Potential Use of Propolis in Phytocosmetic as Phytotherapeutic Constituent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Propolis Collection
2.2. Preparation of Ethanolic Extract of Propolis (EEP)
2.3. Determination of Total Phenolic Content (TPC) and Total Flavonoid Content (TFC)
2.4. Antioxidant Activity
2.4.1. DPPH Assay
2.4.2. ABTS Assay
2.4.3. FRAP Assay
2.4.4. CUPRAC Assay
2.5. Antibacterial Activity
2.5.1. Bacterial Strains
2.5.2. Micro-Dilution Method
2.6. Wound-Healing Activity
2.6.1. Extraction and Propolis Ointment Preparation
2.6.2. Animals
2.6.3. Double Incision Wound Assay
2.7. UFLC/MS-MS Analysis
2.8. Statistical Analysis
3. Results
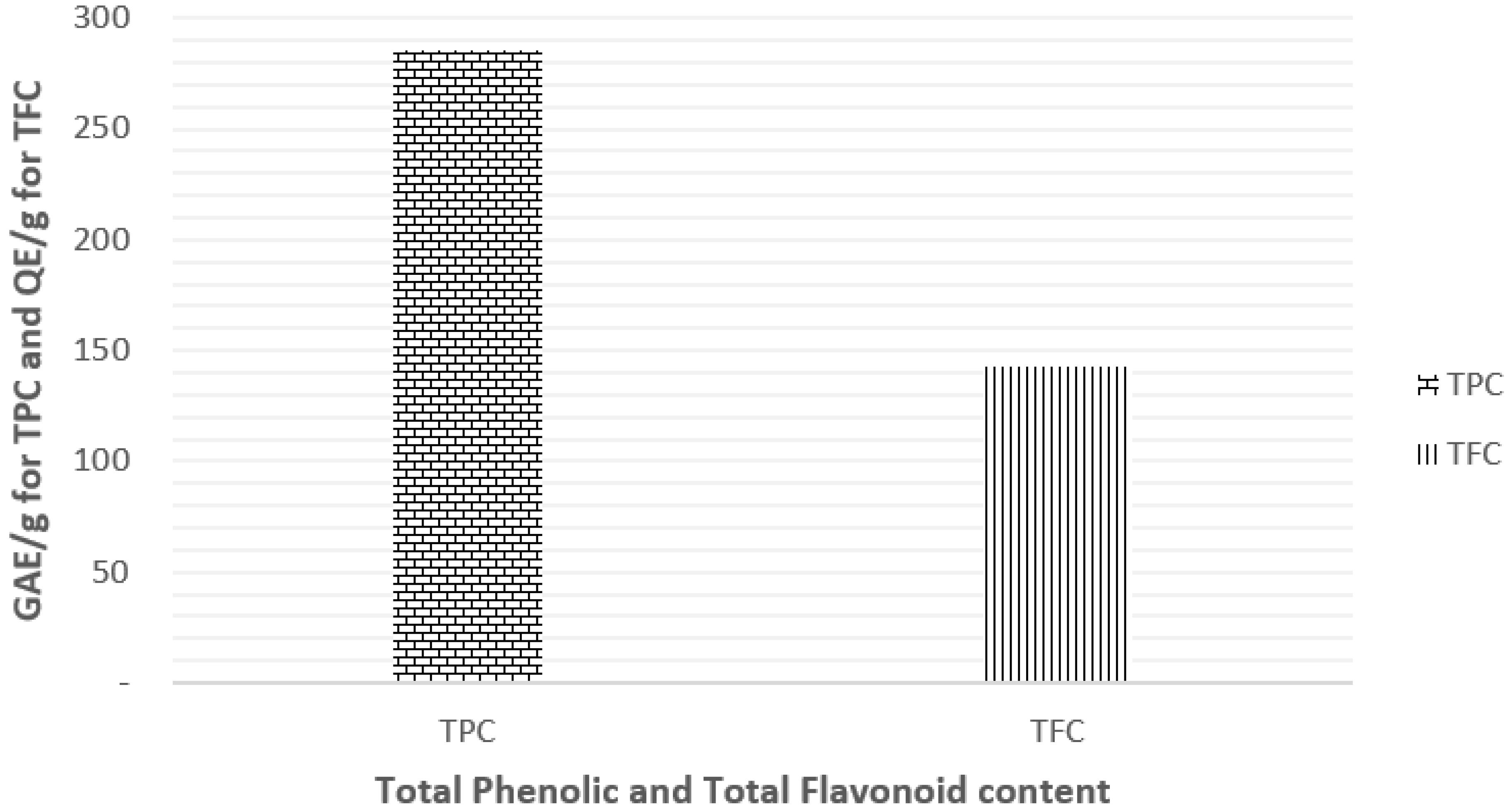
3.1. Determination of Total Phenolic Content (TPC) and Total Flavonoid Content (TFC)
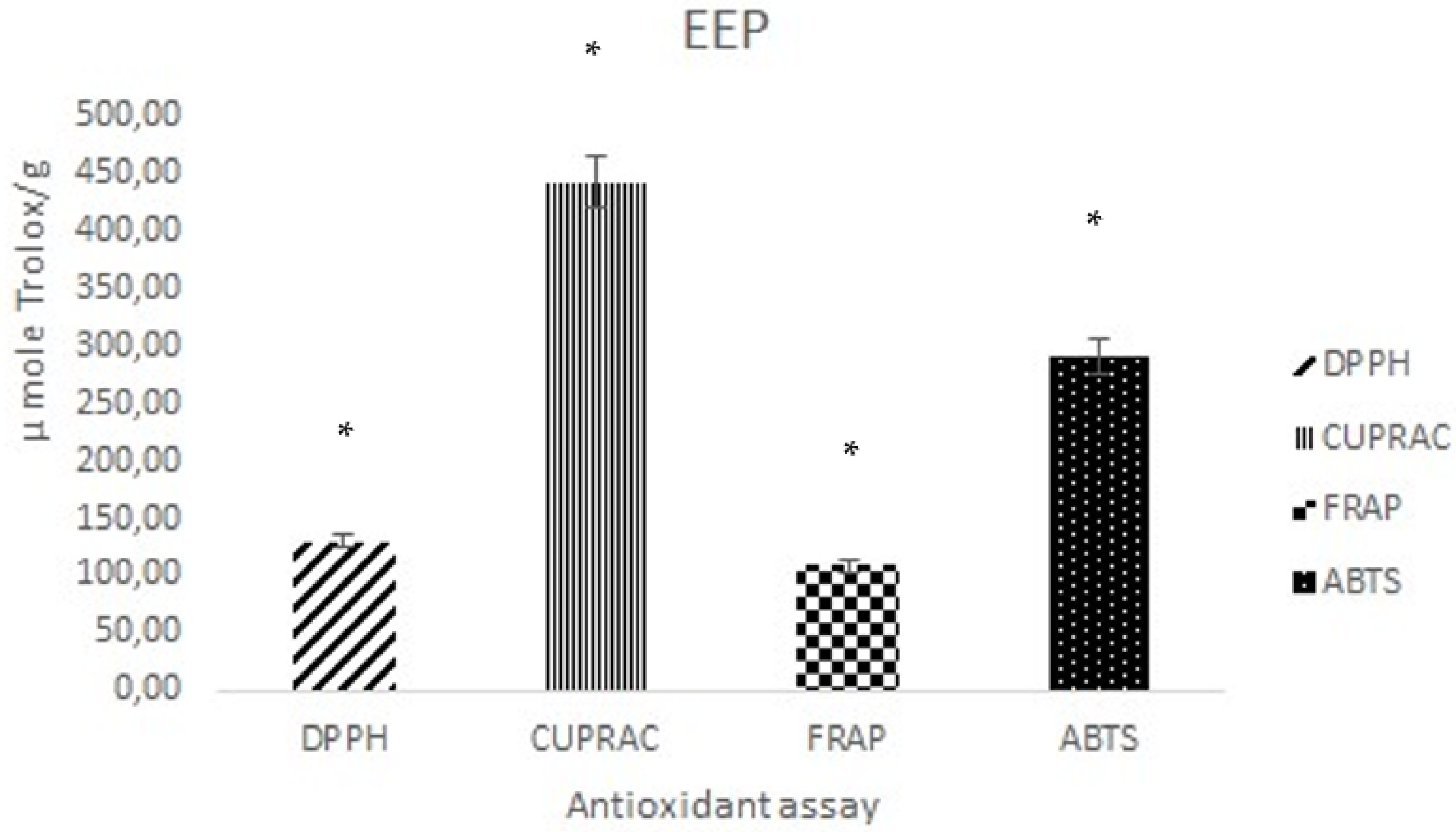
3.2. Antioxidant Activity
3.3. Antibacterial Activity
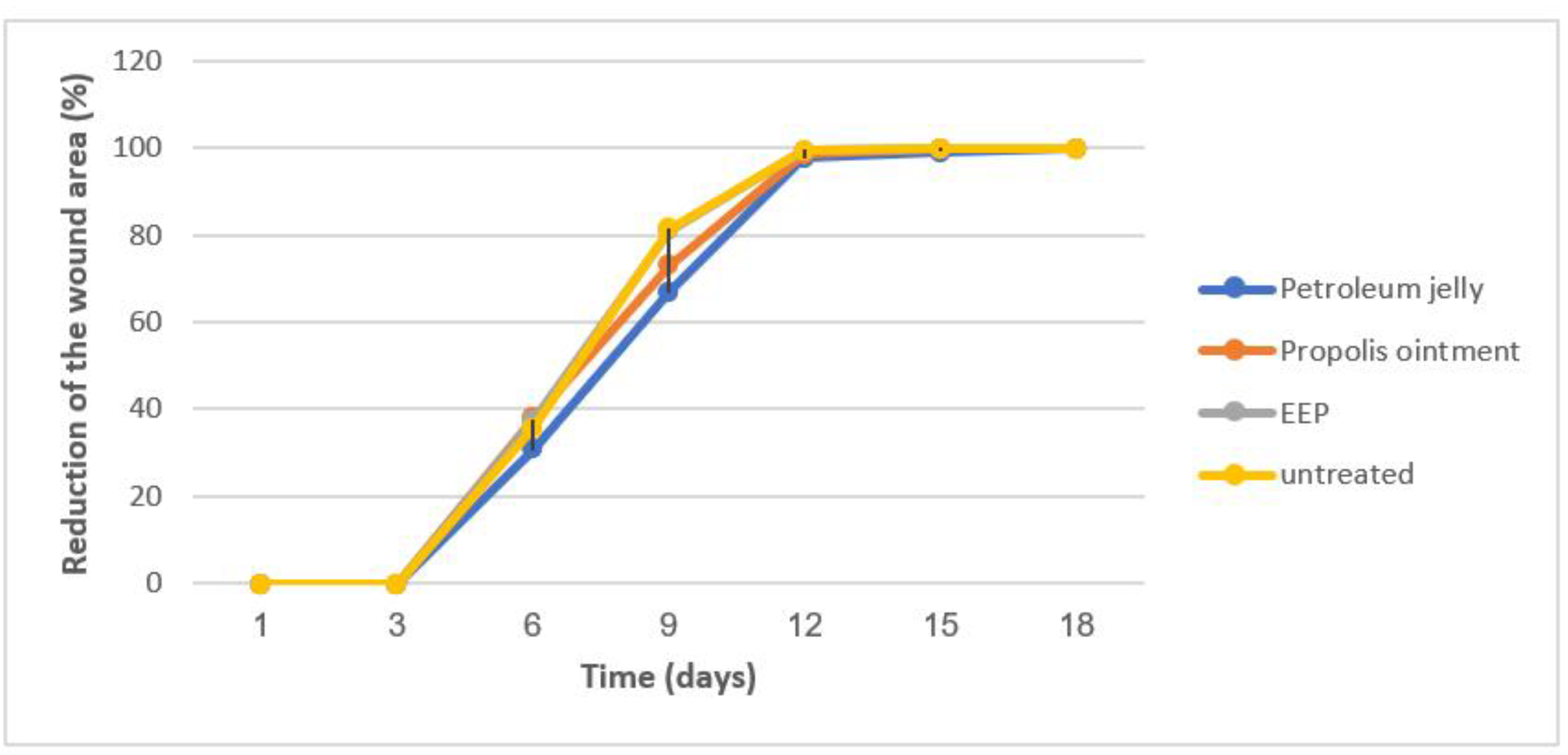
3.4. Wound-Healing Activity
3.5. UFLC/MS-MS Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghisalberti, E.L. Propolis: A review. Bee World 1979, 60, 59–84. [Google Scholar] [CrossRef]
- Valcic, S.; Montenegro, G.; Mujica, A.M.; Avila, G.; Franzblau, S.; Singh, M.P.; Maiese, W.M.; Timmermann, B.N. Phytochemical, morphological, and biological investigations of propolis from Central Chile. Z. Nat. C 1999, 54, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Bankova, V.; Popova, M.; Trusheva, B. New emerging fields of application of propolis. Maced. J. Chem. Chem. Eng. 2016, 35, 1–11. [Google Scholar] [CrossRef]
- Havsteen, B. Flavonoids, a class of natural products of high pharmacological potency. Biochem. Pharm. 1973, 32, 1141–1148. [Google Scholar] [CrossRef]
- Greenaway, W.; Scaysbrook, T.; Whatley, F.R. The composition and plant origins of propolis: A report of work at Oxford. Bee World 1990, 71, 107–118. [Google Scholar] [CrossRef]
- Burdock, G.A. Review of the biological properties and toxicity of bee propolis (propolis). Food Chem. Toxicol. 1998, 36, 347–363. [Google Scholar] [CrossRef]
- Marcucci, M.C. Propolis: Chemical composition, biological properties and therapeutic activity. Apidologie 1995, 26, 83–99. [Google Scholar] [CrossRef]
- De Carvalho, F.M.D.A.; Schneider, J.K.; de Jesus, C.V.F.; de Andrade, L.N.; Amaral, R.G.; David, J.M.; Krause, L.C.; Severino, P.; Soares, C.M.F.; Caramão Bastos, E.; et al. Brazilian red propolis: Extracts production, physicochemical characterization, and cytotoxicity profile for antitumor activity. Biomolecules 2020, 10, 726. [Google Scholar] [CrossRef] [PubMed]
- Batista, C.M.; de Queiroz, L.A.; Alves, Â.V.; Reis, E.C.; Santos, F.A.; Castro, T.N.; Lima, B.S.; Araújo, A.N.S.; Godoy, C.A.P.; Severino, P.; et al. Photoprotection and skin irritation effect of hydrogels containing hydroalcoholic extract of red propolis: A natural pathway against skin cancer. Heliyon 2022, 8, e08893. [Google Scholar] [CrossRef]
- Azemin, A.; Md-Zin, N.B.; Mohd-Rodi, M.M.; Kim-Chee, A.S.; Zakaria, A.J.; Mohd, K.S. Application of metabolite profiling and antioxidant activity in assessing the quality of processed and unprocessed stingless bee’s propolis. J. Fundam. Appl. Sci. 2017, 9, 637–660. [Google Scholar] [CrossRef] [Green Version]
- Farooqui, T.; Farooqui, A.A. Beneficial effects of propolis on human health and neurological diseases. Front. Biosci. 2012, 4, 779–793. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, U.T.; Abdel-Rahman, M.A.; Darwish, M.H.A.; Applegate, T.J.; Cheng, H.W. Behavioral changes and feathering score in heat stressed broiler chickens fed diets containing different levels of propolis. Appl. Anim. Behav. Sci. 2015, 166, 98–105. [Google Scholar] [CrossRef]
- Haščik, P.; Elimam, I.O.; Kročko, M.; Bobko, M.; Kačaniová, M.; Garlík, J.; Šimko, M.; Saleh, A.A. The influence of propolis as supplement diet on broiler meat growth performance, carcass body weight, chemical composition and lipid oxidation stability. Acta Univ. Agric. Etsilvic. Mendel. Brun 2015, 63, 411–418. [Google Scholar] [CrossRef]
- Šegvić-Bubić, T.; Boban, J.; Grubišić, L.; Trumbić, Z.; Radman, M.; Perčić, M.; Čož-Rakovac, R. Effects of propolis enriched diet on growth performance and plasma bio-chemical parameters of juvenile European sea bass (Dicentrarchus labrax L.) under acute low temperature stress. Aquac. Nutr. 2013, 19, 877–885. [Google Scholar] [CrossRef]
- Wafaa, E.; Doaa, I.; El-Murr, A.; Rania, M. Effects of dietary inclusion of black cumin seeds, green tea and propolis extraction growth parameters, body composition and economic efficiency of Nile tilapia, Oreochromis niloticus. World J. Fish Mar. Sci. 2014, 6, 447–452. [Google Scholar] [CrossRef]
- Pobiega, K.; Kraśniewska, K.; Gniewosz, M. Application of propolis in antimicrobial and antioxidative protection of food quality—A review. Trends Food Sci. Technol. 2019, 83, 53–62. [Google Scholar] [CrossRef]
- Torlak, E.; Sert, D. Effectiveness of chitosan-propolis coated polypropylene films against foodborne pathogens. Int. J. Biol. Macromol. 2013, 60, 52–55. [Google Scholar] [CrossRef]
- Costa, S.S.; Druzian, J.I.; Machado, B.A.S.; de Souza, C.O.; Guimarães, A.G. Bi-functional bio based packing of the cassava starch, glycerol, licurinanocellulose and red propolis. PLoS ONE 2014, 9, e112554. [Google Scholar] [CrossRef]
- De Araújo, G.K.P.; de Souza, S.J.; da Silva, M.V.; Yamashita, F.; Gonçalves, O.H.; Leimann, F.V.; Shirai, M.A. Physical, antimicrobial and antioxidant properties of starch-based film containing ethanolic propolis extract. Int. J. Food Sci. Technol. 2015, 50, 2080–2087. [Google Scholar] [CrossRef]
- Bouzabata, A. Contemporary use of phytocosmetics in three districts from North-Eastern Algeria. Pharm. J. 2017, 9, 762–766. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, M.V.; de Moura, N.G., Jr.; Motoyama, A.B.; Ferreira, V.M. A review of the potential therapeutic and cosmetic use of propolis in topical formulations. J. Appl. Pharmacol. Sci. 2019, 10, 131–141. [Google Scholar] [CrossRef]
- Segueni, N.; Keskin, M.; Keskin, Ş.; Benlabed, K.; Kolaylı, S.; Akkal, S. Comparison between phenolic content, antioxidant, and antibacterial activity of Algerian and Turkish propolis. Comb. Chem. High Throughput Screen. 2021, 24, 1679–1687. [Google Scholar] [CrossRef] [PubMed]
- Spanos, G.A.; Wrolstad, R.E. Influence of processing and storage on the phenolic composition of Thompson Seedless Grape Juice. J. Agric. Food Chem. 1990, 38, 1565–1571. [Google Scholar] [CrossRef]
- Dewanto, V.; Wu, X.; Adom, K.; Liu, R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.; Wahile, A.; Murkherjee, K.; PadaSaha, B.; Pulok, K.; Mukherjee, P.K. Antioxidant activity of Nelumbonucifera (sacred lotus) seeds. J. Ethnopharmacol. 2006, 104, 322–327. [Google Scholar] [CrossRef]
- Toor, R.K.; Savage, G.P.; Lister, C.E. Seasonal variations in the antioxidant composition of greenhousegrown tomatoes. J. Food Comp. Anal. 2006, 19, 1–10. [Google Scholar] [CrossRef]
- Deighton, N.; Brennan, R.; Finn, C.; Davies, H. Antioxidant properties of domesticated and wild Rubus species. J. Sci. Food Agric. 2000, 80, 1307–1313. [Google Scholar] [CrossRef]
- Apak, R.; Ozyurek, G.; Ozyurek, M.; Karademir, S.E. Novel total antioxidant Capacity index of dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence on neocuprione: CUPRAC method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Methods for dilution antimicrobial susceptibility tests for bacteria that growaerobically. In Approved Standard, 9th ed.; CLSI: Wayne, PA, USA, 2012. [Google Scholar]
- Peppa, M.; Brem, H.; Ehrlich, P.; Zhang, J.G.; Cai, W.; Li, Z.; Croitoru, C.; Thung, S.; Vlassara, H. Adverse effects of dietary glycotoxins on wound healing in genetically diabetic mice. Diabetes 2003, 52, 2805–2813. [Google Scholar] [CrossRef]
- Subramoniam, A.; Evans, D.A.; Rajasekharan, S.; Nair, G.S. Effect of Hemigraphiscolorata (Blume) HG Hallier leaf on wound healing and inflammation in mice. Indian J. Pharm. 2001, 3, 283–285. [Google Scholar]
- Gültekin-Özgüven, M.; Davarci, F.; Pasli, A.A.; Demir, N.; Özçelik, B. Determination of phenolic compounds by ultra high chromarography-tendem mass spectrometry: Application in nuts. LTW Food Sci. Tech. 2015, 64, 42–49. [Google Scholar] [CrossRef]
- Ribeiro, A.S.; Estanqueiro, M.; Oliveira, M.B.; Sousa Lobo, J.M. Main benefits and applicability of plant extracts in skin care products. Cosmetics 2015, 2, 48–65. [Google Scholar] [CrossRef]
- Fonseca-Santos, B.; Corrêa, M.A.; Chorilli, M. Sustainability, natural and organic cosmetics: Consumer, products, efficacy, toxicological and regulatory considerations. Braz. J. Pharm. Sci. 2015, 51, 17–26. [Google Scholar] [CrossRef]
- Marquele, F.D.; Di Mambro, V.M.; Georgetti, S.R.; Casagrande, R.; Valim, Y.M.; Fonseca, M.J.V. Assessment of the antioxidant activities of Brazilian extracts of propolis alone and in topical pharmaceutical formulations. J. Pharm. Bioiomed. Anal. 2005, 39, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Oršolić, N.; Skurić, J.; Đikić, D.; Stanić, G. Inhibitory effect of a propolis on di-n-propyl disulfide or n-hexyl salycilate-induced skin irritation, oxidative stress and inflammatory responses in mice. Fitoterapia 2014, 93, 18–30. [Google Scholar] [CrossRef]
- Wagh, V.D. Propolis: A wonder bees product and its pharmacological potentials. Adv. Pharmacol. Sci. 2013, 2013, 308249. [Google Scholar] [CrossRef]
- Trusheva, B.; Trunkova, D.; Bankova, V. Different extraction methods of biologically active components from propolis; a preliminary study. Chem. Cent. J. 2007, 1, 1–4. [Google Scholar] [CrossRef]
- Zin, N.B.M.; Azemin, A.; Rodi, M.M.M.; Mohd, K.S. Chemical composition and antioxidant activity of stingless bee propolis from different extraction methods. Int. J. Eng. Tech. 2018, 7, 90–95. [Google Scholar]
- Margeretha, I.; Suniarti, D.F.; Herda, E.; Alim, Z. Optimization and comparative study of different extraction methods of biologically active components of Indonesian propolis Trigona spp. J. Nat. Prod. 2012, 5, 233–242. [Google Scholar]
- Sun, C.; Wu, Z.; Wang, Z.; Zhang, H. Effect of ethanol/water solvents on phenolic profiles and antioxidant properties of Beijing propolis extracts. Evid. Based Complement. Altern. Med. 2015, 9, 595393. [Google Scholar] [CrossRef]
- Ćujić, N.; Šavikin, K.; Janković, T.; Pljevljakušić, D.; Zdunić, G.; Ibrić, S. Optimization of polyphenols extraction from dried chokeberry using maceration as traditional technique. Food Chem. 2016, 194, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Narimane, S.; Demircan, E.; Salah, A.; Ozcelik, B.Ö.; Salah, R. Correlation between antioxidant activity and phenolic acids profile and content of Algerian propolis: Influence of solvent. Pak. J. Pharm. Sci. 2017, 30, 1417–1423. [Google Scholar]
- Bouaroura, A.; Segueni, N.; Erenler, R.; May, A.; Bensouici, C.; Akkal, S.; Rhouati, S. Phytochemical Investigation of Phenolic Constituents and In vitro Evaluation of Antioxidant Activity of Five Algerian Propolis. Curr. Bioact. Compd. 2021, 17, 79–87. [Google Scholar] [CrossRef]
- Kara, Y.; Can, Z.; Kolaylı, S. What Should Be the Ideal Solvent Percentage and Solvent-Propolis ratio in the Preparation of Ethanolic Propolis Extract? Food Anal. Methods 2022, 15, 1707–1719. [Google Scholar] [CrossRef]
- Mello, B.C.; Hubinger, M.D. Antioxidant activity and polyphenol contents in Brazilian green propolis extracts prepared with the use of ethanol and water as solvents in different pH values. Int. J. Food Sci. Technol. 2012, 47, 2510–2518. [Google Scholar] [CrossRef]
- Park, Y.K.; Ikegaki, M. Preparation of water and ethanolic extracts of propolis and evaluation of the preparations. Biosci. Biotechnol. Biochem. 1998, 62, 2230–2232. [Google Scholar] [CrossRef]
- Yusof, N.; Munaim, M.S.A.; VelooKutty, R. Optimization of total phenolic compounds extracted from propolis by ultrasound-assisted extraction. Chem. Eng. Commun. 2021, 208, 564–572. [Google Scholar] [CrossRef]
- Monroy, Y.M.; Rodrigues, R.A.; Rodrigues, M.V.; Sant’Ana, A.S.; Silva, B.S.; Cabral, F.A. Brazilian green propolis extracts obtained by conventional processes and by processes at high pressure with supercritical carbon dioxide, ethanol and water. J. Supercrit. Fluids 2017, 130, 189–197. [Google Scholar] [CrossRef]
- Bakchiche, B.; Temizer, İ.K.; Güder, A.; Çelemli, Ö.G.; Yegin, S.Ç.; Bardaweel, S.K.; Ghareeb, M.A. Chemical composition and biological activities of honeybee products from Algeria. J. Appl. Biotechnol. Rep. 2020, 7, 93–103. [Google Scholar] [CrossRef]
- Boulechfar, S.; Zellagui, A.; Bensouici, C.; Asan-Ozusaglam, M.; Tacer, S.; Hanene, D. Anticholinesterase, anti-α-glucosidase, antioxidant and antimicrobial effects of four Algerian propolis. J. Food Meas. Charact. 2022, 16, 793–803. [Google Scholar] [CrossRef]
- Touzani, S.; Imtara, H.; Katekhaye, S.; Mechchate, H.; Ouassou, H.; Alqahtani, A.S.; Noman, O.N.; Nasr, F.A.; Fearnley, H.; Fearnley, J.; et al. Determination of phenolic compounds in various propolis samples collected from an African and an Asian region and their impact on antioxidant and antibacterial activities. Molecules 2021, 26, 4589. [Google Scholar] [CrossRef] [PubMed]
- Stoia, M.; Cotınghıu, A.; Budin, F.; Oancea, S. Total phenolics contentof Romanian propolis and bee pollen. Victoria 2015, 2, 20. [Google Scholar]
- Dezmirean, D.S.; Mărghitaş, L.A.; Chirilă, F.; Copaciu, F.; Simonca, V.; Bobiş, O.; Erler, S. Influence of geographic origin, plant source and polyphenolic substances on antimicrobial properties of propolis against human and honey bee pathogens. J. Apic. Res. 2017, 56, 588–597. [Google Scholar] [CrossRef]
- Ndiaye, M.; Philippe, C.; Mukhtar, H.; Ahmad, N. The grape antioxidant resveratrol for skin disorders: Promise, prospects, and challenges. Arch. Biochem. Biophys. 2011, 508, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, Y.; Sánchez-Catalán, F.; Rojano, B.; Durango, D.; Gil, J.; Marín-Loaiza, J. Physicochemical characterization and evaluation of antioxidant activity of propolis collected in the atlántic department, Colombia. Rev. UDCA Actual. Divulg. Científica 2012, 15, 303–311. [Google Scholar]
- Da Silva, C.; Prasniewski, A.; Calegari, M.A.; de Lima, V.A.; Oldoni, T.L. Determination of total phenolic compounds and antioxidant activity of ethanolic extracts of propolis using ATR–FT-IR spectroscopy and chemometrics. Food Anal. Methods 2018, 11, 2013–2021. [Google Scholar] [CrossRef]
- Gargouri, W.; Osés, S.M.; Fernández-Muiño, M.A.; Sancho, M.T.; Kechaou, N. Evaluation of bioactive compounds and biological activities of Tunisian propolis. Lwt 2019, 111, 328–336. [Google Scholar] [CrossRef]
- Ozdal, T.; Sari-Kaplan, G.; Mutlu-Altundag, E.; Boyacioglu, D.; Capanoglu, E. Evaluation of Turkish propolis for its chemical composition, antioxidant capacity, anti-proliferative effect on several human breast cancer cell lines and proliferative effect on fibroblasts and mouse mesenchymal stem cell line. J. Apic. Res. 2018, 57, 627–638. [Google Scholar] [CrossRef]
- Bakkaloglu, Z.; Arici, M.; Karasu, S. Optimization of ultrasound-assisted extraction of Turkish propolis and characterization of phenolic profile, antioxidant and antimicrobial activity. Food Sci. Technol. 2021, 41, 687–695. [Google Scholar] [CrossRef]
- Augusto-Obara, T.R.; Oliveira, J.D.; Gloria, E.M.D.; Spoto, M.H.F.; Godoy, K.; Vieira, T.M.F.D.S.; Scheuermann, E. Benefits of superfine grinding method on antioxidant and antifungal characteristic of Brazilian green propolis extract. Sci. Agric. 2019, 76, 398–404. [Google Scholar] [CrossRef]
- Cavalaro, R.I.; Fabricio, L.F.D.F.; Vieira, T.M.F.D.S. Ultrasound-Assisted Extraction of Antioxidants from Baccharis dracunculifolia and Green Propolis. Processes 2020, 8, 1530. [Google Scholar] [CrossRef]
- Irigoiti, Y.; Yamul, D.K.; Navarro, A.S. Co-crystallized sucrose with propolis extract as a food ingredient: Powder characterization and antioxidant stability. LWT 2021, 143, 111164. [Google Scholar] [CrossRef]
- Oldoni, T.L.C.; Oliveira, S.C.; Andolfatto, S.; Karling, M.; Calegari, M.A.; Sado, R.Y.; Maia, F.M.C.; Alencar, S.M.; Lima, V.A. Chemical characterization and optimization of the extraction process of bioactive compounds from propolis produced by selected bees Apismellifera. J. Braz. Chem. Soc. 2015, 26, 2054–2062. [Google Scholar] [CrossRef]
- Andrade, J.K.S.; Denadai, M.; Andrade, G.R.S.; da Cunha Nascimento, C.; Barbosa, P.F.; Jesus, M.S.; Narain, N. Development and characterization of microencapsules containing spray dried powder obtained from Brazilian brown, green and red propolis. Food Res. Int. 2018, 109, 278–287. [Google Scholar] [CrossRef]
- Kasote, D.M.; Pawar, M.V.; Bhatia, R.S.; Nandre, V.S.; Gundu, S.S.; Jagtap, S.D.; Kulkarni, M.V. HPLC, NMR based chemical profiling and biological characterisation of Indian propolis. Fitoterapia 2017, 122, 52–60. [Google Scholar] [CrossRef]
- Przybyłek, I.; Karpiński, T.M. Antibacterial Properties of Propolis. Molecules 2019, 24, 2047. [Google Scholar] [CrossRef] [Green Version]
- Sforcin, J.M. Biological Properties and Therapeutic Applications of Propolis. Phytother. Res. 2016, 30, 894–905. [Google Scholar] [CrossRef]
- Boufadi, Y.M.; Soubhye, J.; Nève, J.; Van Antwerpen, P.; Riazi, A. Antimicrobial effects of six Algerian propolis extracts. Int. J. Food Sci. Technol. 2016, 51, 2613–2620. [Google Scholar] [CrossRef]
- Iyyam Pillai, S.; Palsamy, P.; Subramanian, S.; Kandaswamy, M. Wound healing properties of Indian propolis studied on excision wound-induced rats. Pharm. Biol. 2010, 48, 1198–1206. [Google Scholar] [CrossRef]
- Abu-Seida, A.M. Effect of propolis on experimental cutaneous wound healing in dogs. Vet. Med. Int. 2015, 2015, 672643. [Google Scholar] [CrossRef]
- Stevenson, D.E.; & Hurst, R.D. Polyphenolic phytochemicals–just antioxidants or much more? Cell Mol. Life Sci. 2007, 64, 2900–2916. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Cueva, C.; Moreno-Arribas, M.V.; Martín-Álvarez, P.J.; Bills, G.; Vicente, M.F.; Basilio, A.; Lopez, C.; Requena, T.; Rodriguez, J.M.; Bartolomé, B. Antimicrobial activity of phenolic acids against commensal, probiotic and pathogenic bacteria. Res. Microbiol. 2010, 161, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Campos, F.M.; Couto, J.A.; Figueiredo, A.R.; Tóth, I.V.; Rangel, A.O.; Hogg, T.A. Cell membrane damage induced by phenolic acids on wine lactic acid bacteria. Int. J. Food Microb. 2009, 135, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Melguizo-Rodríguez, L.; de Luna-Bertos, E.; Ramos-Torrecillas, J.; Illescas-Montesa, R.; Costela-Ruiz, V.J.; García-Martínez, O. Potential effects of phenolic compounds that can be found in olive oil on wound healing. Foods 2021, 10, 1642. [Google Scholar] [CrossRef]
- Kahkeshani, N.; Farzaei, F.; Fotouhi, M.; Alavi, S.S.; Bahramsoltani, R.; Naseri, R.; Momtaz, S.; Abbasabadi, Z.; Rahimi, R.; Farzaei, M.H.; et al. Pharmacological effects of gallic acid in health and diseases: A mechanistic review. Iran. J. Basic Med. Sci. 2019, 22, 225–237. [Google Scholar] [CrossRef]
- Yang, D.J.; Moh, S.H.; Son, D.H.; You, S.; Kinyua, A.W.; Ko, C.M.; Song, M.; Yeo, J.; Choi, Y.-H.; Kim, K.W. Gallic Acid Promotes Wound Healing in Normal and Hyperglucidic Conditions. Molecules 2016, 21, 899. [Google Scholar] [CrossRef]
- Phan, T.T.; Wang, L.; See, P.; Grayer, R.J.; Chan, S.Y.; Lee, S.T. Phenolic compounds of Chromolaena odorata protect cultured skincells from oxidative damage: Implication for cutaneous wound healing. Biol. Pharm. Bull 2001, 24, 1373–1379. [Google Scholar] [CrossRef]
- Li, L.; Sun, W.; Wu, T.; Lu, R.; Shi, B. Caffeic acid phenethyl ester attenuates lipopolysaccharide-stimulated proinflammatoryresponses in human gingival fibroblasts via NF-kB and PI3K/Akt signaling pathway. Eur. J. Pharm. 2017, 794, 61–68. [Google Scholar] [CrossRef]
- Melguizo-Rodríguez, L.; Illescas-Montes, R.; Costela-Ruiz, V.J.; Ramos-Torrecillas, J.; de Luna-Bertos, E.; García-Martínez, O.; Ruiz, C. Antimicrobial properties of olive oil phenolic compounds and their regenerative capacity towards fibroblast cells. J. Tissue Viability 2021, 30, 372–378. [Google Scholar] [CrossRef]
- Lin, C.-M.; Chiu, J.-H.; Wu, I.-H.; Wang, B.-W.; Pan, C.-M.; Chen, Y.-H. Ferulic acid augments angiogenesis via VEGF, PDGF and HIF-1α. J. Nutr. Biochem. 2010, 21, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-Y.; Huang, C.-N.; Liao, C.-K.; Chang, H.-M.; Kuan, Y.-H.; Tseng, T.-J.; Yen, K.-J.; Yang, K.-L.; Lin, H.-C. Effects of Rutin on Wound Healing in Hyperglycemic Rats. Antioxidants 2020, 9, 1122. [Google Scholar] [CrossRef] [PubMed]
- Pivec, T.; Kargl, R.; Maver, U.; Bračič, M.; Elschner, T.; Žagar, E.; Gradišnik, L.; Kleinschek, K.S. Chemical Structure-Antioxidant Activity Relationship ofWater-Based Enzymatic Polymerized Rutin and ItsWound Healing Potential. Polymers 2019, 11, 1566. [Google Scholar] [CrossRef]
- Martínez, L.; Jongberg, S.; Ros, G.; Skibsted, L.H.; Nieto, G. Plant derived ingredients rich in nitrates or phenolics for protectionof pork against protein oxidation. Food Res. Int. 2020, 129, 108789. [Google Scholar] [CrossRef] [PubMed]
- Martínez, J.; Nieto, G.; Castillo, J.; Ros, G. Influence of in vitro gastrointestinal digestion and/or grape seed extract addition on antioxidant capacity of meat emulsions. LWT Food Sci. Technol. 2014, 59, 834–840. [Google Scholar] [CrossRef]
- Milla, P.; Peñalver, R.; Nieto, G. Health Benefits of Uses and Applications of Moringa oleifera in Bakery Products. Plants 2021, 10, 318. [Google Scholar] [CrossRef]
- Nieto, G. A review on applications and uses of thymus in the food industry. Plants 2020, 9, 961. [Google Scholar] [CrossRef]
- Martínez, L.; Ros, G.; Nieto, G. Effect of natural extracts obtained from food industry by-products on nutritional quality and shelf life of chicken nuggets enriched with organic Zn and Se provided in broiler diet. Poult. Sci. 2020, 99, 1491–1501. [Google Scholar] [CrossRef]
- Nieto, G.; Martínez, L.; Castillo, J.; Ros, G. Effect of hydroxytyrosol, walnut and olive oil on nutritional profile of Low-Fat Chicken Frankfurters. Eur. J. Lipid Sci. Technol. 2017, 119, 1600518. [Google Scholar] [CrossRef]
| Bacterial Strains | MIC (mg/mL) |
|---|---|
| Standards strains | |
| S. aureus ATCC 25923 | 0.06 ± 0.00 a |
| E. coli ATCC 25922 | 1.00 ± 0.00 b |
| P. aeruginosa ATCC 27853 | 1.10 ± 0.01 b,c |
| Clinical strains | |
| S. aureus | 0.20 ± 0.01 d |
| β hemolytic streptococcus | 0.60 ± 0.01 a |
| α hemolytic streptococcus | >1.40 e |
| non-hemolytic streptococcus | 0.60 ± 0.01 a |
| E. coli | 1.00 ± 0.00 b |
| P. aeruginosa | 1.10 ± 0.01 b,c |
| P. mirabilis | 1.20 ± 0.02 c |
| K. pneumoniae | 1.20 ± 0.02 c |
| Day of Receiving Treatment | Petroleum Jelly Untreated Rats | 30% EEP | Propolis Ointment |
|---|---|---|---|
| 1 | Inflammatory reactions and a skin edema around the wound | ||
| 3 | The wounds were smaller with a formed scab. Edema and reddening at the wound were visually smaller | ||
| 6 | Wounds were covered with poorly formed scrab | Wounds were clean with a correctly developed scab | |
| 9 | No changes when compared to day 6 | Wounds were covered with smaller scabs and on the skin border of the wound there was a pink scar | Dry wounds. Formation of dry scabs |
| 18 | Wounds covered with a light-pink epithelium | Wounds were healed completely. During palpation, the animals did not react defensively | |
| Compounds | Rt (min) | ([M − H]−) | Concentration (ng /mL) |
|---|---|---|---|
| Gallic acid | 7.9 | 169 | 44.25 ± 6.40 |
| Chlorogenic acid | 8.31 | 353 | 48.79 ± 5.01 |
| p-hydroxybenzoic acid | 8.36 | 137 | ND |
| Rutin | 8.43 | 609 | 21.12 ± 3.57 |
| p-coumaric acid | 8.69 | 163 | ND |
| Caffeic acid | 8.78 | 179 | 28.19 ± 4.95 |
| Vanillic acid | 8.8 | 167 | 4.24 ± 3.27 |
| Syringic acid | 8.86 | 197 | 7.69 ± 1.36 |
| Sinapic acid | 9.2 | 223 | 3.32 ± 2.61 |
| Ferulic acid | 9.34 | 193 | 11.48 ± 2.29 |
| Trans cinnamic acid | 10.2 | 147 | 20.10 ± 6.51 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Segueni, N.; Akkal, S.; Benlabed, K.; Nieto, G. Potential Use of Propolis in Phytocosmetic as Phytotherapeutic Constituent. Molecules 2022, 27, 5833. https://doi.org/10.3390/molecules27185833
Segueni N, Akkal S, Benlabed K, Nieto G. Potential Use of Propolis in Phytocosmetic as Phytotherapeutic Constituent. Molecules. 2022; 27(18):5833. https://doi.org/10.3390/molecules27185833
Chicago/Turabian StyleSegueni, Narimane, Salah Akkal, Kadour Benlabed, and Gema Nieto. 2022. "Potential Use of Propolis in Phytocosmetic as Phytotherapeutic Constituent" Molecules 27, no. 18: 5833. https://doi.org/10.3390/molecules27185833
APA StyleSegueni, N., Akkal, S., Benlabed, K., & Nieto, G. (2022). Potential Use of Propolis in Phytocosmetic as Phytotherapeutic Constituent. Molecules, 27(18), 5833. https://doi.org/10.3390/molecules27185833







