in the roots and leaves of the plant. The methods using MFs and their effects on several plants in terms of plant components are summarised in Table 4 and Figure 4.

Table 4.
Effects of MFs on plant components.

Figure 4.
The summary of the effects of MFs on plant components [23,34,91,93,94,100,134,142].
7. Effect of MFs on Gene Expression
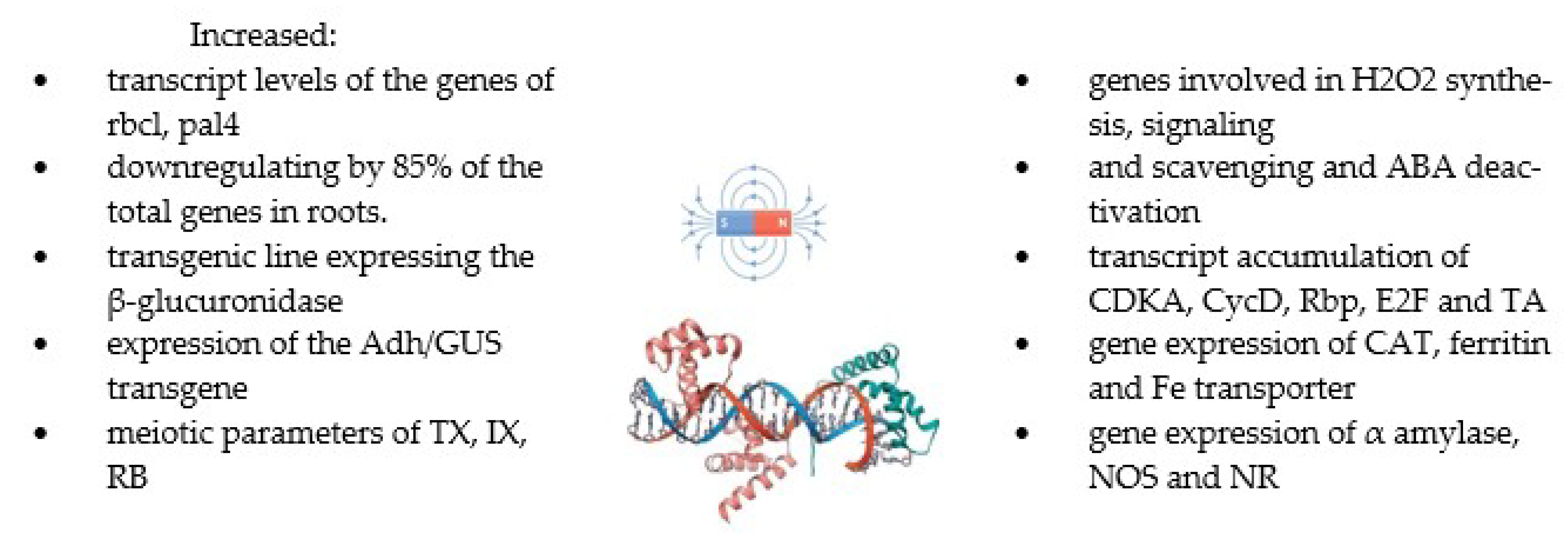
Magnetic fields influence DNA and RNA synthesis and cell proliferation and can cause changes in cellular metabolism and various cellular functions [59,143,146]. In doing so, they activate the cellular stress response as a protective mechanism that induces gene expression in the stress response. Paul et al. (2006) [129] found that MF T values induce the expression of the Adh/GUS transgene in Arabidopsis roots and leaves. Microarray analyses of 8000 genes showed that 114 genes were differentially expressed by more than 2.5-fold compared with the control sample. A static magnetic field of 30 mT increased the relative expression of the CAT and Fe transporter gene, which resulted in the enhancement of the total iron contents of the plants compared with the control. This induced the expression of the ferritin gene and an increase in ferritin content, which is involved in protection against oxidative stress [147]. Catalase is another main H2O2 scavenger, and the expression and activity of its gene increased in treated plants by MFs. However, a reduction in the gene expression of ferritin and CAT in a 20 mT SMF was observed. Ferrous content is superior to the expression of ferritin and CAT genes and is controlled by the expression of the Fe transporter gene. It can be processed by a magnetic field, which may have perturbed chemical reactions [129]. Some studies [42,148,149] showed a higher expression of the α-amylase gene and total amylase activity in seeds treated with SMFs, which resulted in increased seed germination and seedling vigour. This is mainly due to metabolic changes, including gene transcription, protein biosynthesis, and enzymatic activity [150]. The effects of MFs on gene expression have been investigated for very strong fields [129] and near-null and weak fields for GMFs [151,152]. A study by Dhiman and Galland (2018) [62] showed a significant effect of GMF reversal on the gene expression and growth of Arabidopsis seedlings. A similar effect on gene expression in null MFs was demonstrated by Xu et al. [153,154,155], and Agliassa et al. [151]. Another study [27] showed that in roots treated with a magnetic field, approximately half of the 359 downregulated genes changed more than two-fold compared with the control. The growth of chloroplasts was inhibited by a 600 mT SMF. Mohammadi et al. (2018) [63] and Okano et al. [111] showed that the exposure of tobacco cells to SMFs increased the content of reactive oxygen species and radicals (3–6 h of exposure), which modify proteins (through S-glutathionylation and S-nitrosylation) and thus regulate gene expression and activity proteins. Similar relationships were observed on germinated tomato seeds and magnetically treated soybean seedlings [42,156]. Anand et al. (2019) [57] investigated the expression of genes related to the synthesis, scavenging, and signalling of hydrogen peroxide under the influence of a 100 mT MF. The relative expression of genes involved in the production of hydrogen peroxide, i.e., amine oxidase (AO), superoxide dismutase (SOD1 and SOD9), and RACK 1 homologue (ArcA2) was significantly increased in treated seeds by MFs. Amine oxidase played a major role in the production of hydrogen peroxide, which regulates the expression of various genes involved in plant development. The methods using MFs and their effects on several plants in terms of gene expression are summarised in Table 5 and Figure 5.

Table 5.
Effects of MFs on gene expression.
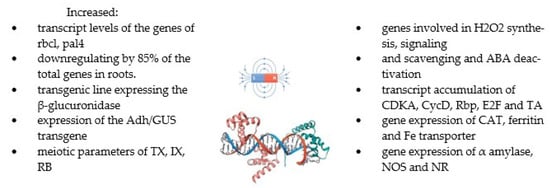
Figure 5.
The summary of the effects of MFs on gene expression [27,42,57,62,63,64,128,143].
8. Possible Mechanisms
Living organisms, including plants, generate various electric fields with which they are associated, such as membrane, electrical, functional, or flow potentials. Therefore, external MFs can influence plant development and metabolism through interactions [157]. In recent years, the following models explaining the mechanisms of the influence of MFs on biological systems have been proposed [18]: quantum oscillator and electronic cyclotron resonance quantum interference of bound ions and electrons, coherent quantum excitations, effects of torsion fields, free-radical mechanisms, parametric and stochastic resonance model phase transitions, etc. The radical-pair mechanism has been proposed to explain the effect of MFs on enzyme-catalysed reactions involving free-radical-pair intermediates [61,158,159]. Several studies [56,64,110] reported the influence of MFs on ROS production, the initiation of oxidative stress, the activity of enzymatic antioxidants, or the expression of their genes. It can also affect the singlet–triplet conversion of free radical pairs, which is driven by the internal magnetic fields produced by nuclear spins [74]. The energy involved in the recombination of radical pairs results from the interaction between the spins of unpaired electrons and the spin of adjacent nuclei; between the spins of the radical pair; and the interaction of the electron’s isolated spin and magnetic field (the Zeeman interaction) causing the direction of the electron’s magnetic moment to oscillate [61]. The magnetosensitive reactions of Arabidopsis plants were elucidated based on the radical-pair mechanism. Upon photoexcitation, a radical pair is formed, and the yield thereof depends on the direction of the MF. In this way, it allows the protein to act as a radical-pair-based magnetic sensor, which depends on the optimal radical-pair lifetime. The induced spin relaxation can also affect the magnetosensitive reactions of plants [159,160].
Binhi and Prato [65] developed the so-called molecular gyroscope mechanism. The essence of this mechanism is the rotation of large fragments of macromolecules or amino acid residues with distributed electric charges under the influence of MFs. The biological effect is related to the reaction yield, the number of gyroscopes that enter this reaction, or those that are in a state of equilibrium.
Vaezzadeh et al. (2006) [161] presented a theoretical model based on the oscillation of ferritin under the influence of MFs. The paramagnetic components of the cell include the concentrations of Fe, Co, and diamagnetic starch [162]. There is a theory that explains the increase in chloroplast content under the influence of MFs [142,163]. Chloroplasts contain Mn2+, which is a paramagnetic substance. Therefore, the MF energy may be absorbed, which affects the mobility and uptake of ions which play important roles in photosynthesis. Goldsworthy [164,165,166] presented the mechanism of changing the membrane potential and the permeability of the cell membrane under the influence of an electric field as a result of the selective removal of Ca2+ from the membrane and its replacement with other cations (mainly K+) [4]. Another model, the so-called ionic cyclotron resonance, was presented as a mechanism to explain the interaction between MFs and the ionic current in the plant cell membrane, which resulted in changes in ion concentration and osmotic pressure [15,135,167]. This mechanism is based on the interaction between the ions circulating in the plane perpendicular to the field and the MF. Binhi and Prato (2017) [65] presented a universal physical model as a mechanism for the interaction of MFs with the magnetic moments of unpaired electrons, paramagnetic ions (e.g., iron, copper, manganese), protons, and other particles. The disruption of the dynamics of the magnetic moment produces a biological effect at the physical and chemical levels.
9. Conclusions and Perspectives
The scientific achievements concerning the influence of MFs on the activity of plants, measured by the dynamics of changes in life processes, changes in the content of pigments and elements, and the structure of plants were reviewed. The various parameters of MF photosynthesis in plants were discussed in this review. Photosynthesis is a complex process, and the effects of magnetopriming have not been fully explored. The literature shows that MFs had a significant impact on photosynthesis efficiency. Research showed an increase in photosynthesis parameters in plants such as the maximum quantum efficiency, the electron transport quantum efficiency, the relative phase amplitude, the photosynthesis rate, and the efficiency index, which contributed to a higher level of light absorption efficiency. The research results presented in the literature review showed that MFs increase the biomass and vigour accumulation indexes, and thus have an impact on the plant yield.
In general, treating plants with SMFs accelerates the formation and accumulation of reactive oxygen species. This is related to the risk of oxidative stress. At the same time, the influence of MFs causes the high activity of antioxidant enzymes, which reduces oxidative stress. Research shows an increase in the activity of SOD, POD, and CAT enzymes of up to 300–400%, depending on the intensity of the MF, application time, and type of plant. The influence of MFs on the structure and development of cells was investigated in terms of the shape of the plasma and the plasma membrane of various biological systems. Medium-intensity (6 mT) SMFs have a strong influence on the shape of the cell and the structure of the cell membrane, thus increasing their permeability, which, in turn, influences the various activities of the metabolic pathways. Significant changes were recorded in the root meristem cells of plants exposed to MFs in terms of increases in their cell density and size. Changes in the ultrastructural organisation of some organelles, a decrease in the volume of the granular component of the nucleus, and the appearance of the nucleolus vacuole in comparison with the control roots were found. Static magnetic fields in the range of 10–100 mT and exposures for 30–360 min significantly increased photosynthetic pigments (chlorophyll, carotenoids). Studies also showed a higher content of proteins, carbohydrates, soluble and reducing sugars, and in some cases, lipids and fatty acid composition in plants under the influence of MFs, compared with the control. The use of magnetic treatments on plants influenced the uptake and accumulation of macroelements (N, P, K, Ca, and Mg) and microelements (Fe, Mn, and Cu) in the roots and shoots of plants. The influence of MFs on gene expression has been proven, and it depends on its intensity and application time. Researchers have shown that about half of the genes are regulated in roots treated with a magnetic field. A 30 mT static magnetic field increased the relative expression of the CAT and Fe transporter gene, which resulted in an increase in iron content in the plants compared with the control. Moreover, the greater expression of the ferritin gene, which is involved in the protection against oxidative stress and catalase as the main scavenger of H2O2, was shown. On the other hand, a higher expression of the α-amylase gene under the influence of MFs resulted in increased seed germination and vigour of seedlings. Research on the effects of magnetic fields on plants should be continued. This allows the development of a technique by which it will be possible to influence the course of biochemical processes related to plant metabolism and also influence enzymatic processes, chemical reactions, the structural system, properties of antioxidant plants, and the content of nutrition components. Owing to this research, it will be possible to discover the sense of sight and sensitivity of plants. There is a need to identify plant magnetoreceptors and study the cellular responses that convert pulses of a biophysical nature into quantum ones.
The current knowledge about the influence of MFs on living organisms is still insufficient, even more so because today’s research shows that many cellular properties can be modified through a static magnetic field.
Author Contributions
Conceptualisation, C.P. and B.S.; formal analysis, C.P.; investigation, B.S. and G.Z.; resources, C.P. and A.P.-S.; data curation, C.P. and E.S.; writing—original draft preparation, C.P. and B.S.; writing—review and editing, A.S., E.S. and A.P.-S.; visualisation, B.S. and M.B.; supervision, B.S., G.Z. and M.B.; project administration, B.S. and A.S.; funding acquisition, C.P. All authors have read and agreed to the published version of the manuscript.
Funding
The project is financed by the program of the Minister of Science and Higher Education named "Regional Initiative of Excellence in the years 2019–2022, project number 026/RID/2018/19, the amount of financing PLN 9 542 500.00.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Radhakrishnan, R. Magnetic field regulates plant functions, growth and enhances tolerance against environmental stresses. Physiol. Mol. Biol. Plants 2019, 25, 1107–1119. [Google Scholar] [CrossRef] [PubMed]
- Novitsky, Y.I.; Novitskaya, G.V.; Kocheshkoiva, T.K.; Nechiporenko, G.A.; Dobrovolskii, M.V. Growth of green onions in a weak permanent magnetic field. Russ. J. Plant Physiol. 2001, 48, 709–715. [Google Scholar] [CrossRef]
- Nossol, B.; Buse, G.; Silny, J. Influence of weak static and 50 Hz magnetic fields on the redox activity of cytochrome-C oxidase. Bioelectromagnetics 1993, 14, 361–372. [Google Scholar] [CrossRef]
- Teixeira da Silva, J.A.; Dobránszki, J. Magnetic fields: How is plant growth and development impacted? Protoplasma 2016, 253, 231–248. [Google Scholar] [CrossRef] [PubMed]
- Dziergowska, K.; Lewandowska, S.; Mech, R.; Pol, M.; Detyna, J.; Michalak, I. Soybean germination response to algae extract and a static magnetic field treatment. Appl. Sci. 2021, 11, 8597. [Google Scholar] [CrossRef]
- Barbic, M. Possible magneto-mechanical and magneto-thermal mechanisms of ion channel activation in magnetogenetics. Elife 2019, 8, e45807. [Google Scholar] [CrossRef]
- Mitrov, P.P.; Kroumova, Z.; Baidanova, V.D. Auxin content of corn and tomato plants following magnetic field treatments. Fiziol No Rastenyata. 1988, 14, 18–23. [Google Scholar]
- Repacholi, M.H.; Greenebaum, B. Interaction of static and extremely low frequency electric and magnetic fields with living systems: Health effects and research needs. Bioelectromagn. J. Bioelectromagn. Soc. Soc. Phys. Regul. Biol. Med. Eur. Bioelectromagn. Assoc. 1999, 20, 133–160. [Google Scholar] [CrossRef]
- Belyavskaya, N. Biological effects due to weak magnetic field on plants. Adv. Space Res. 2004, 34, 1566–1574. [Google Scholar] [CrossRef]
- Dini, L.; Abbro, L. Bioeffects of moderate-intensity static magnetic fields on cell cultures. Micron 2005, 36, 195–217. [Google Scholar] [CrossRef]
- Sear, R.P. Diffusiophoresis in cells: A general nonequilibrium, nonmotor mechanism for the metabolism-dependent transport of particles in cells. Phys. Rev. Lett. 2019, 122, 128101. [Google Scholar] [CrossRef] [PubMed]
- Zablotskii, V.; Polyakova, T.; Dejneka, A. Effects of High Magnetic Fields on the Diffusion of Biologically Active Molecules. Cells 2021, 11, 81. [Google Scholar] [CrossRef] [PubMed]
- Stange, B.; Rowland, R.; Rapley, B.; Podd, J. ELF magnetic fields increase amino acid uptake into Vicia faba L. roots and alter ion movement across the plasma membrane. Bioelectromagn. J. Bioelectromagn. Soc. Soc. Phys. Regul. Biol. Med. Eur. Bioelectromagn. Assoc. 2002, 23, 347–354. [Google Scholar]
- Shine, M.B.; Guruprasad, K.; Anand, A. Effect of stationary magnetic field strengths of 150 and 200 mT on reactive oxygen species production in soybean. Bioelectromagnetics 2012, 33, 428–437. [Google Scholar] [CrossRef]
- Radhakrishnan, R. See pretreatment with magnetic field alters the storage proteins and lipid profiles in harvested soybean seeds. Physiol. Mol. Biol. Plant. 2018, 24, 343–347. [Google Scholar] [CrossRef]
- Cakmak, T.; Dumlupinar, R.; Erdal, S. Acceleration of germination and early growth of wheat and bean seedlings grown under various magnetic field and osmotic conditions. Bioelectromagn. J. Bioelectromagn. Soc. Soc. Phys. Regul. Biol. Med. Eur. Bioelectromagn. Assoc. 2010, 31, 120–129. [Google Scholar] [CrossRef]
- Bhardwaj, J.; Anand, A.; Nagarajan, S. Biochemical and biophysical changes associated with magnetopriming in germinating cucumber seeds. Plant Physiol. Biochem. 2012, 57, 67–73. [Google Scholar] [CrossRef]
- Maffei, M.E. Magnetic field effects on plant growth, development, and evolution. Front. Plant Sci. 2014, 5, 445. [Google Scholar] [CrossRef]
- Mroczek-Zdyrska, M.; Tryniecki, Ł.; Kornarzyński, K.; Pietruszewski, S.; Gagoś, M. Influence of magnetic field stimulation on the growth and biochemical parameters in Phaseolus vulgaris L. J. Microbiol. Biotechnol. Food Sci. 2016, 5, 548. [Google Scholar] [CrossRef]
- Asghar, T.; Jamil, Y.; Iqbal, M.; Abbas, M. Laser light and magnetic field stimulation effect on biochemical, enzymes activities and chlorophyll contents in soybean seeds and seedlings during early growth stages. J. Photochem. Photobiol. B Biol. 2016, 165, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; ul Haq, Z.; Malik, A.; Ayoub, C.M.; Jamil, Y.; Nisar, J. Pre-sowing seed magnetic field stimulation: A good option to enhance bitter gourd germination, seedling growth and yield characteristics. Biocatal. Agric. Biotechnol. 2016, 5, 30–37. [Google Scholar] [CrossRef]
- Deamici, K.M.; Cardias, B.B.; Costa, J.A.V.; Santos, L.O. Static magnetic fields in culture of Chlorella fusca: Bioeffects on growth and biomass composition. Process Biochem. 2016, 51, 912–916. [Google Scholar] [CrossRef]
- Hozayn, M.; Abdallha, M.; Abd, E.M.A.; El-Saady, A.; Darwish, M.; Hozayn, M.; Abdallha, M.; Abd, E.M.A.; El-Saady, A.; Darwish, M. Applications of magnetic technology in agriculture: A novel tool for improving crop productivity (1): Canola. Afr. J. Agric. Res. 2016, 11, 441–449. [Google Scholar] [CrossRef]
- Govindaraj, M.; Masilamani, P.; Albert, V.A.; Bhaskaran, M. Effect of physical seed treatment on yield and quality of crops: A review. Agric. Rev. 2017, 38, 1–14. [Google Scholar] [CrossRef]
- Bauer, L.M.; Costa, J.A.V.; da Rosa, A.P.C.; Santos, L.O. Growth stimulation and synthesis of lipids, pigments and antioxidants with magnetic fields in Chlorella kessleri cultivations. Bioresour. Technol. 2017, 244, 1425–1432. [Google Scholar] [CrossRef]
- Schmiedchen, K.; Petri, A.K.; Driessen, S.; Bailey, W.H. Systematic review of biological effects of exposure to static electric fields. Part II: Invertebrates and plants. Environ. Res. 2018, 160, 60–76. [Google Scholar] [CrossRef]
- Jin, Y.; Guo, W.; Hu, X.; Liu, M.; Xu, X.; Hu, F.; Lan, Y.; Lv, C.; Fang, Y.; Liu, M.; et al. Static magnetic field regulates Arabidopsis root growth via auxin signaling. Sci. Rep. 2019, 9, 14384. [Google Scholar] [CrossRef]
- Harb, A.M.; Alnawateer, B.M.; Abu-Aljarayesh, I. Influence of Static Magnetic Field Seed Treatments on the Morphological and the Biochemical Changes in Lentil Seedlings (Lens Culinaris Medik). Jordan J. Biol. Sci. 2021, 14, 179–186. [Google Scholar]
- Hilal, M.; Hilal, M. Application of magnetic technologies in desert agriculture. II-Effect of magnetic treatments of irrigation water on salt distribution in olive and citrus fields and induced changes of ionic balance in soil and plant. Egypt. J. Soil Sci. 2000, 40, 423–435. [Google Scholar]
- Maheshwari, B.L.; Grewal, H.S. Magnetic treatment of irrigation water: Its effects on vegetable crop yield and water productivity. Agric. Water Manag. 2009, 96, 1229–1236. [Google Scholar] [CrossRef]
- Rathod, G.R.; Anand, A. Effect of seed magneto-priming on growth, yield and Na/K ratio in wheat (Triticum aestivum L.) under salt stress. Indian J. Plant Physiol. 2016, 21, 15–22. [Google Scholar] [CrossRef]
- Taimourya, H.; Oussible, M.; Baamal, L.; Harif, A.; Zaid, E.; Guedira, A.; Smouni, A. Magnetic treatment of culture medium enhance growth and minerals uptake of strawberry (Fragaria × ananassa Duch.) and tomato (Solanum lycopersicum) in Fe deficiency conditions. Int. J. Sci. Eng. Res. 2017, 8, 1414–1436. [Google Scholar]
- Deamici, K.M.; Santos, L.O.; Costa, J.A.V. Magnetic field action on outdoor and indoor cultures of Spirulina: Evaluation of growth, medium consumption and protein profile. Bioresour. Technol. 2018, 249, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Chu, F.J.; Wan, T.J.; Pai, T.Y.; Lin, H.W.; Liu, S.H.; Huang, C.F. Use of magnetic fields and nitrate concentration to optimize the growth and lipid yield of Nannochloropsis oculata. J. Environ. Manag. 2020, 253, 109680. [Google Scholar] [CrossRef]
- Selim, A.F.H.; Selim, D.A. Physio-biochemical behaviour, water use efficiency and productivity of wheat plants exposed to magnetic field. J. Plant Prod. 2019, 10, 185–191. [Google Scholar] [CrossRef]
- Sini Thomas, R.R.; Anand, A. Growth, Na+/K+ Partitioning and Yield of Chickpea Plants Alleviated From Salt Stress by Magnetopriming. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 821–833. [Google Scholar] [CrossRef]
- Huo, S.; Chen, X.; Zhu, F.; Zhang, W.; Chen, D.; Jin, N.; Cobb, K.; Cheng, Y.; Wang, L.; Ruan, R. Magnetic field intervention on growth of the filamentous microalgae Tribonema sp. in starch wastewater for algal biomass production and nutrients removal: Influence of ambient temperature and operational strategy. Bioresour. Technol. 2020, 303, 122884. [Google Scholar] [CrossRef] [PubMed]
- Michalak, I.; Bartniczak, A.; Baśladyńska, S.; Lewandowska, S.; Detyna, J.; Łoziński, M.; Niemczyk, K.; Bujak, H. Cladophora glomerata extract and static magnetic field influences the germination of seeds and multielemental composition of carrot. Ecol. Chem. Eng. 2020, 27, 629–641. [Google Scholar] [CrossRef]
- Buchachenko, A. Why magnetic and electromagnetic effects in biology are irreproducible and contradictory? Bioelectromagnetics 2016, 37, 1–13. [Google Scholar] [CrossRef]
- Gupta, M.K.; Anand, A.; Paul, V.; Dahuja, A.; Singh, A. Reactive oxygen species mediated improvement in vigour of static and pulsed magneto-primed cherry tomato seeds. Indian J. Plant Physiol. 2015, 20, 197–204. [Google Scholar] [CrossRef]
- Shine, M.; Kataria, S.; Guruprasad, K.; Anand, A. Enhancement of maize seeds germination by magnetopriming in perspective with reactive oxygen species. J. Agric. Crop Res. 2017, 5, 66–76. [Google Scholar]
- Raipuria, R.K.; Kataria, S.; Watts, A.; Jain, M. Magneto-priming promotes nitric oxide via nitric oxide synthase to ameliorate the UV-B stress during germination of soybean seedlings. J. Photochem. Photobiol. B Biol. 2021, 220, 112211. [Google Scholar] [CrossRef]
- Shine, M.; Guruprasad, K.; Anand, A. Enhancement of germination, growth, and photosynthesis in soybean by pre-treatment of seeds with magnetic field. Bioelectromagnetics 2011, 32, 474–484. [Google Scholar] [CrossRef]
- Baghel, L.; Kataria, S.; Guruprasad, K.N. Static magnetic field treatment of seeds improves carbon and nitrogen metabolism under salinity stress in soybean. Bioelectromagnetics 2016, 37, 455–470. [Google Scholar] [CrossRef] [PubMed]
- Baghel, L.; Kataria, S.; Jain, M. Mitigation of adverse effects of salt stress on germination, growth, photosynthetic efficiency and yield in maize (Zea mays L.) through magnetopriming. Acta Agrobot. 2019, 72. [Google Scholar] [CrossRef]
- Deamici, K.M.; Cuellar-Bermudez, S.P.; Muylaert, K.; Santos, L.O.; Costa, J.A.V. Quantum yield alterations due to the static magnetic fields action on Arthrospira platensis SAG 21.99: Evaluation of photosystem activity. Bioresour. Technol. 2019, 292, 121945. [Google Scholar] [CrossRef] [PubMed]
- Fatima, A.; Kataria, S.; Prajapati, R.; Jain, M.; Agrawal, A.K.; Singh, B.; Kashyap, Y.; Tripathi, D.K.; Singh, V.P.; Gadre, R. Magnetopriming effects on arsenic stress-induced morphological and physiological variations in soybean involving synchrotron imaging. Physiol. Plant. 2021, 173, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Kataria, S.; Jain, M.; Rastogi, A.; Brestic, M. Static magnetic field treatment enhanced photosynthetic performance in soybean under supplemental ultraviolet-B radiation. Photosynth. Res. 2021, 150, 263–278. [Google Scholar] [CrossRef]
- Sarraf, M.; Deamici, K.M.; Taimourya, H.; Islam, M.; Kataria, S.; Raipuria, R.K.; Abdi, G.; Brestic, M. Effect of Magnetopriming on Photosynthetic Performance of Plants. Int. J. Mol. Sci. 2021, 22, 9353. [Google Scholar] [CrossRef]
- Kataria, S.; Baghel, L.; Guruprasad, K. Pre-treatment of seeds with static magnetic field improves germination and early growth characteristics under salt stress in maize and soybean. Biocatal. Agric. Biotechnol. 2017, 10, 83–90. [Google Scholar] [CrossRef]
- Kataria, S.; Baghel, L.; Guruprasad, K. Alleviation of adverse effects of ambient UV stress on growth and some potential physiological attributes in soybean (Glycine max) by seed pre-treatment with static magnetic field. J. Plant Growth Regul. 2017, 36, 550–565. [Google Scholar] [CrossRef]
- Kataria, S.; Baghel, L.; Jain, M.; Guruprasad, K. Magnetopriming regulates antioxidant defense system in soybean against salt stress. Biocatal. Agric. Biotechnol. 2019, 18, 101090. [Google Scholar] [CrossRef]
- Abdolmaleki, P.; Ghanati, F.; Sahebjamei, H.; Sarvestani, A.S. Peroxidase activity, lignification and promotion of cell death in tobacco cells exposed to static magnetic field. Environ. 2007, 27, 435–440. [Google Scholar] [CrossRef]
- Sahebjamei, H.; Abdolmaleki, P.; Ghanati, F. Effects of magnetic field on the antioxidant enzyme activities of suspension-cultured tobacco cells. Bioelectromagn. J. Bioelectromagn. Soc. Soc. Phys. Regul. Biol. Med. Eur. Bioelectromagn. Assoc. 2007, 28, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Vashisth, A.; Nagarajan, S. Effect on germination and early growth characteristics in sunflower (Helianthus annuus) seeds exposed to static magnetic field. J. Plant Physiol. 2010, 167, 149–156. [Google Scholar] [CrossRef]
- Ghanati, F.; Payez, A. Iron biofortification and activation of antioxidant system of wheat by static magnetic field. Iran. J. Sci. Technol. (Sci.) 2015, 39, 355–360. [Google Scholar]
- Anand, A.; Kumari, A.; Thakur, M.; Koul, A. Hydrogen peroxide signaling integrates with phytohormones during the germination of magnetoprimed tomato seeds. Sci. Rep. 2019, 9, 8814. [Google Scholar] [CrossRef]
- Kataria, S.; Jain, M.; Tripathi, D.K.; Singh, V.P. Involvement of nitrate reductase-dependent nitric oxide production in magnetopriming-induced salt tolerance in soybean. Physiol. Plant. 2020, 168, 422–436. [Google Scholar] [CrossRef]
- Kataria, S.; Rastogi, A.; Bele, A.; Jain, M. Role of nitric oxide and reactive oxygen species in static magnetic field pre-treatment induced tolerance to ambient UV-B stress in soybean. Physiol. Mol. Biol. Plants 2020, 26, 931–945. [Google Scholar] [CrossRef]
- Kotnik, T.; Frey, W.; Sack, M.; Meglič, S.H.; Peterka, M.; Miklavčič, D. Electroporation-based applications in biotechnology. Trends Biotechnol. 2015, 33, 480–488. [Google Scholar] [CrossRef]
- Albuquerque, W.W.C.; Costa, R.M.P.B.; e Fernandes, T.D.S.; Porto, A.L.F. Evidences of the static magnetic field influence on cellular systems. Prog. Biophys. Mol. Biol. 2016, 121, 16–28. [Google Scholar] [CrossRef]
- Dhiman, S.K.; Galland, P. Effects of weak static magnetic fields on the gene expression of seedlings of Arabidopsis thaliana. J. Plant Physiol. 2018, 231, 9–18. [Google Scholar] [CrossRef]
- Mohammadi, F.; Ghanati, F.; Sharifi, M.; Chashmi, N.A. On the mechanism of the cell cycle control of suspension-cultured tobacco cells after exposure to static magnetic field. Plant Sci. 2018, 277, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Shokrollahi, S.; Ghanati, F.; Sajedi, R.H.; Sharifi, M. Possible role of iron containing proteins in physiological responses of soybean to static magnetic field. J. Plant Physiol. 2018, 226, 163–171. [Google Scholar] [CrossRef]
- Binhi, V.N.; Prato, F.S. Biological effects of the hypomagnetic field: An analytical review of experiments and theories. PLoS ONE 2017, 12, e0179340. [Google Scholar]
- Binhi, V.N.; Prato, F.S. Rotations of macromolecules affect nonspecific biological responses to magnetic fields. Sci. Rep. 2018, 8, 13495. [Google Scholar] [CrossRef]
- Binhi, V. Primary physical mechanism of the biological effects of weak magnetic fields. Biophysics 2016, 61, 170–176. [Google Scholar] [CrossRef]
- Binhi, V. Nonspecific magnetic biological effects: A model assuming the spin-orbit coupling. J. Chem. Phys. 2019, 151, 204101. [Google Scholar] [CrossRef] [PubMed]
- Zadeh-Haghighi, H.; Simon, C. Magnetic field effects in biology from the perspective of the radical pair mechanism. arXiv 2022, arXiv:2204.09147. [Google Scholar] [CrossRef]
- Ureta-Leones, D.; García-Quintana, Y.; Vega-Rosete, S.; Pérez-Morell, L.; Bravo-Medina, C.A.; Arteaga-Crespo, Y. Effect of pre-germination treatment with direct magnetic field exposure: A systematic review and meta-analysis. Eur. J. For. Res. 2021, 140, 1029–1038. [Google Scholar] [CrossRef]
- Simkin, A.J.; Faralli, M.; Ramamoorthy, S.; Lawson, T. Photosynthesis in non-foliar tissues: Implications for yield. Plant J. 2020, 101, 1001–1015. [Google Scholar] [CrossRef]
- Foyer, C.H.; Ruban, A.V.; Nixon, P.J. Photosynthesis solutions to enhance productivity. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160374. [Google Scholar] [CrossRef] [PubMed]
- Miyake, C. Molecular mechanism of oxidation of P700 and suppression of ROS production in photosystem I in response to electron-sink limitations in C3 plants. Antioxidants 2020, 9, 230. [Google Scholar] [CrossRef] [PubMed]
- Sarraf, M.; Kataria, S.; Taimourya, H.; Santos, L.O.; Menegatti, R.D.; Jain, M.; Ihtisham, M.; Liu, S. Magnetic field (MF) applications in plants: An overview. Plants 2020, 9, 1139. [Google Scholar] [CrossRef]
- Stirbet, A.; Lazár, D.; Kromdijk, J. Chlorophyll a fluorescence induction: Can just a one-second measurement be used to quantify abiotic stress responses? Photosynthetica 2018, 56, 86–104. [Google Scholar] [CrossRef]
- Baby, S.M.; Narayanaswamy, G.K.; Anand, A. Superoxide radical production and performance index of Photosystem II in leaves from magnetoprimed soybean seeds. Plant Signal. Behav. 2011, 6, 1635–1637. [Google Scholar] [CrossRef] [PubMed]
- Procopio, M.; Link, J.; Engle, D.; Witczak, J.; Ritz, T.; Ahmad, M. Kinetic modeling of the Arabidopsis cryptochrome photocycle: FADHo accumulation correlates with biological activity. Front. Plant Sci. 2016, 7, 888. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, N. Phylogenetic and functional classification of the photolyase/cryptochrome family. Photochem. Photobiol. 2017, 93, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Liu, B.; Su, J.; Liao, J.; Lin, C.; Oka, Y. Cryptochromes orchestrate transcription regulation of diverse blue light responses in plants. Photochem. Photobiol. 2017, 93, 112–127. [Google Scholar] [CrossRef] [PubMed]
- Rredhi, A.; Petersen, J.; Schubert, M.; Li, W.; Oldemeyer, S.; Li, W.; Westermann, M.; Wagner, V.; Kottke, T.; Mittag, M. DASH cryptochrome 1, a UV-A receptor, balances the photosynthetic machinery of Chlamydomonas reinhardtii. New Phytol. 2021, 232, 610–624. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Barshop, W.D.; Bian, M.; Vashisht, A.A.; He, R.; Yu, X.; Liu, B.; Nguyen, P.; Liu, X.; Zhao, X.; et al. The blue light-dependent phosphorylation of the CCE domain determines the photosensitivity of Arabidopsis CRY2. Mol. Plant 2015, 8, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Shameer, S.; Ratcliffe, R.G.; Sweetlove, L.J. Leaf energy balance requires mitochondrial respiration and export of chloroplast NADPH in the light. Plant Physiol. 2019, 180, 1947–1961. [Google Scholar] [CrossRef] [PubMed]
- Hore, P.J.; Mouritsen, H. The radical-pair mechanism of magnetoreception. Annu. Rev. Biophys. 2016, 45, 299–344. [Google Scholar] [CrossRef]
- Ahmad, M.; Galland, P.; Ritz, T.; Wiltschko, R.; Wiltschko, W. Magnetic intensity affects cryptochrome-dependent responses in Arabidopsis thaliana. Planta 2007, 225, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Lv, Y.; Chen, C.; Zhang, Y.; Wei, S. Blue light-dependent phosphorylations of cryptochromes are affected by magnetic fields in Arabidopsis. Adv. Space Res. 2014, 53, 1118–1124. [Google Scholar] [CrossRef]
- Xu, C.; Li, Y.; Yu, Y.; Zhang, Y.; Wei, S. Suppression of Arabidopsis flowering by near-null magnetic field is affected by light. Bioelectromagnetics 2015, 36, 476–479. [Google Scholar] [CrossRef]
- Pooam, M.; Arthaut, L.D.; Burdick, D.; Link, J.; Martino, C.F.; Ahmad, M. Magnetic sensitivity mediated by the Arabidopsis blue-light receptor cryptochrome occurs during flavin reoxidation in the dark. Planta 2019, 249, 319–332. [Google Scholar] [CrossRef]
- Yu, X.; Shalitin, D.; Liu, X.; Maymon, M.; Klejnot, J.; Yang, H.; Lopez, J.; Zhao, X.; Bendehakkalu, K.T.; Lin, C. Derepression of the NC80 motif is critical for the photoactivation of Arabidopsis CRY2. Proc. Natl. Acad. Sci. USA 2007, 104, 7289–7294. [Google Scholar] [CrossRef]
- Burney, S.; Hoang, N.; Caruso, M.; Dudkin, E.A.; Ahmad, M.; Bouly, J.P. Conformational change induced by ATP binding correlates with enhanced biological function of Arabidopsis cryptochrome. FEBS Lett. 2009, 583, 1427–1433. [Google Scholar] [CrossRef][Green Version]
- Shao, W.; Ebaid, R.; Abomohra, A.E.F.; Shahen, M. Enhancement of Spirulina biomass production and cadmium biosorption using combined static magnetic field. Bioresour. Technol. 2018, 265, 163–169. [Google Scholar] [CrossRef]
- Ercan, I.; Tombuloglu, H.; Alqahtani, N.; Alotaibi, B.; Bamhrez, M.; Alshumrani, R.; Ozcelik, S.; Kayed, T.S. Magnetic field effects on the magnetic properties, germination, chlorophyll fluorescence, and nutrient content of barley (Hordeum vulgare L.). Plant Physiol. Biochem. 2022, 170, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Jovanic, B.; Jevtovic, R. Effect of a permanent magnetic field on the optical and physiological properties on green plant leaves. Int. J. Environ. Stud. 2002, 59, 599–606. [Google Scholar] [CrossRef]
- Small, D.P.; Hüner, N.P.; Wan, W. Effect of static magnetic fields on the growth, photosynthesis and ultrastructure of Chlorella kessleri microalgae. Bioelectromagnetics 2012, 33, 298–308. [Google Scholar] [CrossRef]
- Deamici, K.M.; Santos, L.O.; Costa, J.A.V. Magnetic field as promoter of growth in outdoor and indoor assays of Chlorella fusca. Bioprocess Biosyst. Eng. 2021, 44, 1453–1460. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.p.; Li, R.; He, J.M. Magnetic field can alleviate toxicological effect induced by cadmium in mungbean seedlings. Ecotoxicology 2011, 20, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Selim, A.F.H.; El-Nady, M.F. Physio-anatomical responses of drought stressed tomato plants to magnetic field. Acta Astronaut. 2011, 69, 387–396. [Google Scholar] [CrossRef]
- Zandi, P.; Schnug, E. Reactive oxygen species, antioxidant responses and implications from a microbial modulation perspective. Biology 2022, 11, 155. [Google Scholar] [CrossRef]
- Aladjadjiyan, A. Study of the influence of magnetic field on some biological characteristics of Zea mais. J. Cent. Eur. Agric. 2002, 3, 89–94. [Google Scholar]
- Hasanuzzaman, M.; Bhuyan, M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Latef, A.A.H.A.; Dawood, M.F.; Hassanpour, H.; Rezayian, M.; Younes, N.A. Impact of the static magnetic field on growth, pigments, osmolytes, nitric oxide, hydrogen sulfide, phenylalanine ammonia-lyase activity, antioxidant defense system, and yield in lettuce. Biology 2020, 9, 172. [Google Scholar] [CrossRef]
- Jouni, F.J.; Abdolmaleki, P.; Ghanati, F. Oxidative stress in broad bean (Vicia faba L.) induced by static magnetic field under natural radioactivity. Mutat. Res./Genet. Toxicol. Environ. Mutagenesis 2012, 741, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Çelik, Ö.; Büyükuslu, N.; Atak, Ç.; Rzakoulieva, A. Effects of Magnetic Field on Activity of Superoxide Dismutase and Catalase in Glycine max (L.) Merr. Roots. Pol. J. Environ. Stud. 2009, 18, 175–182. [Google Scholar]
- Wang, H.Y.; Zeng, X.B.; Guo, S.Y.; Li, Z.T. Effects of magnetic field on the antioxidant defense system of recirculation-cultured Chlorella vulgaris. Bioelectromagn. J. Bioelectromagn. Soc. Soc. Phys. Regul. Biol. Med. Eur. Bioelectromagn. Assoc. 2008, 29, 39–46. [Google Scholar] [CrossRef]
- Shabrangi, A.; Majd, A.; Sheidai, M. Effects of extremely low frequency electromagnetic fields on growth, cytogenetic, protein content and antioxidant system of Zea mays L. Afr. J. Biotechnol. 2011, 10, 9362–9369. [Google Scholar]
- Novitskii, Y.; Novitskaya, G.; Kocheshkova, T.; Dobrovolskii, M.; Serdyukov, Y. Changes in the content of lipids in the seeds of magnetically-oriented radish types grown in a weak permanent magnetic field. In Doklady. Biochemistry and Biophysics; Springer Nature BV: Berlin, Germany, 2011; Volume 441, p. 248. [Google Scholar]
- Camak, D.T. Assessing the Impact of Low-Head Dams and Life History on Fine Scale Genetic Structure of Three Etheostomatine Darters (Percidae); Southeastern Louisiana University: Hammond, LA, USA, 2012. [Google Scholar]
- Sen, A.; Alikamanoglu, S. Effects of static magnetic field Pre-treatment with and without PEG 6000 or NaCl exposure on wheat biochemical parameters. Russ. J. Plant Physiol. 2014, 61, 646–655. [Google Scholar] [CrossRef]
- Payez, A.; Ghanati, F.; Behmanesh, M.; Abdolmaleki, P.; Hajnorouzi, A.; Rajabbeigi, E. Increase of seed germination, growth and membrane integrity of wheat seedlings by exposure to static and a 10-KHz electromagnetic field. Electromagn. Biol. Med. 2013, 32, 417–429. [Google Scholar] [CrossRef]
- Rajabbeigi, E.; Ghanati, F.; Abdolmaleki, P.; Payez, A. Antioxidant capacity of parsley cells (Petroselinum crispum L.) in relation to iron-induced ferritin levels and static magnetic field. Electromagn. Biol. Med. 2013, 32, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Haghighat, N.; Abdolmaleki, P.; Ghanati, F.; Behmanesh, M.; Payez, A. Modification of catalase and MAPK in Vicia faba cultivated in soil with high natural radioactivity and treated with a static magnetic field. J. Plant Physiol. 2014, 171, 99–103. [Google Scholar] [CrossRef]
- Prasanna, G.L.; Panda, T. Electroporation: Basic principles, practical considerations and applications in molecular biology. Bioprocess Eng. 1997, 16, 261–264. [Google Scholar] [CrossRef]
- Meloni, D.A.; Oliva, M.A.; Martinez, C.A.; Cambraia, J. Photosynthesis and activity of superoxide dismutase, peroxidase and glutathione reductase in cotton under salt stress. Environ. Exp. Bot. 2003, 49, 69–76. [Google Scholar] [CrossRef]
- Kim, S.; Im, W. Static magnetic fields inhibit proliferation and disperse subcellular localization of gamma complex protein3 in cultured C2C12 myoblast cells. Cell Biochem. Biophys. 2010, 57, 1–8. [Google Scholar] [CrossRef]
- Haneda, T.; Fujimura, Y.; Iino, M. Magnetic field exposure stiffens regenerating plant protoplast cell walls. Bioelectromagn. J. Bioelectromagn. Soc. Soc. Phys. Regul. Biol. Med. Eur. Bioelectromagn. Assoc. 2006, 27, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Ghanati, F.; Abdolmaleki, P.; Vaezzadeh, M.; Rajabbeigi, E.; Yazdani, M. Application of magnetic field and iron in order to change medicinal products of Ocimum basilicum. Environ. 2007, 27, 429–434. [Google Scholar] [CrossRef]
- Mroczek-Zdyrska, M.; Kornarzyn´ski, K.; Pietruszewski, S.; Gagos´, M. The effects of low-frequency magnetic field exposure on the growth and biochemical parameters in lupin (Lupinus angustifolius L.). Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2017, 151, 504–511. [Google Scholar] [CrossRef]
- Serdyukov, Y.A.; Novitskii, Y.I. Impact of weak permanent magnetic field on antioxidant enzyme activities in radish seedlings. Russ. J. Plant Physiol. 2013, 60, 69–76. [Google Scholar] [CrossRef]
- Cakmak, T.; Cakmak, Z.E.; Dumlupinar, R.; Tekinay, T. Analysis of apoplastic and symplastic antioxidant system in shallot leaves: Impacts of weak static electric and magnetic field. J. Plant Physiol. 2012, 169, 1066–1073. [Google Scholar] [CrossRef]
- Atak, Ç.; Çelik, Ö.; Olgun, A.; Alikamanoğlu, S.; Rzakoulieva, A. Effect of magnetic field on peroxidase activities of soybean tissue culture. Biotechnol. Biotechnol. Equip. 2007, 21, 166–171. [Google Scholar] [CrossRef]
- Kang, S.G.; Lee, K.E.; Singh, M.; Kumar, P.; Matin, M.N. Rice lesion mimic mutants (LMM): The current understanding of genetic mutations in the failure of ROS scavenging during lesion formation. Plants 2021, 10, 1598. [Google Scholar] [CrossRef]
- Chionna, A.; Dwikat, M.; Panzarini, E.; Tenuzzo, B.; Carla, E.; Verri, T.; Pagliara, P.; Abbro, L.; Dini, L. Cell shape and plasma membrane alterations after static magnetic fields exposure. Eur. J. Histochem. 2003, 47, 299–308. [Google Scholar] [CrossRef]
- Escobar, J.F.; Vaca-González, J.J.; Guevara, J.M.; Garzón-Alvarado, D.A. Effect of magnetic and electric fields on plasma membrane of single cells: A computational approach. Eng. Rep. 2020, 2, e12125. [Google Scholar] [CrossRef]
- Galland, P.; Pazur, A. Magnetoreception in plants. J. Plant Res. 2005, 118, 371–389. [Google Scholar] [CrossRef] [PubMed]
- Bitonti, M.; Mazzuca, S.; Ting, T.; Innocenti, A. Magnetic field affects meristem activity and cell differentiation in Zea mays roots. Plant Biosyst. 2006, 140, 87–93. [Google Scholar] [CrossRef]
- Rosen, A.D. Effect of a 125 mT static magnetic field on the kinetics of voltage activated Na+ channels in GH3 cells. Bioelectromagn. J. Bioelectromagn. Soc. Soc. Phys. Regul. Biol. Med. Eur. Bioelectromagn. Assoc. 2003, 24, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Belyavskaya, N. Ultrastructure and calcium balance in meristem cells of pea roots exposed to extremely low magnetic fields. Adv. Space Res. 2001, 28, 645–650. [Google Scholar] [CrossRef]
- Reszczyn´ska, E.; Hanaka, A. Lipids composition in plant membranes. Cell Biochem. Biophys. 2020, 78, 401–414. [Google Scholar] [CrossRef]
- Bauréus Koch, C.; Sommarin, M.; Persson, B.; Salford, L.; Eberhardt, J. Interaction between weak low frequency magnetic fields and cell membranes. Bioelectromagn. J. Bioelectromagn. Soc. Soc. Phys. Regul. Biol. Med. Eur. Bioelectromagn. Assoc. 2003, 24, 395–402. [Google Scholar] [CrossRef]
- Paul, A.L.; Ferl, R.J.; Meisel, M.W. High magnetic field induced changes of gene expression in arabidopsis. Biomagn. Res. Technol. 2006, 4, 7. [Google Scholar] [CrossRef]
- Poinapen, D.; Toppozini, L.; Dies, H.; Brown, D.C.; Rheinstädter, M.C. Static magnetic fields enhance lipid order in native plant plasma membrane. Soft Matter 2013, 9, 6804–6813. [Google Scholar] [CrossRef]
- Maadane, A.; Merghoub, N.; Ainane, T.; El Arroussi, H.; Benhima, R.; Amzazi, S.; Bakri, Y.; Wahby, I. Antioxidant activity of some Moroccan marine microalgae: Pufa profiles, carotenoids and phenolic content. J. Biotechnol. 2015, 215, 13–19. [Google Scholar] [CrossRef]
- Waghmare, A.G.; Salve, M.K.; LeBlanc, J.G.; Arya, S.S. Concentration and characterization of microalgae proteins from Chlorella pyrenoidosa. Bioresour. Bioprocess. 2016, 3, 16. [Google Scholar] [CrossRef]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Factories 2018, 17, 36. [Google Scholar] [CrossRef] [PubMed]
- Dhawi, F.; Al-Khayri, J.M.; Hassan, E. Static magnetic field influence on elements composition in date palm (Phoenix dactylifera L.). Res. J. Agric. Biol. Sci. 2009, 5, 161–166. [Google Scholar]
- Yaycili, O.; Alikamanoglu, S. The effect of magnetic field on Paulownia tissue cultures. Plant Cell Tissue Organ Cult. 2005, 83, 109–114. [Google Scholar] [CrossRef]
- Ahamed, M.; Elzaawely, A.; Bayoumi, Y. Effect of magnetic field on seed germination, growth and yield of sweet pepper (Capsicum annuum L.). Asian J. Crop Sci. 2013, 5, 286–294. [Google Scholar] [CrossRef]
- Han, S.; Jin, W.; Chen, Y.; Tu, R.; Abomohra, A.E.F. Enhancement of lipid production of Chlorella pyrenoidosa cultivated in municipal wastewater by magnetic treatment. Appl. Biochem. Biotechnol. 2016, 180, 1043–1055. [Google Scholar] [CrossRef]
- Aladjadjiyan, A. The use of physical methods for plant growing stimulation in Bulgaria. J. Cent. Eur. Agric. 2007, 8, 369–380. [Google Scholar]
- Albaqami, M.; Hammad, M.; Pooam, M.; Procopio, M.; Sameti, M.; Ritz, T.; Ahmad, M.; Martino, C.F. Arabidopsis cryptochrome is responsive to Radiofrequency (RF) electromagnetic fields. Sci. Rep. 2020, 10, 11260. [Google Scholar] [CrossRef]
- Kirschvink, J.L.; Kobayashi-Kirschvink, A.; Woodford, B.J. Magnetite biomineralization in the human brain. Proc. Natl. Acad. Sci. USA 1992, 89, 7683–7687. [Google Scholar] [CrossRef]
- Tahir, N.A.R.; Karim, H.F.H. Impact of magnetic application on the parameters related to growth of chickpea (Cicer arietinum L.). Jordan J. Biol. Sci. 2010, 3, 175–184. [Google Scholar]
- Dhawi, F.; Al-Khayri, J.M. Magnetic Fields Induce Changes in Photosynthetic Pigments Content in Date Palm Phoenix dactylifera (L.) Seedlings. Open Agric. J. 2009, 3, 1–5. [Google Scholar] [CrossRef]
- Inhan-Garip, A.; Aksu, B.; Akan, Z.; Akakin, D.; Ozaydin, A.N.; San, T. Effect of extremely low frequency electromagnetic fields on growth rate and morphology of bacteria. Int. J. Radiat. Biol. 2011, 87, 1155–1161. [Google Scholar] [CrossRef]
- Shine, M.; Guruprasad, K. Impact of pre-sowing magnetic field exposure of seeds to stationary magnetic field on growth, reactive oxygen species and photosynthesis of maize under field conditions. Acta Physiol. Plant. 2012, 34, 255–265. [Google Scholar] [CrossRef]
- Racuciu, M.; Creanga, D.; Galugaru, C. The influence of extremely low frequency magnetic field on tree seedlings. Rom J Phys 2008, 35, 337–342. [Google Scholar]
- Atak, Ç.; Emiroǧlu, Ö.; Alikamanoǧlu, S.; Rzakoulieva, A. Stimulation of regeneration by magnetic field in soybean (Glycine max L. Merrill) tissue cultures. J. Cell Mol. Biol. 2003, 2, 113. [Google Scholar]
- Arosio, P.; Ingrassia, R.; Cavadini, P. Ferritins: A family of molecules for iron storage, antioxidation and more. Biochim. Et Biophys. Acta (BBA)-Gen. Subj. 2009, 1790, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Findlay, G.; Hope, A. Electrical properties of plant cells: Methods and findings. In Transport in Plants II; Springer: Berlin/Heidelberg, Germany, 1976; pp. 53–92. [Google Scholar]
- Das, R.K.; Zouani, O.F. A review of the effects of the cell environment physicochemical nanoarchitecture on stem cell commitment. Biomaterials 2014, 35, 5278–5293. [Google Scholar] [CrossRef] [PubMed]
- Racuciu, M.; Creanga, D.; Horga, I. Plant growth under static magnetic field influence. Rom. J. Phys. 2008, 53, 353–359. [Google Scholar]
- Agliassa, C.; Narayana, R.; Christie, J.M.; Maffei, M.E. Geomagnetic field impacts on cryptochrome and phytochrome signaling. J. Photochem. Photobiol. B Biol. 2018, 185, 32–40. [Google Scholar] [CrossRef]
- Agliassa, C.; Narayana, R.; Bertea, C.M.; Rodgers, C.T.; Maffei, M.E. Reduction of the geomagnetic field delays Arabidopsis thaliana flowering time through downregulation of flowering-related genes. Bioelectromagnetics 2018, 39, 361–374. [Google Scholar] [CrossRef]
- Xu, C.; Yin, X.; Lv, Y.; Wu, C.; Zhang, Y.; Song, T. A near-null magnetic field affects cryptochrome-related hypocotyl growth and flowering in Arabidopsis. Adv. Space Res. 2012, 49, 834–840. [Google Scholar] [CrossRef]
- Xu, C.; Yu, Y.; Zhang, Y.; Li, Y.; Wei, S. Gibberellins are involved in effect of near-null magnetic field on Arabidopsis flowering. Bioelectromagnetics 2017, 38, 1–10. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, Y.; Yu, Y.; Li, Y.; Wei, S. Suppression of Arabidopsis flowering by near-null magnetic field is mediated by auxin. Bioelectromagnetics 2018, 39, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Martinez, E.; Florez, M.; Carbonell, M. Stimulatory effect of the magnetic treatment on the germination of cereal seeds. Int. J. Environ. Agric. Biotechnol. 2017, 2, 375–381. [Google Scholar] [CrossRef]
- Trontelj, Z.; Thiel, G.; Jazbinsek, V. Magnetic measurements in plant electrophysiology. In Plant Electrophysiology; Springer: Berlin/Heidelberg, Germany, 2006; pp. 187–218. [Google Scholar]
- Brocklehurst, B. Magnetic fields and radical reactions: Recent developments and their role in nature. Chem. Soc. Rev. 2002, 31, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Kattnig, D.R.; Sowa, J.K.; Solov’yov, I.A.; Hore, P. Electron spin relaxation can enhance the performance of a cryptochrome-based magnetic compass sensor. New J. Phys. 2016, 18, 063007. [Google Scholar] [CrossRef]
- Solov’yov, I.A.; Schulten, K. Reaction kinetics and mechanism of magnetic field effects in cryptochrome. J. Phys. Chem. B 2012, 116, 1089–1099. [Google Scholar] [CrossRef] [PubMed]
- Vaezzadeh, M.; Noruzifar, E.; Faezeh, G.; Salehkotahi, M.; Mehdian, R. Excitation of plant growth in dormant temperature by steady magnetic field. J. Magn. Magn. Mater. 2006, 302, 105–108. [Google Scholar] [CrossRef]
- Penuelas, J.; Llusia, J.; Martínez, B.; Fontcuberta, J. Diamagnetic susceptibility and root growth responses to magnetic fields in Lens culinaris, Glycine soja, and Triticum aestivum. Electromagn. Biol. Med. 2004, 23, 97–112. [Google Scholar] [CrossRef]
- Dao-liang, Y.; Yu-qi, G.; Xue-ming, Z.; Shu-wen, W.; Qin, P. Effects of electromagnetic fields exposure on rapid micropropagation of beach plum (Prunus maritima). Ecol. Eng. 2009, 35, 597–601. [Google Scholar] [CrossRef]
- Goldsworthy, A. Effects of electrical and electromagnetic fields on plants and related topics. In Plant Electrophysiology; Springer: Berlin/Heidelberg, Germany, 2006; pp. 247–267. [Google Scholar]
- Naidu, M.S. High Voltage Engineering; Tata McGraw-Hill Education: New York, NY, USA, 2013. [Google Scholar]
- Asemota, G.N.O. Alternating electromagnetic fields in plantains. Afr. J. Plant Sci. Biotechnol. 2010, 4, 59–75. [Google Scholar]
- Scaiano, J.; Mohtat, N.; Cozens, F.; McLean, J.; Thansandote, A. Application of the radical pair mechanism to free radicals in organized systems: Can the effects of 60 Hz be predicted from studies under static fields? Bioelectromagn. J. Bioelectromagn. Soc. Soc. Phys. Regul. Biol. Med. Eur. Bioelectromagn. Assoc. 1994, 15, 549–554. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).