Synthesis of Starch-Grafted Polymethyl Methacrylate via Free Radical Polymerization Reaction and Its Application for the Uptake of Methylene Blue
Abstract
:1. Introduction
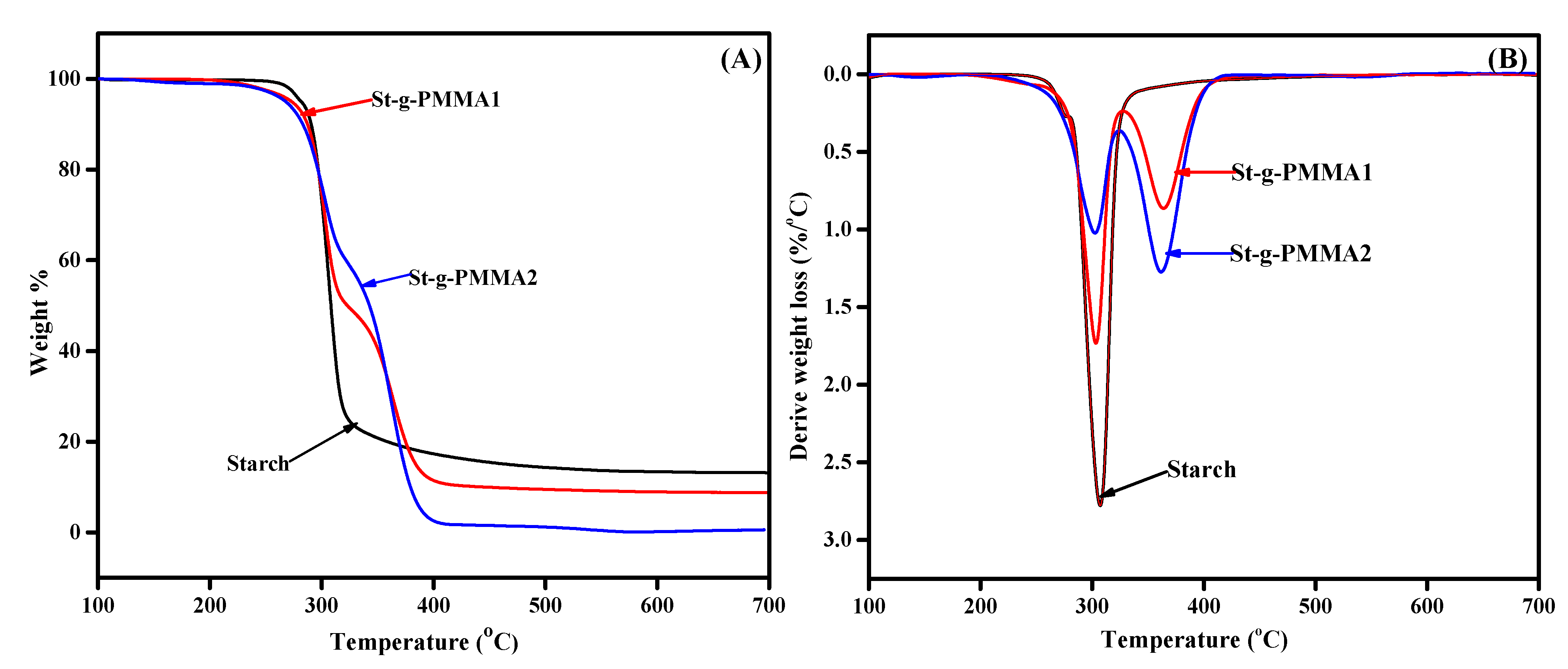
2. Results
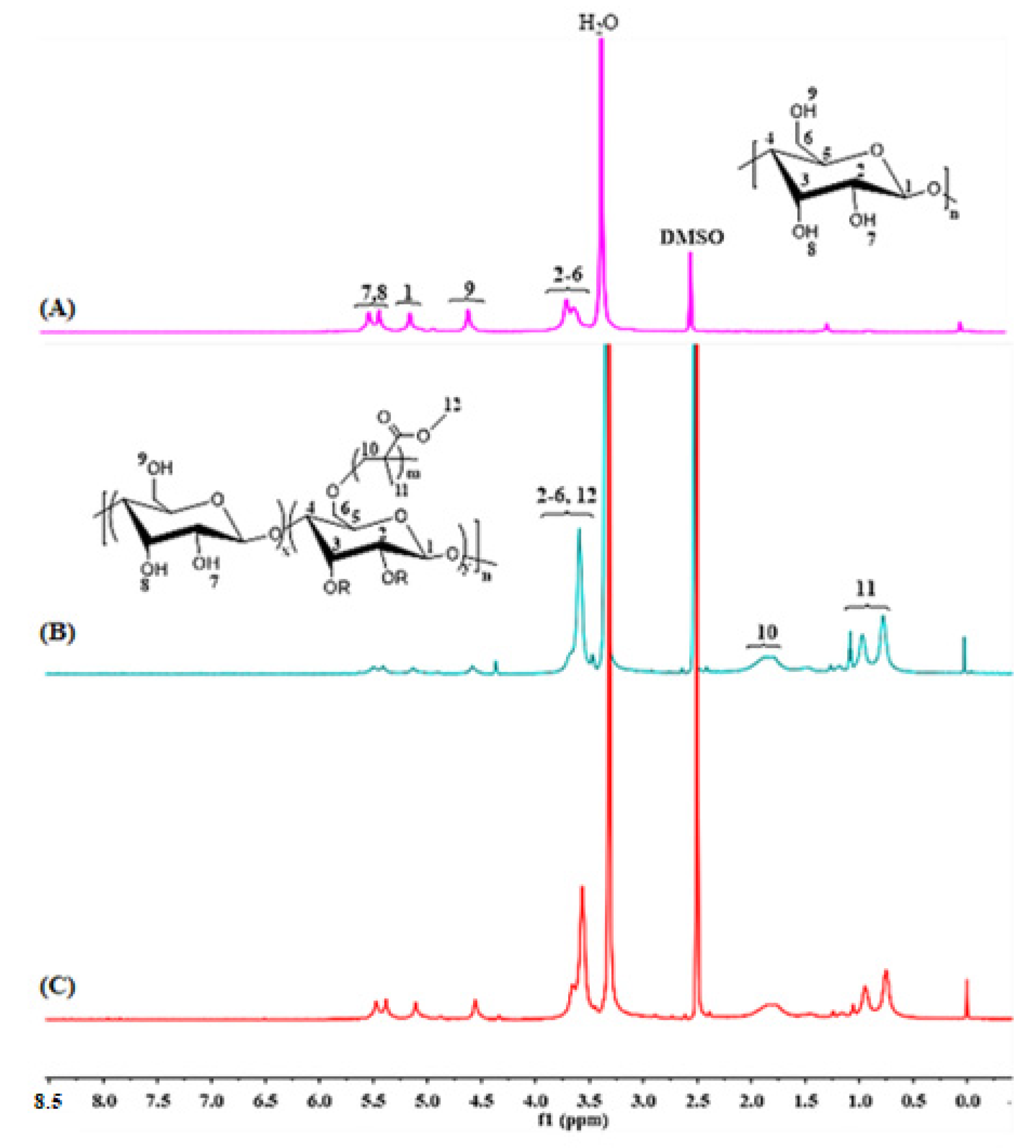
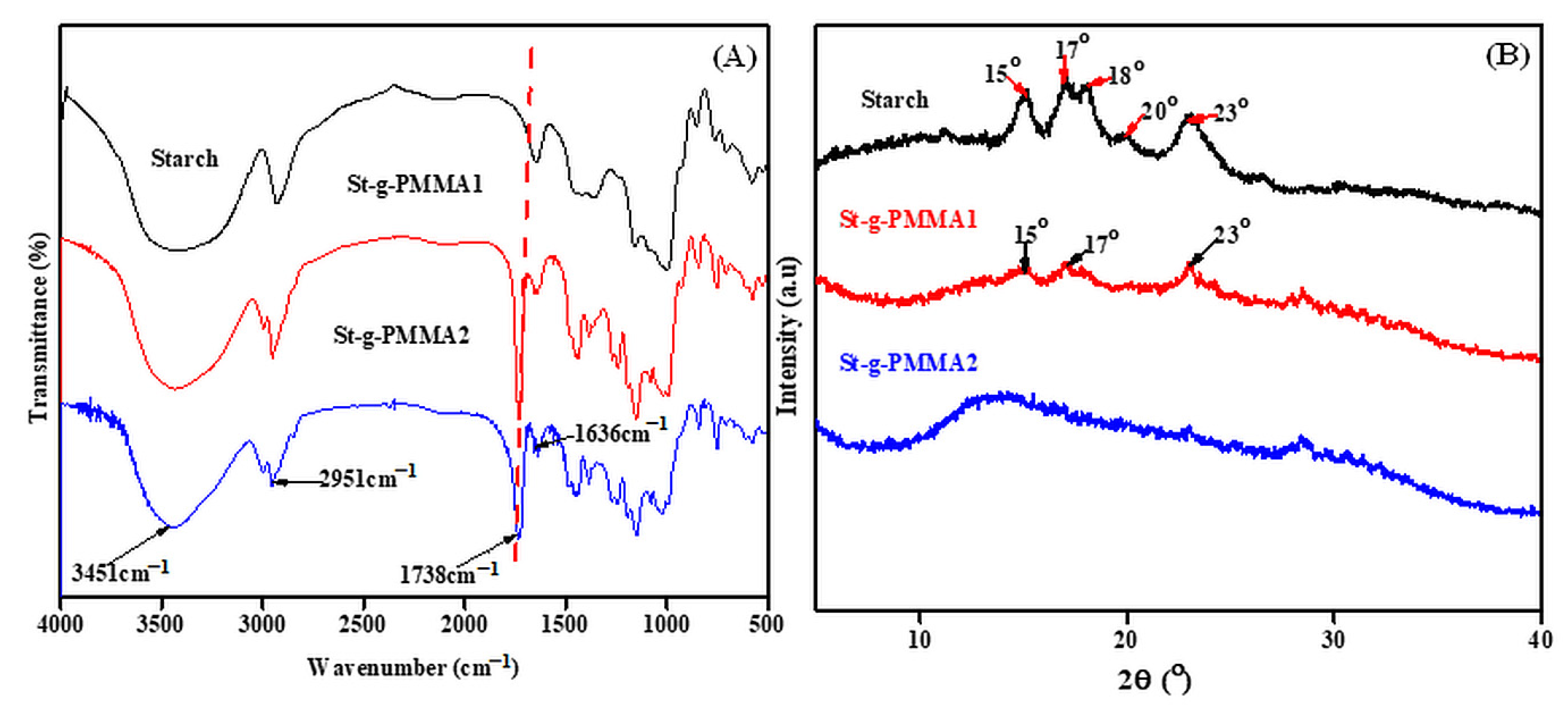
2.1. FT-IR Analysis
2.2. Applications
2.2.1. Effect of Adsorbent Dose
2.2.2. Effect of Initial Concentration of Dye
2.2.3. Effect of Contact Time
2.2.4. Effect of pH
2.3. Kinetic and Isotherm Studies
3. Materials and Methods
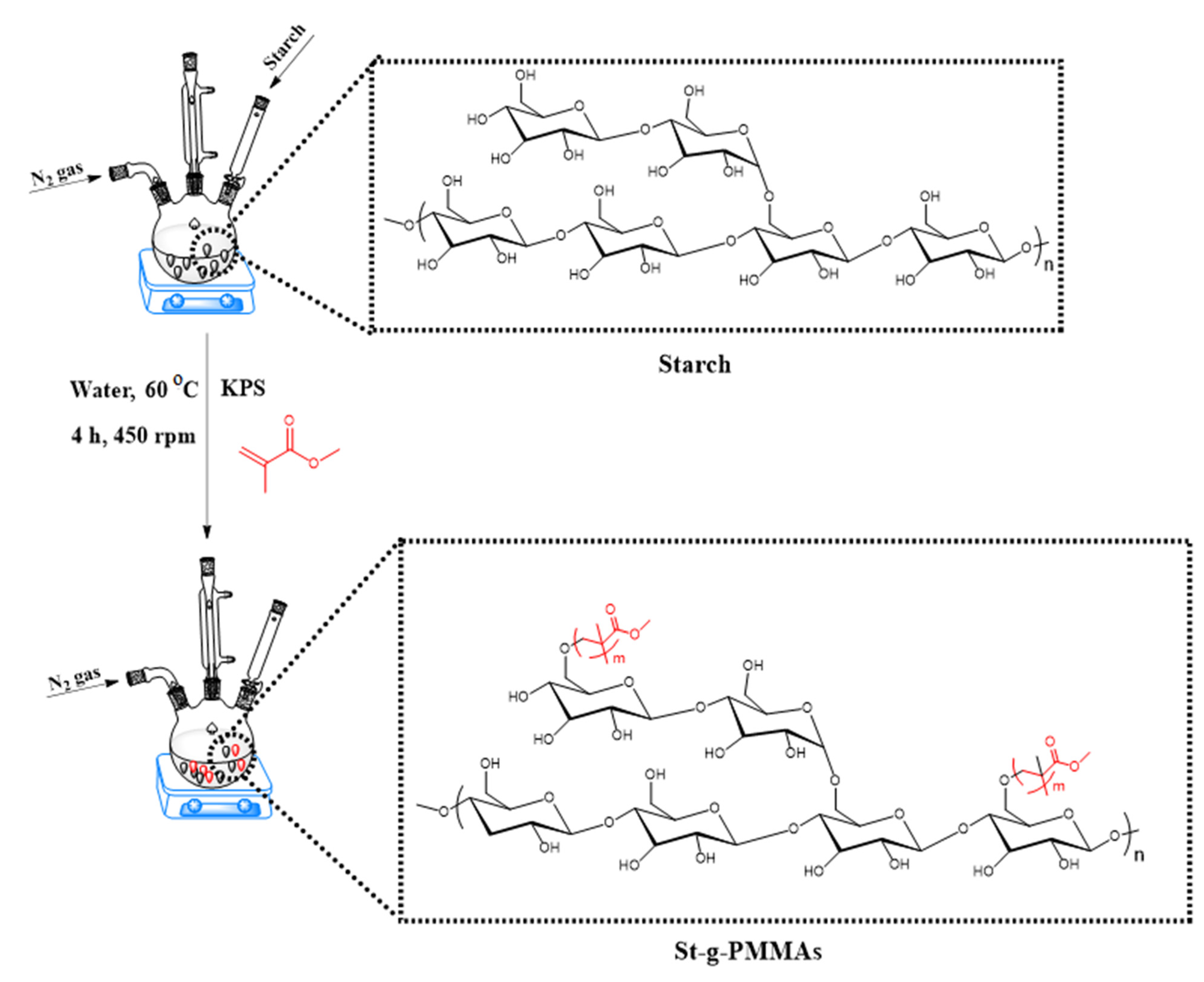
3.1. Synthesis of St-g-PMMA
3.2. Characterization
3.3. Adsorption Experiments
3.3.1. Comparative Adsorption Study
3.3.2. Effect of Adsorbent Dose
3.3.3. Effect of Initial Concentration of Dye
3.3.4. Effect of Contact Time
3.3.5. Effect of pH
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alvarado, N.; Abarca, R.L.; Urdaneta, J.; Romero, J.; Galotto, M.J.; Guarda, A. Cassava Starch: Structural Modification for Development of a Bio-Adsorber for Aqueous Pollutants. Characterization and Adsorption Studies on Methylene Blue. Polym. Bull. 2021, 78, 1087–1107. [Google Scholar] [CrossRef]
- Bhatti, H.N.; Safa, Y.; Yakout, S.M.; Shair, O.H.; Iqbal, M.; Nazir, A. Efficient Removal of Dyes Using Carboxymethyl Cellulose/Alginate/Polyvinyl Alcohol/Rice Husk Composite: Adsorption/Desorption, Kinetics and Recycling Studies. Int. J. Biol. Macromol. 2020, 150, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Hevira, L.; Zilfa; Rahmayeni; Ighalo, J.O.; Aziz, H.; Zein, R. Terminalia Catappa Shell as Low-Cost Biosorbent for the Removal of Methylene Blue from Aqueous Solutions. J. Ind. Eng. Chem. 2021, 97, 188–199. [Google Scholar] [CrossRef]
- Tehrim, A.; Dai, M.; Wu, X.; Umair, M.M.; Ali, I.; Amjed, M.A.; Rong, R.; Javaid, S.F.; Peng, C. Citric Acid Modified Waste Cigarette Filters for Adsorptive Removal of Methylene Blue Dye from Aqueous Solution. J. Appl. Polym. Sci. 2021, 138, 50655. [Google Scholar] [CrossRef]
- Mu, Y.; Ma, H. NaOH-Modified Mesoporous Biochar Derived from Tea Residue for Methylene Blue and Orange II Removal. Chem. Eng. Res. Des. 2021, 167, 129–140. [Google Scholar] [CrossRef]
- Elsayed, M.S.; Ahmed, I.A.; Bader, D.M.D.; Hassan, A.F. Green Synthesis of Nano Zinc Oxide/Nanohydroxyapatite Composites Using Date Palm Pits Extract and Eggshells: Adsorption and Photocatalytic Degradation of Methylene Blue. Nanomaterials 2022, 12, 49. [Google Scholar] [CrossRef] [PubMed]
- Haq, F.; Yu, H.; Wang, L.; Teng, L.; Haroon, M.; Khan, R.U.; Mehmood, S.; Bilal-Ul-Amin; Ullah, R.S.; Khan, A.; et al. Advances in Chemical Modifications of Starches and Their Applications. Carbohydr. Res. 2019, 476, 12–35. [Google Scholar] [CrossRef] [PubMed]
- Esrafili, L.; Firuzabadi, F.D.; Morsali, A.; Hu, M.L. Reuse of Predesigned Dual-Functional Metal Organic Frameworks (DF-MOFs) after Heavy Metal Removal. J. Hazard. Mater. 2021, 403, 123696. [Google Scholar] [CrossRef]
- Khalfa, L.; Sdiri, A.; Bagane, M.; Cervera, M.L. A Calcined Clay Fixed Bed Adsorption Studies for the Removal of Heavy Metals from Aqueous Solutions. J. Clean. Prod. 2021, 278, 123935. [Google Scholar] [CrossRef]
- Yaseen, M.; Kamran, M.; Farid, A.; Ismail, S.; Muzammal, M.; Amir, K.A.; Rashid, S.A. Antibacterial, Hemagglutination, and Insecticidal Activity Studies on the Solvent Extracts of the Roots of Olea ferruginea. Makara J. Sci. 2022, 26, 8. [Google Scholar] [CrossRef]
- Muzammal, M.; Firoz, A.; Ali, H.M.; Farid, A.; Khan, M.A.; Hakeem, K.R. Lumateperone Interact with S-Protein of Ebola Virus and TIM-1 of Human Cell Membrane: Insights from Computational Studies. Appl. Sci. 2022, 12, 8820. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, J.; Fan, Y.; Liu, J. Adsorption of Dyes from Water by Prunella Vulgaris Stem and Subsequent Fungal Decolorization. Korean J. Chem. Eng. 2020, 37, 1445–1452. [Google Scholar] [CrossRef]
- Khan, K.A.; Khan, G.M.; Muzammal, M.; Al Mohaini, M.; Alsalman, A.J.; Al Hawaj, M.A.; Ahmad, A.; Niazi, Z.R.; Shah, K.U.; Farid, A. Preparation of Losartan Potassium Controlled Release Matrices and In-Vitro Investigation Using Rate Controlling Agents. Molecules 2022, 27, 864. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, K.; Kaur, J.; Kumari, A.; Kumari, A.; Chauhan, G.S. Efficient Method of Starch Functionalization to Bis-Quaternary Structure Unit. Int. J. Biol. Macromol. 2015, 80, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Saadullah, M.; Asif, M.; Farid, A.; Naseem, F.; Rashid, S.A.; Ghazanfar, S.; Muzammal, M.; Ahmad, S.; Bin Jardan, Y.A.; Alshaya, H.; et al. A Novel Distachionate from Breynia distachia Treats Inflammations by Modulating COX-2 and Inflammatory Cytokines in Rat Liver Tissue. Molecules 2022, 27, 2596. [Google Scholar] [CrossRef]
- Diyana, Z.N.; Jumaidin, R.; Selamat, M.Z.; Ghazali, I.; Julmohammad, N.; Huda, N.; Ilyas, R.A. Physical Properties of Thermoplastic Starch Derived from Natural Resources and Its Blends: A Review. Polymers 2021, 13, 1396. [Google Scholar] [CrossRef]
- Bortoluz, J.; Ferrarini, F.; Bonetto, L.R.; da Silva Crespo, J.; Giovanela, M. Use of low-cost natural waste from the furniture industry for the removal of methylene blue by adsorption: Isotherms, kinetics and thermodynamics. Cellulose 2020, 27, 6445–6466. [Google Scholar] [CrossRef]
- Imron, M.F.; Ananta, A.R.; Ramadhani, I.S.; Kurniawan, S.B.; Abdullah, S.R.S. Potential of Lemna minor for removal of methylene blue in aqueous solution: Kinetics, adsorption mechanism, and degradation pathway. Environ. Technol. Innov. 2021, 24, 101921. [Google Scholar] [CrossRef]
- Shahnaz, T.; Bedadeep, D.; Narayanasamy, S. Investigation of the adsorptive removal of methylene blue using modified nanocellulose. Int. J. Biol. Macromol. 2022, 200, 162–171. [Google Scholar] [CrossRef]
- Belhassen, R.; Vilaseca, F.; Mutjé, P.; Boufi, S. Thermoplasticized starch modified by reactive blending with epoxidized soybean oil. Ind. Crops Prod. 2014, 53, 261–267. [Google Scholar] [CrossRef]
- Yilmaz, E. Compatibilization of polyvinyl chloride-polymethyl methacrylate polymer blends with maleic anhydride-styrene-methyl methacrylate terpolymer. J. Appl. Polym. Sci. 2022, 139, 51745. [Google Scholar] [CrossRef]
- Krolikowski, T.; Knitter, R.; Stachnik, M. Thermo-mechanic tests using 3d printed elements. Procedia Comput. Sci. 2019, 159, 2551–2559. [Google Scholar] [CrossRef]
- Behera, A. Advanced Plastic Materials. In Advanced Materials; Springer: Berlin/Heidelberg, Germany, 2022; pp. 469–506. [Google Scholar]
- Li, M.; Witt, T.; Xie, F.; Warren, F.J.; Halley, P.J.; Gilbert, R.G. Biodegradation of starch films: The roles of molecular and crystalline structure. Carbohydr. Polym. 2015, 122, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Thakore, I.; Desai, S.; Devi, S. Compatibility and biodegradability of PMMA–starch cinnamate blends in various solvents. J. Appl. Polym. Sci. 2001, 79, 488–496. [Google Scholar] [CrossRef]
- Bhat, D.K.; Kumar, M.S. Biodegradability of PMMA blends with some cellulose derivatives. J. Polym. Environ. 2006, 14, 385–392. [Google Scholar] [CrossRef]
- Haroon, M.; Ullah, R.; Mehmood, S.; Haq, F.; Naeemullah. Synthesis and Characterization of Starch-g-Polymethyl Methacrylate and Their Properties as Adsorbents for Removing Rhodamine 6G from Water. J. Polym. Res. 2021, 28, 330. [Google Scholar] [CrossRef]
- Israr, M.; Pugliese, N.; Farid, A.; Ghazanfar, S.; Di Cerbo, A.; Muzammal, M.; Alamri, A.S.; Basheeruddin Asdaq, S.M.; Ahmad, A.; Khan, K.A. Preparation and Characterization of Controlled-Release Floating Bilayer Tablets of Esomeprazole and Clarithromycin. Molecules 2022, 27, 3242. [Google Scholar] [CrossRef]
- Shen, Q.; Xu, M.H.; Wu, T.; Pan, G.X.; Tang, P.S. Adsorption Behavior of Tetracycline on Carboxymethyl Starch Grafted Magnetic Bentonite. Chem. Pap. 2022, 76, 123–135. [Google Scholar] [CrossRef]
- Varkey, V.; Chandran, A.R.; Jose, E.T.; Paul, I.; Jose, G. Fabrication of Photoluminescent Electrospun Poly(Styrene-Co-Methyl Methacrylate) Nanofibers Integrated with LaPO4:Eu3+for Optical Applications. Mater. Today Proc. 2021, 47, 921–926. [Google Scholar] [CrossRef]
- Haroon, M.; Yu, H.; Wang, L.; Ullah, R.S.; Haq, F.; Teng, L. Synthesis and Characterization of Carboxymethyl Starch-g-Polyacrylic Acids and Their Properties as Adsorbents for Ammonia and Phenol. Int. J. Biol. Macromol. 2019, 138, 349–358. [Google Scholar] [CrossRef]
- Chen, Q.; Yu, H.; Wang, L.; Abdin, Z.U.; Yang, X.; Wang, J.; Zhou, W.; Zhang, H.; Chen, X. Synthesis and Characterization of Amylose Grafted Poly(Acrylic Acid) and Its Application in Ammonia Adsorption. Carbohydr. Polym. 2016, 153, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Farivar, F.; Yap, P.L.; Hassan, K.; Tung, T.T.; Tran, D.N.H.; Pollard, A.J.; Losic, D. Unlocking Thermogravimetric Analysis (TGA) in the Fight against “Fake Graphene” Materials. Carbon 2021, 179, 505–513. [Google Scholar] [CrossRef]
- Siyamak, S.; Laycock, B.; Luckman, P. Synthesis of Starch Graft-Copolymers via Reactive Extrusion: Process Development and Structural Analysis. Carbohydr. Polym. 2020, 227, 115066. [Google Scholar] [CrossRef] [PubMed]
- Alhashem, Y.N.; Farid, A.; Al Mohaini, M.; Muzammal, M.; Khan, M.H.; Dadrasnia, A. Protein Isolation and Separation Techniques of Pasteurella multocidavia One-and Two-Dimensional Gel Electrophoresis. Int. J. Cur. Res. Rev 2022, 14, 1–8. [Google Scholar] [CrossRef]
- Haq, F.; Yu, H.; Wang, L.; Teng, L.; Mehmood, S.; Haroon, M.; Fahad, S.; Uddin, M.A.; Shen, D. Synthesis of carboxymethyl starch grafted polyvinyl imidazole (CMS-g-PVIs) and their role as an absorbent for the removal of phenol. Environ. Eng. Res. 2021, 26, 200327. [Google Scholar] [CrossRef]
- Aziz, T.; Farid, A.; Haq, F.; Kiran, M.; Ullah, A.; Zhang, K.; Li, C.; Ghazanfar, S.; Sun, H.; Ullah, R.; et al. A Review on the Modification of Cellulose and Its Applications. Polymers 2022, 14, 3206. [Google Scholar] [CrossRef]
- Han, R.; Wang, Y.; Han, P.; Shi, J.; Yang, J.; Lu, Y. Removal of methylene blue from aqueous solution by chaff in batch mode. J. Hazard. Mater. 2006, 137, 550–557. [Google Scholar] [CrossRef]
- Yang, S.T.; Chen, S.; Chang, Y.; Cao, A.; Liu, Y.; Wang, H. Removal of methylene blue from aqueous solution by graphene oxide. J. Colloid Interface Sci. 2011, 359, 24–29. [Google Scholar] [CrossRef]
- Pathania, D.; Sharma, S.; Singh, P. Removal of methylene blue by adsorption onto activated carbon developed from Ficus carica bast. Arab. J. Chem. 2017, 10, S1445–S1451. [Google Scholar] [CrossRef]
- Abid, R.; Ghazanfar, S.; Farid, A.; Sulaman, S.M.; Idrees, M.; Amen, R.A.; Muzammal, M.; Shahzad, M.K.; Mohamed, M.O.; Khaled, A.A.; et al. Pharmacological Properties of 4′,5,7-Trihydroxyflavone (Apigenin) and Its Impact on Cell SignalingPathways. Molecules 2022, 27, 4304. [Google Scholar] [CrossRef] [PubMed]
- Laaziz, A.; Kouda, I.; Barhoun, A.; Draoui, K. Kinetic, isotherm and thermodynamic study of methyl orange adsorption on raw clay from north of Morocco. J. Environ. Treat. Tech. 2021, 9, 675–685. [Google Scholar] [CrossRef]
- Alouache, A.; Selatnia, A.; Sayah, H.E.; Khodja, M.; Moussous, S.; Daoud, N. Biosorption of Hexavalent Chromium and Congo Red Dye onto Pleurotus Mutilus Biomass in Aqueous Solutions. Int. J. Environ. Sci. Technol. 2022, 19, 2477–2492. [Google Scholar] [CrossRef]
- Khan, K.A.; Zizzadoro, C.; Di Cerbo, A.; Pugliese, N.; Khan, G.M.; Ghazanfar, S.; Almusalami, E.M.; Muzammal, M.; Alsalman, K.J.; Farid, A. Preparation and In Vitro Evaluation of Controlled-Release Matrices of Losartan Potassium Using Ethocel Grade 10 and Carbopol 934P NF as Rate-Controlling Polymers. Polymers 2022, 14, 2993. [Google Scholar] [CrossRef] [PubMed]
- Belhadri, M.; Sassi, M.; Bengueddach, A. Preparation of Economical and Environmentaly Friendly Modified Clay and Its Application for Copper Removal. J. Water Chem. Technol. 2019, 41, 357–362. [Google Scholar] [CrossRef]
- Pal, A.; Pal, S. Amphiphilic copolymer derived from tamarind gum and poly (methyl methacrylate) via ATRP towards selective removal of toxic dyes. Carbohydr. Polym. 2017, 160, 1–8. [Google Scholar] [CrossRef]
- Wen, Y.; Xie, Z.; Xue, S.; Li, W.; Ye, H.; Shi, W.; Liu, Y. Functionalized polymethyl methacrylate-modified dialdehyde guar gum containing hydrazide groups for effective removal and enrichment of dyes, ion, and oil/water separation. J. Hazard. Mater. 2022, 426, 127799. [Google Scholar] [CrossRef]





| (a) Kinetic Parameters | ||
|---|---|---|
| Order of reaction | Parameters | MB dye |
| Pseudo first order | qe (mg/g) | 1.142 |
| K1 (min−1) | −0.00065 | |
| R2 | 0.86 | |
| Pseudo second order | ||
| qe,cal (mg/g) | 5.32 | |
| K2 (g mg−1·min−1) | 0.302 | |
| R2 | 0.99 | |
| (b) Isotherm Parameters | ||
| Types of isotherm | Parameters | MB dye |
| Langmuir | qmax (mg/g) | 16.28 |
| KL (g mg−1 min−1) RL | 0.867 0.081 | |
| Freundlich | R2 Kf 1/n R2 | 0.92 7.95 −0.70 0.87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yasmeen, U.; Haq, F.; Kiran, M.; Farid, A.; Ullah, N.; Aziz, T.; Haroon, M.; Mehmood, S.; Muzammal, M.; Ghazanfar, S.; et al. Synthesis of Starch-Grafted Polymethyl Methacrylate via Free Radical Polymerization Reaction and Its Application for the Uptake of Methylene Blue. Molecules 2022, 27, 5844. https://doi.org/10.3390/molecules27185844
Yasmeen U, Haq F, Kiran M, Farid A, Ullah N, Aziz T, Haroon M, Mehmood S, Muzammal M, Ghazanfar S, et al. Synthesis of Starch-Grafted Polymethyl Methacrylate via Free Radical Polymerization Reaction and Its Application for the Uptake of Methylene Blue. Molecules. 2022; 27(18):5844. https://doi.org/10.3390/molecules27185844
Chicago/Turabian StyleYasmeen, Uzma, Fazal Haq, Mehwish Kiran, Arshad Farid, Naveed Ullah, Tariq Aziz, Muhammad Haroon, Sahid Mehmood, Muhammad Muzammal, Shakira Ghazanfar, and et al. 2022. "Synthesis of Starch-Grafted Polymethyl Methacrylate via Free Radical Polymerization Reaction and Its Application for the Uptake of Methylene Blue" Molecules 27, no. 18: 5844. https://doi.org/10.3390/molecules27185844
APA StyleYasmeen, U., Haq, F., Kiran, M., Farid, A., Ullah, N., Aziz, T., Haroon, M., Mehmood, S., Muzammal, M., Ghazanfar, S., Alhomrani, M., Alamri, A. S., Asdaq, S. M. B., Alghamdi, S. A., & Ullah, I. (2022). Synthesis of Starch-Grafted Polymethyl Methacrylate via Free Radical Polymerization Reaction and Its Application for the Uptake of Methylene Blue. Molecules, 27(18), 5844. https://doi.org/10.3390/molecules27185844







